Abstract
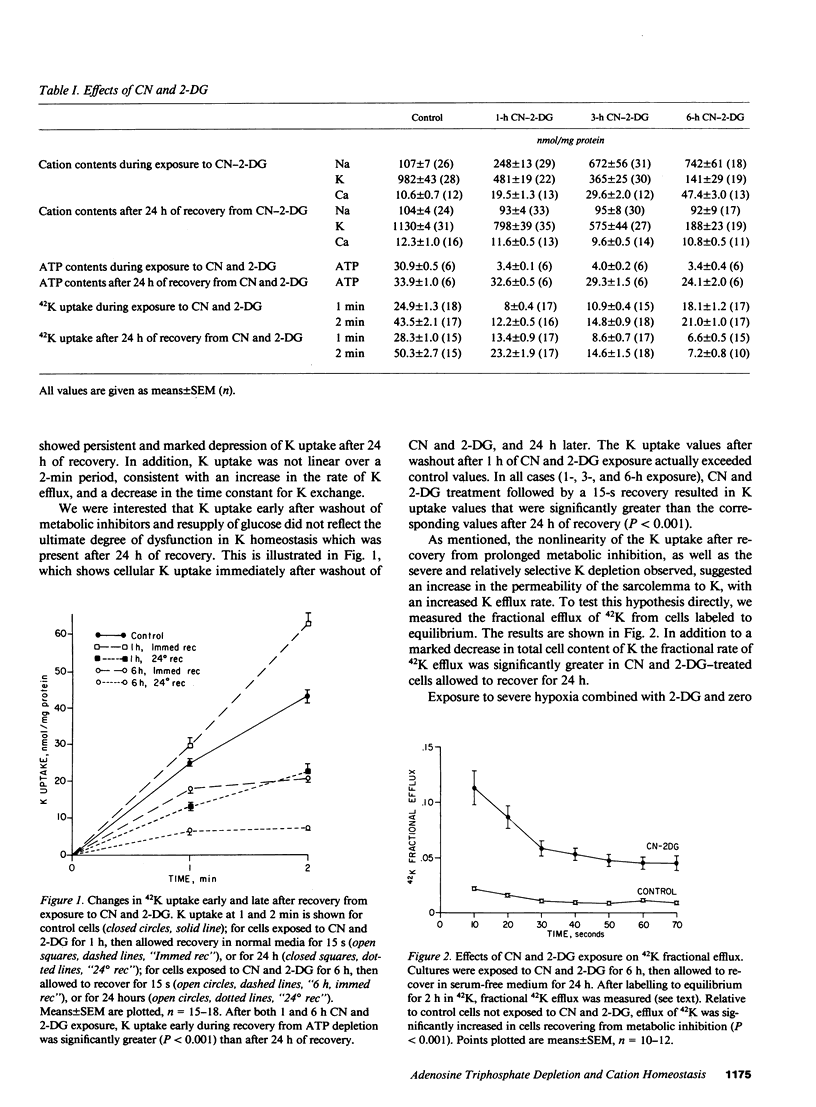
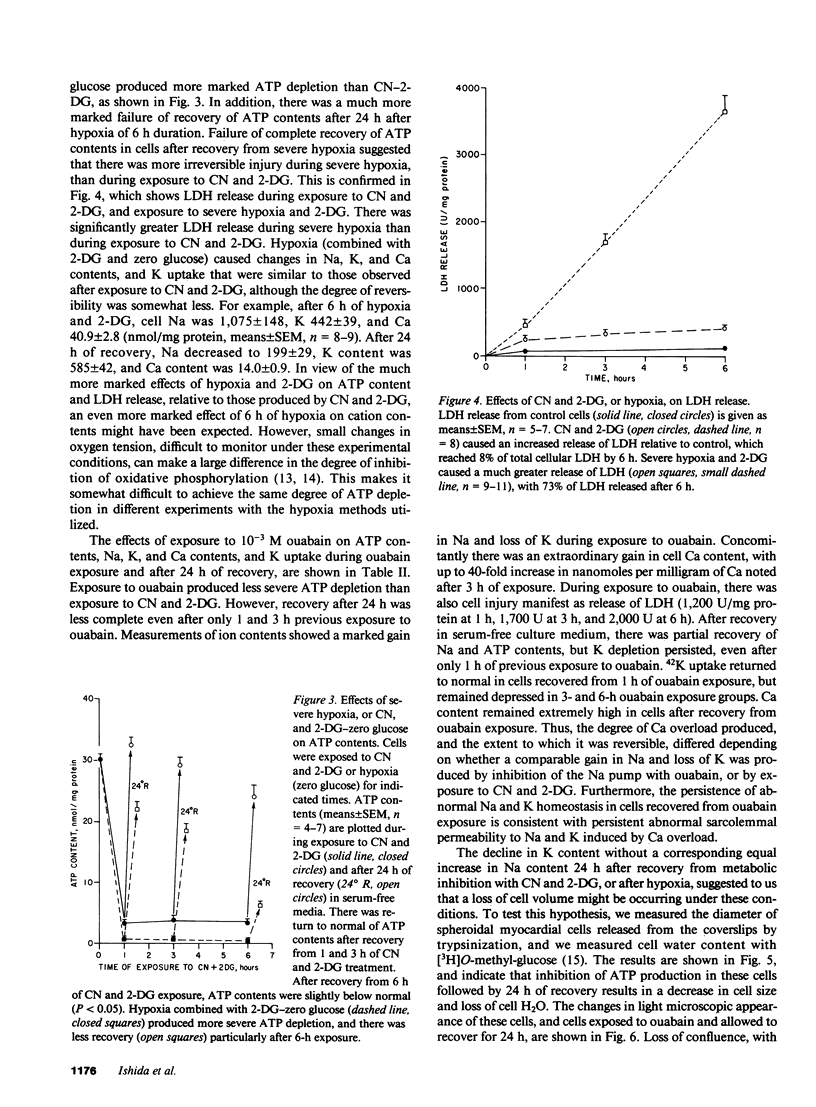
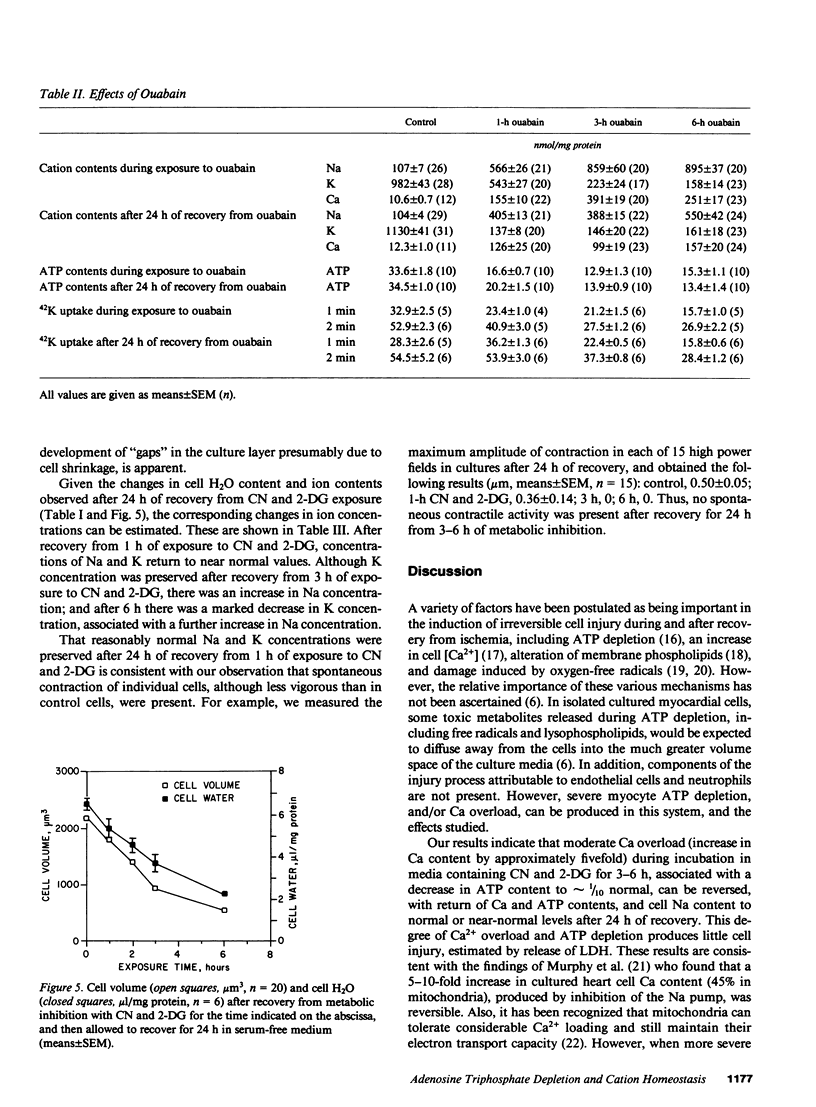
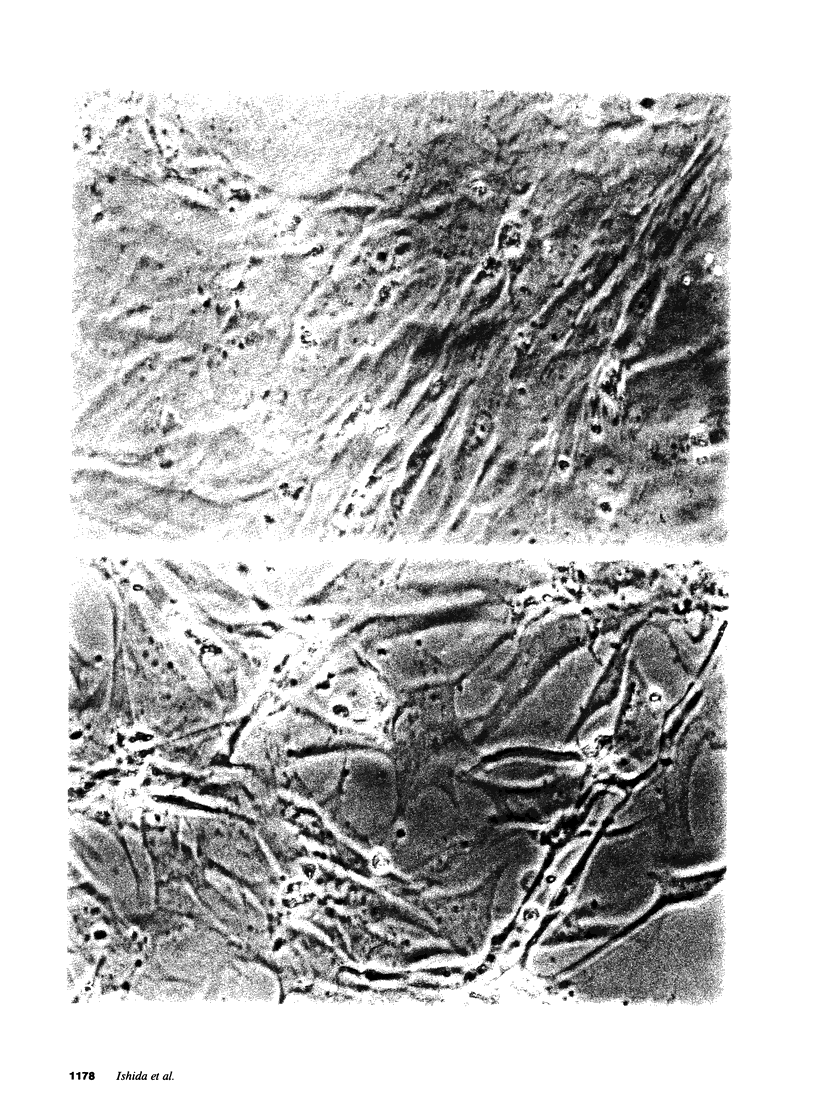
Alterations in cation homeostasis during and after recovery from myocardial ischemia may account for some of the reversible and irreversible components of myocardial cell injury. To investigate possible mechanisms involved, we exposed cultured layers of spontaneously contracting chick embryo ventricular cells to media containing 1 mM cyanide (CN) and 20 mM 2-deoxyglucose (2-DG), and zero glucose for up to 6 h, and then allowed cultured cells to recover in serum-free culture medium for 24 h. Changes in Na, K, and Ca contents, 42K uptake and efflux, ATP content, cell water content, and lactate dehydrogenase (LDH) release were measured, and compared with changes produced by exposure to 10(-3) M ouabain and severe hypoxia. Exposure to CN and 2-DG caused marked increase in cell Na (sevenfold) and Ca (fivefold) contents, and a decrease in K content (one-fifth normal), coincident with ATP depletion to one-tenth normal levels. This produced only slight cell injury, evidenced by increased LDH release. Recovery for 24 h resulted in return to near normal values (expressed in nanomoles per milligram of protein) of Na, Ca, and ATP contents. However, there was failure of cell K content to return to normal, associated with a persistent reduced net uptake of 42K, and an increase in the rate of 42K efflux. These abnormalities in K homeostasis were associated with a decrease in cell volume and water content per milligram of protein. More marked ATP depletion (to 1/100 normal values) was produced by hypoxia plus 2-DG and zero glucose, and was associated with much more severe cell injury manifested by LDH loss. Ouabain exposure resulted in a much greater Ca gain (20-30-fold), relative to increase in Na content, than did either CN and 2-DG or hypoxia; and ouabain effects were not reversible (after a 15-fold or greater increase in Ca content was produced) and were associated with significant LDH release. We conclude that these cells are resistant to cell injury caused by moderately severe Ca overload and ATP depletion produced by exposure to CN and 2-DG. However, metabolic inhibition of ATP production produces persistent abnormalities in K homeostasis, associated with functional abnormalities.
Full text
PDF

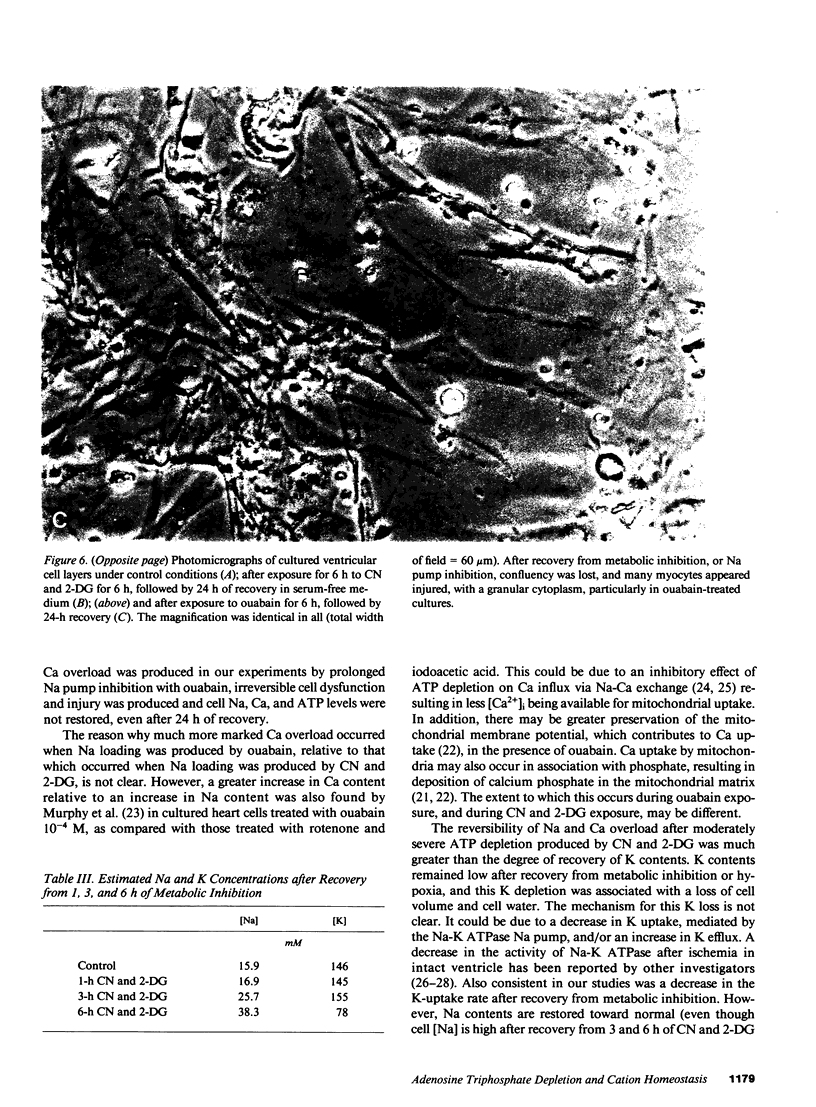


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiton J. F., Chipperfield A. R., Lamb J. F., Ogden P., Simmons N. L. Occurrence of passive furosemide-sensitive transmembrane potassium transport in cultured cells. Biochim Biophys Acta. 1981 Sep 7;646(3):389–398. doi: 10.1016/0005-2736(81)90307-2. [DOI] [PubMed] [Google Scholar]
- Aiton J. F., Simmons N. L. Effect of ouabain upon diuretic-sensitive K+ transport in cultured cells. Evidence for separate modes of operation of the transporter. Biochim Biophys Acta. 1983 Oct 12;734(2):279–289. doi: 10.1016/0005-2736(83)90126-8. [DOI] [PubMed] [Google Scholar]
- Barry W. H., Hasin Y., Smith T. W. Sodium pump inhibition, enhanced calcium influx via sodium-calcium exchange, and positive inotropic response in cultured heart cells. Circ Res. 1985 Feb;56(2):231–241. doi: 10.1161/01.res.56.2.231. [DOI] [PubMed] [Google Scholar]
- Barry W. H., Peeters G. A., Rasmussen C. A., Jr, Cunningham M. J. Role of changes in [Ca2+]i in energy deprivation contracture. Circ Res. 1987 Nov;61(5):726–734. doi: 10.1161/01.res.61.5.726. [DOI] [PubMed] [Google Scholar]
- Barry W. H., Pober J., Marsh J. D., Frankel S. R., Smith T. W. Effects of graded hypoxia on contraction of cultured chick embryo ventricular cells. Am J Physiol. 1980 Nov;239(5):H651–H657. doi: 10.1152/ajpheart.1980.239.5.H651. [DOI] [PubMed] [Google Scholar]
- Barry W. H., Rasmussen C. A., Jr, Ishida H., Bridge J. H. External Na-independent Ca extrusion in cultured ventricular cells. Magnitude and functional significance. J Gen Physiol. 1986 Sep;88(3):393–411. doi: 10.1085/jgp.88.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller G. A., Conroy J., Smith T. W. Ischemia-induced alterations in myocardial (Na+ + K+)-ATPase and cardiac glycoside binding. J Clin Invest. 1976 Feb;57(2):341–350. doi: 10.1172/JCI108285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersohn M. M., Philipson K. D., Fukushima J. Y. Sodium-calcium exchange and sarcolemmal enzymes in ischemic rabbit hearts. Am J Physiol. 1982 May;242(5):C288–C295. doi: 10.1152/ajpcell.1982.242.5.C288. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982 Dec;66(6):1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The regulation of the Na+ -Ca2+ exchanger of heart sarcolemma. Eur J Biochem. 1983 May 16;132(3):451–460. doi: 10.1111/j.1432-1033.1983.tb07383.x. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Sen A., Reynolds R., Chang A., Kim Y., Gunn M. D., Buja L. M., Willerson J. T. Release of arachidonate from membrane phospholipids in cultured neonatal rat myocardial cells during adenosine triphosphate depletion. Correlation with the progression of cell injury. J Clin Invest. 1985 Jun;75(6):1770–1780. doi: 10.1172/JCI111889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M. J., Elz J. S., Nayler W. G. Sarcolemmal enzymes and Na+ -Ca2+ exchange in hypoxic, ischemic, and reperfused rat hearts. Am J Physiol. 1984 Aug;247(2 Pt 2):H237–H243. doi: 10.1152/ajpheart.1984.247.2.H237. [DOI] [PubMed] [Google Scholar]
- Dresdner K. P., Kline R. P., Wit A. L. Intracellular K+ activity, intracellular Na+ activity and maximum diastolic potential of canine subendocardial Purkinje cells from one-day-old infarcts. Circ Res. 1987 Jan;60(1):122–132. doi: 10.1161/01.res.60.1.122. [DOI] [PubMed] [Google Scholar]
- Fink R., Lüttgau H. C. An evaluation of the membrane constants and the potassium conductance in metabolically exhausted muscle fibres. J Physiol. 1976 Dec;263(2):215–238. doi: 10.1113/jphysiol.1976.sp011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganote C. E., Liu S. Y., Safavi S., Kaltenbach J. P. Anoxia, calcium and contracture as mediators of myocardial enzyme release. J Mol Cell Cardiol. 1981 Jan;13(1):93–106. doi: 10.1016/0022-2828(81)90231-5. [DOI] [PubMed] [Google Scholar]
- Hasin Y., Barry W. H. Myocardial metabolic inhibition and membrane potential, contraction, and potassium uptake. Am J Physiol. 1984 Aug;247(2 Pt 2):H322–H329. doi: 10.1152/ajpheart.1984.247.2.H322. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Goknur A. B., Hunter D. R., Hegge J. O., Berkoff H. A. Inhibition of calcium influx in isolated adult rat heart cells by ATP depletion. Circ Res. 1987 Apr;60(4):586–594. doi: 10.1161/01.res.60.4.586. [DOI] [PubMed] [Google Scholar]
- Hess M. L., Manson N. H., Okabe E. Involvement of free radicals in the pathophysiology of ischemic heart disease. Can J Physiol Pharmacol. 1982 Nov;60(11):1382–1389. doi: 10.1139/y82-206. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Vereecke J., van der Heyden G., Carmeliet E. The shortening of the action potential by DNP in guinea-pig ventricular myocytes is mediated by an increase of a time-independent K conductance. Pflugers Arch. 1983 Jun 1;397(4):251–259. doi: 10.1007/BF00580257. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A. Lethal myocardial ischemic injury. Am J Pathol. 1981 Feb;102(2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Kakei M., Noma A., Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Kimura S., Bassett A. L., Gaide M. S., Kozlovskis P. L., Myerburg R. J. Regional changes in intracellular potassium and sodium activity after healing of experimental myocardial infarction in cats. Circ Res. 1986 Feb;58(2):202–208. doi: 10.1161/01.res.58.2.202. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Pariza M. W., Becker J. E., Potter V. R. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975 Oct;68(2):537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Libby P. Long-term culture of contractile mammalian heart cells in a defined serum-free medium that limits non-muscle cell proliferation. J Mol Cell Cardiol. 1984 Sep;16(9):803–811. doi: 10.1016/s0022-2828(84)80004-8. [DOI] [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. The energy-state of mitochondria during the transport of Ca2+. Eur J Biochem. 1980 Sep;110(1):211–216. doi: 10.1111/j.1432-1033.1980.tb04857.x. [DOI] [PubMed] [Google Scholar]
- Murphy E., Aiton J. F., Horres C. R., Lieberman M. Calcium elevation in cultured heart cells: its role in cell injury. Am J Physiol. 1983 Nov;245(5 Pt 1):C316–C321. doi: 10.1152/ajpcell.1983.245.5.C316. [DOI] [PubMed] [Google Scholar]
- Murphy E., Wheeler D. M., LeFurgey A., Jacob R., Lobaugh L. A., Lieberman M. Coupled sodium-calcium transport in cultured chick heart cells. Am J Physiol. 1986 Mar;250(3 Pt 1):C442–C452. doi: 10.1152/ajpcell.1986.250.3.C442. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms D., Jacob R., Horres C. R., Lieberman M. Potassium-chloride cotransport in cultured chick heart cells. Am J Physiol. 1985 Sep;249(3 Pt 1):C337–C344. doi: 10.1152/ajpcell.1985.249.3.C337. [DOI] [PubMed] [Google Scholar]
- Renlund D. G., Gerstenblith G., Lakatta E. G., Jacobus W. E., Kallman C. H., Weisfeldt M. L. Perfusate sodium during ischemia modifies post-ischemic functional and metabolic recovery in the rabbit heart. J Mol Cell Cardiol. 1984 Sep;16(9):795–801. doi: 10.1016/s0022-2828(84)80003-6. [DOI] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver L. H., Houser S. R. Transmembrane potassium fluxes in isolated feline ventricular myocytes. Am J Physiol. 1985 May;248(5 Pt 2):H614–H621. doi: 10.1152/ajpheart.1985.248.5.H614. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Hess M. L. The oxygen free radical system: a fundamental mechanism in the production of myocardial necrosis. Prog Cardiovasc Dis. 1986 May-Jun;28(6):449–462. doi: 10.1016/0033-0620(86)90027-7. [DOI] [PubMed] [Google Scholar]
- Thornhill W. B., Laris P. C. KCl loss and cell shrinkage in the Ehrlich ascites tumor cell induced by hypotonic media, 2-deoxyglucose and propranolol. Biochim Biophys Acta. 1984 Jun 27;773(2):207–218. doi: 10.1016/0005-2736(84)90084-1. [DOI] [PubMed] [Google Scholar]
- Trube G., Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Arch. 1984 Jun;401(2):178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- Weich H. F., Strauss H. W., Pitt B. The extraction of thallium-201 by the myocardium. Circulation. 1977 Aug;56(2):188–191. doi: 10.1161/01.cir.56.2.188. [DOI] [PubMed] [Google Scholar]
- Weiss J., Hiltbrand B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest. 1985 Feb;75(2):436–447. doi: 10.1172/JCI111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Shine K. I. Extracellular K+ accumulation during myocardial ischemia in isolated rabbit heart. Am J Physiol. 1982 Apr;242(4):H619–H628. doi: 10.1152/ajpheart.1982.242.4.H619. [DOI] [PubMed] [Google Scholar]
- Weiss J., Shine K. I. [K+]o accumulation and electrophysiological alterations during early myocardial ischemia. Am J Physiol. 1982 Aug;243(2):H318–H327. doi: 10.1152/ajpheart.1982.243.2.H318. [DOI] [PubMed] [Google Scholar]





