Abstract
Background:
An increase in airway caliber (airway distensibility) with lung inflation is attenuated in COPD. Furthermore, some subjects have a decrease in airway caliber with lung inflation. We aimed to test the hypothesis that airway caliber increases are lower in subjects with emphysema-predominant (EP) compared with airway-predominant (AP) CT scan subtypes. Additionally, we compared clinical and CT scan features of subjects with (airway constrictors) and without a decrease in airway caliber.
Methods:
Based on GOLD (Global Initiative for Chronic Obstructive Lung Disease) stages and CT scan subtypes, we created a control group (n = 46) and the following matched COPD groups (n = 23 each): GOLD-2-AP, GOLD-2-EP, GOLD-4-AP, and GOLD-4-EP. From the CT scans of all 138 subjects, we measured emphysema, lung volumes, and caliber changes in the third and fourth airway generations of two bronchi. We expressed airway distensibility (ratio of airway lumen diameter change to lung volume change from end tidal breathing to full inspiration) as a global or lobar measure based on normalization by whole-lung or lobar volume changes.
Results:
Global distensibility in the third and fourth airway generations was significantly lower in the GOLD-2-EP and GOLD-4-EP groups than in control subjects. In GOLD-2 subjects, lobar distensibility of the right-upper-lobe fourth airway generation was significantly lower in those with EP than in those with AP. In multivariate analysis, emphysema was an independent determinant of global and lobar airway distensibility. Compared with nonconstrictors, airway constrictors experienced more dyspnea, were more hyperinflated, and had a higher percentage of emphysema.
Conclusions:
Distensibility of large- to medium-sized airways is reduced in subjects with an EP CT scan subtype. Emphysema seems to alter airway-parenchyma interdependence.
Trial registry:
ClinicalTrials.gov; No.: NCT00608764; URL: www.clinicaltrials.gov
A defining characteristic of COPD is expiratory airflow limitation due to intrinsic remodeling of the small airways and their dynamic collapse during forced exhalation.1 In a normal lung, inflation results in a predictable increase in airway caliber because of the interdependence of parenchyma and airways (the relative change in airway diameter is linearly related with the cube root of lung volume).2 Emphysema alters this relationship by disrupting airway-parenchymal interdependence. Early work in small animals demonstrated that methacholine-induced bronchoconstriction was increased in elastase models of emphysema,3 suggesting that the bronchoconstrictive effect of airway smooth muscle activation is opposed by the radial traction of the surrounding parenchyma. Scichilone et al4 since have substantiated this observation in lung tissue from subjects with COPD. They showed that the loss of alveolar attachments to airway walls is associated with a decrease in bronchodilatory response to deep inspiration. These data suggest that the burden and possibly the distribution of emphysema in subjects with COPD may influence airway dilation during lung inflation.
CT scanning is a useful tool to assess airway caliber changes.5,6 Furthermore, CT scanning is a recognized technique to assess the presence, extent, and location (ie, lobar level) of emphysema.7 CT scan can be used to classify subjects with COPD into emphysema-predominant (EP) or airway-predominant (AP) subtypes.8,9 For the present investigation, we assumed that parenchymal destruction and disruption of normal tissue interdependence is represented by the EP subtype, whereas intrinsic narrowing of the airways due to inflammation and fibrosis is represented by the AP subtype. We then used these CT scan subtypes to evaluate the effect of structural changes of the lung parenchyma on the ability of the airways to dilate with lung inflation. It is known that the ability of the airways to dilate is attenuated in more severe stages of COPD.10,11 We hypothesized that within a given disease stage, we would observe less dilation of the airways in the EP subtype than in the AP subtype. We refer to the airway caliber change with lung inflation as airway distensibility. More extreme than reduced airway distensibility are airways that paradoxically narrow in lumen size with inflation.12 We also sought to describe clinical and CT scan features of subjects who experienced this decrease in airway caliber. Finally, because emphysema may be unevenly distributed throughout the lungs, we also reasoned that the ability of the airways to dilate may vary according to regional emphysema severity. Better understanding of both emphysema and airway behavior at a regional level may help with understanding structure-function relationships and their heterogeneity. To carry out this investigation, we used data from the COPDGene Study,13 which included volumetric chest CT scans performed at relaxed exhalation and full inflation.
Materials and Methods
Further details of this section are provided in e-Appendix 1.
Study Population
We selected subjects from the COPDGene13 (www.copdgene.org) study based on GOLD (Global Initiative for Chronic Obstructive Lung Disease) stages14 and CT scan subtypes (AP or EP if percent emphysema on CT scan was < 13% or ≥ 25%, respectively)9 to create a control group (n = 46) and the following four matched COPD groups (n = 23 each): GOLD-2-AP, GOLD-2-EP, GOLD-4-AP, and GOLD-4-EP. COPDGene subjects were evaluated in one to two visits, with questionnaires and pulmonary function tests usually done before scanning. Based on prior data showing an association between airway distensibility measured by forced oscillation technique and emphysema after bronchodilation with albuterol,15 we analyzed data in a subset of 73 subjects who had CT imaging performed within 3 h of albuterol inhalation. We called this group the postbronchodilator cohort. COPDGene was approved by the institutional review board at each participating center, and all subjects provided written informed consent. The current analysis was approved by the Partners HealthCare Research Committee (2007P-000554).
Clinical and Physiologic Assessments
Demographic and clinical history data, including dyspnea, spirometry, and 6-min walk test, were collected with standardized instruments.16,17
Airway Distensibility
We expressed airway distensibility as the ratio of absolute change in airway inner diameter to the cube root of absolute change in lung volume from relaxed exhalation to full inflation, based on Wilson et al.2 We calculated this ratio with whole-lung and lobar CT scan measures of volume to obtain global and lobar airway distensibility. A higher ratio is consistent with greater airway distensibility.
CT Scan Examination
All subjects underwent volumetric CT scanning without IV contrast in the supine position at the end of both full inspiration and relaxed exhalation (herein referred to as end tidal breathing). Subjects were given standardized, verbal instructions to take a deep breath and then to exhale in a relaxed manner. Image acquisition parameters by scanner are described in e-Table 1.
CT Scan Airway Analysis
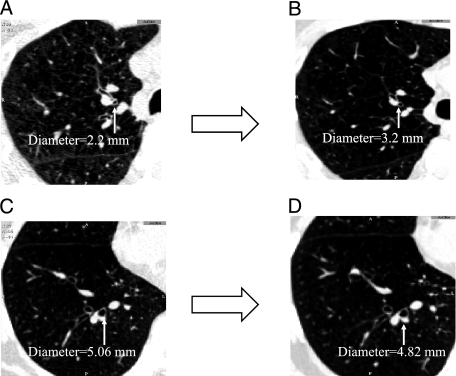
Airway analysis was performed with Airway Inspector (www.airwayinspector.org).18 The third and fourth airway generations (AGs) of the right-upper-lobe (RUL) apical bronchus and the right-lower-lobe (RLL) posterior basal bronchus were identified, matched, and measured on paired inspiratory (at full inspiration) and expiratory (at end tidal breathing) CT scans (Fig 1). In each AG, lumen radius (Ri), lumen area (Ai), and total area (Ao) were measured at three locations and averaged. Lumen diameter (= Ri × 2) and wall area (WA) % (WA% = ([(Ao − Ai)/Ao)] × 100) were calculated. An airway constrictor (AC) was defined as a subject who had a decrease or lack of increase in airway lumen diameter from end tidal breathing to full inspiration in at least one AG in the RUL or RLL.12
Figure 1.
A-D, Volumetric CT scan slices showing changes in airway lumen from end tidal breathing (A, C) to full inspiration (B, D). Measures of matched airways show an increase in airway lumen (A→B) in the fourth airway generation of the right-upper-lobe apical bronchus and a decrease (C→D) in the third airway generation of the right-lower-lobe posterior basal bronchus.
CT Scan Measures of Lung Volumes and Emphysema
Quantitative measures of lung volume and emphysema for the whole lung were performed using Airway Inspector software.19 The total volume of the lung at suspended full inspiration and expiration was measured and expressed as total lung capacity (TLC) % predicted and functional residual capacity (FRC) % predicted.20 Whole-lung emphysema on CT scan (hereafter referred to as emphysema) was calculated as the percentage of voxels with attenuation area < −950 Hounsfield units on inspiratory CT scans.21
Statistical Analysis
Analyses were performed using SAS version 9.2 (SAS Institute, Inc) statistical software. Airway distensibility across groups was compared using analysis of variance with adjustment for multiple comparisons. Clinical and CT scan data were compared between ACs and non-ACs using parametric tests. Pairwise correlation and linear regression analysis were used to assess the relationship between global or lobar airway distensibility and emphysema. Multivariate linear regression was performed to examine determinants of distensibility in the fourth AG. This outcome was chosen because correlation between the fourth AG and emphysema was higher than between the third AG and emphysema. Emphysema was the primary predictor of interest; other covariates were chosen on the basis of previous data.22‐24 Finally, this multivariate analysis was repeated in the postbronchodilator cohort.
Results
Population Description
Baseline characteristics and CT scan data by study group are shown in Table 1. Respiratory medications, CT scan lobar data, and the distribution of airway inner diameter sizes at full inspiration by group are shown in e-Table 2, e-Table 3, and e-Figure 1, respectively. Compared with control subjects, the dyspnea score was significantly higher for each COPD group. As expected, FRC % predicted, TLC % predicted, and emphysema were significantly higher in both the GOLD-2-EP and the GOLD-4-EP groups than in the control subjects. The 6-min walk test only was lower in the GOLD-4 groups than in the control subjects.
Table 1.
—Demographic, Clinical, Physiologic, and CT Scan Data by Study Group
| Characteristic | Control Group (n = 46) | GOLD-2-AP (n = 23) | GOLD-2-EP(n = 23) | GOLD-4-AP(n = 23) | GOLD-4-EP(n = 23) |
| Age, y | 61 ± 9 | 61 ± 9 | 65 ± 7 | 62 ± 9 | 63 ± 8 |
| Male sex | 29 (63) | 14 (61) | 14 (61) | 14 (61) | 14 (61) |
| White race | 35 (76) | 18 (78) | 20 (87) | 14 (61) | 19 (83) |
| BMI, kg/m2 | 29 ± 4 | 29 ± 7 | 25 ± 3a | 30 ± 6 | 23 ± 5a,b |
| Smoking history, pack-y | 41 ± 25 | 46 ± 24 | 50 ± 27 | 56 ± 38 | 57 ± 30 |
| Current smoking status | 17 (37) | 10 (43) | 4 (17) | 7 (30) | 3 (13) |
| mMRC dyspnea score | 1 ± 1 | 2 ± 1a | 2 ± 1a | 3 ± 1a | 3 ± 1a |
| FEV1 % predicted | 98 ± 12 | 65 ± 7a | 60 ± 9a | 24 ± 4a | 20 ± 5a |
| FVC % predicted | 97 ± 13 | 81 ± 10a | 95 ± 9 | 55 ± 13a | 54 ± 16a |
| FEV1/FVC ratio | 0.77 ± 0.1 | 0.62 ± 0.1a | 0.48 ± 0.1a,b | 0.34 ± 0.1a | 0.29 ± 0.1a,b |
| FRC % predicted | 89 ± 21 | 104 ± 28 | 122 ± 32a | 149 ± 24a | 178 ± 23a,b |
| TLC % predicted | 92 ± 13 | 87 ± 13 | 112 ± 16a,b | 106 ± 10a | 123 ± 13a,b |
| 6-min walk test, m | 452 ± 108 | 369 ± 139 | 398 ± 123 | 274 ± 128a | 241 ± 113a |
| Emphysema, % | 1 ± 1 | 2 ± 1 | 29 ± 3a,b | 7 ± 4a | 40 ± 6a,b |
| Third AG lumen diameter at end tidal breathing, mm | 3.71 ± 1.12 | 3.76 ± 0.81 | 3.96 ± 0.73 | 3.41 ± 0.99 | 3.97 ± 1.02 |
| Fourth AG lumen diameter at end tidal breathing, mm | 2.72 ± 0.66 | 2.85 ± 0.57 | 2.88 ± 0.63 | 2.64 ± 0.59 | 3.13 ± 0.73 |
| Third AG WA% at end tidal breathing, % | 68 ± 8 | 67 ± 7 | 70 ± 5 | 70 ± 8 | 65 ± 7 |
| Fourth AG WA% at end tidal breathing, % | 76 ± 6 | 74 ± 5 | 77 ± 5 | 76 ± 5 | 71 ± 6a,b |
Data are presented as mean ± SD or No. (%). AG = airway generation; AP = airway-predominant CT scan subtype; EP = emphysema-predominant CT scan subtype; FRC = functional residual capacity; GOLD = Global Initiative for Chronic Obstructive Lung Disease; mMRC = modified Medical Research Council; TLC = total lung capacity; WA = wall area.
P < .05 vs control group.
P < .05 vs AP CT scan subtype within a GOLD stage.
Airway Distensibility by Groups
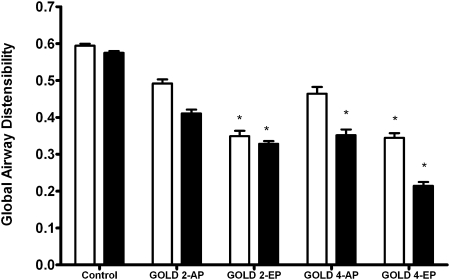
Global airway distensibility across groups is shown in Figure 2. Compared with control subjects, there was a significant decrease in global airway distensibility of the third and fourth AG in both GOLD-2-EP and GOLD-4-EP groups. For a given GOLD stage, global airway distensibility in the third and fourth AG tended to be lower in EP than in AP subjects, but this relationship did not reach statistical significance. Comparable results were obtained when we used predicted values of FRC and TLC instead measured values (e-Figure 2).
Figure 2.
Global airway distensibility (mean ± SEM) of the third (open bars) and the fourth (solid bars) airway generations from end tidal breathing to full inspiration in smoker control subjects and COPD groups based on GOLD stages and CT scan subtypes (AP and EP) is shown. (Global airway distensibility is defined in the Methods section.) *P < .05 vs control subjects. AP = airway-predominant CT scan subtype; EP = emphysema-predominant CT scan subtype; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
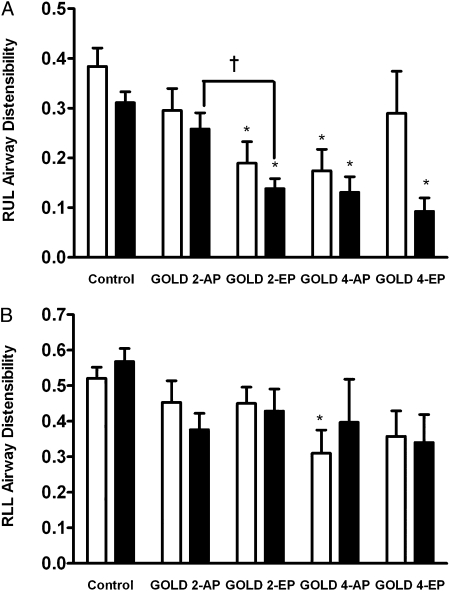
Lobar airway distensibility across groups is shown in Figure 3. Compared with control subjects, RUL airway distensibility in the fourth AG was significantly lower in both EP groups (Fig 3A). Among the subjects in the GOLD-2 groups, lobar airway distensibility in the fourth AG was lower in EP than in AP subjects (mean ± SEM, 0.14 ± 0.02 vs 0.26 ± 0.02; P = .03) (Fig 3A). In contrast, in the RLL, there were no significant differences between groups in airway distensibility except in the third AG, where distensibility was lower in the GOLD-4-AP group than in the control subjects (Fig 3B).
Figure 3.
Lobar airway distensibility of the RUL and RLL in smoker control subjects and COPD groups based on GOLD stages and CT scan subtypes (AP and EP). A, RUL. B, RLL. Mean ± SEM lobar airway distensibility of the third (open bars) and fourth (solid bars) airway generations from end tidal breathing to full inspiration is shown. *P < .05 vs control subjects. †P < .05 vs AP. RLL = right lower lobe; RUL = right upper lobe. See Figure 2 legend for expansion of other abbreviations.
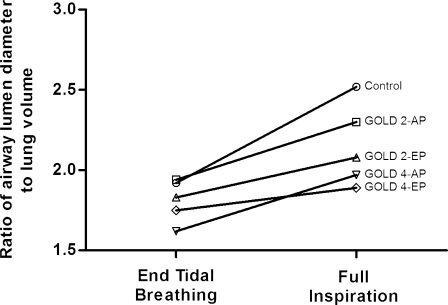
In addition to the analysis provided in earlier figures, we sought to further depict the potential influence of both end tidal and full inflation lung volume on static airway diameter. In Figure 4, we provide ratios for airway diameter at end tidal and full inflation divided by the cube root of lung volume at these expiratory and inspiratory points, respectively. Although there was no statistically significant difference in this ratio at end tidal lung volume, subjects with more severe disease (higher GOLD stage) had diminished airway diameter-to-lung volume ratio at full inflation.
Figure 4.
Ratio of airway lumen diameter to cube root of lung volume at end tidal breathing and at full inspiration across smoker control subjects and study COPD groups (AP and EP). Mean ratio for the fourth airway generation at end tidal breathing and full inspiration for each group is shown. All the groups had similar ratios at end tidal breathing. At full inspiration, however, there was a clear trend toward decreasing ratio with increasing GOLD stage. See Figure 2 legend for expansion of abbreviations.
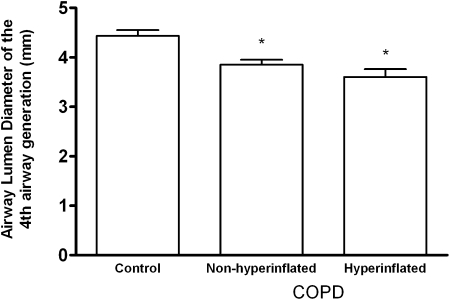
To evaluate whether the change in airway caliber depended on hyperinflation, we divided the entire cohort into three groups as follows: control, nonhyperinflated, and hyperinflated. The presence of hyperinflation was defined as a TLC > 120% predicted.25 Figure 5 shows a significant trend toward decreasing airway caliber of the fourth AG with increasing TLC % predicted.
Figure 5.
Airway lumen diameters (mean ± SEM) of the fourth airway generation at full inspiration for control subjects and subjects with COPD with and without hyperinflation. Total lung capacity % predicted was 92%, 99%, and 131% for control, nonhyperinflated, and hyperinflated groups, respectively. There was a significant trend toward lower airway diameter with increasing total lung capacity % predicted. *P < .001 vs control subjects.
Relationships Between Airway Distensibility and Emphysema
There was a significant inverse association between global airway distensibility and whole-lung emphysema (third AG, r = −0.28; P = .001; fourth AG, r = −0.40; P < .0001). Results were similar at lobar level (e-Table 4). The association between distensibility of the fourth AG and emphysema remained significant after adjustment for age, male sex, BMI, and pack-years of smoking (Table 2). In this model, BMI also was associated with global fourth AG distensibility (β = 0.01, P = .02) and with lobar airway distensibility in the RLL (β = 0.02, P = .002) but not in the RUL (P = .17). WA% of the fourth AG at end tidal breathing was directly associated with airway distensibility. Multivariate regression results were comparable in the postbronchodilator group (e-Table 5). We also explored the determinants of lobar airway distensibility in the fourth AG for the RUL and RLL using the lobar measures of volume and emphysema. In these models, emphysema was the determinant of distensibility in the RUL and WA% in the RLL (Table 2). These analyses in the postbronchodilator group showed similar results for the RUL airway distensibility, but the association between WA% and distensibility did not reach significance in the RLL (e-Table 5).
Table 2.
—Multivariate Linear Regression Models for Global and Lobar Airway Distensibility of the Fourth AG
| Model | Parameter Estimate | 95% CI | P Value |
| Model 1: global airway distensibility | |||
| Whole-lung emphysema, % | −0.005 | −0.008 to −0.002 | .001 |
| WA% of the fourth AG at end tidal breathing, % | 0.010 | 0.003 to 0.02 | .005 |
| Model 2: RUL airway distensibility | |||
| RUL emphysema (%) | −0.003 | −0.005 to −0.001 | .0001 |
| WA% of the fourth AG of the RUL apical bronchus at end tidal breathing, % | 0.003 | −0.002 to 0.007 | .22 |
| Model 3: RLL airway distensibility | |||
| RLL emphysema, % | −0.002 | −0.005 to 0.002 | .42 |
| WA% of the fourth AG of the RLL posterior basal bronchus at end tidal breathing, % | 0.010 | 0.003 to 0.02 | .007 |
Models were adjusted for age, sex, BMI, and pack-years of smoking. R2 was 0.32, 0.27, and 0.24 for models 1, 2, and 3, respectively. RLL = right lower lobe; RUL = right upper lobe. See Table 1 for expansion of other abbreviations.
ACs vs non-ACs
Twenty-three percent of subjects in the entire cohort were ACs. In each group, the percentage of ACs was as follows: control, 7%; GOLD-2-AP, 22%; GOLD-2-EP, 30%; GOLD-4-AP, 39%; and GOLD-4-EP, 35%. A comparison of demographic, clinical, physiologic, and CT scan emphysema data between ACs and non-ACs is shown in Table 3.
Table 3.
—Demographic, Clinical, Physiologic, and CT Scan Data for Airway Constrictors and Non-Airway Constrictors
| Characteristic | Non-Airway Constrictors (n = 106) | Airway Constrictors (n = 32) | P Value |
| Age, y | 62 ± 8 | 62 ± 10 | .8 |
| Male sex | 68 (64) | 17 (53) | .26 |
| White race | 90 (85) | 19 (59) | .002 |
| BMI, k/m2 | 28 ± 6 | 24 ± 4 | .0001 |
| Smoking history, pack-y | 48 ± 27 | 51 ± 34 | .63 |
| Current smoking status | 26 (25) | 15 (47) | .02 |
| mMRC dyspnea score | 2 ± 2 | 3 ± 1 | .01 |
| FEV1 % predicted | 66 ± 33 | 43 ± 25 | .0003 |
| FVC % predicted | 82 ± 22 | 71 ± 22 | .01 |
| FEV1/FVC ratio | 0.6 ± 0.2 | 0.4 ± 0.2 | .0006 |
| FRC % predicted | 112 ± 36 | 152 ± 39 | < .001 |
| TLC % predicted | 99 ± 18 | 108 ± 16 | .03 |
| 6-min walk test, m | 383 ± 139 | 302 ± 144 | .005 |
| Emphysema, % | 12 ± 15 | 19 ± 16 | .02 |
| WA% of third AG at end tidal breathing, % | 69 ± 7 | 65 ± 8 | .008 |
| WA% of fourth AG at end tidal breathing, % | 76 ± 5 | 72 ± 7 | .01 |
Data are presented as mean ± SD or No. (%). See Table 1 legend for expansion of abbreviations.
Discussion
In the present study, we found that the change in airway caliber from end tidal breathing to full inspiration was lower in subjects with moderate or severe EP vs AP COPD. Additionally, we found that airway distensibility remained inversely associated with the extent of emphysema after adjusting for relevant covariates. Finally, we found that paradoxical airway constriction with lung inflation was common and that these ACs had distinct clinical and CT scan features compared with non-ACs.
Airway Distensibility Across Groups
We observed that airway distensibility tended to be diminished in more severe disease and in the EP disease. As published previously, we believe that this decrease in distensibility is due to a combination of intrinsic remodeling of the airway walls and progressive parenchymal destruction.11,15 We extended this knowledge by demonstrating that the association between emphysema and global airway distensibility remained after adjustment for relevant covariates, such age and sex. We believe that this adjustment is important because studies have shown differences in airway diameter changes with lung inflation by sex and age in healthy lungs24 and by age in diseased lungs.23 We also observed that airway distensibility varied by lobe. Airway caliber changes tended to be higher in the RLL than in the RUL regardless of AG and study group. We also found differences in distensibility in the fourth AG of the RUL between the two CT scan subtypes in subjects with moderate and very severe airflow obstruction (Fig 3A). There were not, however, similar differences between CT scan subtypes in the RLL, where there was less emphysema at all GOLD stages (e-Table 1). Prior studies have found that emphysema affects the upper-lung zones more severely than the lower ones.26,27 Although part of the present observation may be attributed to lesser degrees of emphysema in the lower lobes, there also may be differences between the two lobes in the proximity of emphysematous destruction to the measured airways.
Hyperinflation appears to reduce airway caliber. Data supporting this effect can be seen in Figure 5, where the lowest airway caliber was observed among subjects with the most hyperinflated lungs. Clinically, hyperinflation has been suggested as one of the potential mechanisms explaining the impaired bronchodilatory effect of deep inspiration in patients with asthma.22 Unlike patients with asthma, subjects with COPD may have both intrinsic airway disease and reduced lung elastic recoil. Without knowledge of transpulmonary pressure in the present study, it is difficult to ascertain the relative contribution of the latter to airway caliber.
Airway wall properties also play a role in airway distensibility in COPD. In fact, Baldi et al15 demonstrated the relationship between emphysema and distensibility only after albuterol administration. Similarly, we validated this association in the postbronchodilator cohort (e-Table 3). If we assume that the airway tone is removed in this subset of subjects, the changes in airway caliber with lung inflation would be determined by properties of both the parenchyma and the airway wall. We observed significant decrements in airway distensibility of GOLD-2-AP and GOLD-4-AP subjects compared with control subjects with similar amounts of emphysema (Fig 2). Although this may in part indicate that CT scan is not a sensitive measure of the parenchymal properties of emphysema, it may suggest that wall structure (wall thickening, fibrosis, stiffening of noncontractile elements such as collagen, and reduced tidal stretching of airway smooth muscle) also determines airway caliber at full inflation. WA% at end tidal breathing was directly associated with airway distensibility. Although WA% of the surveyed airways at end tidal breathing may not be a direct reflection of mural remodeling, we believe that a thicker airway wall may indicate compacted mural tissue at low lung volume. The more compacted or excess tissue evident in the airway wall at end tidal breathing, the more potential to dilate with lung inflation. Alternatively, as other authors have suggested, a thicker airway may be protective against early airway collapse and hyperinflation.22 Further investigation regarding this observation is needed.
An interesting finding in the present study was the observed association between BMI and airway distensibility, suggesting that subjects with greater body size had more distensible airways. The effect of BMI on airway distensibility is independent of emphysema, age, sex, and pack-years of smoking. One explanation for this association may be the effect of BMI on lung volume. Previous research has suggested that there is a relationship between transmural pressure and airway caliber.28 At high pressures (or presumably high lung volumes), airway distensibility decreases. It is possible that the present findings are due to reduced end tidal and maximal inflation volumes on CT scan because of increased BMI. A subject with a greater BMI may start at a lower lung volume and then maximally inflate to a volume that is still within the linear range of airway distension. In comparison, a subject with lower BMI may start at a higher end tidal volume and then maximally inflate to a volume that exceeds the linear range of airway distension. Despite there being changes in volume, the subject with lower BMI may paradoxically exhibit lower airway compliance (or distensibility).
Clinical and CT Scan Features of ACs
The present data confirm the findings of prior studies that demonstrated a paradoxical decrease in airway caliber with lung inflation12 and extend those findings by describing the clinical and CT scan features of ACs. As expected, the frequency of ACs increased with the severity of airflow obstruction. We also found that compared with their counterparts, ACs were more likely to have lower BMI, lower FEV1 % predicted, worse exercise capacity, thinner airways, more hyperinflation, and more emphysema. These characteristics substantiate the notion that airway constriction is a marker of advanced disease.12,29 An explanation of this paradoxical airway behavior is that longitudinal stretching of airways not supported by radial traction is likely to reduce airway caliber with lung inflation.12
Implications
A practical implication of the study findings is that therapy targeting an increase in airway caliber (eg, bronchodilators) may be less effective in subjects with an EP CT scan subtype. This is consistent with prior work by Han et al,30 who observed diminished bronchodilator response in subjects with greater burdens of emphysema.
Limitations
This study has several limitations. First, although we matched subjects based on center and CT scanner brand, there is center-to-center and scanner-to-scanner variability in CT scan measures of the disease. Second, in order to have adequate sample sizes per group, we included subjects with different spirometry-CT scan sequences because the test order was not standardized. Thus, airway distensibility measures were taken from subjects who may or may not have had preceding treatment with albuterol. However, the findings were replicated in a postbronchodilator cohort. Third, it is known that variability in inspiration level affects CT scan measures of volumes and emphysema. To reduce this potential source of variability, standardized, verbal instructions were given to each subject during CT scan acquisition. Finally, we selected proximal AGs based on an anatomic approach, and consequently, the analysis was based on generations rather than on grouping the airways by sizes. Although this approach limits comparison with prior studies, it may control for the effect of airway location on airway-parenchyma interdependence.
Conclusions
The results demonstrate that COPD airway distensibility is lower in subjects with an EP CT scan subtype. The data support the notion that emphysema alters airway-parenchyma interdependence. A reduction in airway caliber with lung inflation is frequent, and subjects with this phenomenon have clinical and CT scan features of advanced disease.
Supplementary Material
Acknowledgments
Author contributions: Dr Washko had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Diaz: contributed to the creation and final approval of the manuscript.
Dr Come: contributed to the creation and final approval of the manuscript.
Mr Ross: contributed to software development and the creation and final approval of the manuscript.
Dr San José Estépar: contributed to software development and the creation and final approval of the manuscript.
Dr Han: contributed to the creation and final approval of the manuscript.
Dr Loring: contributed to the creation and final approval of the manuscript.
Dr Silverman: contributed as co-principal investigator of COPDGene, facilitated data collection, and contributed to the creation and final approval of the manuscript.
Dr Washko: contributed to the creation and final approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: In the past 3 years, Dr Han has performed speaking for Boehringer Ingelheim GmbH, Pfizer Inc, and GlaxoSmithKline Inc. She also has consulted for Novartis AG; Genentech, Inc; GlaxoSmithKline Inc; Pfizer Inc; Boehringer Ingelheim GmbH; and Medimmune, LLC. Dr Silverman received an honorarium for a talk on COPD genetics in 2006, grant support and consulting fees from GlaxoSmithKline plc for two studies of COPD genetics; an honorarium from Bayer Healthcare Pharmaceuticals for a symposium at the European Respiratory Society Meeting in 2005; and honoraria in 2007 and 2008 and consulting fees from AstraZeneca. Drs Diaz, Come, San José Estépar, Loring, and Washko and Mr Ross have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of this study, collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: This work was performed at Brigham and Women’s Hospital.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/141/3/736/suppl/DC1.
Abbreviations
- AC
airway constrictor
- AG
airway generation
- AP
airway-predominant CT scan subtype
- EP
emphysema-predominant CT scan subtype
- FRC
functional respiratory capacity
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- RLL
right lower lobe
- RUL
right upper lobe
- TLC
total lung capacity
- WA
wall area
Footnotes
Funding/Support: This work was supported by the National Institutes of Health [Grants COPDGene, U01HL089897, U01HL089856; Dr Come, T32HL007633-25, U10HL074428-5; Dr San José Estépar, K25HL104085; and Dr Washko, K23HL089353]. Dr Washko also was supported by an award from the Parker B. Francis Foundation.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 2.Wilson AG, Massarella GR, Pride NB. Elastic properties of airways in human lungs post mortem. Am Rev Respir Dis. 1974;110(6):716–729. doi: 10.1164/arrd.1974.110.6P1.716. [DOI] [PubMed] [Google Scholar]
- 3.Bellofiore S, Eidelman DH, Macklem PT, Martin JG. Effects of elastase-induced emphysema on airway responsiveness to methacholine in rats. J Appl Physiol. 1989;66(2):606–612. doi: 10.1152/jappl.1989.66.2.606. [DOI] [PubMed] [Google Scholar]
- 4.Scichilone N, Bruno A, Marchese R, Vignola AM, Togias A, Bellia V. Association between reduced bronchodilatory effect of deep inspiration and loss of alveolar attachments. Respir Res. 2005;6:55. doi: 10.1186/1465-9921-6-55. Respiratory Research Web site. http://respiratory-research.com/content/6/1/55. Accessed January 4, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown RH, Scichilone N, Mudge B, Diemer FB, Permutt S, Togias A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med. 2001;163(4):994–1001. doi: 10.1164/ajrccm.163.4.2007119. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa M, Makita H, Nasuhara Y, et al. Relationship between improved airflow limitation and changes in airway calibre induced by inhaled anticholinergic agents in COPD. Thorax. 2009;64(4):332–338. doi: 10.1136/thx.2008.103671. [DOI] [PubMed] [Google Scholar]
- 7.Coxson HO, Mayo J, Lam S, Santyr G, Parraga G, Sin DD. New and current clinical imaging techniques to study chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(7):588–597. doi: 10.1164/rccm.200901-0159PP. [DOI] [PubMed] [Google Scholar]
- 8.Boschetto P, Miniati M, Miotto D, et al. Predominant emphysema phenotype in chronic obstructive pulmonary. Eur Respir J. 2003;21(3):450–454. doi: 10.1183/09031936.03.00048703. [DOI] [PubMed] [Google Scholar]
- 9.Mishima M, Hirai T, Itoh H, et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 1999;96(16):8829–8834. doi: 10.1073/pnas.96.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scichilone N, Marchese R, Catalano F, Vignola AM, Togias A, Bellia V. Bronchodilatory effect of deep inspiration is absent in subjects with mild COPD. Chest. 2004;125(6):2029–2035. doi: 10.1378/chest.125.6.2029. [DOI] [PubMed] [Google Scholar]
- 11.Scichilone N, La Sala A, Bellia M, et al. The airway response to deep inspirations decreases with COPD severity and is associated with airway distensibility assessed by computed tomography. J Appl Physiol. 2008;105(3):832–838. doi: 10.1152/japplphysiol.01307.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerveri I, Pellegrino R, Dore R, et al. Mechanisms for isolated volume response to a bronchodilator in patients with COPD. J Appl Physiol. 2000;88(6):1989–1995. doi: 10.1152/jappl.2000.88.6.1989. [DOI] [PubMed] [Google Scholar]
- 13.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 15.Baldi S, Dellacà R, Govoni L, et al. Airway distensibility and volume recruitment with lung inflation in COPD. J Appl Physiol. 2010;109(4):1019–1026. doi: 10.1152/japplphysiol.00147.2010. [DOI] [PubMed] [Google Scholar]
- 16.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85(6):751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 18.Diaz AA, Bartholmai B, San José Estépar R, et al. Relationship of emphysema and airway disease assessed by CT to exercise capacity in COPD. Respir Med. 2010;104(8):1145–1151. doi: 10.1016/j.rmed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Washko GR, Hunninghake GM, Fernandez IE, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(10):897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8(3):492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 21.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152(2):653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 22.Pyrgos G, Scichilone N, Togias A, Brown RH. Bronchodilation response to deep inspirations in asthma is dependent on airway distensibility and air trapping. J Appl Physiol. 2011;110(2):472–479. doi: 10.1152/japplphysiol.00603.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scichilone N, Marchese R, Catalano F, Togias A, Vignola AM, Bellia V. The bronchodilatory effect of deep inspiration diminishes with aging. Respir Med. 2004;98(9):838–843. doi: 10.1016/j.rmed.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Prakash UB, Hyatt RE. Static mechanical properties of bronchi in normal excised human lungs. J Appl Physiol. 1978;45(1):45–50. doi: 10.1152/jappl.1978.45.1.45. [DOI] [PubMed] [Google Scholar]
- 25.O’Donnell DE, Laveneziana P. Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev. 2006;15(100):61–67. [Google Scholar]
- 26.Gurney JW, Jones KK, Robbins RA, et al. Regional distribution of emphysema: correlation of high-resolution CT with pulmonary function tests in unselected smokers. Radiology. 1992;183(2):457–463. doi: 10.1148/radiology.183.2.1561350. [DOI] [PubMed] [Google Scholar]
- 27.Sakai N, Mishima M, Nishimura K, Itoh H, Kuno K. An automated method to assess the distribution of low attenuation areas on chest CT scans in chronic pulmonary emphysema patients. Chest. 1994;106(5):1319–1325. doi: 10.1378/chest.106.5.1319. [DOI] [PubMed] [Google Scholar]
- 28.Brown RH, Mitzner W. Effect of lung inflation and airway muscle tone on airway diameter in vivo. J Appl Physiol. 1996;80(5):1581–1588. doi: 10.1152/jappl.1996.80.5.1581. [DOI] [PubMed] [Google Scholar]
- 29.Corsico A, Milanese M, Baraldo S, et al. Small airway morphology and lung function in the transition from normality to chronic airway obstruction. J Appl Physiol. 2003;95(1):441–447. doi: 10.1152/japplphysiol.01018.2002. [DOI] [PubMed] [Google Scholar]
- 30.Han MK, Wise R, Mumford J, et al. NETT Research Group Prevalence and clinical correlates of bronchoreversibility in severe emphysema. Eur Respir J. 2010;35(5):1048–1056. doi: 10.1183/09031936.00052509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.