Abstract
Background
Alzheimer disease and related disorders (ADRD) are prevalent in older adults, increase the costs of chronic heart failure (CHF) management, and may be associated with undertreatment of cardiovascular disease.
Objective
The purpose of our study was to determine the relationship between comorbid ADRD and CHF medication use and adherence among Medicare beneficiaries with CHF.
Methods
This 2-year (1/1/2006–12/31/2007) cross-sectional study used data from the Chronic Condition Data Warehouse of the Centers for Medicare and Medicaid Services. Medicare beneficiaries with evidence of CHF who had systolic dysfunction and Medicare Parts A, B, and D coverage during the entire study period were included. ADRD was identified based on diagnostic codes using the Chronic Condition Data Warehouse algorithm. CHF evidence-based medications (EBMs) were selected based on published guidelines: angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, selected β-blockers, aldosterone antagonists, and selected vasodilators. Measures of EBMs included a binary measure of any EBM use and medication possession ratio among users.
Results
Of 9827 beneficiaries with CHF and systolic dysfunction, 24.2% had a diagnosis of ADRD. Beneficiaries with ADRD were older (80.8 vs 73.6 years; P < 0.0001) and more likely to be female (69.3% vs 58.1%; P < 0.0001). Overall EBM use was lower in patients with CHF and ADRD compared with patients with CHF but no ADRD (85.3% vs 91.2%; P < 0.0001). Lower use among those with ADRD was consistent across all EBM classes except vasodilators. Among beneficiaries receiving EBM, those with ADRD had a slightly higher mean medication possession ratio for EBM compared with those without ADRD (0.86 vs 0.84; P = 0.0001).
Conclusions
EBM medication adherence was high in this population, regardless of ADRD status. However, patients with ADRD had lower EBM use compared with those without ADRD. Low use of specific EBM medications such as β-blockers was found in both groups. Therefore, interventions targeting increased treatment with specific EBMs for CHF, even among patients with ADRD, may be of benefit and could help reduce CHF-related hospitalizations.
Keywords: dementia, heart failure, Medicare Part D, medication adherence
INTRODUCTION
Chronic heart failure (CHF) is a chronic disease of older adults, whose incidence and prevalence will continue to increase as the population ages.1,2 CHF is costly to diagnose and treat among Medicare beneficiaries, in part because it is the most common reason for hospitalization among older adults.3–5 CHF is classified as an ambulatory care–sensitive condition, which means that with effective outpatient treatment and care, the disease can be managed without hospitalizations.6,7 Well-established evidence demonstrates the benefit of routine use of specific medications in the management of systolic heart failure.8 In addition, patient adherence to these medications has been shown to reduce preventable hospitalizations and mortality.8
As older adults with CHF typically have >1 chronic disease,9,10 understanding the effect of specific comorbid diseases on the benefit of CHF treatment will help clinicians optimize treatment plans for their patients. Alzheimer disease and related disorders (ADRD) are highly prevalent in Medicare beneficiaries with CHF. The presence of ADRD has been associated with undertreatment of cardiovascular diseases, including CHF.11–13 ADRD also increases the costs of CHF management.14,15 For example, Bynum et al15 estimated that the average total costs in 2006 for treating a patient with CHF bot no ADRD was $17,739, whereas these costs increase to $21,315 to treat a patient with CHF and ADRD. Much of these costs are driven by greater numbers of hospitalizations and longer hospital stays in Medicare beneficiaries with CHF and ADRD; however, there is growing evidence that some of these hospitalizations may have been preventable with appropriate outpatient care, a frequent finding in ambulatory care–sensitive condition management.6,11
In the present study, we seek to describe the use of and adherence to CHF medications in patients with CHF with and without ADRD in order to guide management of CHF in patients with comorbid conditions such as ADRD. The objective of this study is to determine the relationship between comorbid ADRD and CHF medication use and adherence among Medicare beneficiaries with CHF and systolic dysfunction. This analysis is unique because it takes advantage of the recently available Medicare Part D prescription drug database and is, to our knowledge, one of the first studies to describe the impact of comorbid ADRD on CHF medication use and adherence in patients with CHF.
METHODS
Data Source and Study Sample
Using a 2-year (2006–2007) cross-sectional design, we identified a cohort of Medicare beneficiaries with CHF from the Chronic Condition Data Warehouse (CCW) database. The CCW is provided by the Centers for Medicare and Medicaid Services and contains Medicare claims data for a 5% random sample of Medicare beneficiaries. The CCW has 21 predefined chronic condition cohorts, including CHF and ADRD, which are determined based on Medicare claims dating back to 1999.
The study sample was restricted to those with Medicare Parts A, B, and D hospital, health care, and prescription drug plan coverage during the entire study period (Part D coverage starting before July 1, 2006) in order to observe all medical claims and prescription events. We excluded individuals in Medicare Advantage plans because these individuals do not have Medicare Parts A and B claims. We also excluded those who died before January 1, 2007, in order to have at least the first year of observation on all individuals in which to capture medication use and adherence.
Beneficiaries with evidence of CHF were identified based on the CCW algorithm, defined as at least 1 inpatient, hospital outpatient, or carrier (physician) claim with a CHF diagnosis.16 To confirm whether beneficiaries had active disease, we required beneficiaries to have at least 1 CHF diagnostic claim between January 1, 2005 and December 31, 2006. We further restricted the sample to those with a 2006 claim indicating systolic dysfunction (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes: 428.2x, 428.4x), because published treatment guidelines for CHF are based on results from clinical trials of patients with systolic dysfunction.8 This study was approved by the institutional review board of the University of Maryland, Baltimore.
Measures
Dementia Status
Beneficiaries were classified as having ADRD using the CCW algorithm for ADRD, defined as at least 1 inpatient, skilled nursing facility, home health agency, hospital outpatient, or carrier (physician) claim with a dementia diagnosis (ICD-9-CM codes: 331.0, 331.1x, 331.2, 331.7, 290.0, 290.1x, 290.2x, 290.3, 290.4x, 294.0, 294.1x, 294.8, 797).16 The CCW definition is based on a study that found a sensitivity of 87% with this algorithm when compared with an Alzheimer disease registry.17 If beneficiaries met the CCW algorithm definition of ADRD anytime during their Medicare entitlement (back to 1999) through the end of 2006, we considered them as having ADRD.
CHF Medications and Adherence
CHF medications were selected based on the American College of Cardiology and the American Heart Association (ACC/AHA) treatment guidelines.8 Medications included those indicated for chronic use in systolic CHF: angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), selected β-blockers (ie, carvedilol, bisoprolol, metoprolol succinate), aldosterone antagonists, and selected vasodilators (ie, combination hydralazine and isosorbide). ACE inhibitors or ARBs and β-blockers are recommended as first-line therapy, and aldosterone antagonists and vasodilators are recommended as additional therapies for selected patients. These evidence-based medications (EBMs) were selected because they have been shown to improve outcomes and/or reduce mortality in randomized clinical trials.8,18–32 We also separately examined any use of other drugs commonly used in heart failure that have not been shown to improve outcomes: diuretics, cardiac glycosides, and selected dihydropyridine calcium channel blockers (ie, amlodipine, felodipine). Although use of calcium channel blockers is not generally recommended in the ACC/AHA treatment guidelines,8 the use of these medications has been demonstrated to be safe in patients with CHF and systolic dysfunction to treat comorbid hypertension or angina.33 Consequently, we included these vasoselective calcium channel blockers in our study.
Two measures pertaining to CHF medications were estimated over the course of the 2- year study period: a binary measure of any use and the medication possession ratio (MPR). The first measure, CHF medication use, is based on presence of at least 1 prescription claim for a CHF medication in a given class and quantifies the prevalence of use. MPR is a measure of medication adherence and is calculated as the ratio of the sum of the days’ supply from all claims for drugs in a given class to the duration of therapy for that class. The duration of therapy is defined as the number of days between the first and last claim in a drug class, plus the last claim’s days’ supply. EBM MPR was calculated by the ratio of the sum of the days’ supply (numerator) to the sum of the durations (denominator) for each of the contributing drug classes. MPR was only assessed among those who received at least 1 prescription for a CHF EBM or for other CHF medications in a given class.
Other Covariates
Additional characteristics in the study included age (as of January 1, 2006), sex, race, and geographic region. General health indicators included evidence of specific comorbid conditions and the number of physician visits during the study period. Comorbidities for which a CCW indicator was available were identified using the CCW definition and were based on evidence in claims from 1999 through 2006; other conditions were identified by the presence of any claim in 2006 with a relevant ICD-9-CM diagnosis code. We also assessed the number of months each beneficiary resided in a long-term care (LTC) facility during the 2-year study period using an algorithm based on Healthcare Common Procedure Coding System codes modified from previous literature.34,35 Medicare coverage variables included original reason for entitlement, dual (Medicaid) eligibility status, and low income subsidy status.
Statistical Analyses
Univariate analyses were used to describe CHF cohort characteristics and medication use and adherence. Bivariate analyses examined the unadjusted relationship between dementia status and medication use and adherence. Multivariate models using generalized estimating equations were used to estimate the adjusted relationships between ADRD and medication use and adherence. EBM use and adherence were modeled as binary variables; for the latter, poor adherence was compared with fair to high adherence. We modeled adherence using 3 different thresholds to define poor adherence: MPR <0.50, MPR <0.80, and MPR <0.90. We used a modified Poisson distribution with a log link to allow estimation of prevalence ratios of drug use or adherence, rather than prevalence odds ratios.36 Prevalence ratios (PRs) with 99% CIs are reported. All analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina).
Because MPR was calculated among those who received any CHF EBM, the MPRs based on only 1 claim per medication class may be inflated. Therefore, we performed a sensitivity analysis restricting our sample to those in whom MPR was calculated based on >1 claim per medication class.
RESULTS
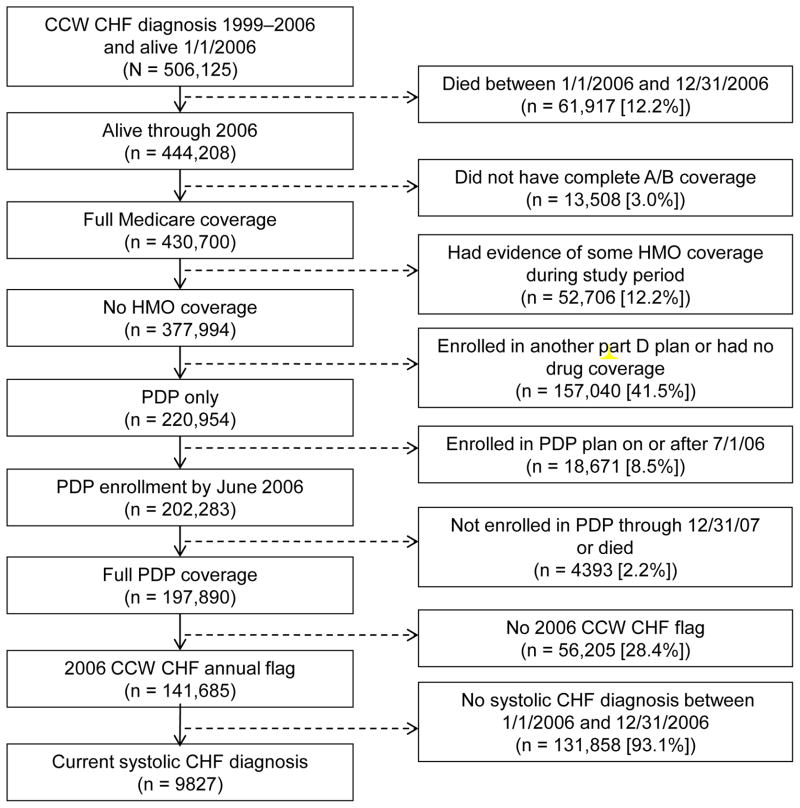
We identified 506,125 Medicare beneficiaries with a CHF diagnosis between 1999 and 2006 based on the CCW algorithm, of whom 9827 met the study inclusion criteria (Figure). The mean (SD) age of the cohort was 75.4 (11.6) years, and 60.7% were female (Table I). More than 80% of beneficiaries in our sample were white, and 54% were receiving low income subsidy for their Part D benefit.
Figure.
Cohort selection flowchart. A/B = Medicare Parts A and B; CCW = Chronic Condition Data Warehouse; CHF = chronic heart failure; HMO = health maintenance organization; Part D = Medicare Part D; PDP = prescription drug plan.
Table I.
Characteristic dysfunction, 2006 to 2007, stratified by Alzheimer disease and related disorders (ADRD) status.
| Characteristic | Total | No ADRD | ADRD | P* | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Sample size | 9827 | 100.0 | 7448 | 75.8 | 2379 | 24.2 | |
|
| |||||||
| Age, y | |||||||
| mean (SD) | 75.4 | (11.6) | 73.6 | (11.7) | 80.8 | (9.1) | <0.0001 |
| <65 | 1378 | 14.0 | 1261 | 16.9 | 117 | 4.9 | <0.0001 |
| 65–74 | 2675 | 27.2 | 2298 | 30.9 | 377 | 15.9 | |
| 75–84 | 3700 | 37.7 | 2696 | 36.2 | 1004 | 42.2 | |
| ≥85 | 2074 | 21.1 | 1193 | 16.0 | 881 | 37.0 | |
| Sex | |||||||
| Male | 3858 | 39.3 | 3121 | 41.9 | 737 | 31.0 | <0.0001 |
| Female | 5969 | 60.7 | 4327 | 58.1 | 1642 | 69.0 | |
| Race | |||||||
| White | 7903 | 80.4 | 5980 | 80.3 | 1923 | 80.8 | 0.530 |
| Black | 1285 | 13.1 | 989 | 13.3 | 296 | 12.4 | |
| Other† | 639 | 6.5 | 479 | 6.4 | 160 | 6.7 | |
| Region | |||||||
| North Central | 2324 | 23.7 | 1808 | 24.3 | 516 | 21.7 | <0.0001 |
| Northeast | 1735 | 17.7 | 1230 | 16.5 | 505 | 21.2 | |
| South‡ | 4105 | 41.8 | 3142 | 42.2 | 963 | 40.5 | |
| West | 1663 | 16.9 | 1268 | 17.0 | 395 | 16.6 | |
| Original reason for Medicare entitlement | |||||||
| Old age/survivor | 7188 | 73.2 | 5236 | 70.3 | 1952 | 82.1 | <0.0001 |
| Disability and/or ESRD | 2639 | 26.9 | 2212 | 29.7 | 427 | 17.9 | |
| CCW medical conditions§ | |||||||
| Acute myocardial infarction | 1873 | 19.1 | 1379 | 18.5 | 494 | 20.8 | 0.015 |
| Atrial fibrillation | 4258 | 43.3 | 3135 | 42.1 | 1123 | 47.2 | <0.0001 |
| Cancer | 1149 | 11.7 | 843 | 11.3 | 306 | 12.9 | 0.041 |
| Chronic kidney disease | 4484 | 45.6 | 3229 | 43.4 | 1255 | 52.8 | <0.0001 |
| COPD | 4824 | 49.1 | 3530 | 47.4 | 1294 | 54.4 | <0.0001 |
| Diabetes | 5468 | 55.6 | 4116 | 55.3 | 1352 | 56.8 | 0.180 |
| Hip fracture | 581 | 5.9 | 290 | 3.9 | 291 | 12.2 | <0.0001 |
| Ischemic heart disease | 8923 | 90.8 | 6761 | 90.8 | 2162 | 90.9 | 0.880 |
| Osteoporosis | 3213 | 32.7 | 2203 | 29.6 | 1010 | 42.5 | <0.0001 |
| Rheumatoid arthritis/osteoarthritis | 4597 | 46.8 | 3218 | 43.1 | 1,384 | 58.2 | <0.0001 |
| Stroke/TIA | 2542 | 25.9 | 1504 | 20.2 | 1,038 | 43.6 | <0.0001 |
| Other medical conditions|| | |||||||
| Anemia (iron deficiency) | 2160 | 22.0 | 1533 | 20.6 | 627 | 26.4 | <0.0001 |
| Hyperlipidemia | 6945 | 70.7 | 5527 | 74.2 | 1418 | 59.6 | <0.0001 |
| Hypertension | 9102 | 92.6 | 6857 | 92.1 | 2245 | 94.4 | 0.0002 |
| Idiopathic cardiomyopathy | 3590 | 36.5 | 2918 | 39.2 | 672 | 28.3 | <0.0001 |
| Peripheral vascular disease | 3674 | 37.4 | 2536 | 34.1 | 1138 | 47.8 | <0.0001 |
| Valvular heart disease | 4689 | 47.7 | 3611 | 48.5 | 1078 | 45.3 | 0.007 |
| No. of outpatient physician visits, 2006 and 2007 | |||||||
| <5 | 834 | 8.5 | 314 | 4.2 | 520 | 21.9 | <0.0001 |
| 5–9 | 875 | 8.9 | 571 | 7.7 | 304 | 12.8 | |
| 10–14 | 1112 | 11.3 | 833 | 11.2 | 279 | 11.7 | |
| ≥15 | 7006 | 71.3 | 5730 | 76.9 | 1276 | 53.6 | |
| No. of months with evidence of an LTC facility stay | |||||||
| 0 | 6310 | 64.2 | 5493 | 73.8 | 817 | 34.3 | <0.0001 |
| 1–6 | 2061 | 21.0 | 1469 | 19.7 | 592 | 24.9 | |
| 7–12 | 561 | 5.7 | 241 | 3.2 | 320 | 13.5 | |
| 13–24 | 895 | 9.1 | 245 | 3.3 | 650 | 27.3 | |
| LIS and dual eligibility | |||||||
| No LIS | 4505 | 45.8 | 3711 | 49.8 | 794 | 33.4 | <0.0001 |
| LIS, dual | 4703 | 47.9 | 3209 | 43.1 | 1494 | 62.8 | |
| LIS, not dual | 619 | 6.3 | 528 | 7.1 | 91 | 3.8 | |
CCW = Chronic Condition Data Warehouse; CHF = chronic heart failure; COPD = chronic obstructive pulmonary disease; ESRD = end-stage renal disease; LIS = low-income subsidy; LTC = long-term care; TIA = transient ischemic attack.
ADRD vs no ADRD (t test used for comparison of means, χ2 used for comparison of proportions).
Other race includes Asian, Hispanic, North American Native, Other, and Unknown.
Puerto Rico and the Virgin Islands are included in the South region.
Medical conditions identified based on the CCW algorithm with evidence between 1999 and 2006.
Medical conditions identified based on evidence in 2006 medical claims.
Among the CHF cohort, 2379 beneficiaries (24.2%) had evidence of an ADRD diagnosis in their Medicare claims. Those with a diagnosis of ADRD were significantly older (80.8 vs. 73.6 years; P < 0.0001) and were more likely to be female (69.0% vs. 58.1%; P < 0.0001) compared with those with no evidence of ADRD. A higher proportion of those with ADRD had other conditions, including atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease, hip fracture, osteoporosis, arthritis, stroke, anemia, and peripheral vascular disease. Certain conditions were less prevalent among those with ADRD compared with those without ADRD: hyperlipidemia, idiopathic cardiomyopathy, and valvular heart disease. On average, patients with ADRD had fewer outpatient physician visits during the study period compared with patients without ADRD. Residence in an LTC facility was more common among individuals with ADRD: just over one fourth of patients with CHF but no ADRD (26.2%) compared with almost two thirds of patients with CHF and ADRD (65.7%) spent ≥1 month in an LTC facility during the study period (P < 0.0001).
Overall, 96.3% of the cohort received at least 1 CHF medication prescription during the study period (Table II). EBMs, diuretics, cardiac glycosides, and selected dihydropyridine calcium channel blockers were taken by 89.7%, 84.2%, 31.0%, and 23.1%, respectively. Compared with those without ADRD, a significantly lower proportion of beneficiaries with ADRD received an EBM (85.3% vs 91.2%; P < 0.0001). Use among those with ADRD was also lower for diuretics and cardiac glycosides compared with those without ADRD, although the differences were less pronounced for these classes. Use of selected dihydropyridine calcium channel blockers was higher among those with ADRD (25.1%) than among those with no ADRD (22.5%) (P = 0.008).
Table II.
Two-year (2006–2007) drug prevalence and adherence among beneficiaries with CHF and systolic dysfunction, stratified by Alzheimer disease and related disorders (ADRD) status.
| Adherence Measure | Total | No ADRD | ADRD | P* | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Sample size | 9827 | 100.0 | 7448 | 75.8 | 2379 | 24.2 | |
|
| |||||||
| Proportion with ≥1 prescription | |||||||
| Any CHF agent | 9467 | 96.3 | 7191 | 96.6 | 2276 | 95.7 | 0.047 |
| EBM | 8819 | 89.7 | 6790 | 91.2 | 2029 | 85.3 | <0.0001 |
| Diuretic | 8270 | 84.2 | 6288 | 84.4 | 1982 | 83.3 | 0.196 |
| Cardiac glycoside | 3042 | 31.0 | 2354 | 31.6 | 688 | 28.9 | 0.014 |
| Calcium channel blocker | 2269 | 23.1 | 1672 | 22.5 | 597 | 25.1 | 0.008 |
| MPR, mean (SD)† | |||||||
| EBM | 0.84 | (0.16) | 0.84 | (0.16) | 0.86 | (0.15) | 0.0001 |
| Diuretic | 0.79 | (0.20) | 0.78 | (0.21) | 0.81 | (0.20) | <0.0001 |
| Cardiac glycoside | 0.86 | (0.18) | 0.87 | (0.17) | 0.86 | (0.18) | 0.161 |
| Calcium channel blocker | 0.87 | (0.18) | 0.87 | (0.18) | 0.88 | (0.17) | 0.591 |
| Proportion with ≥1 prescription of EBM class | |||||||
| ACE inhibitor | 5867 | 59.7 | 4532 | 60.9 | 1335 | 56.1 | <0.0001 |
| ARB | 2862 | 29.1 | 2257 | 30.3 | 605 | 25.4 | <0.0001 |
| ACE inhibitor or ARB | 7576 | 77.1 | 5879 | 78.9 | 1697 | 71.3 | <0.0001 |
| β-Blocker | 5572 | 56.7 | 4427 | 59.4 | 1145 | 48.1 | <0.0001 |
| Aldosterone antagonist | 2350 | 23.9 | 1883 | 25.3 | 467 | 19.6 | <0.0001 |
| Vasodilator | 2641 | 26.9 | 1975 | 26.5 | 666 | 28.0 | 0.157 |
| MPR of EBM classes, mean (SD)† | |||||||
| ACE inhibitor | 0.87 | (0.17) | 0.87 | (0.17) | 0.88 | (0.16) | 0.022 |
| ARB | 0.86 | (0.18) | 0.86 | (0.18) | 0.87 | (0.17) | 0.171 |
| Beta blocker | 0.84 | (0.18) | 0.84 | (0.18) | 0.86 | (0.17) | 0.003 |
| Aldosterone antagonist | 0.85 | (0.20) | 0.84 | (0.20) | 0.86 | (0.19) | 0.082 |
| Vasodilator | 0.86 | (0.18) | 0.86 | (0.18) | 0.86 | (0.17) | 0.665 |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CHF = chronic heart failure; EBM = evidence-based guideline CHF medication; MPR = medication possession ratio.
ADRD vs no ADRD (t test for comparison of means, χ2 for comparison of proportions).
MPR is calculated among those who used the relevant CHF medication class.
Regarding the 5 classes of EBMs, 77.1% of the cohort had at least 1 prescription for an ACE inhibitor or ARB and 56.7% had at least 1 prescription for a β-blocker during the study period. Aldosterone antagonists and vasodilators were used by 23.9% and 26.9% of the cohort, respectively. Use was significantly higher among those without ADRD compared with patients with ADRD for 4 of the 5 EBM classes: ACE inhibitors, ARBs, β-blockers, and aldosterone antagonists. Use of vasodilators was similar among those with and without ADRD.
Compared with beneficiaries without ADRD, those with ADRD had similar but a statistically significant higher mean MPR for EBM (0.86 vs 0.84; P = 0.0001) and diuretics (0.81 vs 0.78; P < 0.0001). This trend of higher adherence among patients with ADRD versus without ADRD was seen in all therapeutic classes of EBMs but was only statistically significant for β-blockers (mean MPR, 0.86 vs 0.84; P = 0.003). EBM adherence was higher among patients residing in LTC facilities in those with and without ADRD. Among patients with no evidence of an LTC facility stay during the study period, mean MPR was 0.84 in patients with and without ADRD, whereas mean MPR was higher among patients with ≥13 months of residence in an LTC facility (mean MPR of 0.89 among patients without ADRD and 0.87 among those with ADRD). This, in combination with the substantially higher proportion of individuals with ADRD who resided in LTC facilities, was the driving force behind the slightly but significantly higher MPRs found among beneficiaries with ADRD.
In the unadjusted model, beneficiaries with CHF and ADRD had lower CHF EBM use (PR = 0.94; 99% CI, 0.91–0.95) compared with beneficiaries with CHF but no ADRD (Table III). The adjusted model showed an attenuated, although still significantly lower, relative prevalence of use for beneficiaries with ADRD (PR = 0.97; 99% CI, 0.95–1.00; P = 0.004). Adjusted PR estimates comparing use for patients with ADRD with those without ADRD ranged from 0.93 to 0.99 for the EBM subclasses, with vasodilators and aldosterone antagonists having the highest and lowest estimates, respectively.
Table III.
Effect of Alzheimer disease and related disorders (ADRD) on chronic heart failure (CHF) evidence-based medication (EBM) use among Medicare beneficiaries with CHF and systolic dysfunction.
| Dependent Variable: Use | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHF EBM | ACE Inhibitor/ARB | β-Blocker | Aldosterone Antagonist | Vasodilator | ||||||
| Independent Variable | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted |
| ADRD prevalence ratio (99% CI) | 0.94 (0.91–0.95) | 0.97 (0.95–1.00) | 0.90 (0.87–0.94) | 0.96 (0.92–1.00) | 0.81 (0.76–0.86) | 0.95 (0.89–1.01) | 0.78 (0.69–0.87) | 0.93 (0.82–1.06) | 1.06 (0.96–1.16) | 0.99 (0.89–1.10) |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker.
Model adjusted for age, sex, race, region, original Medicare entitlement status, low-income subsidy status, comorbidities (acute myocardial infarction, atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease, diabetes, ischemic heart disease, stroke/transient ischemic attack, hyperlipidemia, hypertension, cardiomyopathy, peripheral vascular disease, valvular heart disease, cancer, hip fracture, osteoporosis, osteoarthritis/rheumatoid arthritis, anemia), number of outpatient physician visits, and number of months with evidence of a long-term care facility stay.
Regarding adherence, both unadjusted and adjusted analyses demonstrated a nonsignificant effect of ADRD on poor adherence, defined as MPR <0.5 (unadjusted PR = 0.82 [99% CI, 0.59–1.14]; adjusted PR = 1.12 [99% CI, 0.77–1.62]). Models using different definitions for poor adherence resulted in similar findings. With poor adherence defined as MPR <0.80 and <0.90, the unadjusted models showed a 10% (99% CI, 0.81–0.99) and 7% (99% CI, 0.87–1.00) lower prevalence of poor adherence associated with ADRD, respectively; however, effects of ADRD on the prevalence of poor adherence was nonsignificant in adjusted models: 1.00 (99% CI, 0.90–1.12) and 0.99 (99% CI, 0.92–1.07), respectively (data not shown).
A sensitivity analysis for adherence models was performed among those whose MPR was calculated based on >1 claim per medication class (n = 8589). These results did not differ from the original results, providing unadjusted and adjusted estimates of the effect of ADRD on poor adherence of 0.83 (99% CI, 0.59–1.15) and 1.13 (99% CI, 0.78–1.64), respectively.
DISCUSSION
Using newly available Medicare Part D data, we determined the effect of comorbid dementia on CHF treatment patterns and adherence among a cohort of Medicare beneficiaries. A recent study that analyzed the impact of Part D on medication use and adherence found improved access and adherence to ACE inhibitors, ARBs, and β-blockers for treatment of CHF following the implementation of Part D.37 Our study adds to this by examining CHF medication use and adherence in a nationally representative sample and describing differences between those with and without ADRD. Despite the overall improvements in access observed by Donohue et al,37 our findings demonstrate that beneficiaries with CHF and ADRD were less likely to receive any of the classes of EBMs with the exception of vasodilators and were more likely to receive selected dihydropyridine calcium channel blockers. Overall, this finding is consistent with previous reports of lower use of cardiac medications in patients with ADRD and cardiovascular diseases.12,38–40
Although overall adherence was high among those who received EBM, beneficiaries with ADRD and CHF had slightly higher adherence than beneficiaries with CHF and no ADRD. The higher adherence among patients with ADRD was consistent across all EBM subclasses, although only significant for β-blockers, as well as for diuretics. However, adjusting for confounding factors, including nursing home residence, removed the association between ADRD status and adherence. The higher adherence observed among those with ADRD was likely due to careful administration of medications in LTC settings and assisted living facilities, because we found adherence rates were higher among those residing in nursing homes, and beneficiaries with CHF and ADRD were more likely to reside in nursing homes.
Management of chronic diseases such as CHF in patients with dementia is associated with higher rates of hospitalization and costs compared with those who do not have dementia.11,14,41 Thus, interventions targeting treatment with EBMs for CHF, even among patients with ADRD, may help to reduce CHF-related hospitalizations, particularly since we found adherence to medications in this population to be high.
Our findings should be interpreted with the following study limitations in mind. The cross-sectional study design limits our ability to make causal inferences. Although we did not include individuals with a first diagnosis of ADRD in 2007, CHF medication use may have preceded a dementia diagnosis that occurred late in 2006. However, because dementia is a progressive disease, it is likely that symptoms were present before an official diagnosis and therefore still may have influenced decisions regarding CHF medications. Because this analysis was based on administrative data, measurement and disease ascertainment were limited by information available in such data. We attempted to address this limitation by restricting the study sample to those with a diagnosis of CHF and who had systolic dysfunction (ICD-9-CM codes: 428.2x, 428.4x). This method of identification has a specificity of 97.1% against ejection fractions recorded in the medical record, but it has a low sensitivity (11.8%).42 We chose this definition to avoid capturing patients with rule-out diagnoses that would be included when using broad group CHF diagnosis codes and therefore include only those in whom treatment would be truly indicated; however, the low sensitivity and the fact that a majority of CHF diagnoses in administrative claims are for “unspecified” disease suggests that using ICD-9 codes to identify individuals with systolic dysfunction results in many false-negatives.
Also, the lower use of β-blockers may have been influenced by restricting EBMs to those 3 β-blockers recommended in the guidelines for patients with systolic dysfunction,8 so patients in our cohort identified as nonusers may have been using non–evidence-based β-blockers. An observational study by Kramer et al43 suggested that non-EBM β-blockers are as effective as evidence-based β-blockers in a cohort not limited to those with systolic failure. However, our study was restricted to those with systolic dysfunction; therefore, we chose to include only medications that had demonstrated efficacy in randomized controlled trials for systolic HF and were indicated in the HF treatment guidelines. In addition, we note that there is only 1 randomized controlled trial restricted to treatment of older patients with HF (mean age, 76 years) with nebivolol,44 and the lack of trials focused on use of these drugs in older adults may also have contributed to the low use seen in our cohort.
Another limitation was the lack of ability to determine specific reasons for nonuse of or poor adherence to EBM (eg, patient refusal, history of adverse effects, patient health literacy). Moreover, we observed higher MPR levels for CHF beneficiaries with ADRD, which may be due to residence in LTC facilities or caregiver behavior in those residing in the community. Although we did not have information about caregiver status, we controlled for the higher proportion of those with ADRD in LTC facilities, where a caregiver administers medications. In addition, we chose to use MPR over a measure of proportion of days covered because we were interested in exploring the consistency of medication use for those using medications during their period of drug exposure. It should be noted that MPR may overestimate adherence when a beneficiary switches medications or has therapeutic duplication,45 so adherence results based on MPR may provide upper estimates. However, we calculated the numerator of MPR using days’ supply at a drug level and then “rolled up” MPR to the class level. This reflects the weighted average of the proportion of days that any drug in a given class is available instead of simply adding the days’ supply for all drugs in a given class, thus removing the potential for overestimation due to the numerator. Additionally, adherence was determined by refill patterns, and we did not have information on actual ingestion of the medication. However, adherence as ascertained by prescription refill patterns has been shown to be a valid measure of patient adherence.46–48
Although our study sample was drawn from a random sample of Medicare beneficiaries with CHF who had systolic dysfunction, our findings may not be generalizable to all Medicare patients with CHF due to study exclusion criteria. Specifically, these results cannot be assumed to represent treatment of types of CHF other than systolic dysfunction. In addition, patients included in the analysis were more likely to be younger, of minority race, reside in the South or West, and to have Medicare entitlement due to disability and/or end-stage renal disease compared with those who were excluded (data not shown).
Finally, the large sample size in this study allowed us to calculate precise estimates of the relationship between comorbid ADRD and CHF medication use and adherence among beneficiaries with CHF; however, it remains to be studied if the statistically significant results translate into clinically significant differences that need to be addressed.
CONCLUSIONS
This analysis of CHF medication use and adherence among Medicare beneficiaries documents that patients with CHF and comorbid ADRD are less likely to receive EBM than patients with CHF without comorbid ADRD. However, overall adherence was high among those who did receive CHF EBM, and individuals with ADRD had slightly higher adherence to these medications than those with no ADRD, perhaps due to assistance taking medications that patients with ADRD receive from LTC facilities or caregivers. However, patients with ADRD had lower EBM use in general, and we found low use of specific EBMs such as β-blockers in both groups. Therefore, interventions targeting increased treatment with specific EBMs for CHF, even among patients with ADRD, may be of benefit and help to reduce CHF-related hospitalizations.
With the recent passage of the National Alzheimer’s Project Act (NAPA), a national plan will emerge for the management of ADRD; the optimization and increase in the use of EBM in this population for managing comorbid conditions such as CHF is unknown.49 Future studies, using longitudinal designs and longer follow-up periods as additional years of data become available, are warranted to examine this phenomenon more fully across the management of other chronic diseases in patients with ADRD to help inform chronic disease management in the ADRD population. In particular, future work should determine the effect of evidence-based CHF treatment on patients with comorbid ADRD on individual patient and societal outcomes such as risk of hospitalization and associated costs of hospitalization.
Acknowledgments
This project was supported by the Commonwealth Fund. The authors acknowledge the valuable input from the staff of Lori Walker, Jim Gardner, and Patricia Stewart from Pharmaceutical Research Computing, University of Maryland School of Pharmacy. Dr. Rattinger was supported through the National Institutes of Health (K12HD043489 Building Interdisciplinary Research in Women’s Health [BIRCWH]). Ms. Dutcher is supported through a National Institute on Aging training grant (T32AG000262 Research Training in the Epidemiology of Aging).
Footnotes
CONFLICTS OF INTEREST
Drs. Simoni-Wastila, Gottlieb, and Zuckerman are co-investigators on a grant from the Commonwealth Fund, which funded this project. Dr. Stuart is the principal investigator on a grant from the Commonwealth Fund, which funded this project. Dr. Gottlieb is a consultant for Merck. The Commonwealth Fund played no role in the study concept or design; collection, analysis, or interpretation of data; or manuscript preparation and decision for publication. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–173. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 3.Liao L, Allen LA, Whellan DJ. Economic burden of heart failure in the elderly. Pharmacoeconomics. 2008;26:447–462. doi: 10.2165/00019053-200826060-00001. [DOI] [PubMed] [Google Scholar]
- 4.Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997;133:703–712. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 5.Croft JB, Giles WH, Pollard RA, et al. National trends in the initial hospitalization for heart failure. J Am Geriatr Soc. 1997;45:270–275. doi: 10.1111/j.1532-5415.1997.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 6.Bindman AB, Grumbach K, Osmond D, et al. Preventable hospitalizations and access to health care. JAMA. 1995;274:305–311. [PubMed] [Google Scholar]
- 7.Carter MW, Datti B, Winters JM. ED visits by older adults for ambulatory care-sensitive and supply-sensitive conditions. Am J Emerg Med. 2006;24:428–434. doi: 10.1016/j.ajem.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 9.Masoudi FA, Krumholz HM. Polypharmacy and comorbidity in heart failure. BMJ. 2003;327:513–514. doi: 10.1136/bmj.327.7414.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marengoni A, Rizzuto D, Wang HX, et al. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc. 2009;57:225–230. doi: 10.1111/j.1532-5415.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- 11.Bynum JP, Rabins PV, Weller W, et al. The relationship between a dementia diagnosis, chronic illness, Medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52:187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 12.Lopponen M, Raiha I, Isoaho R, et al. Dementia associates with undermedication of cardiovascular diseases in the elderly: a population-based study. Dement Geriatr Cogn Disord. 2006;22:132–141. doi: 10.1159/000093739. [DOI] [PubMed] [Google Scholar]
- 13.Klarin I, Fastbom J, Wimo A. The use of angiotensin-converting enzyme inhibitors and other drugs with cardiovascular effects by non-demented and demented elderly with a clinical diagnosis of heart failure. A population-based study of the very old. Eur J Clin Pharmacol. 2006;62:555–562. doi: 10.1007/s00228-006-0134-y. [DOI] [PubMed] [Google Scholar]
- 14.Hill JW, Futterman R, Duttagupta S, et al. Alzheimer’s disease and related dementias increase costs of comorbidities in managed Medicare. Neurology. 2002;58:62–70. doi: 10.1212/wnl.58.1.62. [DOI] [PubMed] [Google Scholar]
- 15.Alzheimer’s Association. Characteristics, Costs, and Health Service Use for Medicare Beneficiaries with a Dementia Diagnosis: Report 2: National 20% Sample Medicare Fee-for-Service Beneficiaries. Lebanon, NH: Dartmouth Institute for Health Policy and Clinical Care, Center for Health Policy Research; Jan, 2009. [Google Scholar]
- 16.Chronic Condition Data Warehouse. [Accessed February 24, 2011];Chronic Condition Categories. http://www.ccwdata.org/chronic-conditions/index.htm.
- 17.Taylor DH, Jr, Fillenbaum GG, Ezell ME. The accuracy of Medicare claims data in identifying Alzheimer’s disease. J Clin Epidemiol. 2002;55:929–937. doi: 10.1016/s0895-4356(02)00452-3. [DOI] [PubMed] [Google Scholar]
- 18.Erhardt L, MacLean A, Ilgenfritz J, et al. Fosinopril attenuates clinical deterioration and improves exercise tolerance in patients with heart failure. Fosinopril Efficacy/Safety Trial (FEST) Study Group. Eur Heart J. 1995;16:1892–1899. doi: 10.1093/oxfordjournals.eurheartj.a060844. [DOI] [PubMed] [Google Scholar]
- 19.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 20.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 21.Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 22.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 23.Pitt B, White H, Nicolau J, et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol. 2005;46:425–431. doi: 10.1016/j.jacc.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 24.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 25.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 26.Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA. 2000;283:1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 27.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 28.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 29.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 30.Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314:1547–1552. doi: 10.1056/NEJM198606123142404. [DOI] [PubMed] [Google Scholar]
- 31.Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–310. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 32.Loeb HS, Johnson G, Henrick A, et al. Effect of enalapril, hydralazine plus isosorbide dinitrate, and prazosin on hospitalization in patients with chronic congestive heart failure. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87(6 Suppl):VI78–187. [PubMed] [Google Scholar]
- 33.de Vries RJ, van Veldhuisen DJ, Dunselman PH. Efficacy and safety of calcium channel blockers in heart failure: focus on recent trials with second-generation dihydropyridines. Am Heart J. 2000;139:185–194. doi: 10.1067/mhj.2000.101490. [DOI] [PubMed] [Google Scholar]
- 34.Zuckerman IH, Sato M, Hsu VD, Hernandez JJ. Validation of a method for identifying nursing home admissions using administrative claims. BMC Health Serv Res. 2007;7:202. doi: 10.1186/1472-6963-7-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koroukian SM, Xu F, Murray P. Ability of Medicare claims data to identify nursing home patients: a validation study. Med Care. 2008;46:1184–1187. doi: 10.1097/MLR.0b013e31817925d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 37.Donohue JM, Zhang Y, Lave JR, et al. The Medicare drug benefit (Part D) and treatment of heart failure in older adults. Am Heart J. 2010;160:159–165. doi: 10.1016/j.ahj.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sloane PD, Gruber-Baldini AL, Zimmerman S, et al. Medication undertreatment in assisted living settings. Arch Intern Med. 2004;164:2031–2037. doi: 10.1001/archinte.164.18.2031. [DOI] [PubMed] [Google Scholar]
- 39.Schmader KE, Hanlon JT, Fillenbaum GG, et al. Medication use patterns among demented, cognitively impaired and cognitively intact community-dwelling elderly people. Age Ageing. 1998;27:493–501. doi: 10.1093/ageing/27.4.493. [DOI] [PubMed] [Google Scholar]
- 40.Klarin I, Fastbom J, Wimo A. A population-based study of drug use in the very old living in a rural district of Sweden, with focus on cardiovascular drug consumption: comparison with an urban cohort. Pharmacoepidemiol Drug Saf. 2003;12:669–678. doi: 10.1002/pds.878. [DOI] [PubMed] [Google Scholar]
- 41.Alzheimer’s Association. 2009 Alzheimer’s disease facts and figures. Alzheimers Dement. 2009;5:234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, Glynn RJ, Dreyer NA, et al. Validity of claims-based definitions of left ventricular systolic dysfunction in Medicare patients. Pharmacoepidemiol Drug Saf. 2011;20:700–708. doi: 10.1002/pds.2146. [DOI] [PubMed] [Google Scholar]
- 43.Kramer JM, Curtis LH, Dupree CS, et al. Comparative effectiveness of beta-blockers in elderly patients with heart failure. Arch Intern Med. 2008;168:2422–2428. doi: 10.1001/archinternmed.2008.511. [DOI] [PubMed] [Google Scholar]
- 44.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 45.Martin BC, Wiley-Exley EK, Richards S, et al. Contrasting measures of adherence with simple drug use, medication switching, and therapeutic duplication. Ann Pharmacother. 2009;43:36–44. doi: 10.1345/aph.1K671. [DOI] [PubMed] [Google Scholar]
- 46.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 47.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 48.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 49.Alzheimer’s Association. [Accessed February 24, 2011];National Alzheimer’s Project Act (NAPA) http://www.alz.org/join_the_cause_21243.asp.