Abstract
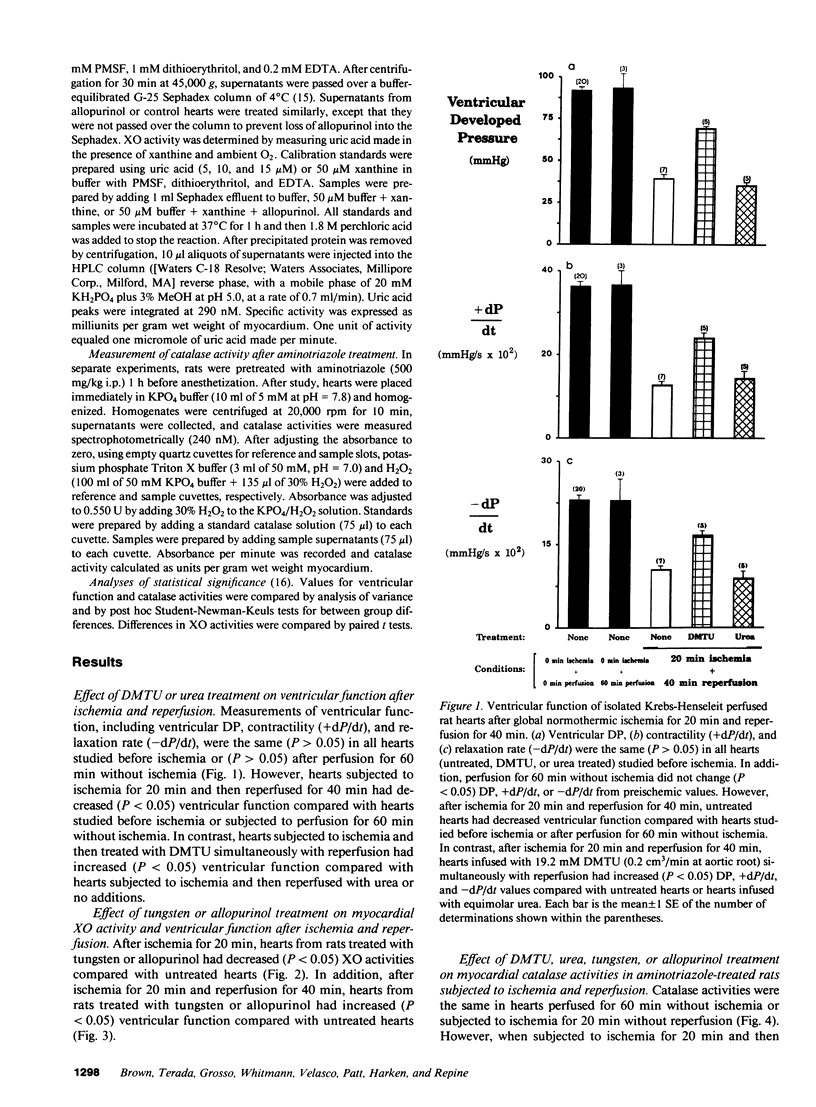
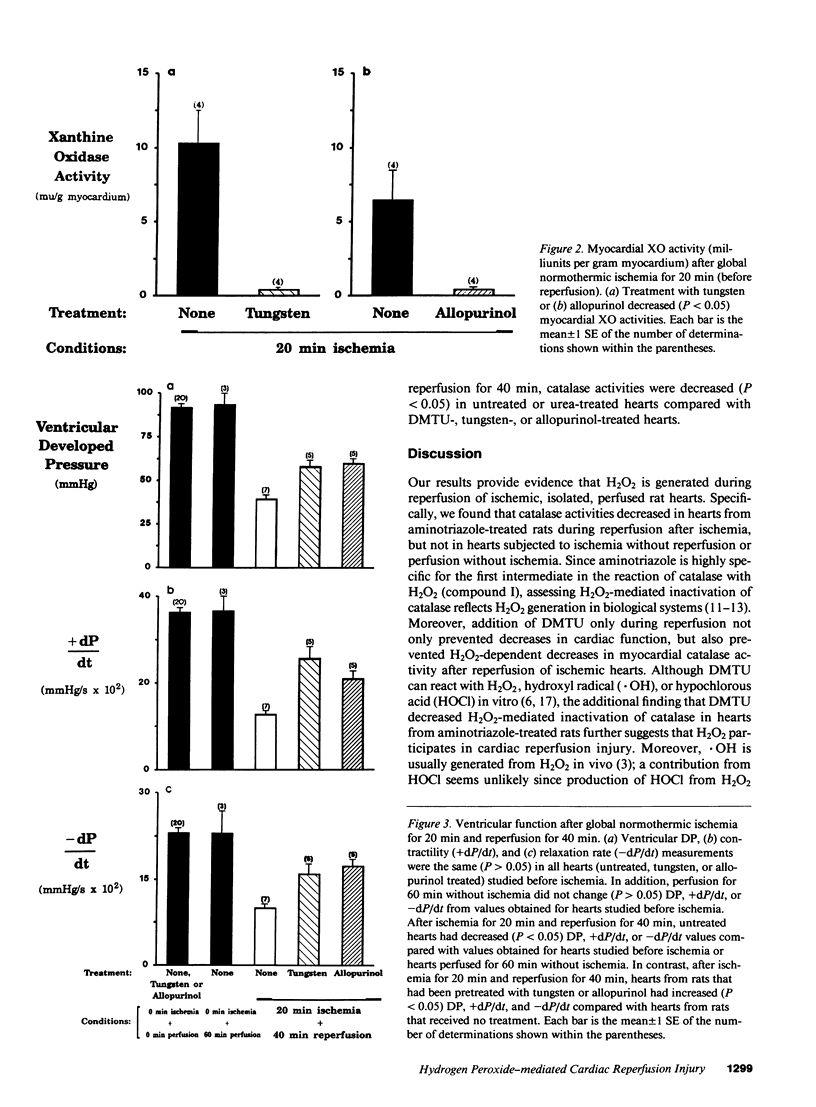
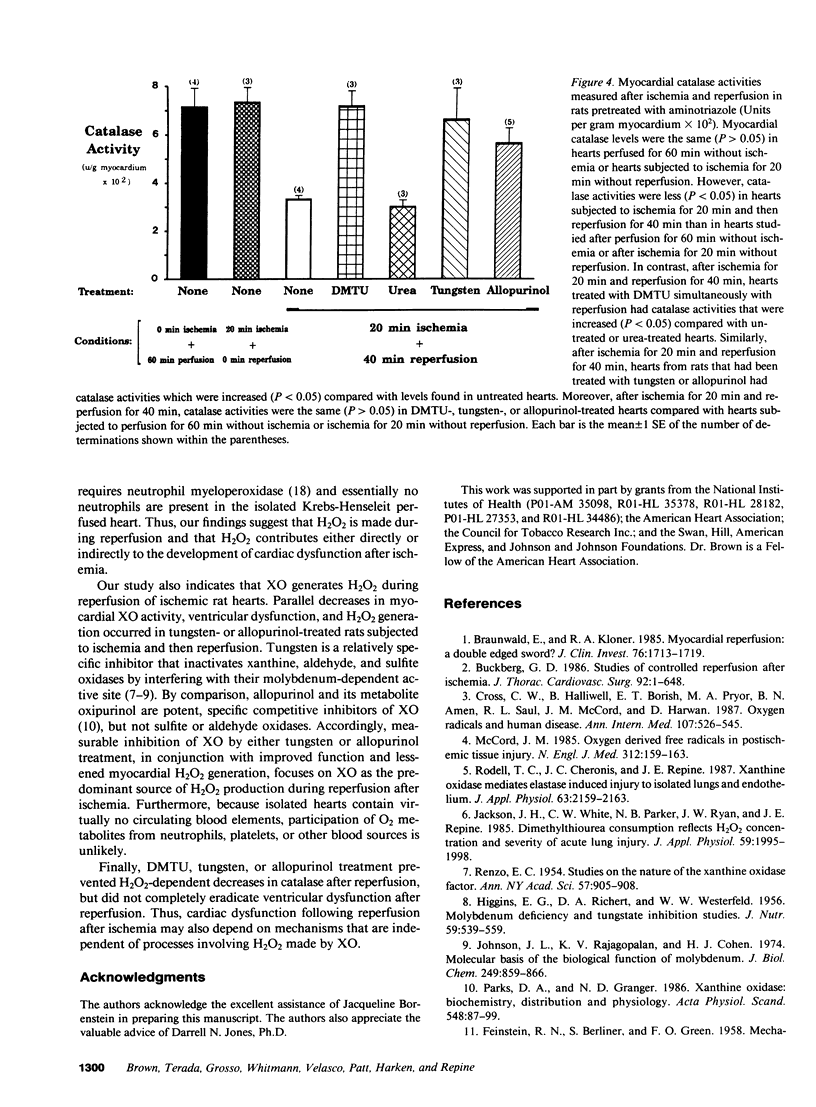
Three lines of investigation indicated that hydrogen peroxide (H2O2) from xanthine oxidase (XO) contributes to cardiac dysfunction during reperfusion after ischemia. First, addition of dimethylthiourea (DMTU), a highly permeant O2 metabolite scavenger (but not urea) simultaneously with reperfusion improved recovery of ventricular function as assessed by ventricular developed pressure (DP), contractility (+dP/dt), and relaxation rate (-dP/dt) in isolated Krebs-Henseleit-perfused rat hearts subjected to global normothermic ischemia. Second, hearts from rats fed tungsten or treated with allopurinol had negligible XO activities (less than 0.5 mU/g wet myocardium compared with greater than 6.0 mU/g in control hearts) and increased ventricular function after ischemia and reperfusion. Third, myocardial H2O2-dependent inactivation of catalase occurred after reperfusion following ischemia, but not after ischemia without reperfusion or perfusion without ischemia. In contrast, myocardial catalase did not decrease during reperfusion of ischemic hearts treated with DMTU, tungsten, or allopurinol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey L., Concepcion W., Shattuck H., Huang L. Method of heart transplantation for treatment of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 1986 Jul;92(1):1–5. [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985 Nov;76(5):1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Catalase-aminotriazole method for measuring secretion of hydrogen peroxide by microorganisms. J Bacteriol. 1969 May;98(2):543–546. doi: 10.1128/jb.98.2.543-546.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- DE RENZO E. C. Studies on the nature of the xanthine oxidase factor. Ann N Y Acad Sci. 1954 May 10;57(6):905–908. doi: 10.1111/j.1749-6632.1954.tb36468.x. [DOI] [PubMed] [Google Scholar]
- FEINSTEIN R. N., BERLINER S., GREEN F. O. Mechanism of inhibition of Catalase by 3-amino-1,2,4-triazole. Arch Biochem Biophys. 1958 Jul;76(1):32–44. doi: 10.1016/0003-9861(58)90116-4. [DOI] [PubMed] [Google Scholar]
- HIGGINS E. S., RICHERT D. A., WESTERFELD W. W. Molybdenum deficiency and tungstate inhibition studies. J Nutr. 1956 Aug 10;59(4):539–559. doi: 10.1093/jn/59.4.539. [DOI] [PubMed] [Google Scholar]
- Jackson J. H., White C. W., Parker N. B., Ryan J. W., Repine J. E. Dimethylthiourea consumption reflects H2O2 concentrations and severity of acute lung injury. J Appl Physiol (1985) 1985 Dec;59(6):1995–1998. doi: 10.1152/jappl.1985.59.6.1995. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Rajagopalan K. V., Cohen H. J. Molecular basis of the biological function of molybdenum. Effect of tungsten on xanthine oxidase and sulfite oxidase in the rat. J Biol Chem. 1974 Feb 10;249(3):859–866. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- Rodell T. C., Cheronis J. C., Ohnemus C. L., Piermattei D. J., Repine J. E. Xanthine oxidase mediates elastase-induced injury to isolated lungs and endothelium. J Appl Physiol (1985) 1987 Nov;63(5):2159–2163. doi: 10.1152/jappl.1987.63.5.2159. [DOI] [PubMed] [Google Scholar]
- Schoutsen B., De Jong J. W., Harmsen E., De Tombe P. P., Achterberg P. W. Myocardial xanthine oxidase/dehydrogenase. Biochim Biophys Acta. 1983 Jul 14;762(4):519–524. doi: 10.1016/0167-4889(83)90055-1. [DOI] [PubMed] [Google Scholar]
- Wasil M., Halliwell B., Grootveld M., Moorhouse C. P., Hutchison D. C., Baum H. The specificity of thiourea, dimethylthiourea and dimethyl sulphoxide as scavengers of hydroxyl radicals. Their protection of alpha 1-antiproteinase against inactivation by hypochlorous acid. Biochem J. 1987 May 1;243(3):867–870. doi: 10.1042/bj2430867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Klein R., Slivka A., Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982 Sep;70(3):598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcester J. The statistical method. N Engl J Med. 1966 Jan 6;274(1):27–36. doi: 10.1056/NEJM196601062740106. [DOI] [PubMed] [Google Scholar]


