Abstract
Objectives
The purpose of our work was to define the complex electrophysiological characteristics seen in second- (2°) and third-degree (3°) atrioventricular block (AVB) and to longitudinally follow the development of atrial and ventricular heart rate and rhythm patterns with a goal of identifying heart rate and rhythm patterns associated with urgent delivery or neonatal pacing.
Background
The electrophysiological characteristics of congenital AVB before birth have not been extensively studied, yet the mortality from this disease is substantial. Along with advances in fetal therapies and interventions, a comprehensive natural history specific to the etiology of AVB, as well as the electrophysiological factors influencing outcome, are needed to best select treatment options.
Methods
Twenty-eight fetuses with AVB were evaluated by fetal magnetocardiography; 21 fetuses were evaluated serially.
Results
Fetuses with 2° AVB and isolated 3° AVB showed: 1) diverse atrial rhythms and mechanisms of atrioventricular conduction during 2° AVB; 2) junctional ectopic tachycardia and ventricular tachycardia during 3° AVB; 3) reactive ventricular and atrial fetal heart rate (FHR) tracings at ventricular rates >56 beats/min; and 4) flat ventricular FHR tracings at ventricular rates <56 beats/min despite reactive atrial FHR tracings. In contrast, fetuses with 3° AVB associated with structural cardiac disease exhibited predominantly nonreactive heart rate tracings and simpler rhythms.
Conclusions
Second-degree AVB, isolated 3° AVB, and 3° AVB associated with structural cardiac disease manifest distinctly different electrophysiological characteristics and outcome. Fetuses with 2° AVB or isolated 3° AVB commonly exhibited complex, changing heart rate and rhythm patterns; all 19 delivered fetuses are alive and healthy. Fetuses with structural cardiac disease and 3° AVB exhibited largely monotonous heart rate and rhythm patterns and poor prognosis. Junctional ectopic tachycardia and/or ventricular tachycardia may be characteristic of an acute stage of heart block.
Because the fetal electrocardiogram is not readily recordable during much of gestation, the pathological progression of atrioventricular (AV) block (AVB) has not been well established, and markers of electrophysiological instability that impact morbidity and mortality in children and adults, such as corrected QT interval prolongation, T-wave alternans, wide QRS escape rhythm, and nonreactive heart rate patterns, have not been adequately characterized. Lengthy evaluation of fetal heart rate (FHR) and rhythm patterns by Doppler and M-mode echocardiography is time consuming and is relatively imprecise. These various factors have contributed to a high rate of Cesarean and premature deliveries.
Prior investigations have demonstrated the efficacy of fetal magnetocardiography (fMCG), the magnetic analog of the fetal electrocardiogram, for assessment of heart rate and rhythm in AVB (1–3); however, these involved few subjects and did not comprehensively assess atrial and ventricular heart rate and rhythm. In this study, serial fMCG recordings were performed on a cohort of fetuses with isolated second- (2°) and third-degree (3°) AVB secondary to maternal SSA and SSB antibodies, or AVB associated with complex congenital cardiac defects. The purposes were to: 1) define the complex electrophysiological characteristics seen in 2° and 3° AVB; and 2) longitudinally follow the development of atrial and ventricular heart rate and rhythm patterns with a goal of identifying heart rate and rhythm patterns associated with urgent delivery or neonatal pacing.
Methods
Patient population
This was a retrospective longitudinal cohort study of 28 fetuses presenting from 1998 to 2006 with 2° and 3° AVB that were evaluated by fMCG. Informed consent was obtained from all subjects.
fMCG recordings
Recordings were made using a 37-channel biomagnetometer (Magnes 4D, Neuro-Imaging Inc., San Diego, California) in a magnetically shielded room. Typically, 5 to 10 recordings each of 10-min duration were obtained.
Signal processing was used to remove maternal interference, and the presence of atrial and ventricular arrhythmias in the rhythm strips was noted. Ventricular FHR tracings were computed from the RR intervals, and actograms (tracings of fetal activity derived from movement-related changes in signal amplitude) were obtained from the instantaneous QRS amplitudes (4). To compute atrial FHR, the fetal QRS complexes were first removed using signal processing. Atrial FHR tracings were computed from the PP intervals and were measured in runs where P-waves were clearest. The PR interval was measured in 2° AVB fetuses during 2:1 and 1:1 conduction, and QRS duration was measured in all fetuses using signal averaging of 20 to 50 complexes to increase the signal-to-noise ratio of the waveforms (5). Atrioventricular conduction in 2° AVB was assessed by plotting PR versus RP for each P-wave.
QRS duration was defined as wide if it exceeded the 95% confidence interval for gestation (approximately 62 to 70 ms) as previously defined in this laboratory in a normal fetal control population (5). Ventricular tachycardia (VT) was defined as paroxysms of a wide-complex rhythm with QRS morphology similar to that of spontaneous premature ventricular complexes and with a relatively regular rate >100 beats/min that exceeded the baseline ventricular rate at all other times, including FHR accelerations. Although faster than the underlying ventricular rhythm, VT was slower than the atrial rhythm, presumably reflecting the effects of acute inflammation or chronic disease on ventricular automaticity. Junctional ectopic tachycardia (JET) was defined as paroxysms of a narrow complex rhythm with a sporadic ventricular rate that exceeded the baseline ventricular rate at all other times, including FHR accelerations. Thus, VT and JET were distinguished on the basis of rhythmicity, as well as QRS morphology. Both rhythms exhibited ventriculoatrial dissociation, and in JET no antegrade sinus capture beats were observed due to impaired AV conduction.
Treatment
Mothers of 16 of 18 fetuses with isolated AVB received dexamethasone, 4 mg orally every day for 3 to 15 weeks. Mothers of 8 fetuses were treated with terbutaline, 2.5 to 7.5 mg given orally every 4 to 6 h for 7 to 11 weeks to maintain FHR >55 beats/min and maternal heart rate <120 beats/min. Fifteen of 28 fetuses were initially studied before any pharmacologic intervention.
Study variables
The primary study variables are tabulated in Tables 1 and 2. Atrial and ventricular reactivity (i.e., FHR acceleration associated with fetal movement) were ascertained by fMCG actocardiography (4). At <32 weeks, atrial acceleration was defined as FHR increases >10 beats/min sustained for >10 s, and at >32 weeks it was defined as FHR increases >15 beats/min sustained for >15 s (6). Ventricular reactivity was defined as sustained FHR increases >5 beats/min coinciding with atrial acceleration. No standard criterion exists for ventricular reactivity in fetuses with AVB at any gestational age or in normal fetuses earlier than about 25 weeks, when FHR monitoring normally is not performed.
Table 1.
Electrophysiological Characteristics of 3° AVB Fetuses
| First fMCG
|
Last fMCG
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fetus # | GA (Weeks) | RX | FHR (beats/min) | FHR Pattern | QRS (ms) | VT/JET | % Ectopy | # fMCG | GA (Weeks) | RX | FHR (beats/min) | FHR Pattern | QRS (ms) | VT/JET | % Ectopy |
| SSA | |||||||||||||||
|
| |||||||||||||||
| 1 | 25 | D | 74 | R | — | — | 0 | 5 | 37 | D | 70 | R | — | — | 0 |
| 2 | 25 | — | 67 | R | 45 | — | 0.7 | 4 | 37 | D | 75 | R | 62 | — | 17 |
| 3* | 26 | D | 71 | — | 44 | VT, JET | 92 | 4 | 30 | D | 50 | NR | 59–71 | — | 0.1 |
| 4* | 28 | D | 63 | R | 50–78 | — | 6.7 | 2 | 34 | D | 50 | NR | 59 | VT | 3.1 |
| 5 | 25 | D | 58 | R | 44 | — | 0 | 3 | 34 | D | 65 | R | 56 | — | 0 |
| 6 | 23 | D | 77 | R | 57 | — | 0 | 2 | 32 | D | 80 | R | 52 | 0 | |
| 7 | 23 | — | 67 | R | 48 | — | 0 | 4 | 37 | — | 68 | R | 59 | — | 0.4 |
| 8 | 26 | — | 53 | NR | 57 | — | 0.8 | 1 | |||||||
| 9 | 27 | D | 62 | R | 57 | — | 0.1 | 2 | 32 | — | 62 | R | 67 | — | 0 |
| 10* | 25 | T | 58 | NR | 51 | — | 0.1 | 3 | 33 | D, T | 60 | NR | 56 | — | 0 |
| 11* | 24 | — | 56 | — | 46 | JET | 89.7 | 6 | 34 | D | 52 | R | 57 | — | <0.1 |
| 12* | 20 | — | 72 | R | 44 | JET | 7 | 6 | 32 | D, T | 54 | NR | 50–83 | JET | 0.2 |
| 13 | 32 | D, T | 65 | R | 46 | — | 0.2 | 1 | |||||||
| 14* | 19 | D | 71 | — | 44 | JET | 91 | 3 | 30 | D | 60 | NR | 48 | — | 1.6 |
| 15* | 27 | D, T | 78 | NR | 75 | — | 0.1 | 2 | 31 | D, T | 64 | NR | 80 | — | |
| Avg | 25 | 65 | 49 | 29% | 20.6 | 3.1 | 34 | 62 | 60 | 14% | 2.2 | ||||
|
| |||||||||||||||
| CSD | |||||||||||||||
|
| |||||||||||||||
| 16* | 25 | — | 53 | NR | 48 | — | 0 | 2 | 31 | T | 63 | NR | 55 | — | 0 |
| 17*† | 23 | — | 64 | NR | 46 | — | 0.1 | 3 | 34 | — | 54 | NR | 57 | — | 0.1 |
| 18*† | 25 | — | 61 | NR | 51 | — | 0.1 | 1 | |||||||
| 19*† | 29 | T | 68 | NR | 61 | JET | 8.4 | 1 | |||||||
| 20*† | 29 | — | 56 | NR | 48 | — | 0 | 2 | 31 | T | 66 | NR | 60 | — | <0.1 |
| 21* | 24 | — | 61 | NR | 54 | — | 0 | 2 | 37 | 62 | NR | 55 | — | 1.5 | |
| 22*† | 23 | — | 61 | R | 56 | — | 0 | 1 | |||||||
| 23* | 21 | T | 63 | NR | 54 | — | 0 | 3 | 36 | T | 60 | NR | 61 | JET | 84 |
| Avg | 25 | 61 | 52 | 13% | 1.1 | 1.8 | 33 | 61 | 58 | 25% | 28.0 | ||||
Fetuses #1 to #15 had third-degree (3°) atrioventricular block (AVB) in association with SSA antibodies. Fetuses #16 to #23 had 3° AVB in association with complex structural disease (CSD); fetuses #16 to #21 had left atrial isomerism, and fetuses #18 and #20 were also twin pregnancies in which the other twin was normal; fetuses #22 and #23 had complex corrected transposition of the great arteries, accompanied by tricuspid atresia in fetus #22.
Fetuses requiring neonatal pacing;
neonatal deaths. Fetus #8 was electively terminated.
D = dexamethasone; FHR = fetal heart rate; FHR pattern = R: reactive; NR: nonreactive; GA = gestational age; QRS = QRS duration; RX = medication; T = terbutaline; VT/JET = ventricular tachycardia or junctional ectopic tachycardia; % ectopy = percent ectopic beats;
fMCG = number of fetal magnetocardiography sessions.
Table 2.
Electrophysiological Characteristics of 2° AVB Fetuses
| First fMCG
|
Last fMCG
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fetus # | GA (Weeks) | RX | FHR (beats/min) | FHR Pattern | QRS (ms) | PR (ms) | % NSR | # fMCG | GA (Weeks) | RX | FHR (beats/min) | FHR Pattern | QRS (ms) | PR (ms) | % NSR |
| 24 | 32 | — | 65 | R | 73–102 | 85 | 0 | 3 | 37 | D | 63 | R | 57 | 42–140 | 0 |
|
| |||||||||||||||
| 25 | 20 | — | 80 | R | 58 | 262 | 8.7 | 4 | 33 | D | 70 | R | 73 | 275–365 | 10.1 |
|
| |||||||||||||||
| 26 | 22 | — | 71 | R | 48 | 141 | 1.3 | 4 | 31 | D | 71 | R | 54 | 125–148 | 4.4 |
|
| |||||||||||||||
| 27 | 24 | — | 68 | R | 56 | 62–125 | 4 | 1 | |||||||
|
| |||||||||||||||
| 28 | 32 | — | 67 | R | 62 | 104 | 74 | 1 | |||||||
Fetus #27 had complex atrioventricular canal defect; fetus #28 had complex corrected transposition of the great arteries, the others had AVB in association with SSA antibodies; none required neonatal pacing.
PR = PR interval; % NSR = percent time in normal sinus rhythm; 2° = second degree; other abbreviations as in Table 1.
The statistical associations between urgent delivery or neonatal pacing (within the first 72 h of birth) and FHR patterns, ventricular ectopy, wide escape rhythm, and tachycardia were evaluated by the Fisher exact test. After birth, infants were paced because of class I indications as outlined in the present pacing recommendations in children (7).
Results
Clinical features
One pregnancy was electively terminated; the 27 other fetuses were delivered at 32 to 38.5 (mean 36) weeks’ gestation. All 17 delivered fetuses with isolated AVB are still living 4 months to 8 years after birth; none have dilated cardiomyopathy. Of 8 fetuses with structural cardiac disease and 3° AVB, 5 died within 2 weeks of birth and 3 are still living 3 months to 3 years after birth. Both fetuses with structural cardiac disease and 2° AVB are alive and healthy.
Rhythms
Five fetuses had 2° AVB (1 with complex AV canal defect and 1 with complex corrected transposition of the great arteries); all others had 3° AVB (8 with structural cardiac defects). Conversions from 2° to 3° AVB or vice versa were not seen in any fetus.
2° AVB
Fetuses with 2° AVB showed extended periods of 2:1 conduction and/or sinus rhythm, during which the PR interval was assessed. Modest PR lengthening consistent with Mobitz type I was observed in only a few instances; the predominant behavior was Mobitz type II. Of the 5 fetuses with 2° AVB, the PR interval was normal (104 ms) in 1, was modestly prolonged (120 to 148 ms) for normal sinus beats in 3, and was markedly prolonged (262 to 365 ms) in 1 with increased left atrial size and endocardial density. This fetus also showed QRS prolongation in the last 3 of 4 sessions.
Intermittent pre-excitation (Fig. 1A) was seen in 1 fetus at 32 and 33 weeks’ gestation. At 37 weeks’ gestation, pre-excitation was no longer present, but transient 3° AVB (Fig. 1B) with accrochage (8), which refers to the tendency of dissociated atrial and ventricular rhythms to synchronize, was noted. Another fetus showed alternating low atrial (Fig. 1C) and high atrial (not shown) rhythms, evidenced by alternation of the PR interval between 2 stable values (125 and 62 ms).
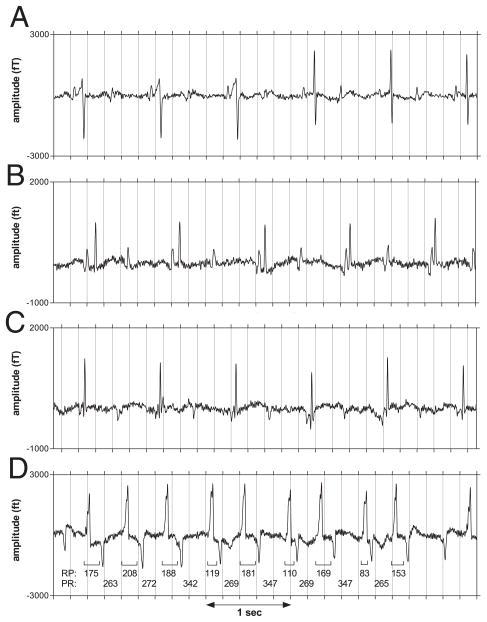
Figure 1. Rhythms in 2° Fetal AVB.
Rhythm strips from second-degree (2°) atrioventricular block (AVB) subjects showing: (A) intermittent pre-excitation with QRS normalization in fetus #24 at 32 weeks’ gestation; (B) varying PR interval due to transient atrioventricular dissociation in fetus #24 at 37 weeks’ gestation; (C) period of low atrial rhythm (PR = 55 ms) in fetus #27 with 2 atrial rhythms at 24 weeks’ gestation; in the high rhythm (not shown), PR interval was approximately 107 ms; and (D) transition of sinus rhythm (1:1 conduction) from stable RP/PR to alternating RP/PR in fetus #25 at 33 weeks’ gestation. After several beats with relatively constant RP/PR, the fourth atrial beat conducts slowly, shortening RP of the succeeding beat. Rather than blocking, the fifth atrial beat not only conducts, it does so more rapidly than the prior beat, prolonging RP of the succeeding beat. The alternating (short–long) RP intervals of the ensuing beats continue to show PR prolongation during the “long” phase of the cycle and conduction at short RP during the “short” phase until block occurs, notably, during the “long” phase. The tracings were processed to remove maternal interference but were not signal averaged.
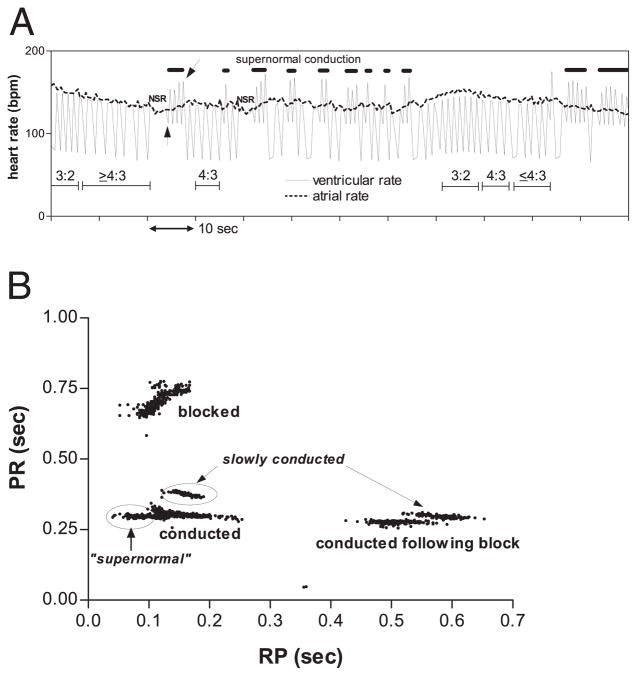
The last fMCG of fetus #25 showed highly reproducible and unusual rhythms, suggestive of “supernormal” conduction (Fig. 1D). Heart rate trends (Fig. 2A) and PR versus RP scatter plots (Fig. 2B) confirmed that the unusual AV conduction seen during “supernormal” conduction (arrow) was not present at other times and often followed periods of 1:1 conduction. The scatter plots also suggested the presence of dual AV nodal pathways at long (450 to 650 ms), as well as typical (100 to 250 ms), RP intervals.
Figure 2. Fetal Heart Rate Patterns and AV Conduction Curves in Supernormal Conduction.
(A) Ventricular and atrial heart rate tracing from last session of fetus #25, showing heart rate pattern changes associated with rhythm transitions. Wenckebach periods, indicated by the thin horizontal bars with conduction ratios above the bars, result in alternation of instantaneous ventricular heart rate between the atrial rate and half the atrial rate. A second pattern of alternation (thick horizontal lines) is due to beat-to-beat alternation of RP and PR, compatible with “supernormal” conduction. The RP and PR interval changes show a paradoxical positive correlation (Fig. 1D), which results in prominent beat-to-beat RR oscillations distinct from those due to Wenckebach periodicity. Usually, this pattern is immediately preceded by several or more beats of 1:1 sinus rhythm with constant RP/PR. The episodes initiate with a long RR interval (upward pointing arrow) and terminate with a short RR interval (slanted arrow) followed by a very long RR interval due to block of the following beat, as exemplified in the rhythm strip in Figure 1D. (B) Scatter plot of PR versus RP for each atrial beat in a 10-min recording during the last session of fetus #25. Atrial beats with RP >170 ms were always conducted, and the probability of conduction varied inversely with RP for 100 ms <RP <170 ms. During episodes of PR alternation (Fig. 1D); however, the data points formed 2 distinct clusters, enclosed by the ovals. One cluster consisted of beats conducted with long PR (slowly conducted); the other consisted of beats that conducted with short RP (“supernormal”). During episodes of PR alternation, beats with RP <100 ms were always conducted. Notice that in the ranges 100 ms <RP <250 ms and 450 ms <RP <650 ms, the conducted beats exhibited 2 distinct PR levels, suggesting the existence of 2 atrioventricular (AV) nodal pathways.
ECTOPY, TACHYARRHYTHMIAS, AND WIDE QRS RHYTHMS
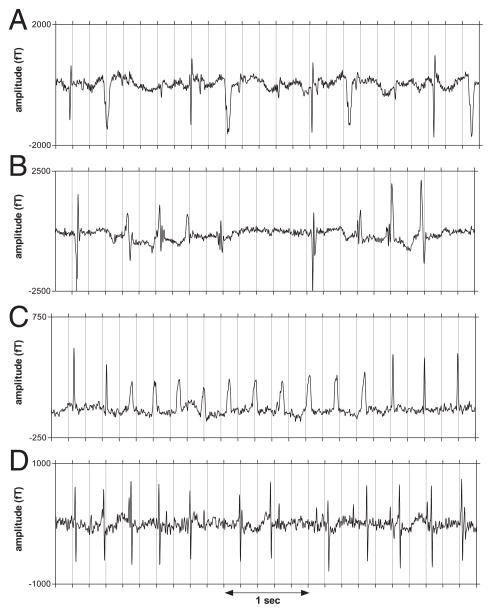
Atrial ectopy was noted in 8 (29%) fetuses, including 1 (fetus #3) with atrial bigeminy. Ventricular ectopy was rarely seen in 2° AVB, but was common in 3° AVB (17 of 23, 74%), including ventricular bigeminy (7 of 23, 30%) (Fig. 3A).
Figure 3. Ectopy and Tachycardia in 3° Fetal AVB.
Rhythm strips from third-degree (3°) atrioventricular block (AVB) fetuses showing: (A) ventricular bigeminy with wide ectopic complexes; (B and C) episodes of nonsustained VT in fetuses #4 and #3, respectively; and (D) junctional ectopic tachycardia in fetus #14 with highly irregular ventricular rhythm at 19 weeks’ gestation.
Nonsustained VT (Figs. 3B and 3C) was seen in fetus #4 at 32 weeks’ gestation and in fetus #3 at 26 weeks’ gestation, which also showed JET during the same session.
Five of the relatively immature fetuses (19 to 29 weeks’ gestation; 4 with isolated 3° AVB, 1 with structural cardiac disease) showed a highly irregular rhythm characterized by JET and very frequent ventricular ectopy (Fig. 3D), which was sporadic, in contrast to the bigeminal rhythms with fixed coupling interval seen in most other patients. This irregular rhythm was seen only in the first 1 or 2 sessions. Four of these fetuses showed the nonreactive heart rate pattern in the first session or when ectopy subsided, suggesting that JET and frequent ectopy are ominous precursors to loss of FHR reactivity; one fetus showed weak reactivity but had a low heart rate (52 to 56 beats/min) in all sessions.
Wide escape rhythms (QRS >70 ms) were seen in 4 of 15 (27%) fetuses with isolated 3° AVB, but were present only intermittently in 3 of the 4. No fetuses with structural cardiac disease showed wide escape rhythms.
3° AVB WITH ISORHYTHMIC AV DISSOCIATION
Whereas conversions from 3° to 2° AVB were not seen in any of our subjects, isorhythmic AV dissociation, which closely mimics 2° AVB, was often seen when the atrial and ventricular rates were in a 2:1 ratio. During periods of isorhythm, which could last up to 30 s, the AV interval was approximately 120 ms and atrial FHR variability was increased due to prominent ventriculophasic sinus arrhythmia (VSA). The atrial and ventricular rhythms were regular at the onset and termination of the isorhythm.
FHR patterns
REACTIVITY
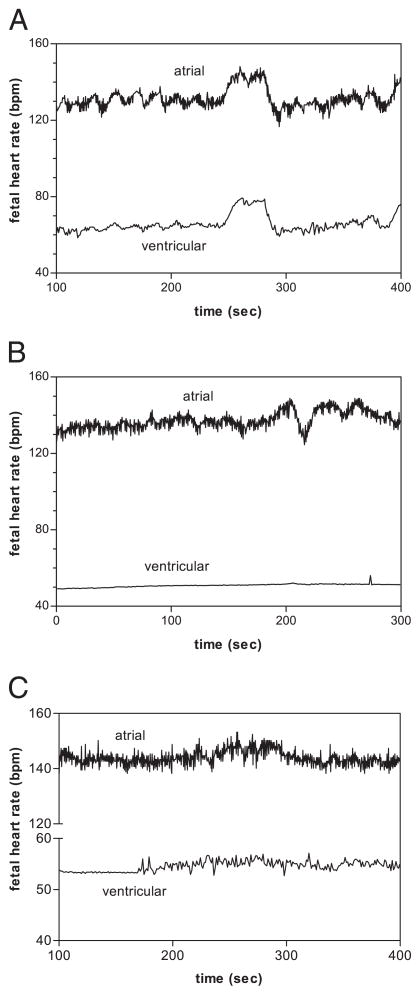
A notable aspect of the FHR tracings is that nearly all of them could be classified into 1 of 2 distinct patterns: a reactive and a nonreactive pattern. In the reactive pattern (Fig. 4A), the atrial and ventricular FHR tracings were reactive and highly correlated, and the amplitude of the ventricular FHR accelerations was strongly associated with mean ventricular heart rate. In the nonreactive pattern (Fig. 4B), ventricular reactivity was so low that the ventricular FHR tracings were almost completely flat, although normal atrial reactivity was present. The dichotomy of the patterns was evident during abrupt transitions between them, which were seen occasionally when the ventricular rate was around 56 beats/min (Fig. 4C).
Figure 4. Fetal Heart Rate Patterns in Fetal AVB.
Atrial and ventricular fetal heart rate tracings from fetus #4 showing (A) reactive pattern at 28 weeks’ gestation and (B) nonreactive pattern at 32 weeks’ gestation. In the reactive pattern, the atrial and ventricular tracings were highly correlated; even variations as small as 3 beats/min (bpm) were often correlated. The degree of ventricular reactivity and the amplitude of the beat-to-beat fetal heart rate variability varied strongly with mean ventricular rate. At rates >70 beats/min, ventricular reactivity was exaggerated and the amplitude of the ventricular accelerations actually exceeded that of the atrial accelerations. As mean ventricular rate declined, the amplitude of the ventricular accelerations and the beat-to-beat fetal heart rate variability progressively decreased. Notice that atrial reactivity is present in the nonreactive pattern. Both patterns showed prominent atrial beat-to-beat variability due to the presence of ventriculophasic sinus arrhythmia. Unlike normal beat-to-beat variability, its amplitude does not diminish during heart rate acceleration. (C) Atrial and ventricular fetal heart rate tracings from fetus #3 at 30 weeks’ gestation showing abrupt transition from the nonreactive to the reactive pattern. AVB = atrioventricular block.
All 2° AVB fetuses showed the reactive pattern. With one exception, fetuses with 3° AVB associated with structural cardiac disease showed the nonreactive pattern throughout the study, regardless of ventricular rate. Generally, the isolated 3° AVB fetuses showed the reactive pattern when ventricular heart rate exceeded about 56 beats/min and showed the nonreactive pattern at lower rates; however, fetuses on terbutaline showed a nonreactive pattern, even when terbutaline elevated the heart rate above 56 beats/min.
VARIABILITY
The atrial FHR tracings showed enhanced beat-to-beat variability due to the continual presence of VSA, which produced 3- to 10-beat/min fluctuations in the atrial FHR tracings. Ventriculophasic sinus arrhythmia was present in both the reactive and nonreactive patterns. Generally, it increased at lower atrial rates and was strongest during periods of isorhythmic AV dissociation, as noted in the preceding text. Although it was larger at late gestational ages, VSA resulted in prominent atrial FHR variability in some subjects before the third trimester, when beat-to-beat FHR variability normally is very small, and it was seen in fetuses with left atrial isomerism, a condition in which sinoatrial node abnormalities are often present.
In the nonreactive pattern, ventricular FHR variability was virtually absent. In the reactive pattern, long-term ventricular FHR variability was present, but decreased with declining heart rate, in proportion to the degree of reactivity. Beat-to-beat ventricular FHR variability was also present but was visibly reduced compared with that of normal subjects. This, however, was due in large part to bradycardia, which precludes resolution of high-frequency FHR variability.
PROGRESSION OF HEART RATE PATTERNS
Seven of 8 fetuses with structural cardiac disease and 3° AVB presented with the nonreactive pattern and remained in this pattern throughout the study.
Of 20 fetuses with 2° or isolated 3° AVB, 14 presented with a reactive FHR pattern, 3 presented with a nonreactive pattern, and 3 presented with ectopy so frequent that it was not possible to determine the underlying FHR pattern. Of the 14 presenting with a reactive pattern, 2 (fetuses #4 and #12) had deteriorated to the nonreactive pattern by the last session. Both showed ominous rhythms during the first session. Fetus #4 showed intermittent QRS broadening (>70 ms) and abrupt transitions to brief periods of a bigeminal escape rhythm with very low rate; fetus #12 showed episodes of JET. No subjects ever reverted from the nonreactive pattern to the reactive pattern.
Association of pacing with FHR and rhythm patterns
A highly statistically significant association was found between neonatal pacing and a nonreactive pattern in the last session (p < 0.001). All 12 unpaced fetuses showed reactive FHR patterns. Of 15 paced fetuses, only fetuses #11 and #22 showed reactive FHR patterns in the last session. Fetus #11 had a very low ventricular rate (52 beats/min) and fetus #22, who had complex structural disease, was not studied after 23 weeks’ gestation.
A statistically significant association was found between neonatal pacing and tachycardia (p < 0.02). The 6 fetuses with JET and/or VT all required neonatal pacing. The 4 fetuses with intermittent wide QRS escape rhythms required neonatal pacing.
Steroid therapy and FHR and rhythm patterns
The 3 fetuses with isolated 2° AVB all received steroid therapy sometime after the first fMCG session. None progressed to 3° AVB, none reverted to sinus rhythm, and the percent time in sinus rhythm did not change appreciably before and after initiation of therapy. Of 4 fetuses with isolated 3° AVB presenting with JET, 2 were already receiving steroid therapy and 2 were not. Two 3° AVB fetuses on steroid therapy deteriorated from a reactive to a nonreactive FHR pattern, which was permanent and was accompanied by VT in 1 case. The lone delivered fetus with isolated AVB that did not receive steroid therapy had 3° AVB and showed a reactive FHR pattern throughout the study.
Discussion
To our knowledge, this is the first longitudinal study of atrial and ventricular heart rate and rhythm patterns in a sizable population of fetuses with AVB assessed by magnetocardiography. We found that 2° AVB, isolated 3° AVB, and 3° AVB associated with structural cardiac disease manifest distinctly different electrophysiological characteristics and that no transitions occurred between 2° and 3° AVB. Fetuses with 2° AVB showed complex, variable rhythm patterns associated with diverse forms of AV conduction and variable atrial rhythms. In fetuses with isolated 3° AVB, disease progression was reflected in the escape rhythm, which could deteriorate to a nonreactive pattern, often in association with intermittent QRS broadening and/or tachycardia. Junctional ectopic tachycardia and frequent ventricular ectopy were early predictors of more severe disease. Remarkably, all patients with isolated AVB are alive and well. In contrast, those with structural cardiac disease and 3° AVB showed nonreactive heart rate tracings, comparatively monotonous rhythm, and high postnatal mortality. This high mortality in association with structural heart disease has been previously reported (9); the nonreactivity implies that the pacemaker is infranodal.
Junctional ectopic tachycardia and VT, which were present in 30% of 3° AVB subjects, were not generally identified before referral despite multiple echocardiograms, in part because of their brief and paroxysmal nature and also because these rhythms were not easily distinguished from intermittent conduction or various forms of ectopy. We speculate that VT, JET, and frequent ectopy may be characteristic of an acute stage of 3° AVB and that their prevalence may relate to the severity of the disease during the acute phase. Our findings suggest that when JET is observed in the fetus with or without AVB, the mother should be tested for SSA and SSB antibodies, as previously suggested by Dubin et al. (10).
It is commonly believed that AVB associated with SSA antibodies occurs gradually and that the degree of block reflects disease severity, but this has not been systematically documented. Our data suggest that the preponderance of injury to the conduction system occurs early and very rapidly and/or that 2° and 3° fetal AVB may have different etiology. The 3° AVB fetuses were already in 3° AVB at presentation, generally before 30 weeks’ gestation. Although it is plausible that these fetuses had gradual progression from 1° to 3° AVB before recognition, our data suggest instead that the onset of 3° AVB was rapid and was often accompanied by frequent ectopy and JET. The 2° AVB fetuses presented at similar gestational ages, yet remained in 2° AVB throughout the study. In contrast, some previous studies have reported a substantial conversion rate from 2° to 3° fetal AVB (9). We speculate that the presence of frequent ectopy and JET, which were common at early gestational ages, and isorhythmic AV dissociation, can lead to overestimation of 2° versus 3° fetal AVB and of conversion from 2° to 3° fetal AVB.
Fetuses with 2° AVB exhibited complex, changing rhythms such as intermittent pre-excitation, alternating high and low atrial rhythms, and variable AV conduction, including rhythm patterns suggestive of multiple AV nodal pathways and/or “supernormal” conduction. “Supernormal” conduction refers not to conduction that is more rapid than normal, but to conduction that is more rapid than expected, pertaining especially to situations when block is expected. It has been invoked previously to explain PR alternation in patients with impaired AV conduction (11,12); however, conclusive evidence is generally difficult to obtain due to such alternative explanations as dual AV nodal pathways, concealed junctional extrasystoles, and “gap” phenomena (13). In this study, the suggestion of “supernormal” conduction in association with PR alternation was supported by analysis of AV conduction curves. The marked PR prolongation during the “long” phase of the cycle strongly suggests the presence of a concealed, slow AV nodal pathway. The consistent conduction at extremely short RP interval during the “short” phase of the cycle, along with the fact that the pattern always terminated with block during the “long” phase, suggests that conduction during the “short” phase occurred through a concealed “supernormal” pathway.
We have previously shown that T-wave abnormalities (QT prolongation, T-wave alternans) (14) and P-wave amplitude elevation (15) are common in fetal bradycardia, including fetal AVB, and may be associated with suboptimal outcome and compensatory hypertrophy, respectively. In this study, the incidence and degree of QRS prolongation was modest; however, fetuses with wide escape rhythms, even when present intermittently, required neonatal pacing. PR prolongation in 2° AVB was also modest in most subjects, implying that detection of disease onset based on assessment of echocardiographic mechanical PR interval will require high measurement precision. Fetal magnetocardiography monitoring and other electrophysiological screening for pre-clinical features of antibody-mediated conduction disease such as atrial or ventricular hypertrophy, T-wave alternans, or QT prolongation before the onset of heart block may supplement the echocardiographic mechanical AV interval measurement in pregnant lupus or antibody-positive patients.
Owing to the ability of fMCG to assess atrial and ventricular FHR simultaneously, it could be seen that beat-to-beat FHR variability was abolished when the ventricular rate fell below about 56 beats/min, and it could be verified that the atrial reactivity remained normal despite the wide-ranging reactivity of the ventricular FHR. It was also observed that VSA caused a marked increase in beat-to-beat atrial FHR variability, which was evident even before the third trimester when beat-to-beat FHR variability normally is very low due to immaturity of the parasympathetic nervous system. This implies that arterial pulsations, which are the source of VSA, exert a rapid and strong influence on sinus rate, although their influence is evident only during heart block and/or AV dissociation.
The use of steroid therapy in fetal AVB associated with SSA antibodies remains controversial. Although we found no evidence that steroid therapy is effective in reversing AVB or nonreactivity, we believe that the improved long-term survival reported by Jaeggi et al. (16) justifies the use of steroid therapy when acute inflammatory findings or poor prognostic indicators such as ventricular dysfunction or endocardial fibroelastosis, arrhythmias, severe QT prolongation, or T-wave alternans are present. A means by which the role of steroids in AVB could be elucidated would involve study of a large number of SSA-positive patients during sinus rhythm, using fMCG to seek the natural history of AVB and the causes of sudden demise. A limitation of the technique, however, is the very low signal amplitude before about 18 weeks’ gestation.
Conclusions
Congenital AVB is not a monotonous and simple rhythm, but one that is electrophysiologically intricate and dynamic. Ventricular reactivity and heart rate variability, key indicators of outcome, are present in most fetuses with isolated AVB and can either continue throughout pregnancy and beyond or can be lost. Frequent ventricular ectopy and junctional tachyarrhythmia at early gestational ages portend a more severe manifestation of the disease.
Acknowledgments
This research was supported by NIH grants R01 HL63174 and R21 HD049022 and by a grant from the Advancing a Healthier Wisconsin Partnership Program.
Abbreviations and Acronyms
- AV
atrioventricular
- AVB
atrioventricular block
- FHR
fetal heart rate
- fMCG
fetal magnetocardiography
- JET
junctional ectopic tachycardia
- VSA
ventriculophasic sinus arrhythmia
- VT
ventricular tachycardia
- 2°
second degree
- 3°
third degree
References
- 1.Menendez T, Achenbach S, Beinder E, et al. Usefulness of magnetocardiography for the investigation of fetal arrhythmias. Am J Cardiol. 2001;88:334–6. doi: 10.1016/s0002-9149(01)01658-7. [DOI] [PubMed] [Google Scholar]
- 2.Van Leeuwen P, Hailer B, Bader W, Geissler J, Trowitzsch E, Gronemeyer D. Magnetocardiography in the diagnosis of fetal arrhythmia. Br J Obstet Gynaecol. 1999;11:1200–8. doi: 10.1111/j.1471-0528.1999.tb08149.x. [DOI] [PubMed] [Google Scholar]
- 3.Wakai RT, Leuthold AC, Cripe L, Martin CB. Assessment of fetal rhythm in complete congenital heart block by magnetocardiography. Pacing Clin Electrophysiol. 2000;23:1047–50. doi: 10.1111/j.1540-8159.2000.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhao H, Wakai RT. Simultaneity of foetal heart rate acceleration and foetal trunk movement determined by foetal magnetocardiogram actocardiography. Phys Med Biol. 2002;47:839–46. doi: 10.1088/0031-9155/47/5/310. [DOI] [PubMed] [Google Scholar]
- 5.Leuthold A, Wakai RT, Martin CB. Noninvasive in utero assessment of PR and QRS intervals from the fetal magnetocardiogram. Early Hum Dev. 1999;54:235–43. doi: 10.1016/s0378-3782(98)00100-5. [DOI] [PubMed] [Google Scholar]
- 6.National Institute of Child Health and Human Development. Electronic fetal heart rate monitoring: research guidelines for interpretation. National Institute of Child Health and Human Development Research Planning Workshop. Am J Obstet Gynecol. 1997;177:1385–90. [PubMed] [Google Scholar]
- 7.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines) J Am Coll Cardiol. 2002;40:1703–19. doi: 10.1016/s0735-1097(02)02528-7. [DOI] [PubMed] [Google Scholar]
- 8.Marriott HJ. Atrioventricular synchronization and accrochage. Circulation. 1956;14:38–43. doi: 10.1161/01.cir.14.1.38. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt KG, Ulmer HE, Silverman NH, Kleinman CS, Copel JA. Perinatal outcome of fetal complete atrioventricular block: a multi-center experience. J Am Coll Cardiol. 1991;17:1360–6. doi: 10.1016/s0735-1097(10)80148-2. [DOI] [PubMed] [Google Scholar]
- 10.Dubin AM, Cuneo BF, Strasburger JF, Wakai RT, Van Hare GF, Rosenthal DN. Congenital junctional ectopic tachycardia and congenital complete atrioventricular block: a shared etiology? Heart Rhythm. 2005;2:313–5. doi: 10.1016/j.hrthm.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Langendorf R. Alternation of A-V conduction time. Am Heart J. 1958;55:181–91. doi: 10.1016/0002-8703(58)90117-0. [DOI] [PubMed] [Google Scholar]
- 12.Pick A, Langendorf R, Katz LN. The supernormal phase of atrioventricular conduction. I. Fundamental mechanisms. Circulation. 1962;26:388–404. doi: 10.1161/01.cir.26.3.388. [DOI] [PubMed] [Google Scholar]
- 13.Mazgalev T, Tchou P. Atrioventricular nodal conduction gap and dual pathway electrophysiology. Circulation. 1995;92:2705–14. doi: 10.1161/01.cir.92.9.2705. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H, Strasburger JF, Cuneo BF, Wakai RT. Fetal cardiac repolarization abnormalities. Am J Cardiol. 2006;98:491–6. doi: 10.1016/j.amjcard.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Strasburger JF, Cuneo BF, Gotteiner NL, Wakai RT. Giant fetal magnetocardiogram P waves in congenital atrioventricular block: a marker of cardiovascular compensation? Circulation. 2004;110:2097–101. doi: 10.1161/01.CIR.0000144302.30928.AA. [DOI] [PubMed] [Google Scholar]
- 16.Jaeggi ET, Fouron JC, Silverman ED, Ryan G, Smallhorn J, Hornberger LK. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation. 2004;110:1542–8. doi: 10.1161/01.CIR.0000142046.58632.3A. [DOI] [PubMed] [Google Scholar]