Abstract
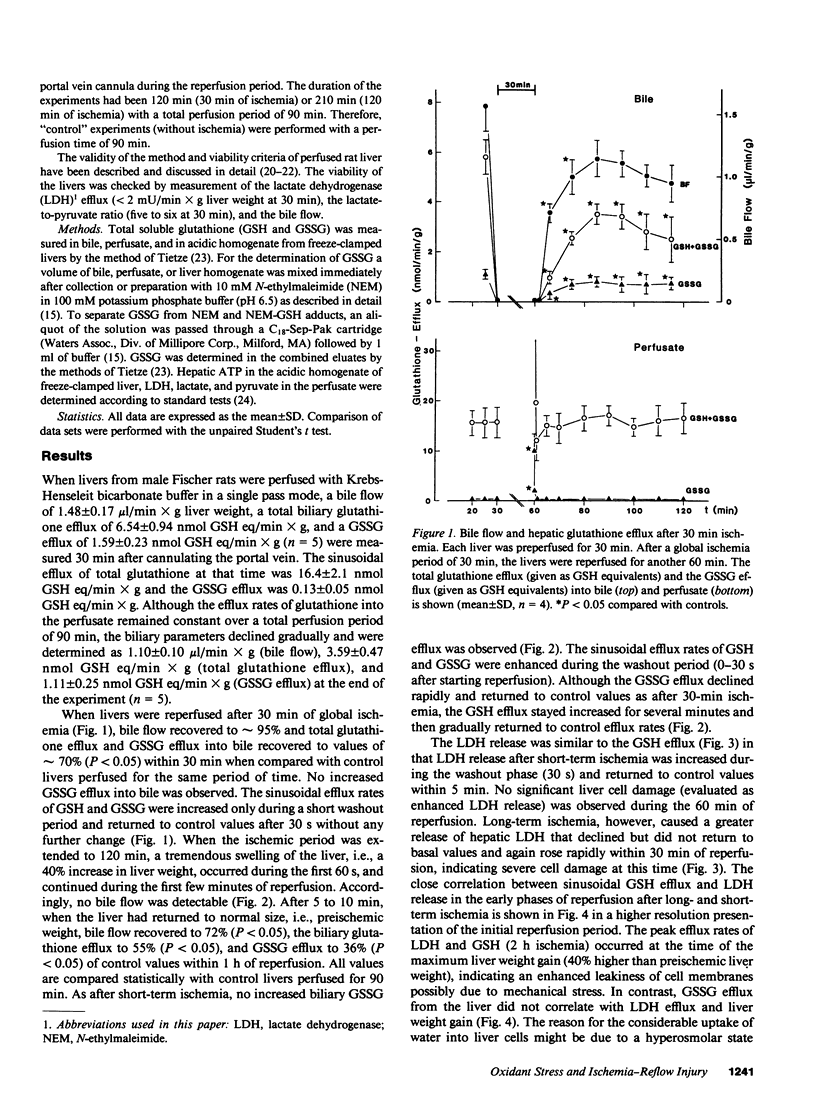
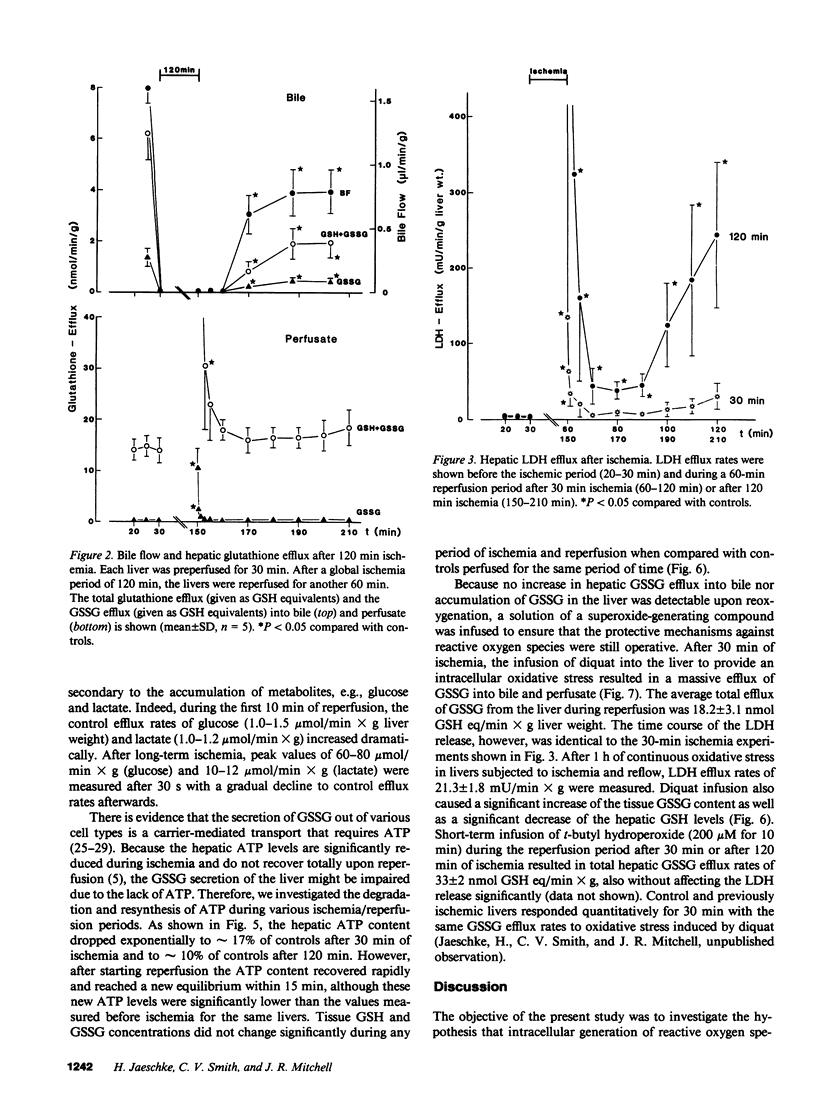
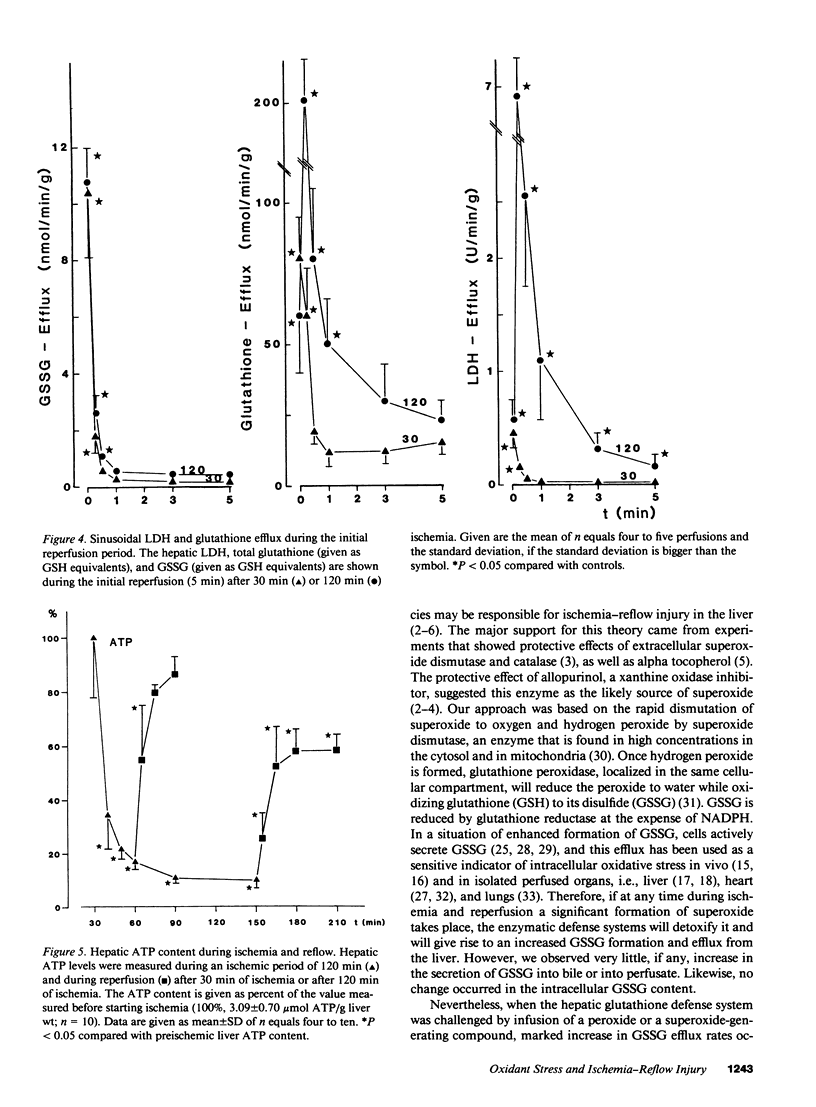
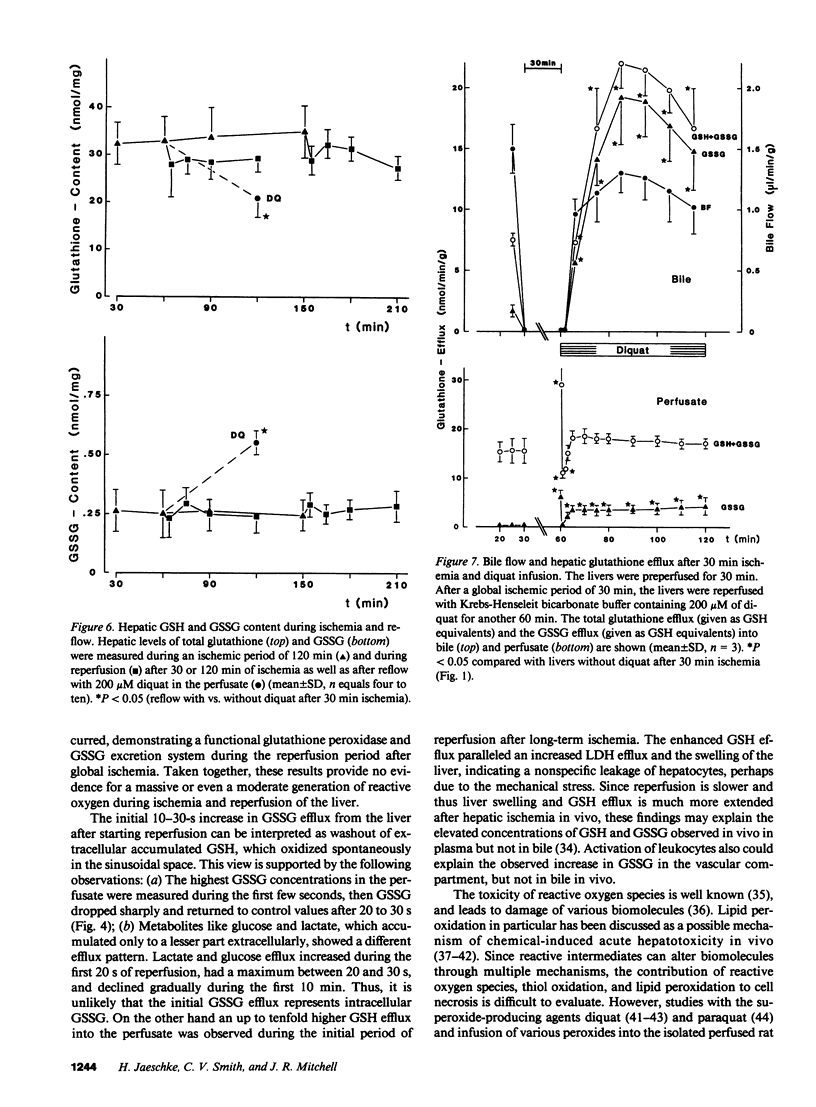
The hypothesis that intracellular generation of reactive oxygen species in hepatocytes or reticuloendothelial cells may cause ischemia-reperfusion injury was tested in isolated perfused livers of male Fischer rats. GSSG was measured in perfusate, bile, and tissue as a sensitive index of oxidative stress. After a preperfusion phase of 30 min, the perfusion was stopped (global ischemia) for various times (30, 120 min) and the liver was reperfused for another 60 min. The bile flow (1.48 +/- 0.17 microliters/min X gram liver weight), the biliary efflux of total glutathione (6.54 +/- 0.94 nmol GSH eq/min X g), and GSSG (1.59 +/- 0.23 nmol GSH eq/min X g) recovered to 69-86% after short-term ischemia and to 36-72% after 2 h of ischemia when compared with values obtained from control livers perfused for the same period of time. During reperfusion, the sinusoidal efflux of total glutathione (16.4 +/- 2.1 nmol GSH eq/min X g) and GSSG (0.13 +/- 0.05 nmol GSH eq/min X g) did not change except for an initial 10-30-s increase during reperfusion washout. No increased GSSG secretion into bile was detectable at any time during reperfusion. The liver content of total glutathione (32.5 +/- 3.5 nmol GSH eq/mg protein) and GSSG (0.27 +/- 0.09 nmol GSH eq/mg protein) did not change significantly during any period of ischemia or reperfusion. We conclude, therefore, that at most only a minor amount of reactive oxygen species were generated during reperfusion. Thus, reactive oxygen species are unlikely to cause ischemia/reperfusion injury in rat liver by lipid peroxidation or tissue thiol oxidation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. D., Jr, Lauterburg B. H., Mitchell J. R. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther. 1983 Dec;227(3):749–754. [PubMed] [Google Scholar]
- Akerboom T. P., Bilzer M., Sies H. The relationship of biliary glutathione disulfide efflux and intracellular glutathione disulfide content in perfused rat liver. J Biol Chem. 1982 Apr 25;257(8):4248–4252. [PubMed] [Google Scholar]
- Akerboom T., Inoue M., Sies H., Kinne R., Arias I. M. Biliary transport of glutathione disulfide studied with isolated rat-liver canalicular-membrane vesicles. Eur J Biochem. 1984 May 15;141(1):211–215. doi: 10.1111/j.1432-1033.1984.tb08177.x. [DOI] [PubMed] [Google Scholar]
- Bartoli G. M., Sies H. Reduced and oxidized glutathione efflux from liver. FEBS Lett. 1978 Feb 1;86(1):89–91. doi: 10.1016/0014-5793(78)80105-7. [DOI] [PubMed] [Google Scholar]
- Brigelius R., Anwer M. S. Increased biliary GSSG-secretion and loss of hepatic glutathione in isolated perfused rat liver after paraquat treatment. Res Commun Chem Pathol Pharmacol. 1981 Mar;31(3):493–502. [PubMed] [Google Scholar]
- Bulkley G. B. The role of oxygen free radicals in human disease processes. Surgery. 1983 Sep;94(3):407–411. [PubMed] [Google Scholar]
- Burk R. F., Lawrence R. A., Lane J. M. Liver necrosis and lipid peroxidation in the rat as the result of paraquat and diquat administration. Effect of selenium deficiency. J Clin Invest. 1980 May;65(5):1024–1031. doi: 10.1172/JCI109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers D. E., Parks D. A., Patterson G., Roy R., McCord J. M., Yoshida S., Parmley L. F., Downey J. M. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol. 1985 Feb;17(2):145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- Gardner T. J., Stewart J. R., Casale A. S., Downey J. M., Chambers D. E. Reduction of myocardial ischemic injury with oxygen-derived free radical scavengers. Surgery. 1983 Sep;94(3):423–427. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Sies H. Cardiac transport of glutathione disulfide and S-conjugate. Studies with isolated perfused rat heart during hydroperoxide metabolism. J Biol Chem. 1984 Mar 25;259(6):3838–3843. [PubMed] [Google Scholar]
- Ishikawa T., Zimmer M., Sies H. Energy-linked cardiac transport system for glutathione disulfide. FEBS Lett. 1986 May 5;200(1):128–132. doi: 10.1016/0014-5793(86)80524-5. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kawakami M., Yamauchi Y., Shimizu S., Nakamura M. Effect of allopurinol on ischemia and reperfusion-induced cerebral injury in spontaneously hypertensive rats. Stroke. 1986 Nov-Dec;17(6):1284–1287. doi: 10.1161/01.str.17.6.1284. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Kleinwaechter C., Wendel A. The role of acrolein in allyl alcohol-induced lipid peroxidation and liver cell damage in mice. Biochem Pharmacol. 1987 Jan 1;36(1):51–57. doi: 10.1016/0006-2952(87)90381-9. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Krell H., Pfaff E. No increase of biliary permeability in ethinylestradiol-treated rats. Gastroenterology. 1983 Oct;85(4):808–814. [PubMed] [Google Scholar]
- Jaeschke H., Krell H., Pfaff E. Quantitative estimation of transcellular and paracellular pathways of biliary sucrose in isolated perfused rat liver. Biochem J. 1987 Feb 1;241(3):635–640. doi: 10.1042/bj2410635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson S. G., Spence T. H., Jr, Lawrence R. A., Hill K. E., Duncan C. A., Johnson K. H. Rat lung glutathione release: response to oxidative stress and selenium deficiency. J Appl Physiol (1985) 1987 Jan;62(1):55–60. doi: 10.1152/jappl.1987.62.1.55. [DOI] [PubMed] [Google Scholar]
- Krell H., Jäschke H., Höke H., Pfaff E. Bile secretion in hemoglobin-free perfused rat liver. Hoppe Seylers Z Physiol Chem. 1984 Sep;365(9):1115–1122. doi: 10.1515/bchm2.1984.365.2.1115. [DOI] [PubMed] [Google Scholar]
- Lauterburg B. H., Smith C. V., Hughes H., Mitchell J. R. Biliary excretion of glutathione and glutathione disulfide in the rat. Regulation and response to oxidative stress. J Clin Invest. 1984 Jan;73(1):124–133. doi: 10.1172/JCI111182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubayashi S., Dohi K., Ochi K., Kawasaki T. Role of free radicals in ischemic rat liver cell injury: prevention of damage by alpha-tocopherol administration. Surgery. 1986 Feb;99(2):184–192. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Moore M., Bellomo G., Mirabelli F., Orrenius S. Demonstration and partial characterization of glutathione disulfide-stimulated ATPase activity in the plasma membrane fraction from rat hepatocytes. J Biol Chem. 1985 Feb 25;260(4):1999–2002. [PubMed] [Google Scholar]
- Nordström G., Seeman T., Hasselgren P. O. Beneficial effect of allopurinol in liver ischemia. Surgery. 1985 Jun;97(6):679–684. [PubMed] [Google Scholar]
- Paller M. S., Hebbel R. P. Ethane production as a measure of lipid peroxidation after renal ischemia. Am J Physiol. 1986 Nov;251(5 Pt 2):F839–F843. doi: 10.1152/ajprenal.1986.251.5.F839. [DOI] [PubMed] [Google Scholar]
- Rao K. S., Recknagel R. O. Early onset of lipoperoxidation in rat liver after carbon tetrachloride administration. Exp Mol Pathol. 1968 Oct;9(2):271–278. doi: 10.1016/0014-4800(68)90041-5. [DOI] [PubMed] [Google Scholar]
- Siems W., Mielke B., Müller M., Heumann C., Räder L., Gerber G. Status of glutathione in the rat liver. Enhanced formation of oxygen radicals at low oxygen tension. Biomed Biochim Acta. 1983;42(9):1079–1089. [PubMed] [Google Scholar]
- Sies H., Gerstenecker C., Menzel H., Flohé L. Oxidation in the NADP system and release of GSSG from hemoglobin-free perfused rat liver during peroxidatic oxidation of glutathione by hydroperoxides. FEBS Lett. 1972 Oct 15;27(1):171–175. doi: 10.1016/0014-5793(72)80434-4. [DOI] [PubMed] [Google Scholar]
- Sies H., Summer K. H. Hydroperoxide-metabolizing systems in rat liver. Eur J Biochem. 1975 Sep 15;57(2):503–512. doi: 10.1111/j.1432-1033.1975.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Sies H. The use of perfusion of liver and other organs for the study of microsomal electron-transport and cytochrome P-450 systems. Methods Enzymol. 1978;52:48–59. doi: 10.1016/s0076-6879(78)52005-3. [DOI] [PubMed] [Google Scholar]
- Smith C. V. Effect of BCNU pretreatment on diquat-induced oxidant stress and hepatotoxicity. Biochem Biophys Res Commun. 1987 Apr 14;144(1):415–421. doi: 10.1016/s0006-291x(87)80526-0. [DOI] [PubMed] [Google Scholar]
- Smith C. V. Evidence for participation of lipid peroxidation and iron in diquat-induced hepatic necrosis in vivo. Mol Pharmacol. 1987 Sep;32(3):417–422. [PubMed] [Google Scholar]
- Smith C. V., Hughes H., Lauterburg B. H., Mitchell J. R. Oxidant stress and hepatic necrosis in rats treated with diquat. J Pharmacol Exp Ther. 1985 Oct;235(1):172–177. [PubMed] [Google Scholar]
- Suttorp N., Toepfer W., Roka L. Antioxidant defense mechanisms of endothelial cells: glutathione redox cycle versus catalase. Am J Physiol. 1986 Nov;251(5 Pt 1):C671–C680. doi: 10.1152/ajpcell.1986.251.5.C671. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Wendel A., Feuerstein S., Konz K. H. Acute paracetamol intoxication of starved mice leads to lipid peroxidation in vivo. Biochem Pharmacol. 1979 Jul 1;28(13):2051–2055. doi: 10.1016/0006-2952(79)90223-5. [DOI] [PubMed] [Google Scholar]
- Xia Y. M., Hill K. E., Burk R. F. Effect of selenium deficiency on hydroperoxide-induced glutathione release from the isolated perfused rat heart. J Nutr. 1985 Jun;115(6):733–742. doi: 10.1093/jn/115.6.733. [DOI] [PubMed] [Google Scholar]



