Abstract
Quinolinic acid (QUIN), an endogenous metabolite of the kynurenine pathway, is involved in several neurological disorders, including Huntington’s disease, Alzheimer’s disease, schizophrenia, HIV associated dementia (HAD) etc. QUIN toxicity involves several mechanisms which trigger various metabolic pathways and transcription factors. The primary mechanism exerted by this excitotoxin in the central nervous system (CNS) has been largely related with the overactivation of N-methyl-D-aspartate receptors and increased cytosolic Ca2+ concentrations, followed by mitochondrial dysfunction, cytochrome c release, ATP exhaustion, free radical formation and oxidative damage. As a result, this toxic pattern is responsible for selective loss of middle size striatal spiny GABAergic neurons and motor alterations in lesioned animals. This toxin has recently gained attention in biomedical research as, in addition to its proven excitotoxic profile, a considerable amount of evidence suggests that oxidative stress and energetic disturbances are major constituents of its toxic pattern in the CNS. Hence, this profile has changed our perception of how QUIN-related disorders combine different toxic mechanisms resulting in brain damage. This review will focus on the description and integration of recent evidence supporting old and suggesting new mechanisms to explain QUIN toxicity.
Keywords: quinolinate, toxic mechanisms, excitotoxicity, oxidative stress, neurodegeneration
Introduction
Is quinolinic acid (QUIN or 2,3-pyridine-dicarboxylic acid) an “enemy at home”? Indeed, it could be: the presence of this endogenous molecule in the brain constitutes a major risk because under normal physiological conditions QUIN is a component of a major metabolic pathway of tryptophan degradation and can modulate some local events in the CNS. However, under pathological conditions, it is capable of inducing a potent neurotoxic pattern by different mechanisms,1,2 therefore representing a latent menace for neurodegeneration by means of the brain’s own “tools”. QUIN is an extensively studied endogenous metabolite of the tryptophan degradation pathway, also known as the kynurenine pathway (KP). Under normal conditions, QUIN is produced as a downstream transient metabolite of tryptophan involved in adenine dinucleotide (NAD+) synthesis as under regular conditions KP catalyzes L-tryptophan into NAD+. In mammals, the majority of tryptophan comes from dietary intake, and is metabolized by the KP. The relevance of this pathway is a topic often reviewed, and is magnified by evidence demonstrating the formation of two major neuroactive metabolites: kynurenic acid (KYNA)—an endogenous N-methyl-D-aspartate receptor (NMDAr) antagonist with the ability of modulating α7 nicotinic receptors—and QUIN—an endogenous NMDAr agonist. In addition, the KP is responsible for the formation of other metabolites exhibiting redox activity, including 3-hydroxykynurenine (3-HK) and 3-hydroxyanthranilic acid (3-HAA). Whilst 3-HK is accepted as a pro-oxidant metabolite, there is also recent evidence showing that both 3-HK and 3-HAA are antioxidants acting as nitric oxide scavengers.3
In this minireview, the findings collected from several groups using QUIN as a toxic tool in neurosciences will be discussed. Particular attention will be given to its excitotoxic, pro-oxidant and energy-disrupting properties. QUIN is a glutamatergic agonist acting on NMDAr, preferentially on discrete populations of these receptors containing the NR2A and NR2B subunits. This metabolite is normally present at nanomolar concentrations in human and rat brains,4 and in nano to micromolar concentrations in cerebrospinal fluid.5 However, under inflammatory conditions, the KP is stimulated by cytokines—particularly by interferon-γ (IFN-γ)—in macrophages. This results in the production of increased levels of QUIN and 3-HK.6 These altered levels have been observed in different inflammatory disorders of the CNS and these diseases also involve overactivation of NMDAr.2
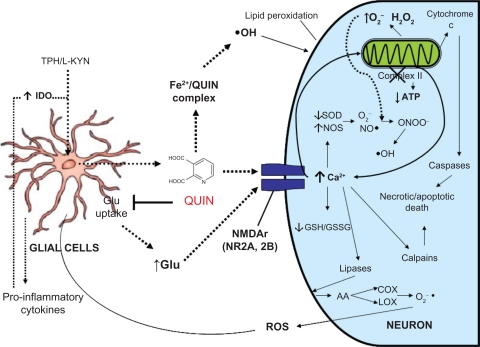
The pattern of toxicity which results from increased QUIN levels is considerably complex with many mechanisms potentially involved. At a primary level, QUIN exerts excessive excitation of NMDAr and recruits enhanced cytoplasmic Ca2+ concentrations, mitochondrial dysfunction, decreased ATP levels, cytochrome c release, selective loss of GABAergic and cholinergic neurons, and oxidative stress.1 Indeed, it may be assumed that most of the toxic cascades triggered or stimulated by QUIN eventually involve the formation of reactive oxygen and nitrogen species (ROS and RNS, respectively), thus leading cells to oxidative damage as part of their degenerative processes (see Fig. 1). Thus, when injected into the brain, QUIN reproduces neurodegenerative events in rodents, resembling those observed in the brains of patients with Huntington’s disease (HD).7
Figure 1.
Schematic representation of the classical proposed mechanisms by which quinolinic acid (QUIN) exerts toxicity in the Central Nervous System. Firstly, increased levels of QUIN in the extracellular domain are achieved after inflammatory-induced glial activation. QUIN can then act in several nonexcluding ways: (1) stimulating NMDAr and, together with other endogenous excitatory agents (glutamate), to induce excitotoxic events further leading to exacerbated intracellular calcium-mediating signaling and recruiting more calcium from internal storages (mitochondria and endoplasmic reticulum). QUIN can then act with other inner toxic signals, including mitochondrial dysfunction, cytochrome c release, reactive oxygen and nitrogen species (ROS/RNS) formation, protease activation, etc. Altogether, the above interactions lead to necrotic and apoptotic cell death. (2) QUIN directly interacts with free iron ions to form toxic complexes that exacerbate ROS/RNS formation, oxidative stress and excitotoxic events already in course. Eventually, these toxic signals can be extended, thus reaching adjacent cells, either glial or neuronal, hence starting a degenerative chain in the brain.
Abbreviations: AA, arachidonic acid; COX, cyclooxygenase; L-KYN, L-kynurenine; LOX, lipooxygenase; SOD, superoxide dismutase.
More recently, there has been intensive research into kynurenines and this has provided new insights into the toxic mechanisms involved in neurodegenerative events with compromised KP metabolism. This review will offer an update on the recent findings on QUIN toxicity in order to bring a brief and “refreshing” perspective on this topic. We will specifically focus on current studies and discuss what areas require further characterization in this paradigm. Although we recognize the relevance of other mechanisms exerted by QUIN as part of its toxic pattern—including mitochondrial dysfunction and inflammatory events—, we will not focus our attention on them in this occasion due to space limitations.
Quinolinate, Excitotoxicity and Oxidative Stress: A Recurrent Relationship
Excitotoxicity is a deleterious process involving increased intracellular calcium concentrations in response to neuronal cell exposure to the persistent action of excitatory amino acids. An augmented Ca2+ influx through the NMDAr-ion channel complex is responsible for the activation of lethal metabolic pathways ultimately activating lysis enzymes (protein kinases, phospholipases, nitric oxide synthase, proteases, etc.). This produces mitochondrial alterations while increasing the ROS/RNS formation, and ultimately leads to apoptotic or necrotic death.1
The exposure of corticostriatal structures to submicromolar concentrations of QUIN has been shown to induce neuronal cell death through an excitotoxic mechanism,2 suggesting that the toxin can trigger multiple toxic cascades even at low concentrations. Moreover, excitotoxicity induced by QUIN has also been related to its ability to increase ROS/RNS formation and further oxidative damage. As accepted elsewhere, the brain is particularly susceptible to the attack of ROS/RNS due to a limited antioxidant defense system, a high demand of oxygen accompanied by a considerable dependency on a redox metabolism, and an enriched content of polyunsaturated fatty acid chains in cell membranes. This is particularly relevant as we now know that a high redox activity is necessary for the development and adequate functioning of the brain. In fact, ROS formation in the CNS should not be conceptualized as a mere toxic response as these species are critical for cellular signaling pathways and oxidative homeostasis, as well as maintaining energy status, metabolite concentrations and developmental and survival responses.8 Therefore, it seems reasonable to consider that the brain is “loaded” with endogenous tools to handle and use ROS for its own benefits when the formation of these species remains at certain controlled levels. However, when toxins like QUIN create a drastic scenario of exacerbated intracellular deleterious signals and aberrant oxidative activity, excessive ROS leads to cell damage and death.
Previous studies have shown that QUIN possesses the ability to form complexes with Fe(II), thereby inducing ROS formation—especially hydroxyl radical (•OH), which in turn is responsible for DNA chain breakdown and lipid peroxidation—.9 This suggests that QUIN may cause additional oxidative events that could be partially independent on its direct capacity to stimulate NMDAr. Moreover, free radicals are known to increase glutamate release from nerve endings,10 also inhibiting its reuptake,11 hence providing an alternative route by which QUIN could potentiate its primary excitotoxic mechanism.
Other studies report that the intrastriatal infusion of QUIN into rodents stimulates lipid peroxidation in this region within 2 h post-lesion,12 and these findings have been correlated with increased extracellular levels of hydroxyl radical in the striatum.13 As a result of these and other findings, the hypothesis that at least a fraction of the oxidative and cell damage induced by QUIN could correspond with components that are independent of NMDAr overactivation formally emerged in the late 90’s—early 2000’s.13,14 Supporting this hypothesis is that QUIN is known to generate a dysregulation in the oxidant/antioxidant ratio by affecting the reduced glutathione:oxidized glutathione (GSH:GSSG) rate, as well as depleting the activity of Cu, Zn-SOD at different times post-lesion.15 In addition, the toxin has been shown to cause the early and time-dependent formation of peroxynitrite (ONOO−) as a key RNS contributing to this paradigm.16 Moreover, these and other alterations induced by QUIN can be prevented by different antioxidants such as melatonin, sodium selenite, L-carnitine, etc.12,14,17 For instance, it has been shown that piruvate, the final metabolite of glycolysis, exhibits antioxidant activity and reduces different markers of oxidative stress (4-hydroxynonenal, 8-hydroxyguanosine and ROS formation) induced by QUIN.18 In parallel, Ganzella and coworkers19 demonstrated that 4 hours after hippocampal infusion of QUIN to rats (36.8 nM i.c.v.) there was an increased formation of ROS which returned to basal levels 24 h after its infusion. However, cell damage persisted 72 h post-lesion. In the same study it was demonstrated that the total antioxidant capacity was increased 8 h post-lesion, which in turn can be considered as a compensatory response possibly contributing to the decreased levels of ROS found at later times. It was also speculated that these toxic pathways and processes initiated by ROS followed routes that were independent of the presence of these species. These and other studies raised considerations about the substantial contribution of oxidative stress, coming from different sources, to the toxic paradigm elicited by QUIN: either from mitochondrial dysfunction, excitotoxic events at membrane and cytoplasmic level, or from direct ROS formation through metal dissociation from proteins and further formation of pro-oxidant complexes.
Other more recent studies have shown that moderate activation of NMDAr by QUIN, combined with mitochondrial dysfunction induced by 3-nitropropionic acid (3-NP), can synergistically augment the striatal degeneration in rodents through the dysregulation of intracellular Ca2+. This suggests a lack of hypersensitization of NMDAr.20 This synergic model is relevant for mimicking some pathological alterations of neurological disorders—such as Huntington’s disease (HD)—combining excitotoxic events and deficient energy metabolism. Based on these findings, it was further demonstrated that mitochondrial dysfunction and oxidative damage are potentiated when these two toxins, 3-NP + QUIN are simultaneously added to synaptosomal fractions and striatal slices at subtoxic concentrations. This noxious synergism is likely to be dependent on protease activation.21
What’s New?
Key insights into QUIN toxicity have recently been described. For example, QUIN not only induces damage to neurons, but also to glial cells. This discovery presents a new perspective on the toxic properties of this agent and highlights the importance of designing therapeutic alternatives for neurological disorders involving these cells. In this regard, the findings of Guillemin and coworkers,22 which showed that QUIN is capable of inducing a certain degree of apoptosis in cultured human astrocytes is particularly relevant for disorders involving high concentrations of QUIN in cerebrospinal fluid, including HAD. Moreover, the recent discovery and characterization of the KP in cultured human neurons and neuroblastoma cells is not only enlightening itself, but also describes for the first time a differential role for the KP in neuronal protection and/or degeneration.23 Interestingly, these authors found that the differential capacity of primary cultures and adult neurons to produce the neuroprotectant picolinic acid (PIC) is in contrast with the metabolic orientation of tumoral cells to synthesize QUIN. These differences establish important clues to understanding the nature of modulatory actions of the KP in neuroprotection or neurotoxicity through immune and tumor regulation. Shortly thereafter, Owe-Young and coworkers24 extended the investigation of the KP to the components of blood-brain-barrier, endothelial cells and pericytes. Under normal conditions endothelial cells produced KYNA and pericytes synthesized PIC. However, under conditions of immune activation both cell types basolaterally secreted high amounts of kynurenine for further conversion to QUIN by perivascular macrophages. Once again, triggering mechanisms such as immune activation through inflammatory processes is likely to stimulate different cell types to produce QUIN, which in turn could account for local neurotoxicity. Braidy and coworkers25 further demonstrated that the concentration of different metabolites from the KP determines whether they stimulate beneficial effects for human glial and neuronal cells (such as NAD+ synthesis) or induce damaging events and cell death. It was concluded from this study that some metabolites like 3-hydroxyanthanilic acid, 3-HK, QUIN and PIC, at low concentrations (100 nM), clearly stimulated NAD+ formation, while above this concentration, the same metabolites induced cell damage. In parallel, the same authors demonstrated that at low concentrations (<50 nM), QUIN is a substrate for NAD+ synthesis, whereas at abnormally high concentrations (>150 nM) this metabolite can mediate astrocytic and neuronal inflammation and damage though a mechanism involving excitotoxicity, induction of nitric oxide synthase (NOS) and further nitric oxide-stimulated oxidative stress.26 Taken together, these studies certainly constitute valuable advances in this area and will change our perception on the role of KP modulation in neurodegenerative disorders.
In 2007, Poeggeler and coworkers27 discovered a key component of the NMDA- and QUIN-induced NMDAr-mediated striatal toxicity. This component was elegantly revealed using dopaminergic agonists and antagonists. When administering dopaminergic agonists—d-amphetamine and apomorphine—, the striatal lesion induced by NMDA and QUIN was enhanced and KYNA levels were decreased. In contrast, pretreatment with the antagonists abolished the potentiated NMDAr-mediated toxicity. As a result of these findings, the modulation of dopaminergic activity should be considered in the future when designing therapeutic strategies against QUIN toxicity. However, the precise magnitude of the dopaminergic contribution to these paradigms is yet to be delineated.
More specifically with regard to QUIN and oxidative stress, a recent and exciting investigation relating to redox modulation at a molecular level in the toxic model evoked by QUIN has been described. Tert-butyl hydroquinone (tBHQ), a well known inducer of nuclear translocation of the transcription nuclear factor Nrf2 (which in turn is responsible for the activation of antioxidant response elements (ARE) and the consequent synthesis of phase 2 antioxidant enzymes) was shown to be able to prevent the lipid peroxidation and mitochondrial dysfunction caused by QUIN in rat striatal slices.28 This effect correlated with a partial recovery in the activity of glutathione-S-transferase, an enzyme involved in the modulation of nuclear Nrf2 translocation. As expected, QUIN itself decreased the nuclear expression of Nrf2, thus suggesting a compromised antioxidant defense in the toxic model. This study is particularly relevant for future considerations in this field as it suggests that QUIN toxicity is associated with a reduction of antioxidant defenses at a molecular level by silencing phase 2 antioxidant enzymes. As a result of these studies, therapeutic alternatives designed to stimulate Nrf2 translocation and further phase 2 enzyme expression should be considered to mitigate the toxic events produced by QUIN.
In addition to these findings, QUIN has also been shown to produce an early increase in the expression of the receptor for advanced glycation end-products (RAGE) in the rat striatum. This can be partially considered a marker of oxidative stress as AGEs result from intense activity of ROS and carbonyl compounds, and RAGE has been shown to be overexpressed in different neurodegenerative conditions.29 Therefore, as QUIN is capable of inducing RAGE upregulation, this could be a novel event accounting for QUIN-induced oxidative stress. Therefore, interesting mechanistic alternatives are now available. Moreover, the recent characterization of a potential contributing role for NAD(P)H oxidase—an enzyme complex catalyzing the formation of superoxide anion from oxygen—to QUIN-induced toxicity in a NMDAr-dependent manner, provides new evidence on the close relationship between excitotoxic and oxidative mechanisms in this model.30
Kumar’s group continues with an extensive search for therapeutic alternatives to ameliorate the deleterious effects produced by QUIN in the rat brain. This group is constantly testing and confirming the effects of targeting the pro-oxidant, inflammatory and metabolic components of this model using drugs directed to ameliorate each toxic event. For example, they recently presented evidence showing that montelukast, a leukotriene receptor antagonist and anti-inflammatory agent, attenuates QUIN-induced neurotoxicity, oxidative stress, aberrant behavior and mitochondrial alterations.31 This group has also shown that statins such as atorvastatin, simvastatin and fluvastatin, can reduce QUIN induced toxicity at the same levels mentioned above.32 Although statins are known to be cholesterol-lowering drugs inducing inhibition of HMG Co-A reductase—the rate-limiting enzyme in cholesterol biosynthesis—, the authors were more interested in exploring the antioxidant and anti-inflammatory potential of these molecules. Other groups have made similar approaches, assessing the positive effects of antioxidant and neuroprotective agents in this toxic paradigm. Some agents exhibiting protective/antioxidant properties against QUIN include the polyamine spermine.33 This was assumed to modulate the polyamine binding site at the NMDAr function and also exert antioxidant effects—, the energy precursor and antioxidant L-carnitine,17 the selective neuronal nitric oxide synthase inhibitor Nomega-Nitro-l-arginine-methyl esther34—acting by recovering the protein tyrosine phosphorylation pattern affected by QUIN—, the antioxidant element selenium35—acting by reducing the proapoptotic signaling derived from NF-κB pathway and stimulating glutathione peroxidase activity—, the free radical scavenger and signaling molecule melatonin,36 and the singlet oxygen and superoxide scavenger 6-hydroxymelatonin,37 among several others. Recently, our group has carried out studies in vitro and in vivo to investigate whether the antioxidant S-allylcysteine can recover the synaptosomes from the toxic insult caused by QUIN, even when this agent is administered in post-lesion schemes.38 The findings of this study suggest that the optimum time window for pharmacological intervention to achieve functional preservation of nerve endings is short, 1 to 3 h post-lesion.
Together, these and other therapeutic approaches against QUIN toxicity highlight the benefits of targeting the different toxic levels exerted by this endogenous metabolite in nerve tissue. However, a more reasonable design of pharmacological alternatives for pathologies involving QUIN as a pathogenic factor should consider a more realistic condition occurring in these disorders: an imbalance between KP metabolites, specifically between QUIN and KYNA. Müller and coworkers39 have addressed this issue by revising and discussing two opposing patterns of type-1 (T-helper 1) and type-2 (T-helper 2) immune responses likely associated with differential activation of KP enzymes. These responses result in either an increased production of KYNA in schizophrenia or decreased KYNA levels in depression, all contrasting with increased levels of QUIN in different neurodegenerative disorders. Furthermore, there is a differential activation of microglia and astrocytes during inflammatory episodes, which adds to this imbalance.
Finally, one of the most exciting and promising recent findings is the demonstration of a differential susceptibility of mice carrying a polyglutamine expanded CAG tract corresponding to mutant Huntingtin (YAC128) to the neurotoxic actions of QUIN during the presymptomatic stages (before obvious phenotypic changes occurr), and a resistance to QUIN toxicity in 10-month-old symptomatic mice.40 These observations could correlate with what may be happening in the brains of HD patients that currently show enhanced levels of QUIN during the first stages of the disease. In addition, presymptomatic mice exhibited increased function of NMDAr, while symptomatic mice presented the opposite. This is a remarkable contribution to the HD and QUIN research fields as it offers an elegant explanation for the dynamic nature of the mutant htt-mediated excitotoxic phenotype and the potential role of altered KP in this disorder. However, the precise mechanisms by which this differential susceptibility is occurring remain to be elucidated.
Conclusions
QUIN has gained considerable relevance in biomedical research, and a series of specific factors contribute to this concept: (1) This is an endogenous metabolite derived from a major catabolic pathway that is present in several mammalian tissues; (2) it has been implicated in cell damage in a wide range of psychiatric, neurological and systemic disorders; (3) QUIN is a molecule with neuroactive properties as a moderate agonist of NMDAr; (4) it is widely employed as an excitotoxic model of neuronal damage in biomedical research; (5) it may also influence a series of toxic mechanisms that could be partially independent of its primary interaction with NMDAr; and finally, (6) the modulation of its endogenous production offers interesting possibilities for a therapeutic intervention in all disorders combining excitotoxicity, oxidative stress, energy depletion and inflammation. Thus, the diversity of toxic mechanisms that have been demonstrated for QUIN suggests a major role for this molecule at different physiologic and physiopathologic levels in the CNS. Together, this toxic profile suggests that QUIN is a metabolite with unique properties as a toxicological tool for research. The characterization of its damaging mechanisms is relevant for the explanation of physiopathologic events occurring in several neurodegenerative disorders.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: AS. Contributed to the writing of the manuscript: VPD and PCM. Jointly developed the structure and arguments for the paper: AS, VPD and PCM. Made critical revisions and approved final version: AS. All authors reviewed and approved of the final manuscript.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Pérez-De La Cruz V, Königsberg M, Santamaría A. Kynurenine pathway and disease: an overview. CNS Neurol Disord Drug Targets. 2007;6:398–410. doi: 10.2174/187152707783399229. [DOI] [PubMed] [Google Scholar]
- 2.Schwarcz R, Guidetti P, Sathyasaikumar KV, Muchowski PJ. Of mice, rats and men: Revisiting the quinolinic acid hypothesis of Huntington’s disease. Prog Neurobiol. 2010;90:230–45. doi: 10.1016/j.pneurobio.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backhaus C, Rahman H, Scheffler S, Laatsch H, Hardeland R. NO scavenging by 3-hydroxyanthranilic acid and 3-hydroxykynurenine: N-nitrosation leads via oxadiazoles to o-quinone diazides. Nitric Oxide. 2008;19:237–44. doi: 10.1016/j.niox.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Meininger V, Guillemin GJ. Recent advances in the treatment of amyotrophic lateral sclerosis. Emphasis on kynurenine pathway inhibitors. Cent Nerv Syst Agents Med Chem. 2009;9:32–9. doi: 10.2174/187152409787601941. [DOI] [PubMed] [Google Scholar]
- 5.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 6.Chiarugi A, Sbarba D, Paccagnini A, Donnini S, Filippi S, Moroni F. Combined inhibition of indoleamine 2,3-dioxygenase and nitric oxide synthase modulates neurotoxin release by interferon-gamma-activated macrophages. J Leukoc Biol. 2000;68:260–6. [PubMed] [Google Scholar]
- 7.McLin JP, Thompson LM, Steward O. Differential susceptibility to striatal neurodegeneration induced by quinolinic acid and kainate in inbred, outbred and hybrid mouse strains. Eur J Neurosci. 2006;24:3134–40. doi: 10.1111/j.1460-9568.2006.05198.x. [DOI] [PubMed] [Google Scholar]
- 8.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. TiBS. 2010;35:505–13. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platenik J, Stopka P, Vejrazka M. Quinolinic acid–iron (II) complexes: slow autooxidation, but enhanced hydroxyl radical production in the Fenton reaction. Free Radic Res. 2001;34:445–59. doi: 10.1080/10715760100300391. [DOI] [PubMed] [Google Scholar]
- 10.Pellegrini-Giampietro DE, Cherici G, Alesiani M, Carla V, Moroni F. Excitatory amino acid release from rat hippocampal slices as a consequence of free radical formation. J Neurochem. 1988;51:1960–3. doi: 10.1111/j.1471-4159.1988.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 11.Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidantvulnerable: a molecular link between oxidative and excitotoxic. neurodegeneration. Trends Pharmacol Sci. 1998;19:328–34. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 12.Santamaría A, Salvatierra-Sánchez R, Vázquez-Román B, et al. Protective effects of the antioxidant selenium on quinolinic acid-induced neurotoxicity in rats: in vitro and in vivo studies. J Neurochem. 2003;86:479–88. doi: 10.1046/j.1471-4159.2003.01857.x. [DOI] [PubMed] [Google Scholar]
- 13.Santamaría A, Jiménez-Capdeville ME, Camacho A, Rodríguez-Martínez E, Flores A, Galván-Arzate S. In vivo hydroxyl radical formation after quinolinic acid infusion into rat corpus striatum. Neuroreport. 2001;12:2693–6. doi: 10.1097/00001756-200108280-00020. [DOI] [PubMed] [Google Scholar]
- 14.Behan WM, McDonald M, Darlington LG, Stone TW. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: protection by melatonin and deprenyl. Br J Pharmacol. 1999;128:1754–60. doi: 10.1038/sj.bjp.0702940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez-Martínez E, Camacho A, Maldonado PD, et al. Effect of quinolinic acid on endogenous antioxidants in rat corpus striatum. Brain Res. 2000;858:436–9. doi: 10.1016/s0006-8993(99)02474-9. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-De La Cruz V, González-Cortés C, Galván-Arzate S, et al. Excitotoxic brain damage involves early peroxynitrite formation in a model of Huntington’s disease in rats: protective role of iron porphyrinate 5,10,15,20-tetrakis (4-sulfonatophenyl) porphyrinate iron (III) Neuroscience. 2005;135:463–74. doi: 10.1016/j.neuroscience.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Silva-Adaya D, Pérez-De La Cruz V, Herrera-Mundo MN, et al. Excitotoxic damage, disrupted energy metabolism, and oxidative stress in the rat brain: antioxidant and neuroprotective effects of L-carnitine. J Neurochem. 2008;105:677–89. doi: 10.1111/j.1471-4159.2007.05174.x. [DOI] [PubMed] [Google Scholar]
- 18.Ryu JK, Choi HB, McLarnon JG. Combined minocycline plus pyruvate treatment enhances effects of each agent to inhibit inflammation, oxidative damage, and neuronal loss in an excitotoxic animal model of Huntington’s disease. Neuroscience. 2006;141:1835–48. doi: 10.1016/j.neuroscience.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 19.Ganzella M, Jardim FM, Boeck CR, Vendite D. Time course of oxidative events in the hippocampus following intracerebroventricular infusion of quinolinic acid in mice. Neurosci Res. 2006;55:397–402. doi: 10.1016/j.neures.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Jacquard C, Trioulier Y, Cosker F, et al. Brain mitochondrial defects amplify intracellular [Ca2+] rise and neurodegeneration but not Ca2+ entry during NMDA receptor activation. FASEB J. 2006;20:1021–3. doi: 10.1096/fj.05-5085fje. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-De La Cruz V, Elinos-Calderón D, Carrillo-Mora P, et al. Time-course correlation of early toxic events in three models of striatal damage: modulation by proteases inhibition. Neurochem Int. 2010;56:834–42. doi: 10.1016/j.neuint.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Guillemin GJ, Wang L, Brew BJ. Quinolinic acid selectively induces apoptosis of human astrocytes: potential role in AIDS dementia complex. J Neuroinflammation. 2005;2:16. doi: 10.1186/1742-2094-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guillemin GJ, Cullen KM, Lim CK, et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27:12884–92. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owe-Young R, Webster NL, Mukhtar M, et al. Kynurenine pathway metabolism in human blood-brain-barrier cells: implications for immune tolerance and neurotoxicity. J Neurochem. 2008;105:1346–57. doi: 10.1111/j.1471-4159.2008.05241.x. [DOI] [PubMed] [Google Scholar]
- 25.Braidy N, Grant R, Brew BJ, Adams S, Jayasena T, Guillemin GJ. Effects of kynurenine pathway metabolites on intracellular NAD+ synthesis and cell death in human primary astrocytes and neurons. Int J Tryptophan Res. 2009;2:63–71. doi: 10.4137/ijtr.s2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braidy N, Grant R, Adams S, Brew BJ, Guillemin GJ. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox Res. 2009;16:77–86. doi: 10.1007/s12640-009-9051-z. [DOI] [PubMed] [Google Scholar]
- 27.Poeggeler B, Rassoulpour A, Wu HQ, Guidetti P, Roberts RC, Schwarcz R. Dopamine receptor activation reveals a novel, kynurenate-sensitive component of striatal N-methyl-D-aspartate neurotoxicity. Neuroscience. 2007;148:188–97. doi: 10.1016/j.neuroscience.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tasset I, Pérez-De La Cruz V, Elinos-Calderón D, et al. Protective effect of tert-butylhydroquinone on the quinolinic acid-induced toxicity in rat striatal slices: role of the Nrf2-antioxidant response element pathway. Neurosignals. 2010;18:24–31. doi: 10.1159/000243650. [DOI] [PubMed] [Google Scholar]
- 29.Cuevas E, Lantz S, Newport G, et al. On the early toxic effect of quinolinic acid: involvement of RAGE. Neurosci Lett. 2010;474:74–8. doi: 10.1016/j.neulet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado PD, Molina-Jijón E, Villeda-Hernández J, Galván-Arzate S, Santamaría A, Pedraza-Chaverrí J. NAD(P)H oxidase contributes to neurotoxicityin an excitotoxic/prooxidant model of Huntington’s disease in rats: protective role of apocynin. J Neurosci Res. 2010;88:620–9. doi: 10.1002/jnr.22240. [DOI] [PubMed] [Google Scholar]
- 31.Kalonia H, Kumar P, Kumar A, Nehru B. Protective effect of montelukast against quinolinic acid/malonic acid induced neurotoxicity: possible behavioral, biochemical, mitochondrial and tumor necrosis factor-α levels alterations in rats. Neuroscience. 2010;171:284–99. doi: 10.1016/j.neuroscience.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 32.Kalonia H, Kumar P, Kumar A. Comparative neuroprotective profile of statins in quinolinic acid induced neurotoxicity in rats. Behav Brain Res. 2010;216:220–8. doi: 10.1016/j.bbr.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 33.Velloso NA, Dalmolin GD, Fonini G, et al. Spermine attenuates behavioral and biochemical alterations induced by quinolinic acid in the striatum of rats. Brain Res. 2008;1198:107–14. doi: 10.1016/j.brainres.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 34.Mallozzi C, Martire A, Domenici MR, Metere A, Popoli P, Di Stasi AM. L-NAME reverses quinolinic acid-induced toxicity in rat corticostriatal slices: Involvement of src family kinases. J Neurosci Res. 2007;85:2770–7. doi: 10.1002/jnr.21178. [DOI] [PubMed] [Google Scholar]
- 35.Santamaría A, Vázquez-Román B, La Cruz VP, et al. Selenium reduces the proapoptotic signaling associated to NF-kappaB pathway and stimulates glutathione peroxidase activity during excitotoxic damage produced by quinolinate in rat corpus striatum. Synapse. 2005;58:258–66. doi: 10.1002/syn.20206. [DOI] [PubMed] [Google Scholar]
- 36.Vega-Naredo I, Poeggeler B, Sierra-Sánchez V, et al. Melatonin neutralizes neurotoxicity induced by quinolinic acid in brain tissue culture. J Pineal Res. 2005;39:266–75. doi: 10.1111/j.1600-079X.2005.00243.x. [DOI] [PubMed] [Google Scholar]
- 37.Maharaj DS, Maharaj H, Antunes EM, et al. 6-Hydroxymelatonin protects against quinolinic acid-induced oxidative neurotoxicity in the rat hippocampus. J Pharm Pharmacol. 2005;57:877–81. doi: 10.1211/0022357056424. [DOI] [PubMed] [Google Scholar]
- 38.Elinos-Calderón D, Robledo-Arratia Y, Pérez-De La Cruz V, et al. Antioxidant strategy to rescue synaptosomes from oxidative damage and energy failure in neurotoxic models in rats: protective role of S-allylcysteine. J Neural Transm. 2010;117:35–44. doi: 10.1007/s00702-009-0299-5. [DOI] [PubMed] [Google Scholar]
- 39.Müller N, Myint AM, Schwarz MJ. The impact of neuroimmune dysregulation on neurprotection and neurotoxicity in psychiatric disorders—relation to drug treatment. Dialogues Clin Neurosci. 2009;11:319–32. doi: 10.31887/DCNS.2009.11.3/nmueller. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham RK, Pouladi MA, Joshi P, et al. Differential susceptibility to excitotoxic stress in YAC128 mouse model of Huntington disease between initiation and progression of disease. J Neurosci. 2009;29:2193–204. doi: 10.1523/JNEUROSCI.5473-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]