Abstract
Background
Streptococcus pneumoniae (Spn) is one of the common bacteria responsible for episodic acute otitis media (AOM; non-otitis prone), recurrent AOM (otitis-prone) and AOM treatment failure (AOMTF) in children.
Objective
From a population of 268 children we sought to compare the serum IgG antibody titers to five different Spn proteins (PhtD, LytB, PcpA, PhtE and Ply) that are vaccine candidates in children with episodic AOM (n=34), who were otitis prone (n=35), and who had AOMTF (n=25) caused by Spn.
Methods
Antibody was quantitated by ELISA.
Results
At their acute AOM visit, anti-PhtD, -LytB, -PhtE and −Ply IgG antibody titers in otitis-prone children were significantly lower compared to non-otitis prone children (p <0.05) and children with AOMTF (p <0.05). Comparing acute to convalescent titers of antibody after AOM we found that otitis-prone, AOMTF and non-otitis prone children had no significant change in geometric mean IgG antibody titers against the five proteins (except for PhtE in children with AOMTF), but detailed analysis showed that about one-third of the children in each cohort had a 2-fold rise in antibody to the studied antigens. While non-otitis prone children had significant increases (p <0.001) between 6 and 24 months of age in anti-PhtD, PcpA, PhtE and Ply IgG antibody titers as a consequence of nasopharyngeal colonization and AOM, otitis-prone children either failed to show rises or the rises were significantly less than the non-otitis prone children.
Conclusion
Otitis-prone and AOMTF children mount less of an IgG serum antibody response than non-otitis prone children to Spn proteins following AOM and nasopharyngeal colonization.
Keywords: S. pneumoniae, PhtD, PhtE, Ply, PcpA, LytB, Acute otitis media, Otitis-prone
Introduction
Streptococcus pneumoniae (Spn) is the most common bacterial pathogen causing acute otitis media (AOM) in children and is commonly carried asymptomatically in the nasopharynx.1,2 Spn also causes pneumonia, bacteremia, and meningitis.3,4 Currently available pneumococcal vaccines induce serotype-specific immunity; and although safe, erosion of efficacy occurs over time as new serotypes emerge to replace those serotypes included in the vaccines.5 Therefore, alternative and complimentary vaccines are being developed based on Spn proteins that contribute to virulence and are common to all serotypes.
Several pneumococcal proteins considered to be potential vaccine candidates include poly-histidine triad proteins, choline-binding proteins, murein hydrolases and non-toxic derivatives of pneumolysin.6,7 This study focuses on five such proteins: PhtD and PhtE (pneumococcal histidine triad proteins), PcpA (a choline binding protein), LytB (a murein hydrolases) and PlyD1 (a non-toxic pneumolysin derivative). Pht proteins have been shown to be involved in the inhibition of complement deposition 8 and elicit protection against pneumococcal systemic infection in an animal model.9 LytB has been shown to be responsible for the cell separation after cell division.10,11 Surface protein PcpA has been shown to elicit protection against lung infection and sepsis in animal model.12 Pneumolysin (Ply) has a wide range of cytotoxic and inhibitory effects on host tissue and immune cells 13 and it has been shown that antibody to Ply may protect against bacteremia.14 The pneumolysin derivative used here (PlyD1) has three point mutations that do not interfere with anti-pneumolysin antibody responses.
In the present study, we compare the development of serum IgG antibodies to PhtD, PhtE, LytB, PcpA and Ply among three groups of 6 to 36 month old children with AOM: 1) an otitis prone group that included children who had 3 or more episodes of AOM in 6 months or 4 or more episodes in a 12 month period; 2) an AOM treatment failure (AOMTF) group that included children who failed to achieve bacterial eradication and resolution of symptoms after at least 48 hours of appropriate antibiotic therapy 15,16 and children whose signs and symptoms of AOM returned within 14 days of completing an antibiotic treatment course; and, 3) a non-otitis prone group that included children who had only one or two episodes of AOM.
Methods
Patient population
The samples collected and analyzed were obtained during a prospective study supported by the National Institutes of Deafness and Communication Disorders, as previously described.17 Children were enrolled from a middle class, suburban socio-demographic pediatric practice in Rochester, NY (Legacy Pediatrics). The study was approved by the University of Rochester and Rochester General Hospital Research Subjects Review Boards and written informed consent was obtained for participation and all procedures.
From a population of 258 children during Jul 2006 to Aug 2009, we identified children with episodic AOM (n=34), children who were otitis prone (n=35), and children who had AOMTF (n=25), with the studied episode caused by Spn. One hundred sixty-eight children were enrolled at age 6 months and followed prospectively until 30 months of age. Serum, Nasopharyngeal (NP) and oropharyngeal (OP) cultures were obtained seven times during the study period at age 6, 9, 12, 15, 18, 24 and 30 months. During the study period whenever children in this group experienced an AOM, serum, NP and OP cultures were obtained along with middle ear fluid (MEF) by tympanocentesis. Convalescent samples of serum were collected three weeks later. Ninety additional children were enrolled at the time of an AOM episode that was either an AOMTF or the child met the definition of otitis prone.
Microbiology
MEF, NP, and OP samples were inoculated into trypticase soy broth, trypticase soy agar with 5% sheep blood plates, and chocolate agar plates. The bacterial isolates from MEF and nasopharyngeal were identified as Spn and identification tests were performed according to instructions described in the 8th edition of Manual of Clinic Microbiology.18
ELISA assay
Protein specific antibody titers were determined by ELISA using purified recombinant proteins (provided by Sanofi Pasteur). The PhtE, PcpA and LytB proteins used here were in a truncated form. The 96-well Nunc-Immulon 4 plates were coated with 0.5μg/ml of individual proteins (100μl/well) in bicarbonate coating buffer (pH 9.4) and incubated overnight at 4°C and ELISA assays were performed as described previously.19 The plates were analyzed at 450 nm on a Spectra max plate reader (Molecular Devices, Sunnyvale, Calif.) using the Softmax end point dilution protocol. The results are reported as end point titers; the starting dilution of sera was 1:4. An in-house positive control serum (mixture of human sera) was run on each plate. The inter-test coefficient of variation was ≤ 30 %.
Statistical analysis
All the statistical analysis was performed on GraphPad Prism 5. Unpaired t test was used to compare the difference among three groups for the IgG antibody analysis. Mann-Whitney test was used to analyze the combined antibody response against five antigens among three groups of children. Paired t test was applied to compare acute vs. convalescence serum samples. One way ANOVA was used to evaluate the antibody rise over time. P values of < 0.05 were considered significant.
Results
Antigen-specific IgG antibody titers against the five Spn proteins in three groups of children at the time of an AOM
Figure 1 shows the IgG antibody titers against PhtD, LytB, PcpA, PhtE and Ply proteins of Spn when measured at the time of an acute AOM caused by Spn in 35 otitis prone children, 25 children with AOMTF and 34 children with their first or second AOM (non-otitis prone group). The IgG antibody titers against PhtD in the otitis prone children were significantly lower compared with age matched non-otitis-prone children (p <0.05). IgG antibody titers to PhtD in AOMTF children were also lower compared with non otitis-prone children but the difference did not achieve significance. IgG antibody titers to LytB in the otitis prone children and AOMTF children were significantly lower compared to non-otitis prone children (p <0.001 for both comparisons). IgG antibody titers to PcpA in the otitis prone and AOMTF children were almost 3 fold lower compared with non-otitis prone children but the difference was not significant among the 3 groups of children due to wide variation in titers of antibody. IgG antibody titers to PhtE in the otitis-prone children and AOMTF children were significantly lower compared to non-otitis prone children (p <0.001). IgG antibody titers to Ply were significantly lower in the otitis prone children (p = 0.006) and AOMTF children (p = 0.02) compared with non-otitis prone children. Lastly, the combination of IgG antibody titers to all the five antigens was significantly lower in the otitis prone (p <0.001) and AOMTF (p <0.001) children compared to non-otitis prone children.
Figure 1.
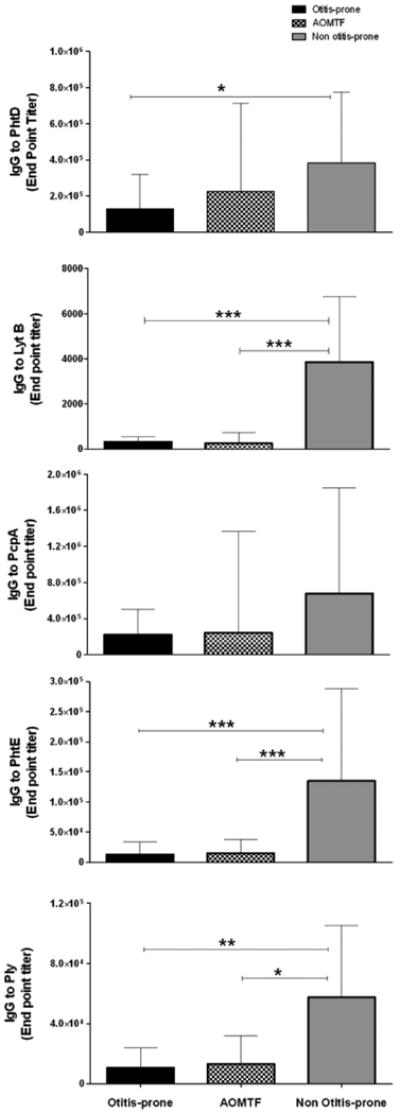
Comparison of IgG antibody in the serum samples of children at their acute visit of AOM in 35 otitis prone, 25 AOMTF and 34 Non-otitis prone children. Note: All the antibody concentrations against five proteins are in end point titers. Lines are shown to indicate significant difference observed between the two groups. *** p value <0.0001, ** p value <0.001, and * p value < 0.05.
Acute and convalescent AOM antibody titers against the five Spn antigens in three groups of children
Twenty-two otitis prone, 13 AOMTF and 20 non-otitis prone children had sufficient paired serum samples obtained at their acute (at the time of AOM) and convalescent stage (3 weeks later) for study. In all three groups of children, geometric mean IgG antibody titers to 4 of the 5 proteins in the acute vs. convalescence stage showed no significant rise in antibody (the exception was PhtE protein in AOMTF children where a significant difference was found, p = 0.04) (Table 1). Wide individual variation of the antibody titers in acute and convalescent stage sera were notable, with about one-third of children in all 3 groups showing two-fold rises in antibody to one or more of the proteins (Table 1); however, in the responders more robust increases in antibody titers were observed in non-otitis prone children (2 to 8 fold) compared to otitis prone children (2 to 3 fold).
Table 1.
Comparison of geometric mean titer of IgG antibody in the serum samples of 22 otitis prone, 13 AOMTF and 20 non-otitis prone children at their acute vs. convalescence stage.
| Acute | Convalescence | >2 fold increase in antibody at convalescence stage | ||
|---|---|---|---|---|
| Proteins | Group (#) of children | |||
| IgG titers (95% Upper & lower confidence interval) | % of children | |||
| Otitis-prone | 1.8×105 (4.1×104-7.92×105) |
1.4×105 (3.9×104-5.1×105) |
24% | |
| PhtD | AOMTF | 7.9×105 (6.3×104-1.0×107) |
8.2×105 (7.7×104-8.7×106) |
15% |
| Non otitis-prone | 3.9×105 (1.2×105-1.3×106) |
6.1×105 (1.8×105-2.0×106) |
35% | |
|
| ||||
| Otitis-prone |
a327 (157-682) |
a275 (115-658) |
20% | |
| LytB | AOMTF |
b260 (30-2275) |
b803 (137-4686) |
33% |
| Non otitis-prone |
a,b4487 (1711-1.1×104) |
a,b5451 (2105-1.4×104) |
33% | |
|
| ||||
| Otitis-prone | 6.6×105 (1.39×105-3.16×106) |
6.8×105 (1.11×105-4.21×106) |
29% | |
| PcpA | AOMTF | 5.1×105 (3.9×104-1.1×107) |
6.9×105 (8.7×104-2.3×107) |
36% |
| Non otitis-prone | 4.8×105 (1.2×105-1.9×106) |
4.6×105 (1.2×105-1.7×106) |
25% | |
|
| ||||
| Otitis-prone |
a1.3×104 (3315-5.8×104) |
a1.4×104 (3474-6.3×104) |
32% | |
| PhtE | AOMTF |
b,
c1.8×104 (3974-8.6×104) |
c2.2×104 (3374-1.4×105) |
23% |
| Non otitis-prone |
a,b1.5×105 (5.2×104-4.5×105) |
a1.1×105 (3.2×104-4.3×105) |
19% | |
|
| ||||
| Otitis-prone |
a1.6×104 (5861-4.4×104) |
8578 (1852-3.9×104) |
40% | |
| Ply | AOMTF | 1.1×104 (2140-6.0×104) |
8534 (1675-4.3×104) |
18% |
| Non otitis-prone |
a6.45×104 (3.4×104-1.2×105) |
5.46×104 (3.0×104-9.6×104) |
0% | |
Significant difference (p value<0.05) found:
Otitis prone vs Non-otitis prone
AOMTF vs Non-otitis prone
Acute vs. convalescence serum
Antibody level in non-otitis prone and otitis-prone children with age
Figure 2 shows the IgG antibody titers against PhtD, LytB, PcpA, PhtE and Ply at the time of routine non-AOM visits in prospectively followed non-otitis prone and otitis prone children at 6-24 months of age. Thirty months of age data are not included in the analysis because too few the children completed this visit. The data shown are from 150 non-otitis prone children and 10 otitis-prone children from the main study cohort. It appears that maternal antibody contributes to the value at 6 months of age based on the observed decrease at 9 months which is then followed by a persistent increase. In the non-otitis prone children the IgG antibody titers rose significantly (p <0.001) between 6 month to 24 months for all the proteins except LytB (p =0.075). In comparison, the otitis prone children did not mount significant changes in IgG antibody titers between 6 to 24 months of age to any of the five proteins (p =0.40 for protein PhtD, p =0.39 for LytB, p =0.11 for PcpA, p =0.09 for PhtE and p =0.42 for Ply). There were only ten children in the otitis prone group for this analysis; this contributed to large confidence intervals surrounding mean antibody titers. Nevertheless, analysis of individual antibody responses from each otitis prone child showed minimal to absent rises for most of the children vs. large rises in antibody in most non-otitis prone children.
Figure 2.
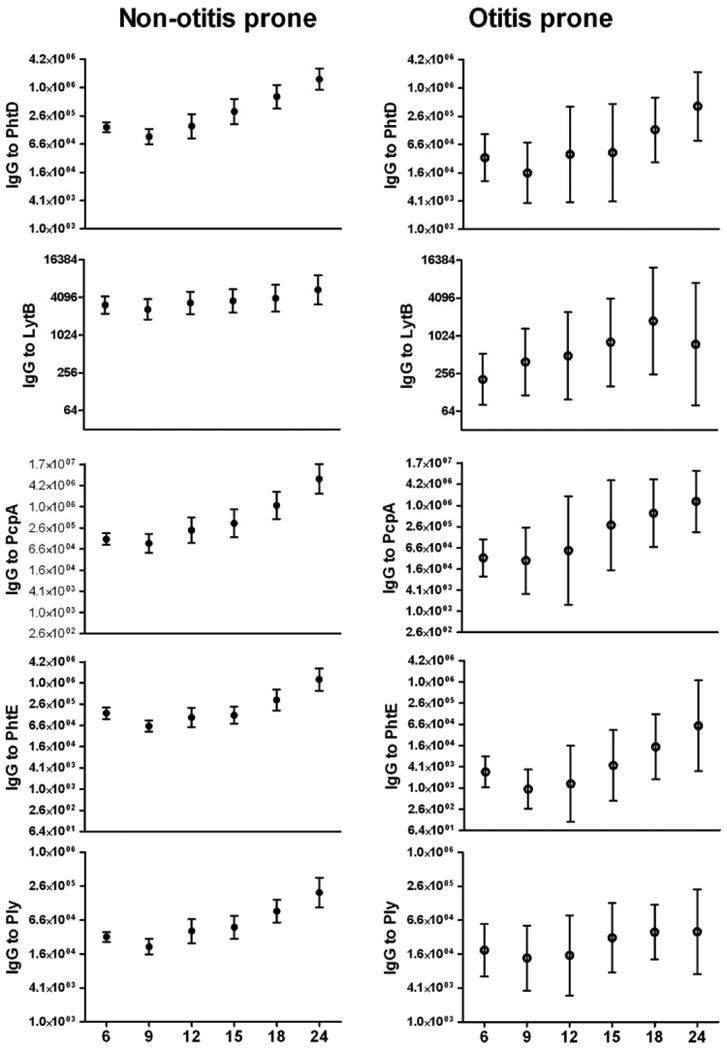
Comparison of IgG antibody titers with age (6-24 months) against five proteins of S. pneumoniae in Non-otitis prone and otitis prone children. The numbers of sera included at 6, 9, 12, 15, 18 and 24 months time points were 107, 88, 65, 61, 55, and 44 respectively for the non-otitis prone children 10, 10, 9, 10, 10 and 4 respectively for the otitis prone children. Significant difference for all the five proteins except LytB (p<0.07), comparing relative rise in IgG serum antibody between 6 to 24 months was found in non-otitis prone children while the difference was not significant in otitis prone children (p= 0.40 for protein PhtD, p = 0.39 for LytB, p = 0.11 for PcpA, p = 0.09 for PhtE and p = 0.42 for Ply).
Discussion
The focus of this study was the serum antibody response to five Spn proteins (PhtD, LytB, PcpA, PhtE and Ply) that are potential vaccine candidates among otitis prone children in comparison to non-otitis prone children. We also studied immune responses in children who met the definition of AOMTF. We found that otitis prone children and children with AOMTF behave similarly and have significantly lower antibody titers to Spn proteins at the onset of AOM compared to age matched non-otitis prone children, suggesting that prior exposures to Spn did not elicit or elicited a less robust adaptive immune response as reflected in serum antibody titers. We also found that otitis prone children increase the amount of serum antibody to the 5 Spn antigens significantly more slowly than non-otitis prone children as a consequence of NP colonization and AOM events; this observation is consistent with an impaired immune response among otitis prone children following otopathogen exposure. This study adds specific new information on the same cohort of children that we have studied regarding development of serum antibody to Non-typeable Haemophilus influenzae (NTHi) proteins D, P6 and OMP26 in otitis prone children.19
The antigen specific immune responses observed against Spn confirm and extend the observations of others for otitis prone children, contradict some earlier reports and provide much new data. Freijd et al20 found significantly lower serum anti-Spn polysaccharide antibody to serotypes 6A and 23 but not to serotype 3 among otitis prone children. Prellner et al21 failed to observe detectable serum anti-Spn polysaccharide antibody to serotypes 6A, 19 and 23 in 60% of the otitis prone children in their study. Even at 6 years of age the anti 6A antibody titers in otitis prone children were lower than non-otitis prone children. Hotomi et al22 observed lower antibody response to NTHi OMP P6 and Spn polysaccharide (23 valent Spn vaccine as antigen) in 55% and 48% of otitis prone children respectively. Yamanaka and Faden in their 1993 studies 23,24 and Bernstein et al25 found similar diminished serum and/or mucosal antibody titers to NTHi, in otitis prone children. To our knowledge this is the first report of serum antibody responses to Spn proteins in otitis prone and in AOMTF children.
Our observations regarding anti- PhtD, LytB, PcpA, PhtE and Ply antibody responses in otitis prone children supports the generally held notion that otitis prone children have a specific immunologic deficiency in antibody response to Spn and other otopathogens antigens when the exposure occurs via the natural NP route. Specifically, in 1979 Giebink et al 26 reported that children with recurrent AOM had polymorphonuclear leukocyte dysfunction. Freijd et al found lower IgG2 titers in otitis prone children.27 Veenhoven et al also reported lower serum IgG, IgA, IgM, IgG1 and IgG2 in otitis-prone children.28 In contrast, Berman et al found no significant differences in total IgG or subclasses IgG1, IgG2, IgG3 or IgG4 in otitis prone children.29
The mechanism for the immune dysfunction among otitis prone children is a topic under study by our group. We have recently observed that otitis prone children have a deficiency in functional T helper and T memory cells in response to Spn and NTHi antigens (manuscript submitted). We studied these children for responses to parenteral vaccination with diphtheria, tetanus and pertussis and found significant reduced antibody titers to vaccine in otitis prone children (unpublished results). Prellner et al 30 also found lower antibody responses to rubella but not to tetanus and diphtheria vaccine in otitis prone children. Wiertsma et al 31 found no difference in otitis prone and non-otitis prone children against diphtheria, tetanus and Haemophilus influenzae type b (Hib) vaccine. The mixed results of these various reports leaves the question open as to whether the immune dysfunction in otitis prone children occurs only following natural exposure to otopathogens or may also occur following parenteral vaccination. Adequate immune responses to Spn conjugate vaccines have been observed to occur in otitis prone children32,33; those results encourage continued studies of PhtD, LytB, PcpA, PhtE and Ply as potential parenteral immunogens in otitis prone children.
Comparing acute and convalescent antibody titers to the studied Spn proteins we found no significant difference of overall geometric mean (GM) titers in 3 groups of children. This is largely due to large variation in individual child immune responses. Most likely these results are due to differences in the length of NP carriage of Spn before AOM infection ensued. Those with longer carriage may achieve a peak in antibody response before the onset of AOM and show steady or falling antibody titers in acute to convalescent sera. Other children may have a brief time of NP carriage before the onset of AOM and show rising acute to convalescent antibody titers. It is not possible to distinguish the immune response to NP colonization versus AOM since responses to both events occur simultaneously. However, we have data to suggest that AOM does not enhance the serum antibody responses to NTHi or Spn antigens since levels of antibody are not significantly higher post AOM compared to post colonization (Pichichero et al, manuscript submitted). We also interpret these results to indicate that the different antigens elicit different antibody response profiles, possibly reflecting their different antigenicity in young children. Similar observations of this variability in acute to convalescent antibody titers surrounding an AOM event have been made by our group on NTHi proteins and by other groups regarding Spn polysaccharide responses.17,34,35 Soininen et al studied the natural development of antibodies to Spn polysaccharide types 1, 6B, 11A, 14, 19F and 23F associated with NP colonization and AOM in a cohort of 329 children followed during their first 2 years of life.36 Antibodies increased modestly but significantly over time; serotypes 11A and 14 were more immunogenic at a younger age. They found that antibody titers were equal after NP colonization or AOM. However in a later study involving the same children Soininen et al described the findings as indicating that antibody rises > 2 fold were relatively infrequent following AOM with variation attributable to age of the child and the serotype of Spn.35
Several of the antigens studied here have been evaluated by other investigators in other populations. Rapola et al 34 found that children (2 months to 2 years) with prior Spn colonization had high anti-Ply and −PspA (pneumococcal surface adhesion A) antibody titers in acute sera regardless of whether the child had experienced a prior AOM or only detected NP carriage. Musher et al14 found higher anti-Ply antibody among non-bacteremic adults than in bacteremic pneumonia. Zhang et al37 and Obaro et al38 reported antibody responses against choline binding protein A (CbpA), Ply and PsaA in children following NP carriage. Holmlund et al39 showed that Spn exposure induced antibody responses to PhtB and PhtE in children during their first 2 years of life; however, the antibody concentrations were not significantly associated with protection from Spn AOM. None of these earlier studies evaluated PhtD, LytB, PcpA, PhtE and Ply in otitis prone children.
The acquisition of antibody over time has been previously studied to antigens expressed by Spn and NTHi.22,23,28,40,41 Antibodies to PhtD, CbpA and LytC have been measured previously in infants (6 weeks to 10 months old) in relation to Spn NP colonization with an increase to all 3 antigens detected.42 In the current study, we found that otitis prone children failed to demonstrate or had a significantly slower age related rise in antibody to all five Spn proteins. A similar finding was previously made by Yamanaka and Faden for P6 of NTHi 23 and Kaur et al for Protein D, P6 and OMP26 of NTHi.19
In conclusion, our results provide further information on the immunologic response of otitis prone children. We observed immunologic hyporesponsiveness in otitis prone children against Spn antigens. Children with AOMTF behave immunologically similar to otitis prone children. The administration of a vaccine composition comprising at least one or more of PhtD, PhtE, PcpA, LytB and detoxified pneumolysin (e.g., PlyD1) by the parenteral route (optionally, with an adjuvant) may prove useful to mitigate the immunological hyporesponsiveness noted following natural exposure to Spn.
Acknowledgments
This study was supported by Sanofi pasteur and NIH NIDCD RO1 08671. We thank Sally Thomas, LPN, CCRC, the nurses and staff of Legacy Pediatrics and the collaborating pediatricians from Sunrise Pediatrics, Westfall Pediatrics, Lewis Pediatrics and Long Pond Pediatrics and the parents who consented and the children who participated in this study.
References
- 1.Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11:S7–11. doi: 10.1097/00006454-199208001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Luotonen J, Herva E, Karma P, Timonen M, Leinonen M, Makela PH. The bacteriology of acute otitis media in children with special reference to Streptococcus pneumoniae as studied by bacteriological and antigen detection methods. Scand J Infect Dis. 1981;13:177–183. doi: 10.3109/inf.1981.13.issue-3.04. [DOI] [PubMed] [Google Scholar]
- 3.Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 4.Denny FW, Loda FA. Acute respiratory infections are the leading cause of death in children in developing countries. Am J Trop Med Hyg. 1986;35:1–2. doi: 10.4269/ajtmh.1986.35.1. [DOI] [PubMed] [Google Scholar]
- 5.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics. 2005;116:e408–e413. doi: 10.1542/peds.2004-2338. [DOI] [PubMed] [Google Scholar]
- 6.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 7.Paton JC, Andrew PW, Boulnois GJ, Mitchell TJ. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu Rev Microbiol. 1993;47:89–115. doi: 10.1146/annurev.mi.47.100193.000513. [DOI] [PubMed] [Google Scholar]
- 8.Ogunniyi AD, Grabowicz M, Mahdi LK, et al. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 2009;23:731–738. doi: 10.1096/fj.08-119537. [DOI] [PubMed] [Google Scholar]
- 9.Adamou JE, Heinrichs JH, Erwin AL, et al. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect Immun. 2001;69:949–958. doi: 10.1128/IAI.69.2.949-958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Las RB, Garcia JL, Lopez R, Garcia P. Purification and polar localization of pneumococcal LytB, a putative endo-beta-N-acetylglucosaminidase: the chain-dispersing murein hydrolase. J Bacteriol. 2002;184:4988–5000. doi: 10.1128/JB.184.18.4988-5000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia P, Gonzalez MP, Garcia E, Lopez R, Garcia JL. LytB, a novel pneumococcal murein hydrolase essential for cell separation. Mol Microbiol. 1999;31:1275–1281. doi: 10.1046/j.1365-2958.1999.01238.x. [DOI] [PubMed] [Google Scholar]
- 12.Glover DT, Hollingshead SK, Briles DE. Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect Immun. 2008;76:2767–2776. doi: 10.1128/IAI.01126-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marriott HM, Mitchell TJ, Dockrell DH. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr Mol Med. 2008;8:497–509. doi: 10.2174/156652408785747924. [DOI] [PubMed] [Google Scholar]
- 14.Musher DM, Phan HM, Baughn RE. Protection against bacteremic pneumococcal infection by antibody to pneumolysin. J Infect Dis. 2001;183:827–830. doi: 10.1086/318833. [DOI] [PubMed] [Google Scholar]
- 15.Pelton SI, Leibovitz E. Recent advances in otitis media. Pediatr Infect Dis J. 2009;28:S133–S137. doi: 10.1097/INF.0b013e3181b6d81a. [DOI] [PubMed] [Google Scholar]
- 16.Pichichero ME, Casey JR, Hoberman A, Schwartz R. Pathogens causing recurrent and difficult-to-treat acute otitis media, 2003-2006. Clin Pediatr (Phila) 2008;47:901–906. doi: 10.1177/0009922808319966. [DOI] [PubMed] [Google Scholar]
- 17.Pichichero ME, Kaur R, Casey JR, Sabirov A, Khan MN, Almudevar A. Antibody Response to Haemophilus influenzae Outer Membrane Protein D, P6, and OMP26 After Nasopharyngeal Colonization and Acute Otitis Media in Children. Vaccine. 2010;28:7184–7192. doi: 10.1016/j.vaccine.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruoff KL, Whiley RA, Beighton D. Streptococcus. In: Murray PR, Barron EJ, Pfaller MA, Jorgensen JH, Yolken RH, editors. Manual of clinical microbiology. Washington D.C: American Society for Microbiology; 2003. pp. 405–421. [Google Scholar]
- 19.Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine. 2011;29:1023–1028. doi: 10.1016/j.vaccine.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freijd A, Hammarstrom L, Persson MA, Smith CI. Plasma anti-pneumococcal antibody activity of the IgG class and subclasses in otitis prone children. Clin Exp Immunol. 1984;56:233–238. [PMC free article] [PubMed] [Google Scholar]
- 21.Prellner K, Kalm O, Pedersen FK. Pneumococcal antibodies and complement during and after periods of recurrent otitis. Int J Pediatr Otorhinolaryngol. 1984;7:39–49. doi: 10.1016/s0165-5876(84)80052-x. [DOI] [PubMed] [Google Scholar]
- 22.Hotomi M, Yamanaka N, Saito T, et al. Antibody responses to the outer membrane protein P6 of non-typeable Haemophilus influenzae and pneumococcal capsular polysaccharides in otitis-prone children. Acta Otolaryngol. 1999;119:703–707. doi: 10.1080/00016489950180667. [DOI] [PubMed] [Google Scholar]
- 23.Yamanaka N, Faden H. Antibody response to outer membrane protein of nontypeable Haemophilus influenzae in otitis-prone children. J Pediatr. 1993;122:212–218. doi: 10.1016/s0022-3476(06)80115-0. [DOI] [PubMed] [Google Scholar]
- 24.Yamanaka N, Faden H. Local antibody response to P6 of nontypable Haemophilus influenzae in otitis-prone and normal children. Acta Otolaryngol. 1993;113:524–529. doi: 10.3109/00016489309135857. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein JM, Bronson PM, Wilson ME. Immunoglobulin G subclass response to major outer membrane proteins of nontypable Haemophilus influenzae in children with acute otitis media. Otolaryngol Head Neck Surg. 1997;116:363–371. doi: 10.1016/S0194-59989770275-4. [DOI] [PubMed] [Google Scholar]
- 26.Giebink GS, Mills EL, Huff JS, Cates KL, Juhn SK, Quie PG. Polymorphonuclear leukocyte dysfunction in children with recurrent otitis media. J Pediatr. 1979;94:13–18. doi: 10.1016/s0022-3476(79)80342-x. [DOI] [PubMed] [Google Scholar]
- 27.Freijd A, Oxelius VA, Rynnel-Dagoo B. A prospective study demonstrating an association between plasma IgG2 concentrations and susceptibility to otitis media in children. Scand J Infect Dis. 1985;17:115–120. doi: 10.3109/00365548509070430. [DOI] [PubMed] [Google Scholar]
- 28.Veenhoven R, Rijkers G, Schilder A, et al. Immunoglobulins in otitis-prone children. Pediatr Res. 2004;55:159–162. doi: 10.1203/01.PDR.0000099776.66136.39. [DOI] [PubMed] [Google Scholar]
- 29.Berman S, Lee B, Nuss R, Roark R, Giclas PC. Immunoglobulin G, total and subclass, in children with or without recurrent otitis media. J Pediatr. 1992;121:249–251. doi: 10.1016/s0022-3476(05)81197-7. [DOI] [PubMed] [Google Scholar]
- 30.Prellner K, Harsten G, Lofgren B, Christenson B, Heldrup J. Responses to rubella, tetanus, and diphtheria vaccines in otitis-prone and non-otitis-prone children. Ann Otol Rhinol Laryngol. 1990;99:628–632. doi: 10.1177/000348949009900808. [DOI] [PubMed] [Google Scholar]
- 31.Wiertsema SP, Sanders EA, Veenhoven RH, et al. Antibody levels after regular childhood vaccinations in the immunological screening of children with recurrent otitis media. J Clin Immunol. 2004;24:354–360. doi: 10.1023/B:JOCI.0000029114.84417.45. [DOI] [PubMed] [Google Scholar]
- 32.Breukels MA, Rijkers GT, Voorhorst-Ogink MM, Zegers BJ, Sanders LA. Pneumococcal conjugate vaccine primes for polysaccharide-inducible IgG2 antibody response in children with recurrent otitis media acuta. J Infect Dis. 1999;179:1152–1156. doi: 10.1086/314705. [DOI] [PubMed] [Google Scholar]
- 33.Barnett ED, Pelton SI, Cabral HJ, et al. Immune response to pneumococcal conjugate and polysaccharide vaccines in otitis-prone and otitis-free children. Clin Infect Dis. 1999;29:191–192. doi: 10.1086/520151. [DOI] [PubMed] [Google Scholar]
- 34.Rapola S, Kilpi T, Lahdenkari M, Makela PH, Kayhty H. Antibody response to the pneumococcal proteins pneumococcal surface adhesin A and pneumolysin in children with acute otitis media. Pediatr Infect Dis J. 2001;20:482–487. doi: 10.1097/00006454-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Soininen A, Lahdenkari M, Kilpi T, Makela PH, Kayhty H. Antibody response to pneumococcal capsular polysaccharides in children with acute otitis media. Pediatr Infect Dis J. 2002;21:186–192. doi: 10.1097/00006454-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Soininen A, Pursiainen H, Kilpi T, Kayhty H. Natural development of antibodies to pneumococcal capsular polysaccharides depends on the serotype: association with pneumococcal carriage and acute otitis media in young children. J Infect Dis. 2001;184:569–576. doi: 10.1086/322794. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, Bernatoniene J, Bagrade L, et al. Serum and mucosal antibody responses to pneumococcal protein antigens in children: relationships with carriage status. Eur J Immunol. 2006;36:46–57. doi: 10.1002/eji.200535101. [DOI] [PubMed] [Google Scholar]
- 38.Obaro SK, Adegbola RA, Tharpe JA, et al. Pneumococcal surface adhesin A antibody concentration in serum and nasopharyngeal carriage of Streptococcus pneumoniae in young African infants. Vaccine. 2000;19:411–412. doi: 10.1016/s0264-410x(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 39.Holmlund E, Simell B, Jaakkola T, et al. Serum antibodies to the pneumococcal surface proteins PhtB and PhtE in Finnish infants and adults. Pediatr Infect Dis J. 2007;26:447–449. doi: 10.1097/01.inf.0000261198.90649.04. [DOI] [PubMed] [Google Scholar]
- 40.Faden H. The microbiologic and immunologic basis for recurrent otitis media in children. Eur J Pediatr. 2001;160:407–413. doi: 10.1007/s004310100754. [DOI] [PubMed] [Google Scholar]
- 41.Soininen A, Pursiainen H, Kilpi T, Kayhty H. Natural development of antibodies to pneumococcal capsular polysaccharides depends on the serotype: association with pneumococcal carriage and acute otitis media in young children. J Infect Dis. 2001;184:569–576. doi: 10.1086/322794. [DOI] [PubMed] [Google Scholar]
- 42.Holmlund E, Quiambao B, Ollgren J, et al. Antibodies to pneumococcal proteins PhtD, CbpA, and LytC in Filipino pregnant women and their infants in relation to pneumococcal carriage. Clin Vaccine Immunol. 2009;16:916–923. doi: 10.1128/CVI.00050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


