Abstract
Purpose of the review
To critically assess recently published literature on predicting asthma exacerbations in children, while also providing general recommendations for future research in this field.
Recent findings
Current evidence suggests that every effort should be made to provide optimal treatment to achieve adequate asthma control, as this will significantly reduce the risk of severe disease exacerbations. Children who have had at least one asthma exacerbation in the previous year are at highest risk for subsequent exacerbations, regardless of disease severity and/or control. Although several tools and biomarkers to predict asthma exacerbations have been recently developed, these approaches need further validation and/or have only had partial success in identifying children at risk.
Summary
Although considerable progress has been made, much remains to be done. Future studies should clearly differentiate severe asthma exacerbations due to inadequate asthma control from those occurring in children whose asthma is well controlled, utilize standardized definitions of asthma exacerbations, and use a systematic approach to identify the best predictors after accounting for the multiple dimensions of the problem. Our ability to correctly predict the development of severe asthma exacerbations in an individual child should improve in parallel with increased knowledge and/or understanding of the complex interactions among genetic, environmental (e.g., viral infections) and lifestyle (e.g., adherence to treatment) factors underlying these events.
Keywords: Childhood asthma, asthma exacerbations, prediction, risk factors, biomarkers
Introduction
Asthma, the most common chronic disease of childhood [1], affects almost 7 million children in the United States. In this country, childhood asthma is the cause of ~700,000 emergency department (ED) visits, ~200,000 hospital admissions, and almost 200 deaths [2,3]. Direct costs from disease exacerbations accounted for over 63% of the estimated $15.5 billion total asthma costs in the U.S. in 2002 [4]. At an individual level, having an exacerbation is one of the most distressing events related to childhood asthma.
Identifying children at high risk for asthma exacerbations is important because it could lead to personalized and improved disease management and, ultimately, decrease suffering, morbidity and healthcare costs. In this review, we assess recently published literature on predicting asthma exacerbations in childhood, with particular emphasis on articles published in the last two years. We also provide general recommendations for future research in this field.
Definition of Asthma Exacerbations
Guidelines and recommendations for clinical trials and clinical practice have increasingly focused on how to best assess asthma control. Although this term encompasses a global assessment of asthma symptoms, lung function, medication use, and frequency of exacerbations, there is no uniform definition of adequate asthma control. Likewise, the definitions of asthma severity and asthma exacerbations have also varied widely among different studies and guidelines [5,6].
In an attempt to standardize the definitions of asthma exacerbations for clinical trials and clinical practice, the American Thoracic Society (ATS) and the European Respiratory Society (ERS) published a consensus statement in 2009 [7••]. Severe asthma exacerbations were described as “events that require urgent action on the part of the patient and physician to prevent a serious outcome, such as hospitalization or death”. Severe asthma exacerbations were defined as the occurrence of either a) an asthma-related hospitalization or visit to the ED or an urgent care facility leading to treatment with systemic (oral, intramuscular, or intravenous) corticosteroids or b) use of systemic corticosteroids for at least three days.
The ATS/ERS consensus statement also defined moderate asthma exacerbations as the occurrence of at least one of the following events for at least two days (without the need for use of systemic corticosteroids): deterioration in symptoms, deterioration in lung function, and/or increased rescue bronchodilator use. According to this definition, ER visits for asthma not requiring systemic corticosteroids should be classified as moderate disease exacerbations.
Risk Factors for Asthma Exacerbations
Poor asthma control can lead to severe asthma exacerbations [8•]. Adequate management of persistent asthma includes treatment with controller medications such as inhaled corticosteroids (ICS), which have been consistently shown to reduce the risk of severe disease exacerbations [9–11]. However, children with persistent asthma and at least one severe exacerbation in the prior year have a twofold increased risk of subsequent severe exacerbations despite use of controller medications [12], suggesting inter-individual (e.g., genetic) susceptibility.
Viral infections have been implicated in most (>80%) asthma exacerbations in children [13•,14]. Although many viruses have been recovered from asthmatics, rhinovirus has been most commonly (≥65%) identified in proven viral-induced asthma exacerbations in children aged 2–17 years [14]. Allergen exposure can cause disease exacerbations in children with atopic asthma [15], and viral infections and allergen exposure likely have synergistic effects on exacerbations in these children [16]. Other risk factors for asthma exacerbations include non-white race [8], younger age [11], season [12], ETS exposure [17], and outdoor air pollution [18•].
Assessment of Asthma Control
Because poor disease control is a risk factor for severe asthma exacerbations (see below), standardized measures of asthma control are important for both clinical purposes and research studies of asthma exacerbations The Childhood Asthma Control Test (C-ACT) [19••] is among the validated instruments included in the current Guidelines for the Diagnosis and Management of Asthma from the National Heart, Lung and Blood Institute (NHLBI) [20••] to assess asthma control in children ages 4–11 years. The C-ACT comprises 7 questions (3 are completed by the child’s parent and 4 are completed by the child and the parent together) that produce a score that can range from 0 to 27, where higher scores indicate better control of asthma symptoms. A score lower than 20 indicates poor control and correlates with lower FEV1, need for change in patients’ therapy, and the specialists’ rating of asthma severity. Similarly, the ACT can be used for children ages 12–18 years, with a score ranging from 5 to 25 (with higher scores also indicating better disease control). Scores from the ACT assess symptoms over a ~4-week period, and are therefore flexible, relatively dynamic, and easy to administer. However, while the AUC (area under the ROC [receiver operating characteristics] curve) in the initial study was 0.79, the sensitivity of the C-ACT was only 0.70, with a positive predictive value (PPV) of 70%. Moreover, there have been concerns that the C-ACT and the ACT may underestimate the proportion of children with uncontrolled asthma as defined by guidelines from the Global Initiative for Asthma (GINA) [21•].
Predicting Asthma Exacerbations
We will review recent efforts that have taken different approaches to try to improve our ability to predict asthma exacerbations in children.
Predictive Models Using Clinical Variables
Findings from three recent studies [11,12,22••] emphasize that a history of a recent severe asthma exacerbation is a strong risk factor for subsequent exacerbations, regardless of disease severity or use of controller medications. Covar et al. examined factors associated with severe disease exacerbations (defined as use of systemic corticosteroids or ≥1 visit to the ED or ≥1 hospitalization for asthma) among 285 children with mild to moderate persistent asthma who were randomized to treatment with one of the following approaches: ICS alone, ICS and salmeterol or montelukast [12]. After adjustment for treatment arm and other covariates, the use of ≥1 course of systemic corticosteroids in the year prior to the study was associated with a twofold increased risk of a severe asthma exacerbation during the 48-week course of the trial (95% confidence interval [CI] for odds ratio [OR]=1.4–3.1, P=0.0008). In another study, Haselkorn et al. examined factors associated with severe exacerbations (defined as recent use of systemic corticosteroids) in an observational longitudinal study of children (6 to 11 years old) with severe or difficult to treat asthma [22••]. After accounting for race and other covariates, both a history of ≥1 severe asthma exacerbation (OR=2.0, 95% CI=1.5–2.6), P <0.001) and very poor asthma control (OR=1.4, 95% CI=1.1–1.8, P=0.01) were associated with increased risk of an exacerbation during 1 year of follow-up. Similarly, Wu et al. showed that a history of ≥1 ED visit or hospitalization for asthma in the prior year was associated with increased risk of ≥1 severe disease exacerbation over four years in children with mild to moderate persistent asthma in the Childhood Asthma Management Program (CAMP) study, even after accounting for treatment arm and other covariates [11]. Of note, however, 22% of children without prior history of a severe asthma exacerbation had subsequent exacerbations during follow-up.
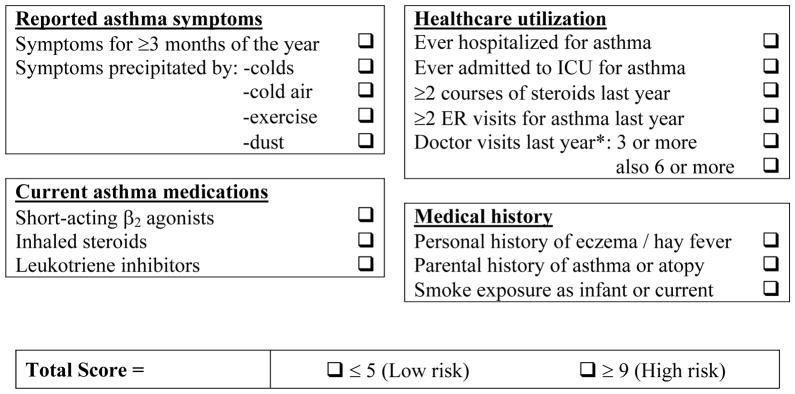
We recently aimed to construct a clinical score to predict severe asthma exacerbations based on questions that could be easily administered in a general pediatrician’s office [23••]. The resulting questionnaire consisted of yes/no questions (Figure 1), with a final score between 0 and 17. In our initial confirmatory sample of Costa Rican children ages 6 to 14 years, each 1-point increase in the score represented a 1.6-fold increment in the odds of an exacerbation in the year prior to the study (95% CI for OR=1.3–2.0, P<0.0001), with an AUC of 0.75. When applied to North American children enrolled in a longitudinal study, the AUC to predict exacerbations up to a year later was 0.69. Importantly, we also provided a cut-off score for children at low risk, with a positive predictive value (PPV) for “no hospitalizations” of 94–99%. While imperfect, this score is promising, as it could allow clinicians to identify children at low and high risk for a severe asthma exacerbation. Before any application, however, this score needs to be prospectively validated in a clinical setting.
Figure 1. Asthma Exacerbation Clinical Score.
Score instructions: One point is assigned for each question answered as yes. The score is calculated by adding all points (total score range, 0–17). For “Doctor visits last year,” one point is assigned for ≥3 visits, and one more point (two total) if the patient also had ≥6 visits.
Reproduced with permission from Forno et al., Chest 2010 (23).
Predictive Models Using Healthcare Data
Researchers have tried to construct predictive models to identify children at high risk for asthma exacerbations using large databases maintained by managed care organizations. In a recent study of healthcare data from 11,779 patients with asthma who were older than 11 years, O’Connor et al. confirmed previously reported associations between suboptimal asthma control and/or history of recent asthma exacerbations with subsequent disease exacerbations [24•]. In another study, Schatz et al. used computerized pharmacy data to create a scale for the use of β-agonists, which correlated with the risk of ED visits, hospitalizations, and courses of systemic steroids [25•].
To the best of our knowledge, only one study has created a computer-based classification tree to identify individual children at risk [26••]. In that study, Lieu et al. used variables in a large managed care database to create two classification trees for prediction of asthma hospitalizations, with AUCs of 0.75 and 0.79. Interestingly, the trees involved only 2–3 questions referring to a history of hospitalization in the prior 6 months, the number of units of β-agonist medication used, and the number of physicians prescribing asthma medications to the patient. However, these algorithms identified only 2–7% of the population as high-risk, with high specificity (94%) but low sensitivity (32%). An additional limitation of this method is that its implementation would require access to unified medication refill information linked with a detailed medical record.
Predictive Models Using Objective Measures of Lung Function
FEV1 is one of the criteria used to classify asthma severity in children, and a reduced FEV1 has been associated with asthma attacks (defined as ≥1 episode of wheezing or shortness of breath) in retrospective cohort studies of children [27] and adults [28]. However, two longitudinal studies of North American children who had mild to moderate persistent asthma and were enrolled in a clinical trial showed no association between FEV1% predicted at baseline and severe asthma exacerbations over 1 to 4 years of follow-up (11,12). In one of these studies, baseline FEV1/FVC was an independent risk factor for severe asthma exacerbations over 4 years of follow-up [11]. This is interesting, as a reduced FEV1/FVC –and not FEV1 –was associated with increased disease severity [29] and increased airway responsiveness [30] in cross-sectional studies of children with asthma in the U.S. Northeast [29] and Costa Rica [30].
Spirometric measures of lung function are not easily obtainable in certain clinical settings and/or geographic areas, and thus, although controversial [31], peak expiratory flow (PEF) has been proposed as a surrogate measure of FEV1 [32]. Thamrin et al. combined serial PEF measurements with the power of computer-based simulation to predict the risk of asthma exacerbations in adults [33••]. They used 64 days of PEF data from 309 asthmatics, used computers to simulate 5,000 PEF data points per patient, and used those models to predict risk of subsequent exacerbations. When exacerbations were defined as a drop of PEF below 80% predicted, the models were highly efficient, with AUC of 0.79–0.85; however, the AUC was substantially lower (0.61–0.66) when trying to predict clinically defined exacerbations.
Predictive Models Using Biomarkers
Identifying a biomarker of severe asthma exacerbations is attractive but has proven elusive. Exhaled nitric oxide (FeNO), a marker of eosinophilic airway inflammation, has been a more effective risk marker of asthma exacerbations in adults [34] than in children [35•,36]. Sputum eosinophils have shown promising results in a handful of studies in adults and children with asthma [37•]. However, sputum eosinophilia may not be a practical risk marker of asthma exacerbations in children because of difficulties in obtaining an adequate sputum sample and uncertain reference values for sputum eosinophil count in childhood [38].
Among 57 children with asthma, Wedes et al. found that an elevated level of urine bromotyrosine (a side product of eosinophil peroxidase) at baseline was associated with a fourfold higher risk of disease exacerbations (95% CI for RR=1.1–14.7, P=0.03) over 6 weeks of follow-up [39••]. Further studies will be required to confirm these findings and, if appropriate, to determine how to use urinary bromotyrosine in a clinical setting (e.g. what constitutes a significant increase/decrease in urinary levels, and how they correlate with risk of exacerbations over extended periods of follow-up). A more complex approach using arrays of ~20–40 metabolites detected by nuclear magnetic resonance spectroscopy (NMR) on urine samples has recently shown promising results in differentiating children with asthma from healthy controls, and stable outpatient asthma versus those presenting to the ED for an asthma exacerbation [40••].
Serum vitamin D level is a novel and modifiable potential risk marker for severe asthma exacerbations. In a cross-sectional study of 616 Costa Rican children, Brehm et al. showed that serum 25(OH)D level was inversely associated with having had ≥1 hospitalization for asthma in the previous year (OR for each log10 unit increase in serum 25(OH)D = 0.05; 95% CI=0.004–0.71) [41••]. Conversely, vitamin D deficiency (a 25(OH)D level <20 ng/ml) was associated with nearly tenfold increased odds of a severe exacerbation in the year prior to the study. In a follow-up longitudinal study of North American children, the same group reported that a baseline serum 25(OH)D ≤30 ng/ml was associated with ≥1 severe asthma exacerbation during four years of follow-up (OR=1.5, 95% CI=1.1–2.0) [42••]. These findings are supported by findings from a small clinical trial [43•] and may be mediated by effects of vitamin D on responsiveness to ICS [42••,44] and/or viral illnesses associated with asthma exacerbations [45]. Ongoing longitudinal studies and clinical trials should help ultimately establish whether serum vitamin D level should be routinely measured, and when and in whom vitamin D supplementation should be used to reduce the risk of severe asthma exacerbations.
Genetics
Asthma has a significant hereditary component. A genetic contribution to severe asthma exacerbations has not been formally tested but is plausible, given inter-individual susceptibility to exacerbations among children with asthma. Xu and colleagues used genome-wide genotypic data (~550,000 single nucleotide polymorphisms [SNPs]) to attempt to identify children at risk for severe asthma exacerbations [46]. A predictive panel was first constructed using data from 417 children, and then validated in a second sample of 164 children. In the validation sample, adding a panel of 160 SNPs increased the AUC for a model using clinical parameters only from 0.54 to 0.66. Consistent with other attempts to use relatively few SNPs as predictors of disease risk, this approach did not achieve optimal results but could be further refined once our understanding of how to best assess interactions among genetic variation and environmental factors is improved.
Gene expression is more “proximal” to a disease phenotype, and thus of great interest. A recent study identified two distinct gene expression signatures (one for innate immunity [including IFNα1, IFNβ1, toll-like receptors, and IL-15] and another for adaptive immunity [including genes related to B- and T-cell antigen receptors]) associated with severe asthma exacerbations [47••]. In a multivariate analysis, only two clinical factors were associated with the specific gene expression signatures: body mass index (BMI) and time between collection of the baseline blood sample and the exacerbation. These findings suggest that increased BMI is associated with a ‘different type’ of asthma exacerbation, and that the two gene expression signatures of interest may be related to different degrees of acuity of asthma exacerbations.
Conclusions and future directions
Substantial effort has been invested in how to best identify children at risk for asthma exacerbations. Although considerable progress has been made, much remains to be done. Currently available data suggest that every effort should be made to provide optimal treatment to achieve adequate asthma control, as this will significantly reduce the risk of severe disease exacerbations. Among children on anti-inflammatory medications whose asthma is well controlled, those who have had at least one disease exacerbation in the previous year are at highest risk for subsequent exacerbations. It should be recognized, however, that severe asthma exacerbations do occur among children with optimal asthma control and no recent history of exacerbations.
Novel and promising biomarkers for severe asthma exacerbations (e.g., serum vitamin D) must be rigorously assessed before their use is recommended for clinical purposes.
Future studies should clearly differentiate severe asthma exacerbations due to inadequate asthma control from those occurring in children whose asthma is well controlled, utilize standardized definitions of asthma exacerbations, and use a systematic approach to identify the best predictors after accounting for the multiple dimensions of the problem (e.g. medical history and clinical variables, genetic/genomic information, biomarkers, and viral infections).
As for other complex diseases or their complications, predictive models of asthma exacerbations are intended to estimate average risk for a population of interest and thus cannot perfectly predict risk for a given individual (which is 0% or 100% –the event will either occur or not). Ultimately, our ability to correctly predict the development of severe asthma exacerbations for an individual patient should improve in parallel with increased knowledge and/or understanding of the complex interactions among genetic, environmental (e.g., viral infections, allergen exposure) and lifestyle (e.g., adherence with prescribed treatment) factors underlying these events.
Table 1.
Risk Factors and Current Approaches to Predicting Asthma Exacerbations in Children
Exacerbation risk factors
|
Key Points.
Adequate asthma control reduces the risk of severe asthma exacerbations in children
Children who have had at least one asthma exacerbation in the previous year are at highest risk for subsequent exacerbations, regardless of disease severity or control
Novel prediction tools using clinical information, healthcare data and/or serial measurements of lung function have been developed but need further (e.g., prospective) validation
Promising biomarkers to predict asthma exacerbations include urine metabolites and serum vitamin D levels
Current tools to predict asthma exacerbations in childhood have had only partial success, and future studies (utilizing standardized definitions) will be more likely to identify accurate prediction tools if they account for the multiple dimensions of the problem.
Acknowledgments
Sources of support: Grant HL079966 from the U.S. National Institutes of Health
References
- 1.WHO. [Accessed 9/7/2011, 2011];Asthma fact sheet. Available at: http://www.who.int/mediacentre/factsheets/fs307/en/index.html.
- 2.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006 Aug 26;368(9537):733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 3.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National Surveillance for Asthma --- United States, 1980--2004. Morb Mortal Wkly Rep Surveill Summ. 2007;56(8):18–54. [PubMed] [Google Scholar]
- 4.Dilley JA, Pizacani BP, Macdonald SM, Bardin J. The Burden of Asthma in Washington State. 2005. DOH Pub No. 345–201. [Google Scholar]
- 5.National Asthma Education and Prevention Program. Expert Panel Report 2: Guidelines for the diagnosis and management of asthma. 1997. [Google Scholar]
- 6.Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, et al. A new perspective on concepts of asthma severity and control. Eur Respir J. 2008 Sep;32(3):545–554. doi: 10.1183/09031936.00155307. [DOI] [PubMed] [Google Scholar]
- 7••.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009 Jul 1;180(1):59–99. doi: 10.1164/rccm.200801-060ST. This important consensus statement proposes standard definitions and classification of asthma exacerbations. [DOI] [PubMed] [Google Scholar]
- 8•.Haselkorn T, Fish JE, Zeiger RS, Szefler SJ, Miller DP, Chipps BE, et al. Consistently very poorly controlled asthma, as defined by the impairment domain of the Expert Panel Report 3 guidelines, increases risk for future severe asthma exacerbations in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2009;11;124(5):895–902.e4. doi: 10.1016/j.jaci.2009.07.035. Identifies risk factors for severe asthma exacerbations based on a large prospective cohort of children, adolescents and adults with asthma. [DOI] [PubMed] [Google Scholar]
- 9.Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000 Oct 12;343(15):1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 10.O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001 Oct 15;164(8 Pt 1):1392–1397. doi: 10.1164/ajrccm.164.8.2104102. [DOI] [PubMed] [Google Scholar]
- 11.Wu AC, Tantisira K, Li L, Schuemann B, Weiss ST, Fuhlbrigge AL, et al. Predictors of Symptoms Are Different From Predictors of Severe Exacerbations From Asthma in Children. Chest. 2011 July 01;140(1):100–107. doi: 10.1378/chest.10-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covar RA, Szefler SJ, Zeiger RS, Sorkness CA, Moss M, Mauger DT, et al. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J Allergy Clin Immunol. 2008 Oct;122(4):741–747. e4. doi: 10.1016/j.jaci.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010 Sep 4;376(9743):826–834. doi: 10.1016/S0140-6736(10)61380-3. Reviews the role of viral infections on asthma exacerbations in children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson DJ, Lemanske RF., Jr The role of respiratory virus infections in childhood asthma inception. Immunol Allergy Clin North Am. 2010 Nov;30(4):513–22. vi. doi: 10.1016/j.iac.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray CS, Simpson A, Custovic A. Allergens, viruses, and asthma exacerbations. Proc Am Thorac Soc. 2004;1(2):99–104. doi: 10.1513/pats.2306027. [DOI] [PubMed] [Google Scholar]
- 16.Bossios A, Gourgiotis D, Skevaki CL, Saxoni-Papageorgiou P, Lotvall J, Psarras S, et al. Rhinovirus infection and house dust mite exposure synergize in inducing bronchial epithelial cell interleukin-8 release. Clin Exp Allergy. 2008 Oct;38(10):1615–1626. doi: 10.1111/j.1365-2222.2008.03058.x. [DOI] [PubMed] [Google Scholar]
- 17.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004 Apr;113(4 Suppl):1007–1015. [PubMed] [Google Scholar]
- 18•.Samoli E, Nastos PT, Paliatsos AG, Katsouyanni K, Priftis KN. Acute effects of air pollution on pediatric asthma exacerbation: evidence of association and effect modification. Environ Res. 2011 Apr;111(3):418–424. doi: 10.1016/j.envres.2011.01.014. Outdoor air pollution increases the risk of asthma exacerbations in childhood. [DOI] [PubMed] [Google Scholar]
- 19••.Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007 Apr;119(4):817–825. doi: 10.1016/j.jaci.2006.12.662. Original study for the C-ACT, one of the standard tools to assess asthma control in children that is included in the NHLBI asthma guidelines. [DOI] [PubMed] [Google Scholar]
- 20••.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007 Nov;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. Current NHLBI and NAEPP guidelines for the diagnosis and treatment of asthma. [DOI] [PubMed] [Google Scholar]
- 21•.Koolen BB, Pijnenburg MW, Brackel HJ, Landstra AM, van den Berg NJ, Merkus PJ, et al. Comparing Global Initiative for Asthma (GINA) criteria with the Childhood Asthma Control Test (C-ACT) and Asthma Control Test (ACT) Eur Respir J. 2011 Sep;38(3):561–566. doi: 10.1183/09031936.00173710. Evaluates the effectiveness of the C-ACT to identify children whose asthma is poorly controlled according to GINA criteria. [DOI] [PubMed] [Google Scholar]
- 22••.Haselkorn T, Zeiger RS, Chipps BE, Mink DR, Szefler SJ, Simons FE, et al. Recent asthma exacerbations predict future exacerbations in children with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2009;124(5):921–927. doi: 10.1016/j.jaci.2009.09.006. One of the most significant risk factors for a future severe asthma exacerbation is a history of a recent severe exacerbation. [DOI] [PubMed] [Google Scholar]
- 23••.Forno E, Fuhlbrigge A, Soto-Quiros ME, Avila L, Raby BA, Brehm J, et al. Risk factors and predictive clinical score for asthma exacerbations in childhood. Chest. 2010 Nov;138(5):1156–65. doi: 10.1378/chest.09-2426. Construction and initial validation of a checklist-type questionnaire and clinical score to predict asthma exacerbations in children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.O’Connor RD, Bleecker ER, Long A, Tashkin D, Peters S, Klingman D, et al. Subacute lack of asthma control and acute asthma exacerbation history as predictors of subsequent acute asthma exacerbations: evidence from managed care data. J Asthma. 2010;47(4):422–428. doi: 10.3109/02770901003605332. Use of managed care clinical data to predict risk of future exacerbations. [DOI] [PubMed] [Google Scholar]
- 25•.Schatz M, Zeiger RS, Vollmer WM, Mosen D, Apter AJ, Stibolt TB, et al. Validation of a beta-agonist long-term asthma control scale derived from computerized pharmacy data. J Allergy Clin Immunol. 2006 May;117(5):995–1000. doi: 10.1016/j.jaci.2006.01.053. Use of pharmacy data on medication refills to predict risk of asthma exacerbations. [DOI] [PubMed] [Google Scholar]
- 26••.Lieu TA, Quesenberry CP, Sorel ME, Mendoza GR, Leong AB. Computer-based models to identify high-risk children with asthma. Am J Respir Crit Care Med. 1998 Apr;157(4 Pt 1):1173–1180. doi: 10.1164/ajrccm.157.4.9708124. To the best of our knowledge, the only study to build and validate a computer-based decision tree to classify children at high risk for asthma exacerbations. [DOI] [PubMed] [Google Scholar]
- 27.Fuhlbrigge AL, Kitch BT, Paltiel AD, Kuntz KM, Neumann PJ, Dockery DW, et al. FEV(1) is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol. 2001 Jan;107(1):61–7. doi: 10.1067/mai.2001.111590. [DOI] [PubMed] [Google Scholar]
- 28.Kitch BT, Paltiel AD, Kuntz KM, Dockery DW, Schouten JP, Weiss ST, et al. A single measure of FEV1 is associated with risk of asthma attacks in long-term follow-up. Chest. 2004 Dec;126(6):1875–82. doi: 10.1378/chest.126.6.1875. [DOI] [PubMed] [Google Scholar]
- 29.Ramsey CD, Celedon JC, Sredl DL, Weiss ST, Cloutier MM. Predictors of disease severity in children with asthma in Hartford, Connecticut. Pediatr Pulmonol. 2005;39(3):268–75. doi: 10.1002/ppul.20177. [DOI] [PubMed] [Google Scholar]
- 30.Ly NP, Soto-Quiros ME, Avila L, Hunninghake GM, Raby BA, Laskey D, et al. Paternal asthma, mold exposure, and increased airway responsiveness among children with asthma in costa rica. Chest. 2008 Jan;133(1):107–14. doi: 10.1378/chest.07-2130. [DOI] [PubMed] [Google Scholar]
- 31.Chan-Yeung M, Chang JH, Manfreda J, Ferguson A, Becker A. Changes in peak flow, symptom score, and the use of medications during acute exacerbations of asthma. Am J Respir Crit Care Med. 1996 Oct;154(4 Pt 1):889–893. doi: 10.1164/ajrccm.154.4.8887581. [DOI] [PubMed] [Google Scholar]
- 32.Bellia V, Cibella F, Coppola P, Greco V, Insalaco G, Milone F, et al. Variability of peak expiratory flow rate as a prognostic index in asymptomatic asthma. Respiration. 1984;46(3):328–333. doi: 10.1159/000194707. [DOI] [PubMed] [Google Scholar]
- 33••.Thamrin C, Zindel J, Nydegger R, Reddel HK, Chanez P, Wenzel SE, et al. Predicting future risk of asthma exacerbations using individual conditional probabilities. J Allergy Clin Immunol. 2011;127(6):1494–1502. doi: 10.1016/j.jaci.2011.01.018. This study uses serial measurements of peak expiratory flow and computer simulation techniques to identify asthmatics at high risk for exacerbations based on deviation from their baseline PEF. [DOI] [PubMed] [Google Scholar]
- 34.Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SA, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010 May 15;181(10):1033–1041. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Cabral ALB, Vollmer WM, Barbirotto RM, Martins MA. Exhaled nitric oxide as a predictor of exacerbation in children with moderate-to-severe asthma: a prospective, 5-month study. Annals of Allergy, Asthma & Immunology. 2009;103(3):206–211. doi: 10.1016/S1081-1206(10)60183-4. This prospective study evaluates the utility of exhaled nitric oxide (FeNO) to predict risk of exacerbations in asthmatic children. [DOI] [PubMed] [Google Scholar]
- 36.Stern G, de Jongste J, van der Valk R, Baraldi E, Carraro S, Thamrin C, et al. Fluctuation phenotyping based on daily fraction of exhaled nitric oxide values in asthmatic children. J Allergy Clin Immunol. 2011;128(2):293–300. doi: 10.1016/j.jaci.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 37•.Garcia-Marcos L, Brand PL. The utility of sputum eosinophils and exhaled nitric oxide for monitoring asthma control with special attention to childhood asthma. Allergol Immunopathol (Madr) 2010;38(1):41–46. doi: 10.1016/j.aller.2009.10.006. Evaluates the use of sputum eosinophil counts as biomarker for risk of exacerbations. [DOI] [PubMed] [Google Scholar]
- 38.Li AM, Tsang TW, Lam HS, Sung RY, Chang AB. Predictors for failed dose reduction of inhaled corticosteroids in childhood asthma. Respirology. 2008;13(3):400–407. doi: 10.1111/j.1440-1843.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 39••.Wedes SH, Wu W, Comhair SAA, McDowell KM, DiDonato JA, Erzurum SC, et al. Urinary Bromotyrosine Measures Asthma Control and Predicts Asthma Exacerbations in Children. J Pediatr. doi: 10.1016/j.jpeds.2011.01.029. In Press. Corrected Proof. Identifies a novel biomarker measured in urine samples from asthmatic children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Saude EJ, Skappak CD, Regush S, Cook K, Ben-Zvi A, Becker A, et al. Metabolomic profiling of asthma: Diagnostic utility of urine nuclear magnetic resonance spectroscopy. J Allergy Clin Immunol. 2011;3;127(3):757–764.e6. doi: 10.1016/j.jaci.2010.12.1077. Measuring urinary levels of multiple metabolites, the authors identify specific profiles associated with asthma and asthma exacerbations. [DOI] [PubMed] [Google Scholar]
- 41.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009 May 1;179(9):765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010 Jul;126(1):52–8. e5. doi: 10.1016/j.jaci.2010.03.043. These two studies (41 and 42) identify vitamin D as a potential biomarker of risk for severe asthma exacerbations in children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Majak P, Olszowiec-Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011 May;127(5):1294–1296. doi: 10.1016/j.jaci.2010.12.016. Small clinical trial reporting that vitamin D supplementation reduces the risk of asthma exacerbations in children. [DOI] [PubMed] [Google Scholar]
- 44.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedón JC. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127(3):741–749. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010 May;91(5):1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 46.Xu M, Tantisira K, Wu A, Litonjua A, Chu J, Himes B, et al. Genome Wide Association Study to Predict Severe Asthma Exacerbations in Children using Random Forests Classifiers. BMC Medical Genetics. 2011;12(1):90. doi: 10.1186/1471-2350-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47••.Bjornsdottir US, Holgate ST, Reddy PS, Hill AA, McKee CM, Csimma CI, et al. Pathways activated during human asthma exacerbation as revealed by gene expression patterns in blood. PLoS One. 2011;6(7):e21902. doi: 10.1371/journal.pone.0021902. This study identified gene expression ‘signatures’ (involving innate and acquired immunity pathways) for increased risk of asthma exacerbations. [DOI] [PMC free article] [PubMed] [Google Scholar]