Summary
Ependymoma is a central nervous system tumor associated with a poor prognosis due to limited efficacy of current medical treatment modalities, often resulting in multiple surgical re-resections with each tumor recurrence. As traditional chemotherapeutic regimens have proved unsuccessful in long-term control of subtotally resected ependymoma, other agents targeting the tumor microenvironement including the angiogenic factors supplying neovascularization have recently been used.
Anti-angiogenic agents such as bevacizumab are routinely used in adult patients with recurrent glioma. Selective intra-arterial cerebral infusion (SIACI) of biological agents within tumor-supplying cerebral vasculature has recently been re-examined as a means to avoid the systemic side-effects associated with intravenous use of bevacizumab. This technical paper describes the first reported use of SIACI for delivery of two targeted biologic agents, bevacizumab and cetuximab in a pediatric patient utilizing the basilar artery to selectively administer the drugs to the tumor microenvironment. We believe this method for therapeutic delivery will both broaden treatment options and better refine treatment methodology as the multi-modality treatment approach often required to treat patients with pediatric ependymomas and other intracranial malignancies evolves.
Key words: bevacizumab, infusions, intra-arterial, ependymoma, basilar artery
Introduction
Advances in adjuvant chemotherapy and radiotherapy have improved overall survival rates in childhood central nervous system (CNS) malignancies, but multiply recurrent disease presents difficult challenges for treating oncologists. Ependymoma is a CNS tumor which accounts for 10% of pediatric tumors and is typically managed with aggressive surgery followed by radiotherapy. The two-year overall survival following disease recurrence is between 20% and 49%; 40-60% of all children diagnosed eventually succumb to this disease. The development of clinical trials using targeted therapeutics tailored to patient-specific tumor biology is becoming imperative due to the frequency of radiation and chemotherapy resistance.
Ependymoma is the third most common type of brain tumor affecting children comprising 30% of tumors in children less than three years of age and 9% of primary CNS tumors in patients under 18. The current standard of care for treating ependymoma is surgical resection followed by radiotherapy, with the exception of children under three years of age due to the long-term effects of radiation in this age group. The response rate of chemotherapy in treating this disease is variable between younger and older children with no single agent having a distinct therapeutic advantage over any other.
Newer agents targeting tumor angiogenesis are being studied. Bevacizumab (Avastin), the most studied of these is a monoclonal antibody targeting soluble vascular endothelial growth factor (VEGF). This agent has shown promise in inhibiting tumor progression in multiple human clinical trials on adult patients with CNS disease. In fact, bevacizumab, recognized as a standard of care for treatment of recurrent malignant supratentorial tumors, has demonstrated a six month progression-free survival (PFS) of 50%. The over-expression of the receptor for VEGF in ependymoma had been demonstrated, suggesting that bevacizumab may have efficacy in these tumors. Moreover, a recent study reported six out of eight adult patients having response to a bevacizumab-containing treatment scheme. Similarly, vascular endothelial-derived growth factor receptor (VEGFR) overexpression has been associated with a poorer prognosis in pediatric ependymoma.
We postulated that synergistic utilization of both bevacizumab and cetuximab would achieve significant inhibition of VEGFR and EGFR translating into tumor growth restriction at the molecular level. We employed super-selective intra-arterial cerebral infusion for drug delivery to selectively infuse the arterial supply of the tumor bed with bevacizumab and cetuximab. The purpose of using this technique as opposed to intravenous peripheral injection is to directly target the VEGF and EGF receptors on the tumor while minimizing systemic toxicity. We describe the super-selective balloon-assisted intra-basilar artery infusion of bevacizumab and cetuximab in a pediatric patient with multiply recurrent posterior fossa ependymoma who had failed all available salvage therapy. We discuss the technical aspects of the selective catheterization as well as the oncologic rational of intra-arterial dual drug delivery. This is the first reported case of super-selective chemotherapeutic treatment of a recurrent posterior fossa tumor in the pediatric literature. Tumor-directed therapy is becoming more routine as genetic profiling of tumor specimens becomes quicker and cheaper and the availability of targeted agents increases. The need to develop safe techniques of super-selective administration and match biologic agents to tumor variability will be paramount as this technique evolves. This report demonstrates the safety and conceptual framework of directed intra-arterial therapy for recurrent pediatric CNS malignancies.
Materials and Methods
Case History
A 16-year-old right-handed male presented at age three years with a posterior fossa mass. He underwent near-total resection of the mass followed by five cycles of cyclophosphamide, etoposide, cisplatin and vincristine, as well as stereotactic radiation therapy. He was monitored with serial MRI scans for his stable residual disease.
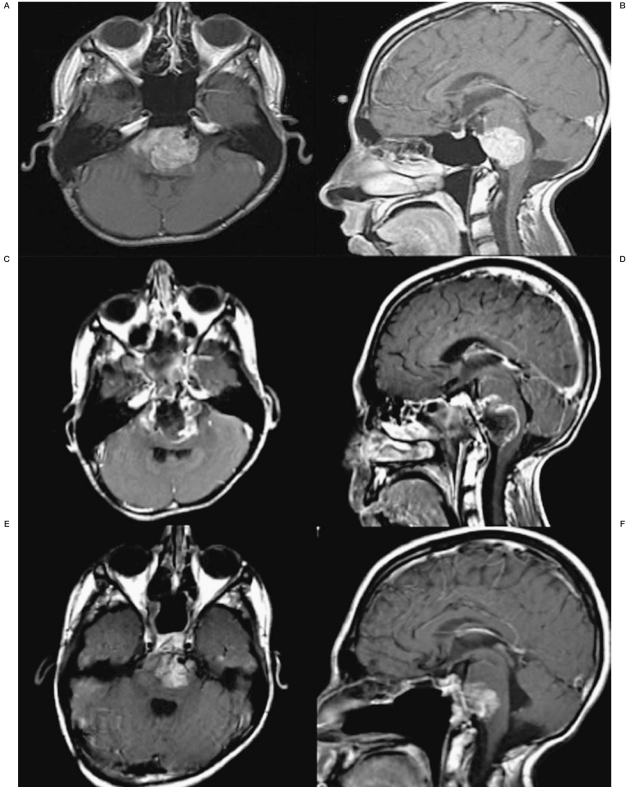
At age 16 years, he presented to the Weill Cornell Brain Tumor Center with headaches and a left hemiparesis. Computer tomography (CT) demonstrated a small pontine hemorrhage. Subsequent magnetic resonance imaging identified a ventral intrinsic ponto-medullary lesion causing severe brainstem compression consistent with disease recurrence (Figure 1A,B). Previous radiation exposure, the size of the lesion and adherence to the brainstem precluded further external radiation and palliative chemotherapy was thought to be ineffective, thus decompressive surgery was offered; the patient underwent an endoscopic transclival near total resection (Figure 1C,D).
Four months later, after fully recovering from the previous neurological deficits, the patient experienced speech and swallowing difficulty, headache and return of the left-sided hemiparesis. An MRI scan identified tumor recurrence. He began a Phase 1 trial (PBTC-024) of MK0752 but this was discontinued when the patient's left-sided hemiparesis and lower cranial nerve function worsened to the point that he could not walk and required intubation for airway protection. He underwent re-resection through the transclival approach to decompress the ventral brainstem and MRI again demonstrated near total resection. With no further protocols available, we offered inclusion in an adult protocol for recurrent intracranial disease utilizing dual agent super-selective intra-arterial delivery of bevacizumab and cetuximab. This protocol had been previously approved by the Institutional Review Board (IRB) at Weill Cornell Medical College.
Figure 1.
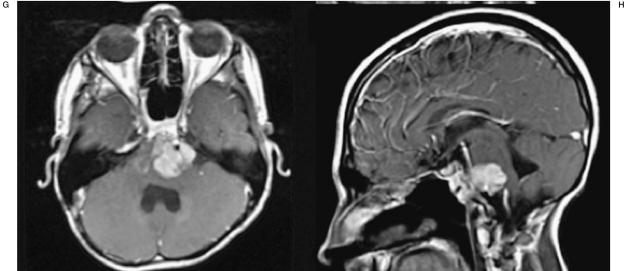
Axial and sagittal T1 post-contrast MRI images prior to and during treatment with SIACI of bevacizumab and cetuximab. A,B) Pre-transclival surgical resection showing tumor burden. C,D) 1-month post-transclival surgical resection approximately 1-month before the first SIACI chemotherapy treatment. E,F) 1-month post first SIACI of chemotherapy treatment. G,H) 1-month post second SIACI of chemotherapy treatment showing stable disease.
Technical aspects of the balloon-assisted super-selective intra-arterial procedure
After placing the patient in a supine position and sterile prepping of the bilateral groin region, the right common femoral artery was accessed with a 19 gauge needle, which was exchanged for a 6 French sheath for the remainder of the procedure.
The sheath was connected to a continuous heparin saline flush. Under fluoroscopic guidance, a 6 French Envoy catheter was advanced over a 0.035" angled Glide guidewire through the abdominal and thoracic aorta into the left vertebral artery. PA and lateral projections of the cranium demonstrated anterograde flow into the left vertebral artery, basilar artery, and accompanying branches, including the posterior cerebral arteries.
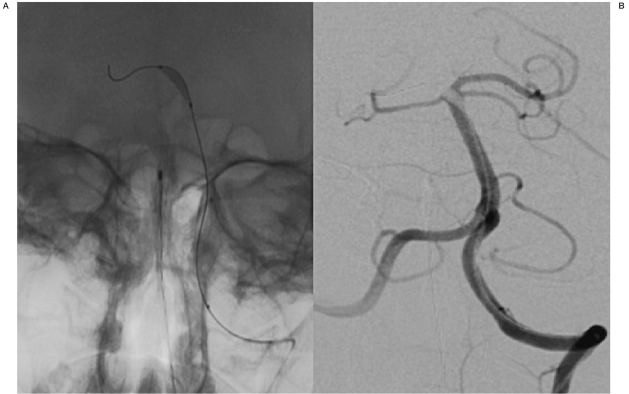
Using the roadmap technique an Excelsior SL 10 microcatheter with a 45 degree angle was advanced over a Synchro 2 soft microwire into the basilar artery along with a hyperglide 3×10 balloon(Figure 2-A). This was positioned into the basilar tip and right posterior cerebral artery. The balloon was then inflated and PA and lateral projections of the left vertebral artery indicated anterograde flow within the basilar artery (Figure 2B).
Figure 2.
A) AP x-ray of the Excelsior SL-10 microcatheter with a 45-degree angle over a Synchro-2 soft microwire with a 3×10 Hyperglide balloon. B) AP left vertebral artery angiogram visualizing the 3×10 Hyperglide balloon positioned into the basilar tip and right posterior cerebral artery. Of note is the anterograde flow throughout the vertebro-basilar system after balloon inflation.
The microwire within the microcatheter was then removed and infusion of 10 cc of 25% mannitol was initiated, this lasted for two minutes. Following this, PA and lateral projections of the basilar artery demonstrated normal anterograde flow with no evidence of vascular injury. Next, 25.8 mL (15 mg/kg) of bevacizumab solution was infused for four minutes via the microcatheter while the balloon was partially inflated.
Post-infusion angiography showed normal anterograde flow through the basilar artery and bilateral posterior cerebral arteries and showed no evidence of vascular injury. The same process was repeated with 72 mL of cetuximab (100 mg/m2) and post infusion angiography again demonstrated normal anterograde flow through the basilar artery including the posterior cerebral arteries and bilateral superior cerebellar arteries confirming no evidence of vascular injury.
Under fluoroscopic guidance, the microcatheter and microwire were removed as well as the balloon and its respective microwire.
PA and lateral projections via the guide catheter demonstrated normal anterograde flow through the basilar artery, bilateral posterior cerebral arteries, and bilateral superior cerebellar arteries. Once again, there was no evidence of vascular injury. After the sheath and guide catheter were removed hemostasis was established by manual compression.
Results
Two months following treatment, MRI demonstrated a decrease in the residual disease (Figure 1E,F). Positron emission tomography (PET) scan also confirmed decreased activity within the tumor. As a result of his positive tumor response, the patient received two more dual agent infusions one month and three months after the first treatment, respectively. The patient is still alive with disease.
Discussion
A standard of care for recurrent pediatric ependymoma has yet to be established and the search for an optimal treatment scheme continues. A multidisciplinary approach is most often utilized to achieve disease control. In many cases, optimizing surgical resection based on tumor location along with the use of systemic chemotherapy and radiation therapy serve as the mainstay for the current treatment arms for this disease. With poor response rates to chemotherapy, biological agents are being studied to augment multiple surgical resections as the primary means of achieving disease control in multiply recurrent disease.
Extent of surgical resection followed by directed radiation therapy (RT) is believed to be one of the most important factors in managing this disease, resulting in five year PFS at 50-60%. Recent evidence from Toronto's Hospital for Sick Children and the Children's Hospital of Philadelphia (CHOP) support these data showing that children up to the age of 16 years with infratentorial ependymoma have an overall survival (OS) of 87% (n=9) with gross total resection (GTR) compared to 30% (n=32) for those with subtotal resection (STR). A similar trend was reported by a study at CHOP showing five year PFS among patients with intracranial ependymoma up to 20 years of age at 60% (n=23) for patients with GTR versus 21% (n=5) with STR. The data from these studies highlight the importance of surgical resection in correlation to long-term PFS and OS.
Disease control utilizing adjuvant systemic chemotherapy and radiation therapy in combination have failed to show active response in ependymomas. The children's cancer group (CCG) performed a prospective study following patients with ependymoma up to the age of 16 years demonstrating no improvement in outcomes of patients treated with post-operative craniospinal radiation and one year of the adjuvant chemotherapy treatment scheme of lomustine (CCNU), vincristine, and prednisone 19. Furthermore, the OS for those two groups was at 36% and 39%, respectively. The role of chemotherapy in the treatment of ependymoma remains unconvincing when using conventional agents.
The dismal response rate with a single agent (12.9%) and combination chemotherapy agents (17.4%) further illustrates the necessity for molecular characterization of the genomic variability of ependymoma and subsequent identification of potential target sites for super-selective drug delivery. While bevacizumab has shown to be active against ependymoma in adult data, there are limitations concerning systemic toxicity when using this agent. Recent phase II trials with IV bevacizumab have reported systemic toxicity relating to infections, wound-healing problems, and embolic events in 9.5%, 3.6%, and 2.4%, respectively. Furthermore, at least a Grade 3 toxicity was identified in 46.4% of patients receiving IV bevacizumab when used as a single agent therapy.
Considering the systemic toxicity of newer molecularly targeted agents, the use of an intra-arterial drug delivery system has been shown to be effective in cerebral disease in both animal glioma models as well as humans. These new drug delivery systems are being refined in order to effectively place these agents into the tumor bed and maintain a localized high concentration via an intra-arterial route. Studies describing techniques for blood-brain barrier disruption with mannitol, and concurrent use of intra-arterially delivered chemotherapeutic agents have allowed for relative circumvention of previously mentioned systemic toxicities 21.
We report here the first case of balloon–assisted SIACI of bevacizumab and cetuximab for a case of multiply recurrent pediatric ependymoma. In this particular case, the patient had been afforded multi-modality treatments for the past 13 years including multiple resections and numerous cycles of chemotherapy but still had explosive disease recurrence. The patient was offered SIACI of bevacizumab and cetixumab as a salvage therapy with a view to achieve a relative measure of disease control. We hoped to improve the patient's quality of life as each recurrence resulted in brainstem compression and severe lower cranial nerve dysfunction with associated speech and swallowing difficulty. We postulated that utilizing both bevacizumab and cetuximab would allow for synergistic growth inhibition of the tumor at the molecular level. After his first treatment with SIACI of dual chemotherapy agents, the patient experienced resolution of his lower cranial nerve dysfunction and returned to normal activities of daily living. While initial post-operative MRI scans demonstrated the tumor to be responsive to this treatment scheme, more recent scans have shown stable, residual disease after subsequent SIACIs of dual agent chemotherapy (Figure 1G,H).
Conclusion
Tailored agents that are able to act on target sites from molecularly characterized tumor specimens and super-selective therapeutic delivery will enhance future treatment paradigms focused on achieving maximal response rates. Endovascular techniques and disruption of the BBB allow for localized infusion of chemotherapy agents. This technique, as a delivery method for molecularly targeted agents, must be studied prospectively in the pediatric population as it may change the current dynamic in which chemotherapeutics are delivered for both primary and recurrent disease. We believe a larger cohort study will prove this technique to be of value in delivering biological agents as both a mainstay treatment and for augmentation of surgical resection, reinforcing the importance of a multi-modality approach to treating recurrent ependymomas.
References
- 1.Wright KD, Gajjar A. New chemotherapy strategies and biological agents in the treatment of childhood ependymoma. Childs Nerv Syst. 2009;25(10):1275–82. doi: 10.1007/s00381-009-0809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karajannis M, Allen JC, Newcomb EW. Treatment of pediatric brain tumors. J Cell Physiol. 2008;217:584–589. doi: 10.1002/jcp.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson P.L, Zeltzer PM, Boyett JM, et al. Survival and prognostic factors following radiation therapy and chemotherapy for ependymomas in children: a report of the Children’s Cancer Group. J Neurosurg. 1998;88:695–703. doi: 10.3171/jns.1998.88.4.0695. [DOI] [PubMed] [Google Scholar]
- 4.Sutton LN, Goldwein J, Perilongo G, et al. Prognostic factors in childhood ependymomas. Pediatr Neurosurg. 1990;16:57–65. doi: 10.1159/000120509. [DOI] [PubMed] [Google Scholar]
- 5.Goldwein JW, Glauser TA, Packer RJ, et al. Recurrent intracranial ependymomas in children. Survival, patterns of failure, and prognostic factors. Cancer. 1990;66:557–563. doi: 10.1002/1097-0142(19900801)66:3<557::aid-cncr2820660325>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Mason WP, Goldman S, Yates AJ, et al. Survival following intensive chemotherapy with bone marrow reconstitution for children with recurrent intracranial ependymoma a report of the Children’s Cancer Group. J Neurooncol. 1998;37:135–143. doi: 10.1023/a:1005980206723. [DOI] [PubMed] [Google Scholar]
- 7.Strother DR PI FP, Hunter JV, Woo SY, et al. Principles and practice of pediatric oncology. New York: Lipincott Williams & Wilkins; 2003. Tumors of the central nervous system; pp. 778–785. [Google Scholar]
- 8.Bouffet E, Foreman N. Chemotherapy for intracranial ependymomas. Childs Nerv Syst. 1999;15:563–570. doi: 10.1007/s003810050544. [DOI] [PubMed] [Google Scholar]
- 9.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nature reviews. Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 10.Kerbel R.S. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 11.Riina HA, Knopman J, Greenfield JP, et al. Balloon-assisted superselective intra-arterial cerebral infusion of bevacizumab for malignant brainstem glioma. A technical note. Interventional Neuroradiology. 2010;16:71–76. doi: 10.1177/159101991001600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 13.Green RM, Cloughesey TF, Stupp R, et al. Bevacizumab for recurrent ependymoma. Neurology. 2009;73:1677–16780. doi: 10.1212/WNL.0b013e3181c1df34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pediatric Brain Tumor Consortium; National Cancer Institute (NCI) ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. MK0752 in treating young patients with recurrent or refractory cns cancer. [cited 20110725]. Available from: URL of the record NLM Identifier: NCT00572182. [Google Scholar]
- 15.Horn B, Heideman R, Geyer R, et al. A multi-institutional retrospective study of intracranial ependymoma in children: identification of risk factors. J Pediatr Hematol Oncol. 1999;21:203–211. doi: 10.1097/00043426-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Pollack IF, Gertszten PC, Martinez AJ, et al. Intracranial ependymomas of childhood: long-term outcome and prognostic factors. Neurosurgery. 1995;37:655–666. doi: 10.1227/00006123-199510000-00008. discussion 666-667. [DOI] [PubMed] [Google Scholar]
- 17.Merchant TE. Current management of childhood ependymoma. Oncology. 2002;16:629–642. 644 discussion 645-646, 648. [PubMed] [Google Scholar]
- 18.Nazar GB, Hoffman HJ, Becker LE, et al. Infratentorial ependymomas in childhood: prognostic factors and treatment. J Neurosurg. 1990;72:408–417. doi: 10.3171/jns.1990.72.3.0408. [DOI] [PubMed] [Google Scholar]
- 19.Evans AE, Anderson JR, Lefkowitz-Boudreaux IB, et al. Adjuvant chemotherapy of childhood posterior fossa ependymoma: cranio-spinal irradiation with or without adjuvant CCNU, vincristine, and prednisone: a Childrens Cancer Group study. Med Pediatr Oncol. 1996;27(1):8–14. doi: 10.1002/(SICI)1096-911X(199607)27:1<8::AID-MPO3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.Bouffet E, Capra M, Bartels U. Salvage chemotherapy for metastatic and recurrent ependymoma of childhood. Childs Nerv Syst. 2009;25(10):1293–1301. doi: 10.1007/s00381-009-0883-x. [DOI] [PubMed] [Google Scholar]
- 21.Gobin YP, Cloughesey TF, Chow KL, et al. Intraarterial chemotherapy for brain tumors by using a spatial dose fractionation algorithm and pulsatile delivery. Radiology. 2001;218(3):724–732. doi: 10.1148/radiology.218.3.r01mr41724. [DOI] [PubMed] [Google Scholar]