Abstract
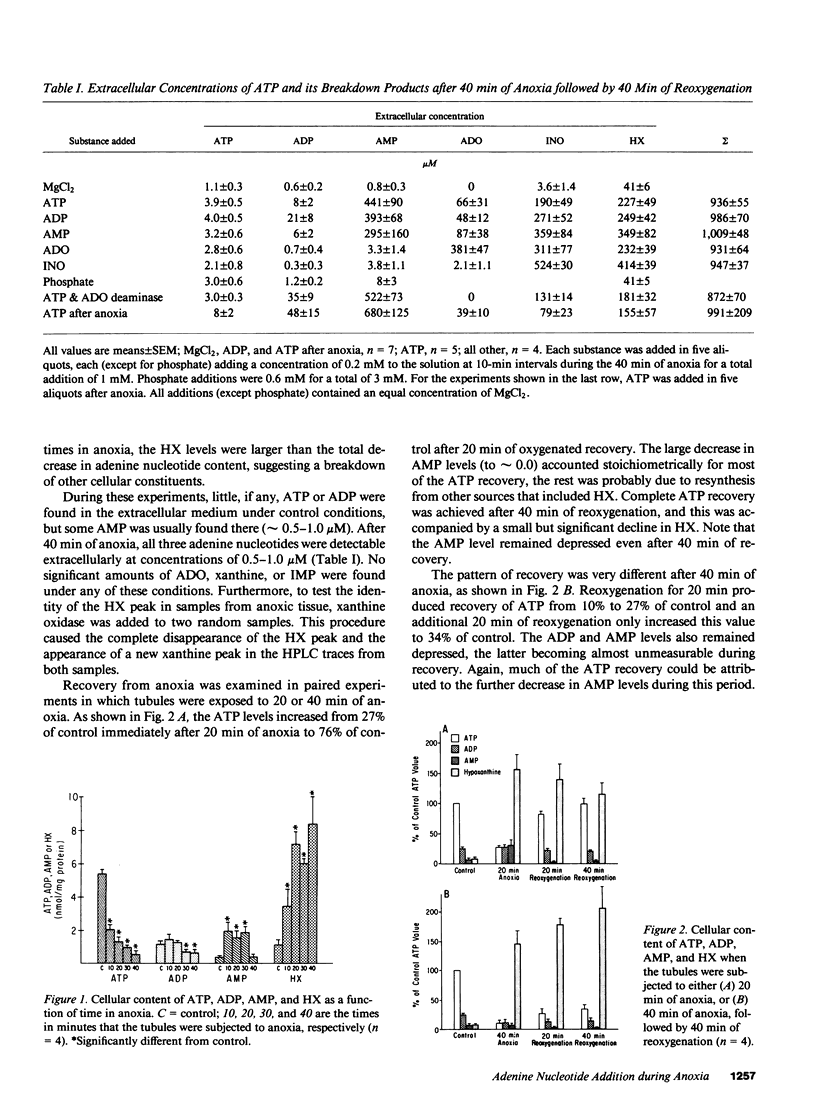
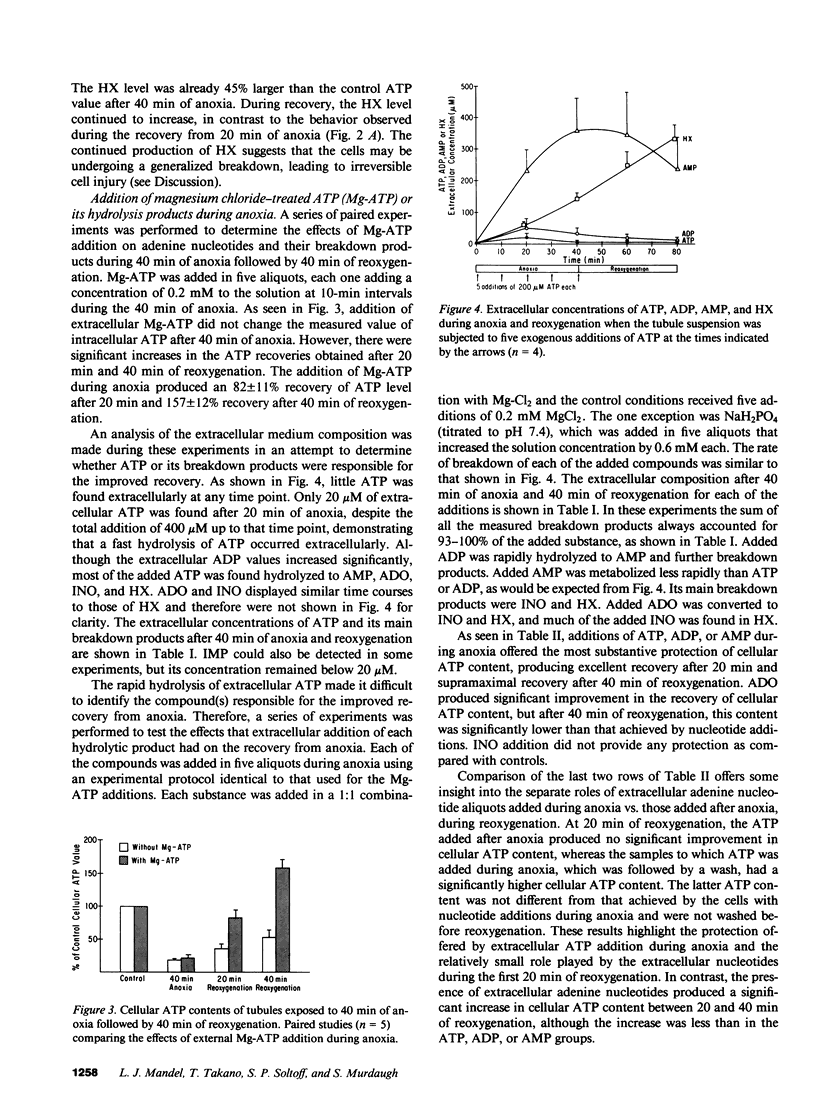
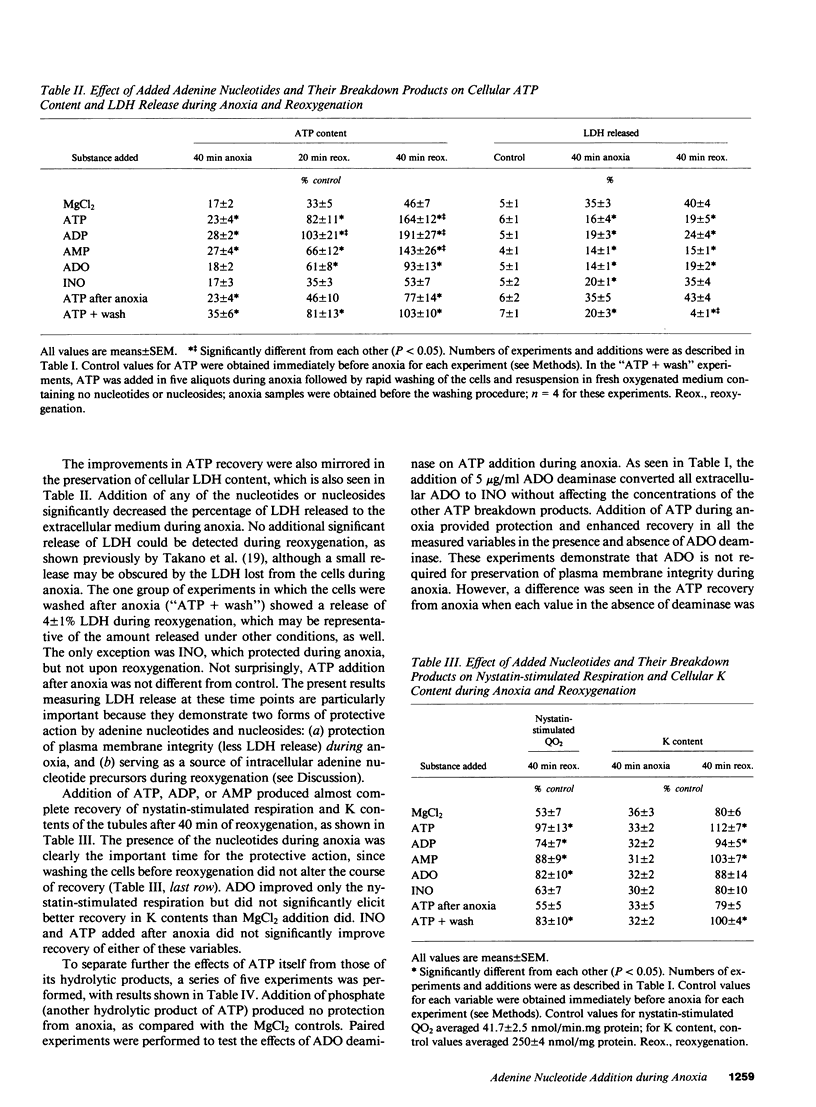
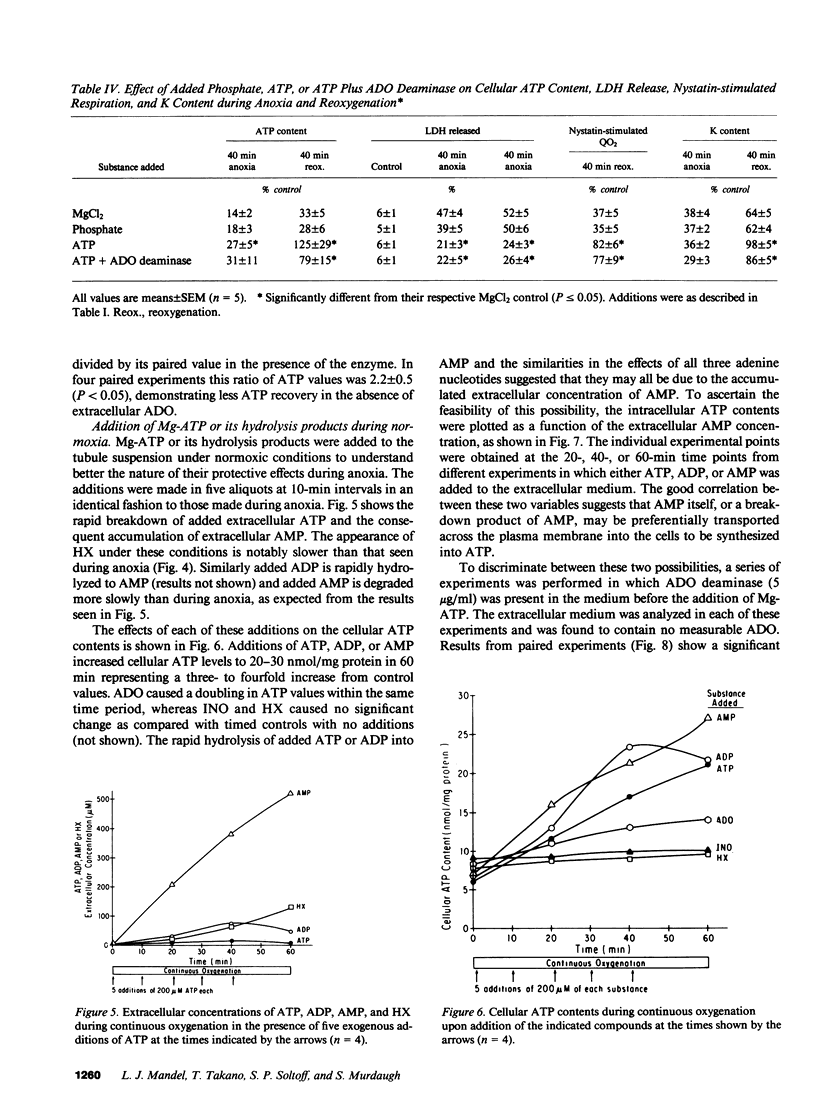
When a suspension of rabbit proximal tubules is subjected to anoxia, ATP falls by 80-90% during 40 min of anoxia, and upon reoxygenation (reox) the cells only recover 25-50% of their initial ATP. Addition of Mg-ATP (magnesium chloride-treated ATP), Mg-ADP, or Mg-AMP (five aliquots of 200 nmol/ml added 10 min apart) during anoxia causes complete recovery of ATP levels, and respiratory and transport function after 40 min of reox. Similar additions of adenosine (ADO), or inosine (INO), or Mg-ATP only during reox are less effective. Lactate dehydrogenase (LDH) release after 40 min of anoxia is 30-40% under control conditions, only 10-15% when adenine nucleotides or ADO are added during anoxia, and 20% when INO is added, suggesting that these additions may stabilize the plasma membrane during anoxia and help preserve cellular integrity. During reox, recovery may depend on the entry of ATP precursors and, therefore, we explored the mechanism whereby exogenous ATP increases the intracellular ATP content. Additions of Mg-ATP, Mg-ADP, or Mg-AMP to continuously oxygenated tubules increase cellular ATP content three- to fourfold in 1 h. The added ATP and ADP are rapidly degraded to AMP, and more slowly to ADO, INO, and hypoxanthine. Furthermore, the ATP-induced increase in cellular ATP is abolished by the exogenous addition of adenosine deaminase, which converts extracellular ADO to INO. These results suggest that the increase in cellular ATP requires extracellular ADO. The ADO obtained from the breakdown of AMP may be preferentially transported into the renal cells to be resynthesized into cellular AMP and ATP.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnasco S., Good D., Balaban R., Burg M. Lactate production in isolated segments of the rat nephron. Am J Physiol. 1985 Apr;248(4 Pt 2):F522–F526. doi: 10.1152/ajprenal.1985.248.4.F522. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Soltoff S. P., Storey J. M., Mandel L. J. Improved renal cortical tubule suspension: spectrophotometric study of O2 delivery. Am J Physiol. 1980 Jan;238(1):F50–F59. doi: 10.1152/ajprenal.1980.238.1.F50. [DOI] [PubMed] [Google Scholar]
- Buhl M. R. Purine metabolism in ischaemic kidney tissue. Dan Med Bull. 1982 Jan;29(1):1–26. [PubMed] [Google Scholar]
- Buhl M. R. The postanoxic regeneration of 5'-adenosine nucleotides in rabbit kidney tissue during in vitro perfusion. Scand J Clin Lab Invest. 1976 Mar;36(2):175–181. [PubMed] [Google Scholar]
- Buhl M. R. The predictive value of 5'-adenine nucleotide depletion and replenishment in ischaemic rabbit kidney tissue. Int Urol Nephrol. 1979;11(4):325–333. doi: 10.1007/BF02086820. [DOI] [PubMed] [Google Scholar]
- Burke T. J., Arnold P. E., Schrier R. W. Prevention of ischemic acute renal failure with impermeant solutes. Am J Physiol. 1983 Jun;244(6):F646–F649. doi: 10.1152/ajprenal.1983.244.6.F646. [DOI] [PubMed] [Google Scholar]
- Busch E. W., von Borcke I. M., Martinez B. Abbauwege und Abbaumuster der Purinnucleotide in Herz-, Leber-, und Nierengewebe von Kaninchen nach Kreislaufstillstand. Biochim Biophys Acta. 1968 Sep 24;166(2):547–556. [PubMed] [Google Scholar]
- Fernando A. R., Armstrong D. M., Griffiths J. R., Hendry W. F., O'Donoghue E. P., Perrett D., Ward J. P., Wickham J. E. Enhanced preservation of the ischaemic kidney with inosine. Lancet. 1976 Mar 13;1(7959):555–557. doi: 10.1016/s0140-6736(76)90356-1. [DOI] [PubMed] [Google Scholar]
- GERLACH E., DEUTICKE B., DREISBACH R. H., ROSARIUS C. W. ZUM VERHALTEN VON NUCLEOTIDEN UND IHREN DEPHOSPHORYLIERTEN ABBAUPRODUKTEN IN DER NIERE BEI ISCHAEMIE UND KURZZEITIGER POST-ISCHAEMISCHER WIEDERDURCHBLUTUNG. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963 Nov 11;278:296–315. [PubMed] [Google Scholar]
- Gaudio K. M., Ardito T. A., Reilly H. F., Kashgarian M., Siegel N. J. Accelerated cellular recovery after an ischemic renal injury. Am J Pathol. 1983 Sep;112(3):338–346. [PMC free article] [PubMed] [Google Scholar]
- Gerlach E., Marko P., Zimmer H. G., Pechan I., Trendelenburg C. Different response of adenine nucleotide synthesis de novo in kidney and brain during aerobic recovery from anoxia and ischemia. Experientia. 1971 Aug;27(8):876–878. doi: 10.1007/BF02135716. [DOI] [PubMed] [Google Scholar]
- Hanley M. J., Davidson K. Prior mannitol and furosemide infusion in a model of ischemic acute renal failure. Am J Physiol. 1981 Nov;241(5):F556–F564. doi: 10.1152/ajprenal.1981.241.5.F556. [DOI] [PubMed] [Google Scholar]
- Harris S. I., Balaban R. S., Barrett L., Mandel L. J. Mitochondrial respiratory capacity and Na+- and K+-dependent adenosine triphosphatase-mediated ion transport in the intact renal cell. J Biol Chem. 1981 Oct 25;256(20):10319–10328. [PubMed] [Google Scholar]
- Hull-Ryde E. A., Cummings R. G., Lowe J. E. Improved method for high energy nucleotide analysis of canine cardiac muscle using reversed-phase high-performance liquid chromatography. J Chromatogr. 1983 Jul 8;275(2):411–417. doi: 10.1016/s0378-4347(00)84388-1. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Rennke H., Levinsky N. G. Recovery of proximal tubular function from ischemic injury. Am J Physiol. 1984 Feb;246(2 Pt 2):F159–F166. doi: 10.1152/ajprenal.1984.246.2.F159. [DOI] [PubMed] [Google Scholar]
- Le Hir M., Angielski S., Dubach U. C. Properties of an ecto-5'-nucleotidase of the renal brush border. Ren Physiol. 1985;8(6):321–327. doi: 10.1159/000173064. [DOI] [PubMed] [Google Scholar]
- Le Hir M., Dubach U. C. Concentrative transport of purine nucleosides in brush border vesicles of the rat kidney. Eur J Clin Invest. 1985 Jun;15(3):121–127. doi: 10.1111/j.1365-2362.1985.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Balaban R. S. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol. 1981 May;240(5):F357–F371. doi: 10.1152/ajprenal.1981.240.5.F357. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Murphy E. Regulation of cytosolic free calcium in rabbit proximal renal tubules. J Biol Chem. 1984 Sep 25;259(18):11188–11196. [PubMed] [Google Scholar]
- Meyskens F. L., Williams H. E. Adenosine metabolism in human erythrocytes. Biochim Biophys Acta. 1971 Jun 30;240(2):170–179. doi: 10.1016/0005-2787(71)90654-x. [DOI] [PubMed] [Google Scholar]
- Morgan E. J. The Distribution of Xanthine Oxidase I. Biochem J. 1926;20(6):1282–1291. doi: 10.1042/bj0201282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller W., Guder W. G., Gstraunthaler G., Kotanko P., Jehart I., Pürschel S. Compartmentation of ATP within renal proximal tubular cells. Biochim Biophys Acta. 1984 Oct 12;805(2):152–157. doi: 10.1016/0167-4889(84)90162-9. [DOI] [PubMed] [Google Scholar]
- RICHERT D. A., WESTERFELD W. W. Xanthine oxidase in different species. Proc Soc Exp Biol Med. 1951 Feb;76(2):252–254. doi: 10.3181/00379727-76-18452. [DOI] [PubMed] [Google Scholar]
- Rothwell D., Bartley J., James M. Preservation of the ischaemic canine kidney with inosine. Urol Res. 1981;9(2):75–78. doi: 10.1007/BF00256680. [DOI] [PubMed] [Google Scholar]
- Siegel N. J., Glazier W. B., Chaudry I. H., Gaudio K. M., Lytton B., Baue A. E., Kashgarian M. Enhanced recovery from acute renal failure by the postischemic infusin of adenine nucleotides and magnesium chloride in rats. Kidney Int. 1980 Mar;17(3):338–349. doi: 10.1038/ki.1980.39. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Active ion transport in the renal proximal tubule. I. Transport and metabolic studies. J Gen Physiol. 1984 Oct;84(4):601–622. doi: 10.1085/jgp.84.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Active ion transport in the renal proximal tubule. III. The ATP dependence of the Na pump. J Gen Physiol. 1984 Oct;84(4):643–662. doi: 10.1085/jgp.84.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpio B. E., Hull M. J., Baue A. E., Chaudry I. H. Comparison of effects of ATP-MgCl2 and adenosine-MgCl2 on renal function following ischemia. Am J Physiol. 1987 Feb;252(2 Pt 2):R388–R393. doi: 10.1152/ajpregu.1987.252.2.R388. [DOI] [PubMed] [Google Scholar]
- THORN W., LIEMANN F. [Metabolite concentrations in the kidneys and para-aminohippuric clearance after acute ischemia and in recovery after ischemia]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1961;273:528–542. doi: 10.1007/BF00363052. [DOI] [PubMed] [Google Scholar]
- Takano T., Soltoff S. P., Murdaugh S., Mandel L. J. Intracellular respiratory dysfunction and cell injury in short-term anoxia of rabbit renal proximal tubules. J Clin Invest. 1985 Dec;76(6):2377–2384. doi: 10.1172/JCI112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble M. E., Coulson R. Adenosine transport in perfused rat kidney and renal cortical membrane vesicles. Am J Physiol. 1984 Jun;246(6 Pt 2):F794–F803. doi: 10.1152/ajprenal.1984.246.6.F794. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Bernard D. B., Donohoe J. F., Levinsky N. G. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978 Jul;14(1):31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- Weidemann M. J., Hems D. A., Krebs H. A. Effects of added nucleotides on renal carbohydrate metabolism. Biochem J. 1969 Oct;115(1):1–10. doi: 10.1042/bj1150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. M. Oxygen deprivation-induced injury to isolated rabbit kidney tubules. J Clin Invest. 1985 Sep;76(3):1193–1208. doi: 10.1172/JCI112075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. R., Arnold P. E., Burke T. J., Schrier R. W. Mitochondrial calcium accumulation and respiration in ischemic acute renal failure in the rat. Kidney Int. 1984 Mar;25(3):519–526. doi: 10.1038/ki.1984.48. [DOI] [PubMed] [Google Scholar]



