Summary
Percutaneous injection of embolization material within head and neck tumors is being described as an alternative or adjunct to transarterial embolization. Access in these reports is by computed tomography (CT) guidance, which is cumbersome given the need to transport the patient from the CT scanner to angiography suite. We describe a case of direct percutaneous onyx embolization of juvenile nasal angiofibroma following endoscopic access in the angiography suite including self-sustained onyx combustion during surgical electrocautery.
Key words: juvenile nasal angiofibroma, onyx, embolization
Introduction
Juvenile nasopharyngeal angiofibromas (JNA) are the most common benign tumor of the nasopharynx primarily affecting young adolescent males with a multimodality approach being employed towards treatment 1-2. Preoperative embolization with the transarterial technique has been described as a means to limit operative blood loss, facilitate tumor removal, and reduce mortality and morbidity 1-4. Percutaneous embolization with a variety of agents has been described as providing the advantage of direct access to the tumor vasculature to allow for more efficient tumor devascularization 5-12. Onyx (ev3, Plymouth, MN, USA) has been described more recently as a percutaneous agent providing the potential advantage of delayed polymerization allowing for deeper penetration particularly within larger tumors 5-9,12. To our knowledge, the use of onyx in the treatment of JNA is limited 5-6. We present a case report describing direct endoscopic intratumoral use of onyx for the treatment of JNA.
Case Report
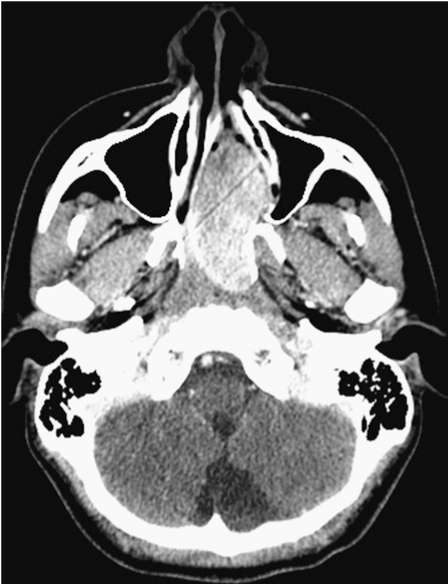
A 16-year-old male status post prior embolization and surgical removal of a nasopharyngeal angiofibroma nine months prior presented with recurrent epistaxis. CT demonstrated an avidly enhancing mass lesion with the left nasal cavity which demonstrated significant mass effect upon the nasal septum, antrum and pterygoid plates (Figure 1). Preoperative CT angiogram demonstrated multiple vessels supplying the mass from the bilateral maxillary, ascending pharyngeal and facial arteries. Findings were consistent with recurrence of a JNA. Given the need for more extensive devascularization, the decision was made for percutaneous onyx embolization with endoscopic access by otolaryngology.
Figure 1.
Contrast-enhanced CT demonstrated avidly enhancing mass within the left nasal cavity consistent with recurrent juvenile nasal angiofibroma.
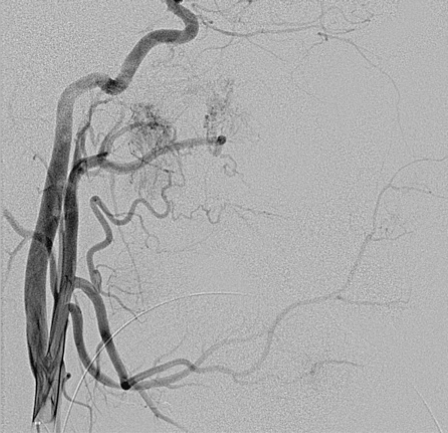
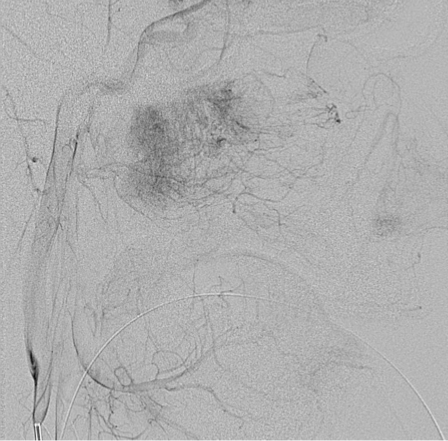
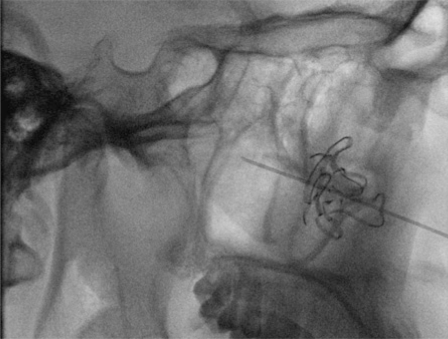
A transfemoral approach was used to insert a 6F guiding catheter into the carotid and vertebral arteries. Preembolization angiography using a biplane angiography unit demonstrated vascular supply via the bilateral maxillary, ascending pharyngeal and facial arteries (Figures 2 and 3). The tumor was accessed endoscopically with a micropuncture needle and blood return was achieved (Figure 4). A Marathon microcatheter (ev3, Plymouth, MN, USA) was advanced through the micropuncture and attached with a luer lock. Contrast was injected through the microcatheter for confirmation of position and then flushed with saline and dimethyl sulfoxide. Under fluoroscopic guidance and roadmapping, embolization was performed with Onyx 18. To access the posterior aspect of the tumor, another micropuncture needle was inserted endoscopically and similarly embolized with Onyx 18. A total of 12 ml of Onyx 18 was injected (Figure 5). Subsequently, embolization was then performed with polyvinyl alcohol particles of 150-250 micrometers (Boston Scientific, Natick, MA, USA) of the left sphenopalatine artery. A follow-up angiogram demonstrated greater than 95% vascular occlusion (Figure 6). Total intraprocedural time including endoscopic access and needle placement was approximately three hours. Following, the embolization procedure, the tumor was removed as a two stage operation with approximately 200 ml of blood loss noted (Figure 7). Histology following resection demonstrated the onyx to be within the lumen of the blood vessels and not within the interstitium (Figure 8).
Figure 2.
A left common carotid artery injection demonstrates vascular supply via maxillary, ascending pharyngeal and facial arteries.
Figure 3.
Later phase imaging from a left common carotid artery demonstrates the extent of tumor supplied from branches from the left external carotid artery.
Figure 4.
Lateral fluoroscopic projection demonstrates direct insertion of the micropuncture needle within the tumor bed.
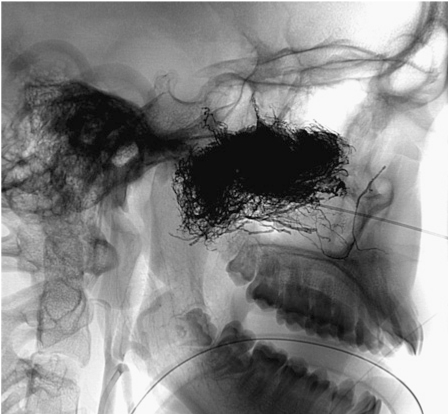
Figure 5.
Lateral fluoroscopic projection demonstrates the embolized tumor within the onyx cast.
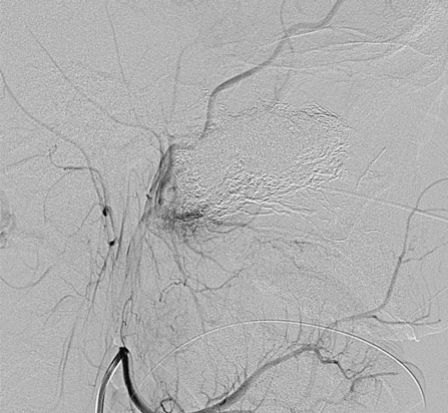
Figure 6.
A left external carotid injection demonstrates a small residual tumor blush along the postero-inferior aspect of the tumor bed.
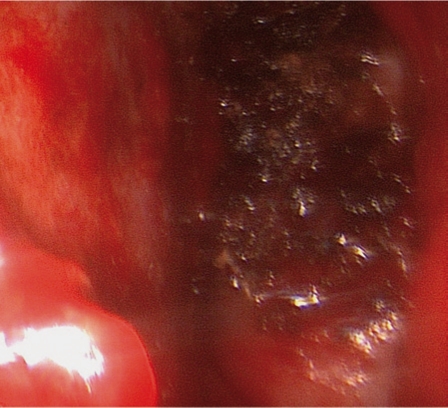
Figure 7.
Endoscopic view of the tumor during resection.
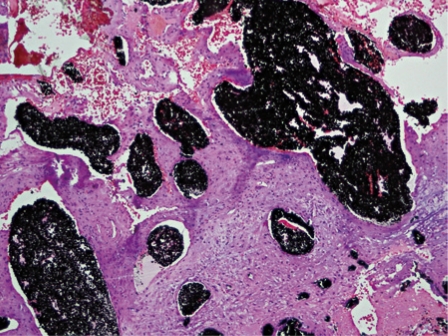
Figure 8.
Photograph following hematoxylin and eosin staining demonstrates onyx distributed diffusely throughout the vessel lumens.
Discussion
Given the propensity of hypervascular tumors of the head and neck to bleed, endovascular techniques have become ever more prevalent as a preoperative measure to reduce intraoperative blood loss, shorten surgical time, facilitate tumor removal, and reduce mortality and morbidity 1-4. Transarterial embolization of hypervascular tumors of the head and neck have been well documented 2-4. However, this technique remains time consuming with long radiation exposure times given the multiple tortuous feeding vessels which are encountered. Also, the potential anastomoses between the several external carotid arteries with internal carotid and vertebral arteries portend additional risk.
One of the primary advantages of a percutaneous approach is to allow for easier access to the tumor, thus allowing for occlusion of the tumor vasculature rather than the arterial feeders. Consequently, this method may provide a more effective devascularization than the endovascular technique. Pierot et al. and Casasco et al. first described direct percutaneous injection of hypervascular head and neck tumors utilizing glue in 1994 13-14. Since then multiple additional reports have described the utility of cyanoacrylate with promising results 10.
The technical feasibility and success of such percutaneous approaches, as well as low rates of complications are well documented. Nevertheless, severe complications, including glue migration into the middle cerebral artery and the ophthalmic artery, have been reported 15. In addition, Krishnamoorthy et al. reported evidence of delayed stroke secondary to glue migration following direct injection of a carotid body tumor 16. Thus, it is especially important to recognize potential feeding vessels and anastomoses that exist between the internal and external carotid arteries as well as between the external carotid and vertebral arteries. Many authors have demonstrated the use of nondetachable balloons to prevent untoward migration of embolic agent 7-8,10,16. With our case, preembolization angiography and parenchymography did not demonstrate feeders from the internal carotid artery. Thus, temporary balloon occlusion was not felt to be necessary. Moreover, the delayed polymerization of Onyx allows for a slower and potentially more controlled injection. Nevertheless, knowledge of the regional anatomy is crucial for safe injection of onyx.
More recently, onyx has been utilized for the percutaneous treatment of head and neck tumors often in conjunction with an endovascular approach utilizing particle embolization 5-9,12. Recently, Gemmete et al. described direct access to the tumor obtained under CT guidance 5. We felt that a direct endoscopic approach would provide for easier access in cases which allow direct endoscopic visualization.
Of note, spark showers and self-sustained combustion occurred intraoperatively. This electrocautery-induced combustion has been reported in both in vivo and in vitro settings. The tantalum powder is hypothesized as the source of flammability. To our knowledge, there have only been limited reports of this potentially dangerous property of Onyx with our case being the first outside of the central nervous system 17. Schirmer et al. further investigated this property of onyx and found sparking and self-contained combustion to be more readily producible using monopolar rather than bipolar electrocautery 17. This is especially relevant to otolaryngology as the surgery is generally performed with monopolar electrocautery. Furthermore, given the larger quantities of onyx for tumor embolization, surgeons should take greater caution with electrocautery. However, distinct advantages of Onyx do exist including angiographically equivalent if not superior embolization.
Moreover, the physical characteristics of onyx lend itself to have a spongy consistency allowing for better surgical handling. Akin et al. demonstrated the histologic findings following intravascular embolization with Onyx in a swine model 18. To our knowledge, no study exists demonstrates the pathologic findings following percutaneous embolization. Following resection, the Onyx was distributed diffusely throughout the vessel lumens without being within the interstitium. Thus, in theory, it can be suggested that the percutaneous technique with onyx embolization does at least provide for histologically similar findings as does embolization with endovascular technique.
In conclusion, percutaneous endoscopic tumor embolization provides an easier alternative to CT guided needle placement. Furthermore, the percutaneous technique has been shown to provide as an alternative to the endovascular technique and may allow for a more complete devascularization as demonstrated angiographically and intraoperatively with histologically similar results. However, given the combustion of Onyx and larger quantities utilized, surgeons should use refrain from monopolar electrocautery during resection.
References
- 1.Roche PH, Paris J, Regis J, et al. Management of invasive juvenile nasopharyngeal angiofibromas: the role of a multimodality approach. Neurosurgery. 2007;61:768–77. doi: 10.1227/01.NEU.0000298905.71259.BB. [DOI] [PubMed] [Google Scholar]
- 2.Moulin G, Chagnaud C, Gras R, et al. Juvenile nasopharyngeal angiofibroma: comparison of blood loss during removal in embolized group versus nonembolized group. Cardiovasc Intervent Radiol. 1995;18:158–161. doi: 10.1007/BF00204142. [DOI] [PubMed] [Google Scholar]
- 3.Valavanis A. Preoperative embolization of the head and neck: indications, patient selections, goals, and precautions. Am J Neuroradiol. 1986:7943–7952. [PMC free article] [PubMed] [Google Scholar]
- 4.Persky MS, Setton A, Niimi Y, et al. Combined endovascular and surgical treatment of head and neck paragangliomas: a team approach. Head Neck. 2002;24:423–431. doi: 10.1002/hed.10068. [DOI] [PubMed] [Google Scholar]
- 5.Gemmete JJ, Chaudhary N, Pandey A, et al. Usefulness of percutaneously injected ethylene-vinyl alcohol copolymer in conjunction with standard endovascular embolization techniques for preoperative devascularization of hypervascular head and neck tumors: technique, initial experience, and correlation with surgical observations. Am J Neuroradiol. 2010;31:961–966. doi: 10.3174/ajnr.A1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman B, Bublik M, Ruiz J, et al. Endoscopic embolization with onyx prior to resection of JNA: a new approach. Int J Ped Otorhinolaryngol. 2011;75:53–56. doi: 10.1016/j.ijporl.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Wanke I, Jackel MC, Goericke S, et al. Percutaneous embolization of carotid paragangliomas using solely onyx. Am J Neuroradiol. 2009;30:1594–1597. doi: 10.3174/ajnr.A1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozyer U, Harman A, Yildirim E, et al. Devascularization of head and neck paragangliomas by direct percutaneous embolization. Cardiovasc Intervent Radiol. 2010;33:967–975. doi: 10.1007/s00270-010-9803-4. [DOI] [PubMed] [Google Scholar]
- 9.Quadros RS, Gallas S, Delcourt C, et al. Preoperative embolization of a cervicodorsal paraganglioma by direct percutaneous injection of onyx and endovascular delivery of particles. Am J Neuroradiol. 2006;27:1907–1909. [PMC free article] [PubMed] [Google Scholar]
- 10.Abud DG, Mounayer C, Benndorf G, et al. Intratumoral injection of cyanoacrylate glue in head and neck paragangliomas. Am J Neuroradiol. 2004;25:1457–1462. [PMC free article] [PubMed] [Google Scholar]
- 11.Chaloupka JC, Mangla S, Huddle DC, et al. Evolving experience with direct puncture therapeutic embolization for adjunctive and palliative management of head and neck hypervascular neoplasms. Laryngoscope. 1999;109:1864–1872. doi: 10.1097/00005537-199911000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Gobin YP, Muratama Y, Milanes K, et al. Head and neck hypervascular lesions: embolizations with ethylene vinyl alcohol copolymer- laboratory evaluation in swine and clinical evaluation in humans. Radiology. 2001;221:309–317. doi: 10.1148/radiol.2212001140. [DOI] [PubMed] [Google Scholar]
- 13.Pierot L, Boulin A, Castaings L, et al. Embolization by direct puncture of hypervascularized oral tumors. Ann Otolaryngol Chir Cervicofac. 1999;111:403–409. [PubMed] [Google Scholar]
- 14.Casasco A, Herbreteau D, Houdart E, et al. Devascularization of craniofacial tumors by percutaneous tumor puncture. Am J Neuroradiol. 1994;15:1233–1239. [PMC free article] [PubMed] [Google Scholar]
- 15.Casasco A, Houdart E, Biondi A, et al. Major complications of percutaneous embolization of skull-base tumors. Am J Neuroradiol. 1999;20:179–181. [PubMed] [Google Scholar]
- 16.Krishnamoororthy T, Gupta AK, Rajan JE, et al. Stroke from delayed embolization of polymerized glue following percutaneous direct injection of a carotid body tumor. Korean J Radiol. 2007;8:249–253. doi: 10.3348/kjr.2007.8.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schirmer CM, Seriis V, Malek AM. Electrocautery-induced ignition of spark showers and self-sustained combustion on onyx ethylene-vinyl alcohol copolymer. Oper Neurosurg. 2006;59:413–419. doi: 10.1227/01.NEU.0000240683.15391.99. [DOI] [PubMed] [Google Scholar]
- 18.Akin ED, Perkins E, Ross IB. Surgical handling characteristics of an ethylene vinyl alcohol copolymer compared with n-butyl cyanoacrylate used for embolization of vessels in an arteriovenous malformation resection model in swine. J Neurosurg. 2003;98:366–370. doi: 10.3171/jns.2003.98.2.0366. [DOI] [PubMed] [Google Scholar]