Abstract
Background
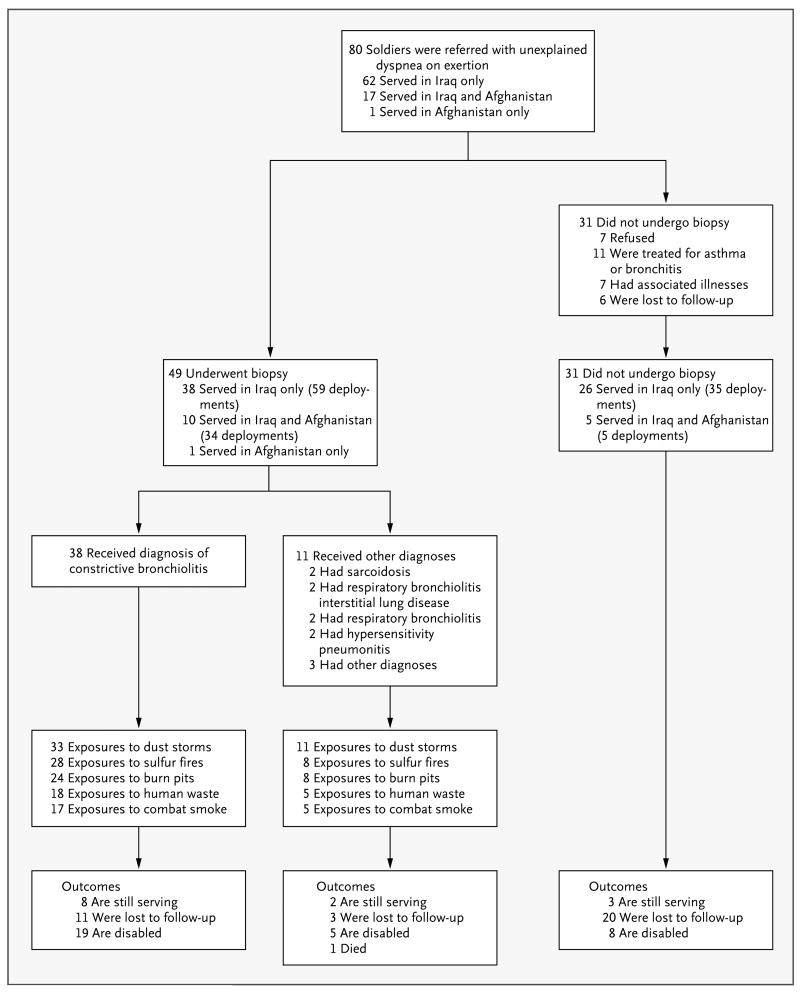
In this descriptive case series, 80 soldiers from Fort Campbell, Kentucky, with inhalational exposures during service in Iraq and Afghanistan were evaluated for dyspnea on exertion that prevented them from meeting the U.S. Army's standards for physical fitness.
Methods
The soldiers underwent extensive evaluation of their medical and exposure history, physical examination, pulmonary-function testing, and high-resolution computed tomography (CT). A total of 49 soldiers underwent thoracoscopic lung biopsy after noninvasive evaluation did not provide an explanation for their symptoms. Data on cardiopulmonary-exercise and pulmonary-function testing were compared with data obtained from historical military control subjects.
Results
Among the soldiers who were referred for evaluation, a history of inhalational exposure to a 2003 sulfur-mine fire in Iraq was common but not universal. Of the 49 soldiers who underwent lung biopsy, all biopsy samples were abnormal, with 38 soldiers having changes that were diagnostic of constrictive bronchiolitis. In the remaining 11 soldiers, diagnoses other than constrictive bronchiolitis that could explain the presenting dyspnea were established. All soldiers with constrictive bronchiolitis had normal results on chest radiography, but about one quarter were found to have mosaic air trapping or centrilobular nodules on chest CT. The results of pulmonary-function and cardiopulmonary-exercise testing were generally within normal population limits but were inferior to those of the military control subjects.
Conclusions
In 49 previously healthy soldiers with unexplained exertional dyspnea and diminished exercise tolerance after deployment, an analysis of biopsy samples showed diffuse constrictive bronchiolitis, which was possibly associated with inhalational exposure, in 38 soldiers.
Reports of respiratory symptoms have been common among soldiers who have served in the Middle East, beginning in the 1990s and more recently in soldiers returning from Iraq and Afghanistan.1,2 Epidemiologic studies in the United States, England, and Australia have documented an increased incidence of respiratory disorders in soldiers who served in the Middle East, as compared with soldiers who were deployed elsewhere.2-5 A 2009 study of 46,000 military personnel showed an association between the development of respiratory symptoms and service in Iraq, as well as an association with service inland versus at sea.2 These reports suggest that some soldiers serving in the Middle East have inhalational injury during deployment, but no studies have identified pathological correlates to these respiratory symptoms and disorders. Respiratory conditions such as acute eosinophilic pneumonia, asthma, and allergic rhinitis have been associated with service in the Middle East but do not fully account for the increased incidence of respiratory symptoms.6-8
A group of soldiers from Blanchfield Army Hospital in Fort Campbell, Kentucky, were referred to Vanderbilt University Medical Center for evaluation of exercise intolerance because of exertional dyspnea after returning from duty in Iraq or Afghanistan. The soldiers were unable to achieve the fitness standard for the 2-mile run that all had met before deployment. Many of the soldiers reported having been exposed to smoke from a fire in a large sulfur mine near Mosul, Iraq, during the summer of 2003, but several of the soldiers reported having had no specific exposure. The cause of the persistent dyspnea was not apparent in these otherwise healthy soldiers.
Methods
Study Design
From February 2004 through December 2009, we evaluated 80 soldiers with respiratory symptoms who were referred because of exercise intolerance (Fig. 1). All the soldiers underwent extensive evaluation of occupational and environmental exposures. Of these soldiers, 49 were referred for videoassisted thoracoscopic lung biopsy at the discretion of the treating physician. The remaining 31 soldiers were not referred for biopsy for the reasons outlined in Figure 1. The 38 soldiers who had biopsy findings of constrictive bronchiolitis are the focus of this analysis. We compared the results on pulmonary-function and cardiopulmonary-exercise testing for these soldiers with results for historical military control subjects.9 We updated all medical histories, evaluation of symptoms, and disability ratings of the entire group of 80 soldiers by means of a survey in the fall of 2010. The institutional review board at Vanderbilt University Medical Center approved the study.
Figure 1. Characteristics and Outcomes of 80 Soldiers Referred for the Evaluation of Shortness of Breath on Exertion.
Among the three soldiers in whom disorders other than those listed here were diagnosed, one had peribronchial scarring, one had an endobronchial stricture, and one had a necrotizing granuloma surrounded by normal lung parenchyma.
Pulmonary-Function and Cardiopulmonary-Exercise Testing
We measured results on spirometry, lung volumes, and diffusing capacities (Medgraphics) according to the guidelines of the American Thoracic Society and interpreted the findings using the standards of Crapo and colleagues.10,11 Cardiopulmonary-exercise testing was performed with the use of an electronically braked cycle ergometer (Ergoline) and consisted of a single bout of incremental exercise to exhaustion.12 We measured pulmonary gas exchange and minute ventilation using a commercially available metabolic cart (CareFusion). Adequate effort on testing was defined as either a peak heart rate of at least 85% of the predicted value or a peak respiratory exchange ratio of 1.00 or more.
High-Resolution Computed Tomography
Of the 38 soldiers with constrictive bronchiolitis, 37 underwent helical computed tomography (CT) with reconstruction into 3-mm, contiguous, standard algorithmic images and also high-resolution images with a slice thickness of 1 mm every 10 mm, without the use of intravenous contrast material. Transaxial images with a thickness of 1.25 mm at 10-mm increments were obtained in the supine position on expiration and in the prone position on inspiration and were reconstructed with a high-resolution algorithm with the use of Philips iCT128 slice or Philips Brilliance 64 with essence technology scanners (Koninklijke Philips Electronics).
Pathological Analysis of Lung Tissue
Two pulmonary pathologists examined the lungbiopsy samples in an unblinded fashion with hematoxylin and eosin and Movat's pentachrome connective-tissue staining. Masson's trichrome connective-tissue staining was also used in many of the specimens. The case definition for constrictive bronchiolitis was the presence of extrinsic narrowing of the luminal wall caused by subepithelial fibrosis, smooth-muscle hypertrophy in membranous bronchioles (non-cartilaginous airways having a complete fibromuscular wall), or both in a patient with otherwise normal lung parenchyma. Constrictive bronchiolitis was defined as an increase in wall thickness of more than 20%, as compared with normal thickness.13
Each membranous bronchiole was examined for the presence of luminal narrowing, subepithelial fibrosis or smooth-muscle hypertrophy, peribronchial inflammation (the presence of any number of lymphocytes or plasma cells within bronchiolar submucosa or adventitia), peribronchial pigment deposition, polarizable material, luminal granulation, mucus plugging, peribronchial eosinophils, bronchial-associated lymphoid tissue, and abnormalities of intima and media in adjacent pulmonary arteries. All slides were also examined under polarized light with the use of the microscope's built-in polarizer and analyzer filters.
Statistical Analysis
We used Welch's t-test (which does not assume equal variance for the two groups) to compare the results on pulmonary-function and cardiopulmonary-exercise testing for the 38 soldiers with constrictive bronchiolitis with the results for the military control subjects, a group of healthy soldiers who were evaluated by Morris et al.9 The mean proportion of airway involvement was estimated, and 95% confidence intervals were calculated with the use of nonparametric bootstrap analysis.
Results
Patients
Among the 38 soldiers (35 men and 3 women) whose biopsy samples showed constrictive bronchiolitis, the median age was 33 years (range, 23 to 44). (Additional demographic data are provided in Section 2 in the Supplementary Appendix, available with the full text of this article at NEJM.org.) All 38 soldiers had met the requirements of the Army Physical Fitness Test wearing combat gear before deployment. These requirements vary according to age, with the time for a 2-mile run ranging from 16.5 minutes for soldiers who are 23 years of age to 19.5 minutes for those who are 48 years of age.14 Of the 38 soldiers, 25 were lifetime nonsmokers, 7 were active smokers, and 6 were former smokers (Table 1).
Table 1. Exposure History and Evaluation Summary for 38 Soldiers with Constrictive Bronchiolitis.
| Variable | Value no. (%) |
|---|---|
| Smoking status | |
| Current | 6 (16) |
| Former | 7 (18) |
| Never | 25 (66) |
| Exposure history | |
| Sulfur-mine fire in 2003 | 28 (74) |
| Incinerated solid waste | 24 (63) |
| Incinerated human waste | 18 (47) |
| Dust storms | 33 (87) |
| Combat smoke | 17 (45) |
| Chest radiography | |
| Normal | 37 (97) |
| Lingular nodule | 1 (3) |
| Computed tomography* | |
| Normal | 25 (68) |
| Mild air trapping | 6 (16) |
| Multiple nodules <1 cm | 2 (5) |
| Solitary nodule <1 cm | 1 (3) |
| Pleural thickening | 1 (3) |
| Bibasilar scarring | 1 (3) |
| Apical bullae | 1 (3) |
| Pulmonary-function testing | |
| Normal | 13 (34) |
| Normal with low carbon monoxide diffusing capacity | 19 (50) |
| Obstructive | 2 (5) |
| Restrictive | 3 (8) |
| Mixed obstructive and restrict ive | 1 (3) |
The results are for 37 soldiers who underwent high-resolution computed tomography.
In this group of soldiers, 28 had served in northern Iraq in 2003 and reported having been exposed to smoke from a sulfur-mine fire near Mosul. The plume had levels of sulfur dioxide as high as 125 ppm and extended for miles over the area where most of the Fort Campbell troops and other soldiers had been barracked. Notably, 11 of the soldiers who underwent biopsy reported having had no exposure to the sulfur-mine fire or any other unique exposure. Thirty-three soldiers reported exposure to dust storms, 24 reported exposure to incinerated solid waste in burn pits, and 18 reported exposure to incinerated human waste. The soldiers served in a variety of positions, including helicopter pilots, flight engineers, infantry members, communication specialists, fuelers, mechanics, and military police. Four soldiers had a history of childhood asthma or allergic rhinitis, three had gastroesophageal reflux disease, one had essential hypertension, and one had remote cutaneous sarcoidosis. The results of physical examination of the chest were normal in all the soldiers.
Imaging Studies
Results on posteroanterior and lateral chest radiography were normal in 37 soldiers and revealed a lingular nodule in 1 soldier (Table 1). Among the 37 soldiers who underwent high-resolution CT, 25 had normal results; 6 soldiers had mild air trapping, 1 had a single nodule, 2 had multiple nodules, 1 had basilar scarring, 1 had pleural thickening, and 1 had apical bullae (Table 1, and Section 3 in the Supplementary Appendix).
Pulmonary-Function and Cardiopulmonary-Exercise Testing
Spirometric values, lung volumes, and measures of carbon monoxide diffusing capacity were within normal limits in 13 soldiers; 19 had an isolated low carbon monoxide diffusing capacity with normal spirometric values and lung volumes. Three soldiers met the criteria for restriction, with two of these soldiers also having a low carbon monoxide diffusing capacity. Two soldiers met the criteria for obstruction, with one also having a low carbon monoxide diffusing capacity.10 One soldier had mixed obstruction and restriction with a reduced carbon monoxide diffusing capacity. Bronchoprovocation with methacholine was not part of the protocol but had been performed before study enrollment in 12 of the 38 soldiers, with 1 positive result. (Details on testing are provided in Sections 4 and 5 in the Supplementary Appendix.)
Soldiers with biopsy-proven constrictive bronchiolitis had significantly lower measures of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), ratio of FEV1 to FVC, and carbon monoxide diffusing capacity than did control subjects (Table 2). Cardiopulmonary-exercise testing was performed in 30 of the 38 soldiers. Patients with constrictive bronchiolitis had mean levels of maximal oxygen consumption and an anaerobic threshold that were at the lower limit of the normal range (maximal oxygen consumption, 80% of the predicted value; anaerobic thresh old, 40% of the predicted maximal oxygen con sum p tion). The mean maximal oxygen consump tion was significantly lower in the soldiers than in control subjects (85.1±15.2% vs. 105.4± 14.3% of the predicted value, P<0.001) and the anaerobic thresh old was also reduced (45.0±9.5% vs. 78.2±15.3% of the predicted value, P<0.001). The level of effort was adequate for all soldiers, with lower peak heart rates in the soldiers than in the control subjects but with an adequate respiratory exchange ratio. The mean breathing reserve (maximum voluntary ventilation minus peak minute ventilation) was normal in the soldiers (59.8±24.3 liters) and did not differ significantly from that of the control subjects (Table 2).
Table 2. Demographic and Clinical Characteristics of 38 Soldiers with Constrictive Bronchiolitis, as Compared with Military Control Subjects.*.
| Characteristic | Control Subjects | Soldiers | P Value† | Between-Group Difference (95% CI)‡ |
|---|---|---|---|---|
| Age (yr) | 27.3±7.5 | 33.4±6.1 | <0.001 | 6.1 (3.4 to 8.7) |
| Body-mass index§ | 25.7±3.3 | 28.9±3.6 | <0.001 | 3.2 (1.8 to 4.6) |
| Pulmonary-function testing | ||||
| FEV1 (% of predicted value) | 99.1±9.2 | 86.7±13.3 | <0.001 | −12.4 (−17.3 to −7.6) |
| FVC (% of predicted value) | 101.6±10.7 | 90.7±13.2 | <0.001 | −10.9 (−15.9 to −5.9) |
| Ratio of FEV1 to FVC | 97.4±5.0 | 79.1±7.6 | <0.001 | −18.3 (−21.0 to −15.6) |
| Total lung capacity (% of predicted value) | 99.6±12.0 | 96.1±15.5 | 0.23 | −3.5 (−9.3 to −2.3) |
| Carbon monoxide diffusing capacity (% of predicted value) | 90.6±12.6 | 73.4±15.4¶ | <0.001 | −17.2 (−23.2 to −11.3) |
| Cardiopulmonary-exercise testing║ | ||||
| Maximal oxygen consumption (% of predicted value) | 105.4±14.3 | 85.1±15.2 | <0.001 | −20.3 (−26.9 to −13.8) |
| Ventilator anaerobic threshold (% of maximal oxygen consumption) | 78.2±15.3 | 45.0±9.5 | <0.001 | −33.2 (−38.2 to −28.1) |
| Respiratory exchange ratio | NA | 1.10±0.20 | NA | NA |
| Maximal heart rate (% of predicted value) | 95.2±5.7 | 87.2±9.5 | <0.001 | −8.0 (−11.7 to −4.2) |
| Respiratory rate (breaths/min) | 44.5±6.7 | 34.4±7.7 | <0.001 | −10.1 (−13.3 to −6.9) |
| Ratio of minute ventilation to carbon dioxide production | 31.9±4.0 | 28.3±3.5 | <0.001 | −3.6 (−5.2 to −2.0) |
| Dyspnea index at maximal effort | 66.5±10.7 | 61.5±12.6 | 0.06 | −5.0 (−10.3 to 0.3) |
| Maximum voluntary ventilation minus peak minute ventilation (liter/min) | 50.9±31.7 | 59.8±24.3 | 0.13 | 8.9 (−2.7 to 20.6) |
Plus–minus values are means ±SD. Data for military control subjects were derived from reports regarding 69 asymptomatic active-duty soldiers who were evaluated at an army tertiary care center in 2002.9 FEV1 denotes forced expiratory volume in 1 second, FVC forced vital capacity, and NA not available.
P values were calculated with the use of Welch's t-test.
The values are for the comparison between soldiers with constrictive bronchiolitis and military control subjects.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Carbon monoxide diffusing capacity could not be measured in one soldier.
Cardiopulmonary-exercise testing was performed in 30 soldiers.
Lung-Biopsy Findings
Lacy black pigment was noted on the visceral pleural surface during biopsy in 37 of the soldiers. Thirty-eight of the biopsy samples revealed constrictive bronchiolitis. Several other histologic features were also frequently present (Table 3). Figure 2 shows representative pathological images for soldiers in whom constrictive bronchiolitis was diagnosed. At low magnification, biopsy samples showed scattered small densities, which at higher magnification were identifiable as membranous bronchioles with variable mural thickening, mixed airway-wall inflammation, and peribronchiolar deposition of grayish black pigment. Membranous bronchioles contained hypertrophic mural smooth muscle or fibrous thickening with luminal narrowing in 64% of small airways (95% confidence interval, 58 to 71). Thirty-seven of the biopsy samples showed the deposition of grayish black peribronchiolar pigment, with 36 showing polarizable material within the pigment. The results of culture of lung-biopsy samples to identify bacteria, fungus, or acid-fast bacilli were all negative. Alveolar structures and larger airways were otherwise normal in all 38 soldiers. Of the 49 soldiers who underwent lung biopsy, 11 had pathological changes other than constrictive bronchiolitis (Fig. 1). (The distribution of bronchiolar lesions is shown in Fig. 1 in the Supplementary Appendix.)
Table 3. Pathological Features of Biopsy Samples Obtained from 38 Soldiers with Constrictive Bronchiolitis.
| Variable | No. of Patients |
|---|---|
| Bronchiolar luminal constriction* | 38 |
| Predominant constrictive stroma | |
| Smooth muscle | 7 |
| Fibrous tissue | 3 |
| Mixed | 28 |
| Pigment deposition | 37 |
| Polarizable material within pigment | 36 |
| Peribronchiolar inflammation | 34 |
| Hypertensive-type arterial change | 28 |
| Respiratory bronchiolitis | 27 |
| Prominent bronchial-associated lymphoid tissue | 19 |
| Mucus plugging | 13 |
| Eosinophils in bronchiolar wall | 7 |
| Luminal granulation | 2 |
| Obliteration of bronchioles | 0 |
On average, luminal constriction was observed in 64% of sampled terminal and respiratory bronchioles (95% confidence interval, 57.6 to 71.0 on nonparametric bootstrap analysis).
Figure 2. Constrictive Bronchiolitis, Arteriopathy, and Peribronchial Pigment Deposition.
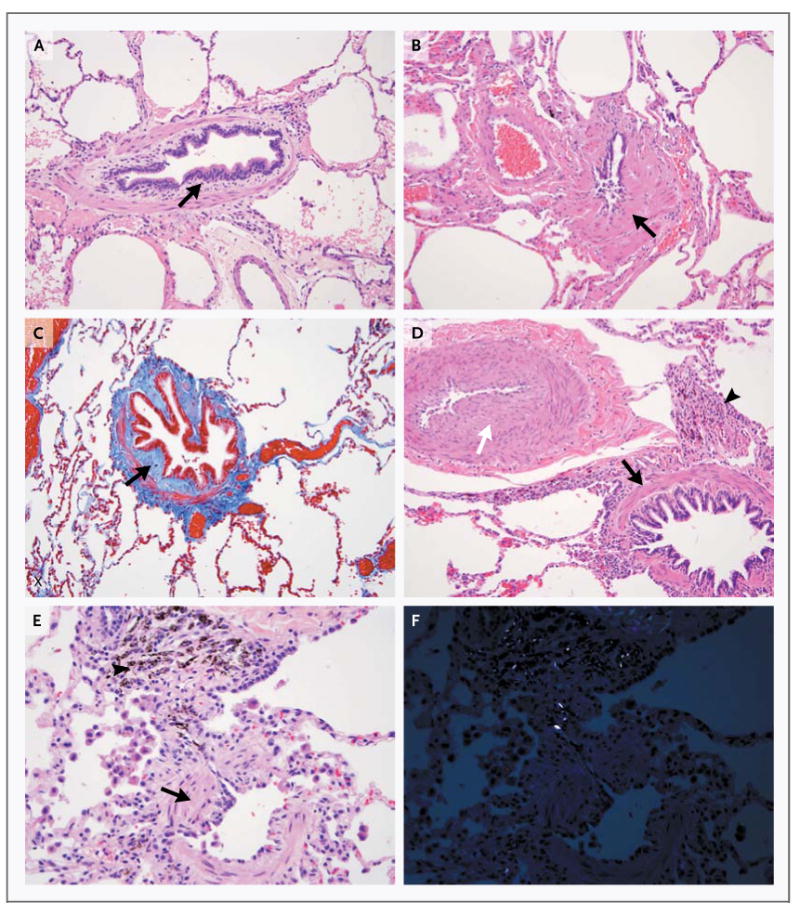
The photomicrographs show some of the pathological features seen in the 38 soldiers in whom constrictive bronchiolitis was diagnosed. The disorder was associated with subepithelial fibrosis (Panel A, arrow; hematoxylin and eosin), smooth-muscle hypertrophy (Panel B, arrow; hematoxylin and eosin), fibrosis between the epithelium and the muscle layer (Panel C, arrow; stained red with Masson's trichrome), smooth-muscle hypertrophy (Panel D, black arrow) with marked intimal fibrosis and medial hypertrophy of the adjacent pulmonary artery (white arrow) and peribronchiolar pigment deposition (arrowhead; hematoxylin and eosin), and smooth-muscle hypertrophy (Panel E, arrow) with adjacent pigment deposition (arrowhead; hematoxylin and eosin). Panel F shows the field shown in Panel E with the pigment refringent under polarized light.
Follow-up
In 2010, a total of 50 of the 80 soldiers responded to a follow-up survey. In addition, some of the data regarding the disability and service status of the 30 soldiers who did not respond to the survey were available from earlier encounters. Of the 38 soldiers with constrictive bronchiolitis, 19 (50%) had left the service with a disability rating, 8 (21%) were still serving despite their inability to complete a 2-mile run within the regulation time, and 11 (29%) were lost to follow-up. Twenty-two soldiers (58%) reported having shortness of breath after climbing one flight of stairs and having had limited job opportunities because of respiratory symptoms. Figure 1 shows outcome data for all 80 soldiers, including alternative diagnoses and reasons that they did not undergo lung biopsy. Since December 2009, constrictive bronchiolitis has been diagnosed in an additional 9 soldiers in our study group. Their profiles have not been added to this data set.
Discussion
Our study suggests that there is a strong association between constrictive bronchiolitis and exercise limitation in a cohort of soldiers who served in the Middle East. Constrictive bronchiolitis, a very rare finding in otherwise healthy, young adults, is most commonly reported in patients with rheumatologic disorders or in those who have undergone organ transplantation. The disorder is also associated with inhalational exposure to nitrogen dioxide, sulfur dioxide, inorganic dust, fly ash, and the diacetyl used in the manufacture of microwave popcorn.15-20
The majority of biopsy samples obtained from soldiers in our study showed polarizable material consistent with the inhalation of particulate matter, even though most of the soldiers were lifelong nonsmokers. Most of the biopsy samples also showed thickening of the arteriolar wall or occlusion in adjacent arterioles, a finding also seen with toxic inhalation.21
The soldiers who were initially evaluated in this series had prolonged exposure to toxic levels of sulfur dioxide associated with the Mosul sulfurmine fire, and we expected that the finding of constrictive bronchiolitis would be limited to this group. Over time, however, a number of soldiers without exposure to the sulfur-mine fire presented with similar exercise limitations. This group causes particular concern, since their potential toxic exposures are shared by most personnel who were deployed to Iraq and Afghanistan. These common exposures include open-air burn pits, in which solid waste was routinely incinerated in close proximity to living quarters, and desert dust storms of such severity that they obscured visibility. The presenting symptoms, smoking histories, evaluations, and biopsy samples of the 10 soldiers who did not report exposure to the sulfurmine fire were indistinguishable from those of the 28 soldiers who did report such exposure.
The diagnosis of constrictive bronchiolitis is challenging, especially in the absence of known predisposing conditions. Typically, patients have nonspecific respiratory symptoms and have an exercise limitation that is disproportionate to findings on pulmonary-function testing,15,16,21 which are frequently normal or mildly abnormal with both obstructive and restrictive patterns.17
In the soldiers in our study, results on pulmonary-function testing tended toward the lower limit of the normal range, as compared with population control subjects, but were significantly lower than those in a group of historical military control subjects.9 Ideally, we would have compared pulmonary function before deployment with measures after deployment, but only one of the soldiers in our study had undergone spirometry before deployment. His post-deployment FEV1 and FVC measurements were much lower than his predeployment values, with the FEV1 dropping from 5.09 liters to 3.91 liters (a decrease from 116% to 94% of the predicted value) and the FVC dropping from 5.77 liters to 4.58 liters (a decrease from 107% to 89% of the predicted value). Despite this accelerated decline in lung function, the results of post-deployment pulmonary-function testing remained within the normal range for this soldier.
Radiologic imaging generally did not suggest the presence of constrictive bronchiolitis among the soldiers in our study. Only a few soldiers had high-resolution CT showing the centrilobular nodularity or expiratory air trapping that can be associated with constrictive bronchiolitis. Several studies have reported normal imaging in patients with constrictive bronchiolitis because of the absence of associated alveolar disease.22-25
We cannot estimate the absolute prevalence of histologic bronchiolitis among soldiers, since the results of analyses of biopsy samples from an asymptomatic group of soldiers who have served in the theater of war have not been reported. The comparison between findings in the soldiers in our study and those in historical military controls is weakened because the control group was slightly younger and had a lower mean body-mass index than the soldiers in our study, a limitation that was attenuated by comparisons of the percent predicted values for the two groups. Despite these differences in demographics, the comparisons between the soldiers in our study and historical military control subjects were more appropriate than comparisons with the general population, given the standards of physical fitness required by military service.
Additional studies are needed to evaluate the particulate matter observed in many biopsy samples obtained from these soldiers. The correlation between the composition of the particulate matter with environmental exposures could lead to enhanced protective measures for soldiers in future deployments in the Middle East and elsewhere.
In summary, we found a high prevalence of constrictive bronchiolitis, an otherwise rare illness, in the 80 soldiers we evaluated. Evaluation for constrictive bronchiolitis should be considered among returning veterans who report having exercise limitations and who have essentially normal results on imaging and physiological studies.
Supplementary Material
Acknowledgments
Supported in part by a Clinical and Translational Science Award (UL1-RR024975) to Vanderbilt University Medical Center from the National Center for Research Resources, National Institutes of Health.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Medinger AE, Chan TW, Arabian A, Rohatgi PK. Interpretive algorithms for the symptom-limited exercise test: assessing dyspnea in Persian Gulf veterans. Chest. 1998;113:612–8. doi: 10.1378/chest.113.3.612. [DOI] [PubMed] [Google Scholar]
- 2.Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD, Ryan MAK. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170:1433–44. doi: 10.1093/aje/kwp287. [DOI] [PubMed] [Google Scholar]
- 3.The Iowa Persian Gulf Study Group. Self-reported illness and health status among Gulf War veterans: a population-based study. JAMA. 1997;277:238–45. [PubMed] [Google Scholar]
- 4.Coker WJ, Bhatt BM, Blatchley NF, Graham JT. Clinical findings for the first 1000 Gulf War veterans in the Ministry of Defence's medical assessment programme. BMJ. 1999;318:290–4. doi: 10.1136/bmj.318.7179.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelsall HL, Sim MR, Forbes AB, et al. Respiratory health status of Australian veterans of the 1991 Gulf War and the effects of exposure to oil fire smoke and dust storms. Thorax. 2004;59:897–903. doi: 10.1136/thx.2003.017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shorr AF, Scoville SL, Cersovsky SB, et al. Acute eosinophilic pneumonia among US military personnel deployed in or near Iraq. JAMA. 2004;292:2997–3005. doi: 10.1001/jama.292.24.2997. [DOI] [PubMed] [Google Scholar]
- 7.Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31:67–71. doi: 10.2500/aap.2010.31.3383. [DOI] [PubMed] [Google Scholar]
- 8.Idem. Increased allergic rhinitis rates among U.S. military personnel after deployment to the Persian Gulf. J Allergy Clin Immunol. 2008;121 1:S230. [Google Scholar]
- 9.Morris MJ, Grbach VX, Deal LE, Boyd SY, Morgan JA, Johnson JE. Evaluation of exertional dyspnea in the active duty patient: the diagnostic approach and the utility of clinical testing. Mil Med. 2002;167:281–8. [PubMed] [Google Scholar]
- 10.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 11.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–64. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society/American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 13.Fiqueira De Mello GC, Ribeiro Carvalho CR, Adib Kairalla R, et al. Small airway remodeling in idiopathic interstitial pneumonias: a pathological study. Respiration. 2010;79:322–32. doi: 10.1159/000235722. [DOI] [PubMed] [Google Scholar]
- 14.Army physical fitness test scorecard: two-mile run score chart. http://www.usma.edu/dpe/testing/apft_scorecard%20(da%20form%20705).pdf.
- 15.Devakonda A, Raoof S, Sung A, Travis WD, Naidich D. Bronchiolar disorders: a clinical-radiological diagnostic algorithm. Chest. 2010;137:938–51. doi: 10.1378/chest.09-0800. [DOI] [PubMed] [Google Scholar]
- 16.Visscher DW, Myers JL. Bronchiolitis: the pathologist's perspective. Proc Am Thorac Soc. 2006;3:41–7. doi: 10.1513/pats.200512-124JH. [DOI] [PubMed] [Google Scholar]
- 17.Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347:330–8. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- 18.Boswell RT, McCunney RJ. Bronchiolitis obliterans from exposure to incinerator fly ash. J Occup Environ Med. 1995;37:850–5. doi: 10.1097/00043764-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Wright JL, Churg A. Morphology of small-airway lesions in patients with asbestos exposure. Hum Pathol. 1984;15:68–74. doi: 10.1016/s0046-8177(84)80332-9. [DOI] [PubMed] [Google Scholar]
- 20.Wright JL, Cagle P, Churg A, Colby TV, Myers J. Diseases of the small airways. Am Rev Respir Dis. 1992;146:240–62. doi: 10.1164/ajrccm/146.1.240. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz MI, King TE. Interstitial lung disease. 4th. Hamilton, ON, Canada: B.C. Decker; 2003. p. 796. [Google Scholar]
- 22.McLoud TC, Epler GR, Colby TV, Gaenster EA, Carrington CB. Bronchiolitis obliterans. Radiology. 1986;159:1–8. doi: 10.1148/radiology.159.1.3952294. [DOI] [PubMed] [Google Scholar]
- 23.Breatnach E, Kerr I. The radiology of cryptogenic obliterative bronchiolitis. Clin Radiol. 1982;33:657–61. doi: 10.1016/s0009-9260(82)80395-4. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka N, Newell JD, Brown KK, Cool CD, Lynch DA. Collagen vascular diseaserelated lung disease: high-resolution computed tomography findings based on the pathologic classification. J Comput Assist Tomogr. 2004;28:351–60. doi: 10.1097/00004728-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Lee ES, Gotway MB, Reddy GP, Golden JA, Keith FM, Webb WR. Early bronchiolitis obliterans following lung transplantation: accuracy of expiratory thin-section CT for diagnosis. Radiology. 2000;216:472–7. doi: 10.1148/radiology.216.2.r00au21472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.