Abstract
Purpose
To evaluate the safety and efficacy of IMC-A12, a human monoclonal antibody (mAb) that blocks insulin-like growth factor receptor-1 (IGF-1R), as monotherapy or in combination with cetuximab in patients with metastatic refractory anti–epidermal growth factor receptor (EGFR) mAb colorectal cancer.
Methods
A randomized, phase II study was performed in which patients in arm A received IMC-A12 10 mg/kg intravenously (IV) every 2 weeks, while patients in arm B received this same dose of IMC-A12 plus cetuximab 500 mg/m2 IV every 2 weeks. Subsequently, arm C (same combination treatment as arm B) was added to include patients who had disease control on a prior anti-EGFR mAb and wild-type KRAS tumors. Archived pretreatment tumor tissue was obtained when possible for KRAS, PIK3CA, and BRAF genotyping, and immunohistochemistry was obtained for pAKT as well as IGF-1R.
Results
Overall, 64 patients were treated (median age, 61 years; range, 40 to 84 years): 23 patients in arm A, 21 in arm B, and 20 in arm C. No antitumor activity was seen in the 23 patients treated with IMC-A12 monotherapy. Of the 21 patients randomly assigned to IMC-A12 plus cetuximab, one patient (with KRAS wild type) achieved a partial response, with disease control lasting 6.5 months. Arm C (all patients with KRAS wild type), however, showed no additional antitumor activity. Serious adverse events thought possibly related to IMC-A12 included a grade 2 infusion-related reaction (2%; one of 64 patients), thrombocytopenia (2%; one of 64 patients), grade 3 hyperglycemia (2%; one of 64 patients), and grade 1 pyrexia (2%, one of 64 patients).
Conclusion
IMC-A12 alone or in combination with cetuximab was insufficient to warrant additional study in patients with colorectal cancer refractory to EGFR inhibitors.
INTRODUCTION
The type 1 insulin-like growth factor receptor (IGF-1R) is a member of a family of transmembrane tyrosine kinases that includes the insulin receptor and the insulin receptor–related receptor.1 IGF-1R is activated by two high affinity binding ligands, insulin-like growth factor (IGF) 1 and IGF-2.2 The principal pathways for transduction of the IGF signal are the mitogen-activated protein kinase and phosphatidylinositol 3-kinase (PI3K)/Akt pathways.2 A large number of preclinical and clinical studies have implicated the IGF-1R and its ligands, IGF-1 and IGF-2, in the development and progression of cancer.2–4
IMC-A12 is a recombinant fully human immunoglobulin G1 monoclonal antibody that specifically targets the human IGF-1R.5–6 Preclinical studies demonstrate that IGF is a strong mitogen in colorectal cancer (CRC).4 IGF-1R mediated signaling may also mediate resistance to epidermal growth factor receptor (EGFR) inhibition, and combined IGF-1R and EGFR inhibition has resulted in enhanced growth inhibition in selected preclinical models.7–9
Cetuximab is a human-murine monoclonal antibody that targets the EGFR.10 Cetuximab had a 17% to 23% response rate when combined with irinotecan in patients whose tumors had progressed during patient treatment with irinotecan and an approximate 9% to 11% single-agent response rate.11–13 Among patients with wild-type KRAS CRC, the single-agent response rate is modest (17% v 0% in unselected patients) with panitumumab monotherapy,14 and it is 13% with cetuximab monotherapy.15 After tumor progression on standard cytotoxic agents and cetuximab or related antibodies occurs, there are no active options for patients. We hypothesized on the basis of preclinical data that the anti–IGF-1R monoclonal antibody (mAb) IMC-A12 might have antitumor activity, either alone or in combination with cetuximab, in these patients.
METHODS
This was a multicenter, phase II trial in patients with metastatic CRC. The trial was approved by the institutional review board at each center, and it was conducted in accordance with the US Department of Health and Human Services guidelines.
Patient Selection
Eligible patients had pathologic confirmation of CRC, with measurable disease according to RECIST (Response Evaluation Criteria in Solid Tumors), and documentation of previous progression on at least one anti-EGFR mAb-containing regimen. Previous progression was defined as any enlargement of measurable or assessable lesion or lesions, or as the development of any unequivocal new lesion, during or within 6 weeks of receiving cetuximab or panitumumab, which was believed by the treating physician to represent clinical progression. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 or 1, to be age 18 years or older, and to have a life expectancy greater than 3 months. Adequate bone marrow and kidney function were required, and bilirubin ≤ 1.5 times the upper limit of normal was required. Patients were excluded if they had received prior IGF-receptor–directed agents or had inadequately controlled diabetes mellitus, defined by fasting blood glucose ≥ 120 mg/dL.
Therapy
Patients in arm A received IMC-A12 at a dose of 10 mg/kg intravenously (IV) over 1 hour every 2 weeks, whereas patients in arm B received IMC-A12 at a dose of 10 mg/kg IV over 1 hour every 2 weeks plus cetuximab 500 mg/m2 IV over 2 hours every 2 weeks. Subsequently, a nonrandomized arm C was added in which patients whose tumors were prospectively demonstrated to have wild-type KRAS (defined as absence of exon 2 mutations) were assigned to the same combination treatment as arm B.
Treatment on each arm was continued until progression of disease or unacceptable toxicity occurred or until the patient withdrew consent. The National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0 were used to assess toxicity. Although hematologic toxicity was not expected to be associated with IMC-A12, IMC-A12 was to be withheld for grade 3 hematologic toxicity and was to be restarted when toxicity had resolved to grade ≤ 2 at a dose of 8 mg/kg. For grade 4 toxicities believed possibly or likely to be related to IMC-A12, IMC-A12 was withheld until resolution of the toxicity and then was reduced to 6 mg/kg when restarted. There was no dose reduction of cetuximab for hematologic toxicity.
Consistent with standard practice, cetuximab was to be withheld for the first incidence of grade 3 acne-like rash, but it was not reduced for this first incidence. If the rash again subsequently reached grade 3, cetuximab was withheld until the rash improved to grade ≤ 2 and then was reduced by 50 mg/m2.
For the assessment of hyperglycemia (ie, diabetes), the NCI-CTCAE endocrine terms were utilized. For grade 3 hyperglycemia (ie, symptoms interfering with the activities of daily living or insulin indicated), IMC-A12 was held if symptoms were present or if glucose was ≥ 300 mg/dL; IMC-A12 was resumed when the patient was asymptomatic, glucose was consistently less than 220 mg/dL, and the patient was on a stable insulin regimen. In such occurrences, the IMC-A12 dose was reduced by 20%. For grade 4 hyperglycemia, IMC-A12 therapy was withheld until the patient was asymptomatic, glucose was consistently less than 220 mg/dL, and the patient was on a stable insulin regimen. When treatment was resumed, the IMC-A12 dose was reduced by 20%.
All other grade 3 or 4 nonhematologic toxicities (with the exception of an infusion-related reaction or acneiform rash) for IMC-A12 and cetuximab were treated with dose reduction on recovery, return to baseline, or to grade 1.
Evaluation Criteria
Computed tomography scans or magnetic resonance imaging scans of measurable lesions were obtained at baseline and every 6 weeks. Responses were categorized according to standard RECIST (Response Evaluation Criteria in Solid Tumors; version 1.0) criteria. Response and progression were determined by a reference radiologist. The primary objective of the study was to evaluate the effect of IMC-A12 with or without cetuximab on objective response rate. Secondary objectives were evaluation of progression-free survival (PFS) and overall survival (OS).
Blood samples from 10 patients in arm A and 10 patients in arm B were collected before and after the initial dose and were collected on day 1 before IMC-A12 infusion (before cetuximab for patients randomly assigned to arm B). Samples were collected at 0 hours (ie, predose), at the end of the IMC-A12 infusion, and then 1 hour, 1 week (ie, 168 hours), and 2 weeks (ie, 336 hours, immediately before second infusion) post dose. Thereafter, collections were performed before and after treatment during weeks 3, 5, 7, 9, and 11.
Correlative Laboratory Analysis of Tissue Samples
Genotyping.
All slides were reviewed for appropriate tumor content by a reference pathologist before analysis and sequencing. Sections (5 to 6 μm thick) cut from paraffin-embedded tumor samples from 19 patients were evaluated for KRAS, BRAF, and PIK3CA mutations by using a chip-based matrix-assisted laser desorption-time-of-flight mass spectrometer (Sequenom, San Diego, CA), as published previously.16,17
Immunohistochemical analysis.
Nineteen prearchival tissue samples (arms A + B) were tested by immunohistochemistry (IHC) for pAKT and IGF-1R. IHC for pAKT and IGF-1R were performed by using rabbit polyclonal antibodies to IGF-1R (Ventana, Tucson, AZ) and phospho-Akt (Cell Signaling, Danvers, MA). The staining was scored on the basis of the intensity (0 to 3+) and the percentage of tumor cells stained.
Statistical Considerations
Patients in arms A and B were randomly assigned through a central registration phone number. The initial planned sample size of the first stage of a Simon two-stage design was a total of 40 patients (20 patients in each arm); if two or more (of the first 20 patients) had an objective response in an arm, then enrollment would be extended to a total of 36 patients in that arm. Because of rapid patient accrual, a total of 45 patients were randomly assigned at three centers to the first stage of the trial. As noted, after the protocol amendment, study arm C was added, and 20 additional patients were enrolled to this combination arm.
The response proportion of single-agent IMC-A12 was deemed unacceptable if it was lower than 7% and promising if ≥ 22%. Given the historically low response rates in this population of patients with metastatic CRC, the combination of IMC-A12 plus cetuximab was also deemed unacceptable and promising at objective response rates of 7% and 22%, respectively. On the basis of Simon's two-stage design, with type I and type II error rates of 10%, at the end of the stage 2, a regimen was to be considered promising if five or more patients among the 36 treated in that arm experienced an objective response. The Kaplan-Meier method was used to estimate OS and PFS.
The initial planned statistical analysis was that, for each tested marker, a Fisher's exact test would be employed to test for associations between response and the activation of the marker and/or mutation. Given the lack of antitumor efficacy, however, such calculations were not performed. Descriptive results, however, are provided.
RESULTS
Patient Characteristics
Sixty-four patients were treated on this trial at three participating institutions. Patient characteristics are outlined in Table 1. All patients had received prior anti-EGFR therapy, and the majority of patients received at least three prior chemotherapy regimens.
Table 1.
Patient Demographic and Clinical Characteristics of Treated Population
| Characteristic | Arm A:IMC-A12 (n = 23) |
Arm B: IMC-A12 +C (n = 21) |
Arm C: IMC-A12 + C and KRAS Wild Type (n = 20) |
All Treatments (N = 64) |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Sex | ||||||||
| Female | 14 | 61 | 9 | 43 | 10 | 50 | 33 | 52 |
| Male | 9 | 39 | 12 | 57 | 10 | 50 | 31 | 48 |
| Age, years | ||||||||
| Median | 59 | 63 | 62 | 61 | ||||
| Range | 41-79 | 40-84 | 44-70 | 40-84 | ||||
| ECOG performance status | ||||||||
| 0 | 3 | 13 | 5 | 24 | 10 | 50 | 18 | 28 |
| 1 | 20 | 87 | 16 | 76 | 10 | 50 | 46 | 72 |
| Prior cetuximab | 21 | 91 | 21 | 100 | 20 | 100 | 61 | 95 |
| Prior panitumumab* | 7 | 30 | 2 | 10 | 2 | 10 | 11 | 17 |
| Prior FU, oxaliplatin, irinotecan | 23 | 100 | 21 | 100 | 20 | 100 | 64 | 100 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FU, fluorouracil.
Only two patients on trial received panitumumab alone; all other patients also received cetuximab.
Toxicity
All three treatment arms were well tolerated. Tables 2 and 3 show the related toxicities (grades 2 and 3) by treatment arm. There were no grade 4 or 5 related events. Serious adverse events thought to be potentially related to IMC-A12 included a grade 2 infusion-related reaction (2%; one of 64 patients), grade 3 thrombocytopenia (2%; one of 64 patients), grade 3 hyperglycemia (2%; one of 64 patients), and grade 1 pyrexia (2%; one of 64 patients). Although the incidence of clinical hyperglycemia/diabetes was modest, 47 (75%) of 63 patients for whom data were available had laboratory glucose elevations at least one CTCAE grade greater than baseline at some point during the study; the majority of these were not associated with symptoms and did not require specific clinical management. In addition, the initial glucose before study entry was measured while patients were fasting; subsequent measurements were nonfasting.
Table 2.
Reported Grades 2 and 3 Toxicities Possibly, Probably, or Definitely Related to IMC-A12 by Treatment Arm, Excluding Hyperglycemia
| Toxicity attributable to IMC-A12 | Patients by Treatment Arm and Grade |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMC-A12 (n = 23) |
IMC-A12 + Cetuximab (n = 21) |
IMC-A12 + Cetuximab and Wild Type (n = 20) |
All Treatments (N = 64) |
|||||||||||||||
| 2 |
3 |
2 |
3 |
2 |
3 |
2 |
3 |
|||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | 95% CI | No. | % | 95% CI | |
| Fatigue | 0 | 0 | 1 | 4 | 1 | 5 | 0 | 0 | 1 | 5 | 0 | 0 | 2 | 3 | 0 to 11 | 1 | 2 | 0 to 8 |
| Nausea | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 2 | 3 | 0 to 11 | 0 | 0 | 0 |
| Vomiting | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 2 | 3 | 0 to 11 | 0 | 0 | 0 |
| Anorexia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 1 | 2 | 0 to 8 |
| Constipation | 1 | 4 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 to 11 | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 to 8 | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 1 | 2 | 0 to 8 |
| Anemia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 to 8 |
| Myalgia/muscle leg pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 1 | 2 | 0 to 8 | 0 | 0 | 0 |
| Infusion-related reaction | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 to 8 | 0 | 0 | 0 |
| Pyrexia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 1 | 2 | 0 to 8 | 0 | 0 | 0 |
| Headache | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 15 | 0 | 0 | 3 | 5 | 1 to 13 | 0 | 0 | 0 |
| Dizziness | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 to 8 |
| Dysgeusia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 1 | 2 | 0 to 8 | 0 | 0 | 0 |
Table 3.
Reported Incidence of Clinical Hyperglycemia/Diabetes
| Hyperglycemia | Arm A:IMC-A12 (n = 23) |
Arm B: IMC-A12 + Cetuximab (n = 21) |
Arm C: IMC-A12 + Cetuximab and KRAS Wild Type (n = 20) |
Total |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Grade 1 | 0 | 0 | 0 | 0 | ||||
| Grade 2 | 0 | 1 | 5 | 1 | 5 | 2 | 3 | |
| Grade 3 | 1 | 4 | 0 | 0 | 1 | 2 | ||
| Grade 4 | 0 | 0 | 0 | 0 | ||||
| Grade 5 | 0 | 0 | 0 | 0 | ||||
Efficacy
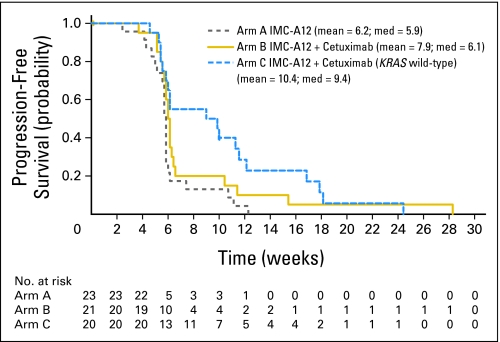
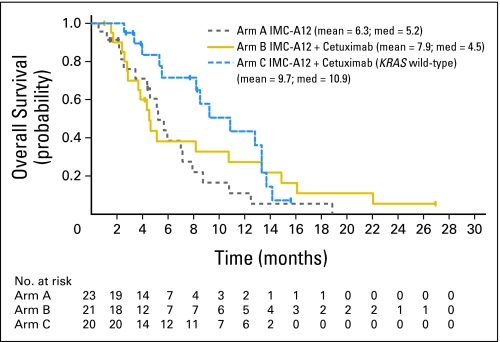
The objective response rates in arms A, B, and C were 0 (0%) of 23 patients (95% CI, 0% to 15%), 1 (5%) of 21 patients (95% CI, 0% to 24%), and 0 (0%) of 20 patients (95% CI, 0% to 17%), respectively. Median PFS times in the study arms A, B, and C were 5.9 weeks, 6.1 weeks, and 9.4 weeks, respectively. With a median follow-up of 5.1 months, the median OS times in the study arms A, B, and C were 5.2 months, 4.5 months, and 10.9 months, respectively. PFS and OS curves are shown in Figures 1 and 2, respectively.
Fig 1.
Kaplan-Meier estimates of progression-free survival (intent-to- treat population).
Fig 2.
Kaplan-Meier estimates of overall survival (intent-to-treat population).
Correlative Assessments
Sequenom testing.
In addition to using the polymerase chain reaction DxS kit (Qiagen, Manchester, United Kingdom) to test for KRAS (particularly for eligibility and enrollment to arm C), we used a chip-based matrix-assisted laser desorption-time-of-flight mass spectrometer in arms A and B; results of this are listed in Table 4. The one patient with confirmed response (in arm B) was KRAS wild type for not only exons 2 but also exons 3 and 4 (patient 12). This patient was also wild type for known hot-spot mutations in NRAS, BRAF, and PIK3CA.
Table 4.
Results of Sequenom Testing for KRAS, BRAF, and PIK3CA Genotyping in Cohorts A and B and IHC for pAKT by Nuclear Staining and IGF-1R by Membrane/Cytoplasmic Staining
| Sample | KRAS | KRAS Mutation Location | BRAF | Location of Tissue Sample | IGF-1R Membrane/Cytoplasmic Staining |
pAKT Nuclear Staining |
Study Arm | PFS (days) | OS (days) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | Cell Staining (%) | Intensity | Cell Staining (%) | ||||||||
| 1 | Mutated | G13D | WT | Liver metastasis | 1 | 30 | 0 | 0 | A | 35 | 328 |
| 2 | WT | N/A | V600E | Primary | 1 | 30 | 2 | 20 | A | 33 | 159 |
| 3 | WT | N/A | WT | Primary | 1 | 30 | 0 | 0 | A | 41 | 213 |
| 4 | WT | N/A | WT | Liver metastasis | 2 | 70 | 0 | 0 | B | 36 | 85 |
| 5 | Mutated | G12D | WT | Primary | 1 | 20 | 1 | 40 | A | 36 | 173 |
| 6 | Mutated | G13D | WT | Primary | 1 | 30 | 1 | 40 | A | 41 | 241 |
| 7 | WT | N/A | WT | Primary | 1 | 60 | 1 | 40 | A | 41 | 140 |
| 8 | WT | N/A | WT | Lung metastasis | 2 | 60 | 1 | 30 | B | 38 | 820+ |
| 9 | WT | N/A | WT | Primary | I | I | I | I | A | 41 | 77 |
| 10 | WT | N/A | WT | Primary | 0 | 0 | 1 | 30 | B | 42 | 112 |
| 11 | Mutated | G12D | WT | Liver metastasis | 2 | 70 | 0 | 0 | A | 29 | 266 |
| 12 | WT | N/A | WT | Primary | I | I | I | I | B | 198 | 453 |
| 13 | Mutated | G12V | WT | Primary | 0 | 0 | 1 | 20 | B | 43 | 156 |
| 14 | Mutated | G12D | WT | Lung metastasis | I | I | I | I | A | 43 | 574 |
| 15 | WT | N/A | WT | Primary | I | I | I | I | B | 40 | 249 |
| 16 | WT | N/A | WT | Primary | I | I | I | I | A | 78 | 380 |
| 17 | Mutated | G13D | WT | Lymph node metastasis | I | I | I | I | B | 36 | 87 |
| 18 | Mutated | G12D | WT | Primary | I | I | I | I | A | 40 | 71 |
| 19 | WT | N/A | WT | Primary | I | I | I | I | A | 40 | 133 |
Abbreviations: IHC, immunohistochemistry; IGF-1R, type 1 insulin-like growth factor receptor; PFS, progression-free survival; OS, overall survival; WT, wild type; N/A, not applicable; I, insufficient material.
IHC testing.
Nineteen prearchival paraffin tissue samples were also tested for IGF-1R and pAKT staining, and the results of 11 (because eight had insufficient material) are noted in Table 4. Overall, eight of 11 tumors had no staining or only 1+ positive staining for IGF-1R; similar results were found with pAKT expression. Three tumors showed modest to strong staining (2+) in at least 60% of the tumor cells for IGF-1R expression; one tumor showed strong staining (2+) in at least 20% of the tumor cells for pAKT; no patients had a tumor that stained 2+ or more for both pAKT and IGF-1R expression. In addition, there appeared to be no difference in the IHC expression of either IGF-1R or pAKT among KRAS wild type versus KRAS mutant type tumors.
Pharmacokinetic differences.
Preliminary noncompartmental pharmacokinetic analysis indicated that secondary IMC-A12 pharmacokinetic parameters were similar to those observed in a phase I study evaluating IMC-A12 monotherapy administered every other week. Also, within this study, IMC-A12 pharmacokinetic behavior was comparable between patients receiving IMC-A12 monotherapy versus those receiving IMC-A12 in combination with cetuximab.
DISCUSSION
In this phase II trial, IMC-A12, alone or in combination with cetuximab, did not demonstrate meaningful antitumor activity in advanced, anti-EGFR antibody-refractory CRC. Of 64 patients treated, no responses were observed in 63. One patient did achieve a durable partial response to IMC-A12 and cetuximab. Before study entry, this patient with CRC and pulmonary metastases had experienced disease progression on both an oxaliplatin- and an irinotecan-containing regimen before being treated with the combination of irinotecan and cetuximab. He experienced documented disease control to the cetuximab/irinotecan regimen, and then he experienced disease progression after 8.5 months, manifested as growth of pulmonary lesions on computed tomography scan and an increase in serum carcinoembryonic antigen levels. Four weeks after disease progression occurred on this combination, the patient was then enrolled in arm B and received the combination of cetuximab and IMC-A12. The schedule of cetuximab on this trial of 500 mg/m2 every 2 weeks differed from the weekly cetuximab 250 mg/m2 he had previously received; we consider it extremely unlikely, however, that this modified dose and schedule constituted the basis for the therapeutic efficacy. It is unclear at this time whether the continuation of cetuximab contributed to the observed response or whether single-agent IGF-1R inhibition would have been sufficient to cause the tumor regression observed. In addition, as the patient was treated with cetuximab immediately after progression on a cetuximab-containing regimen, it is unlikely that the response observed would have been seen with cetuximab treatment alone. On retrospective analysis of archived tumor tissue, the tumor of this patient was KRAS wild type.
Given the unequivocal durable response observed in this patient and the recent data demonstrating that the clinical activity of EGFR-directed therapies is confined to patients whose tumors are KRAS wild type, we amended the study to add a third treatment arm that consisted of patients whose tumors were KRAS wild type and who had experienced a prior response of at least 24 weeks' duration. In this genetically and clinically selected population, no additional responses were observed and only a single patient exhibited stable disease of at least 12 weeks. Although PFS and OS were higher on arm C, this arm was selected to include KRAS wild-type disease plus a response to prior cetuximab for at least 5.5 months; the improved outcomes in this nonrandomized arm may be a reflection of more indolent disease biology.
Given the promising preclinical data with IGF-1R-directed therapies,1–3,6 why was the response rate to the combination of IMC-A12 and cetuximab so low in CRC? KRAS is a key downstream effector of EGFR and other receptor tyrosine kinases. KRAS mutations are found in approximately 40% to 50% of CRC, most commonly at the G12/G13 codons.18–20 These mutations result in impaired intrinsic and guanosine 5′-triphosphatase activating proteins–mediated guanosine 5′-triphosphate hydrolysis and, thus, lead to constitutive expression of high levels of activated RAS–guanosine 5′-triphosphate. Several retrospective assessments of KRAS status in phase III, randomized trials of anti-EGFR–directed therapies have confirmed that activity with these agents is restricted to patients with KRAS wild-type tumors.21–22
As noted previously, however, the objective response rate is relatively low among patients with wild-type KRAS CRC.14,23 These data indicate that KRAS wild-type status is necessary but not sufficient for response with these agents. More recent data suggest that BRAF mutations may also confer resistance to therapy;18 in our study, one of 19 tumor specimens (Table 4) tested retrospectively for I mutation was positive for the V600E mutation.
As the mitogen-activated protein kinase pathway is one of the key effectors of both EGFR- and IGF-1R–mediated cell growth, it would be reasonable to hypothesize that, as was observed with cetuximab, the clinical efficacy of IMC-A12 may also be restricted to patient whose tumors are wild type for KRAS and BRAF. Consistent with this hypothesis, the one patient who did respond to the combination of IMC-A12 and cetuximab had a tumor that was wild type for KRAS, NRAS, BRAF, and PIK3CA. Given the low response rate observed, the results of the study suggest that RAS wild-type status may be required but not sufficient to confer IGF-1R dependence.
A second possible explanation for the limited efficacy observed in this trial is that IGF-1R may not have been highly activated in the patients enrolled. To address this possibility, we determined the level of expression of IGF-1R by IHC. We also assessed for AKT pathway activation by performing IHC for pAKT expression. In this analysis, we observed modest to strong staining for IGF-1R in only three of 11 tumors. Notably, the one responding patient exhibited only 1+ staining for IGF-1R and had no detectable staining for phosphorylated AKT. It should be highlighted that total IGF-1R IHC expression may be a poor indicator of the degree of IGF-1R activation.
In summary, our correlative data and the limited clinical efficacy of IMC-A12 in this study suggest that additional preclinical work will be required to identify predictors of IGF-1R dependence in CRCs, possibly with emphasis on downstream signaling elements, such as AKT and/or PI3K. Novel assays and methodologies will also be needed which can, with high sensitivity and specificity, identify tumors with high levels of IGF-1R activation. This will be a challenge, as IGF-1R activation is typically ligand-dependent in CRC, and mutation and amplification of this receptor in human tumors has not been reported with high frequency.
Our data indicate that the safety of the anti-EGFR and anti-IGF-1R combination does not constitute a major impediment to proceeding with additional studies in different tumor types. No evidence of synergistic toxicity was observed, and the toxicities encountered appear to be comparable to the toxicities that would have been expected from the individual agents alone.
The interpatient pharmacokinetic differences between arms A and B patients appear to be minor and unlikely to be clinically relevant. Importantly, it is unlikely that dose adjustment would be necessary when administering IMC-A12 to patients with mild or moderate diabetes mellitus, although all patients enrolled on the trial had to have a fasting glucose less than 120 mg/dL, which suggests that this selected population had better glucose control.
In conclusion, IMC-A12 alone and in combination with cetuximab was inactive in all but one of 64 patients treated. Study modification to assess a more select population of patients who were KRAS wild type and who had experienced a prolonged response to an anti-EGFR–directed therapy, however, failed to elicit evidence of additional clinical activity. This negative trial does not preclude the potential usefulness of IGF-1R inhibitors when used in combination with active chemotherapy or other targeted pathway inhibitors.
Footnotes
Supported in part by ImClone Systems LLC, A Wholly-Owned Subsidiary of Eli Lilly and Company.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
Clinical trial information can be found for the following: NCT00503685.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Employment or Leadership Position: Jonathan Schwartz, ImClone Systems (C); Kumari Chandrawansa, ImClone Systems (C); Aruna Dontabhaktuni, ImClone Systems (C); Hagop Youssoufian, ImClone Systems (C) Consultant or Advisory Role: Diane Lauren Reidy, Novartis (C), Vogel Farina (C), Pfizer (C); Marwan G. Fakih, ImClone Systems (C); Leonard B. Saltz, ImClone Systems (C), Eli Lilly (C), Roche (C), Genentech (C), Chugai (C), Bristol-Myers Squibb (C), Merck (C), Delcath (C), OSI Pharmaceuticals (C) Stock Ownership: Jonathan Schwartz, ImClone Systems; Kumari Chandrawansa, ImClone Systems; Aruna Dontabhaktuni, ImClone Systems; Hagop Youssoufian, ImClone Systems Honoraria: Diane Lauren Reidy, Pfizer; Muhammad Wasif Saif, Bristol-Myers Squibb Research Funding: Diane Lauren Reidy, Merck; Muhammad Wasif Saif, ImClone Systems; Joel Randolph Hecht, ImClone Systems; Leonard B. Saltz, ImClone Systems, Eli Lilly, Roche, Genentech, Bristol-Myers Squibb, Merck, Bayer Pharmaceuticals, Pfizer, Amgen, Biothera, Synta, Plexxicon Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Diane Lauren Reidy, Efsevia Vakiani, Marwan G. Fakih, Muhammad Wasif Saif, Jonathan Schwartz, Kumari Chandrawansa, Hagop Youssoufian, David B. Solit, Leonard B. Saltz
Financial support: Hagop Youssoufian
Administrative support: Diane Lauren Reidy, Jonathan Schwartz, Hagop Youssoufian, Leonard B. Saltz
Provision of study materials or patients: Diane Lauren Reidy, Marwan G. Fakih, Muhammad Wasif Saif, Joel Randolph Hecht, Ellen Hollywood, Jonathan Schwartz, Hagop Youssoufian, Leonard B. Saltz
Collection and assembly of data: Diane Lauren Reidy, Efsevia Vakiani, Noah Goodman-Davis, Ellen Hollywood, Jinru Shia, Jonathan Schwartz, Kumari Chandrawansa, Leonard B. Saltz
Data analysis and interpretation: Diane Lauren Reidy, Efsevia Vakiani, Jinru Shia, Jonathan Schwartz, Kumari Chandrawansa, Aruna Dontabhaktuni, David B. Solit, Leonard B. Saltz
Manuscript writing: Diane Lauren Reidy, Efsevia Vakiani, Marwan G. Fakih, Muhammad Wasif Saif, Joel Randolph Hecht, Noah Goodman-Davis, Ellen Hollywood, Jinru Shia, Jonathan Schwartz, Kumari Chandrawansa, Aruna Dontabhaktuni, Hagop Youssoufian, David B. Solit, Leonard B. Saltz
Final approval of manuscript: Diane Lauren Reidy, Efsevia Vakiani, Marwan G. Fakih, Muhammad Wasif Saif, Joel Randolph Hecht, Noah Goodman-Davis, Ellen Hollywood, Jinru Shia, Jonathan Schwartz, Kumari Chandrawansa, Aruna Dontabhaktuni, Hagop Youssoufian, David B. Solit, Leonard B. Saltz
REFERENCES
- 1.Adams TE, Epa VC, Garrett TP, et al. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci. 2000;57:1050–1093. doi: 10.1007/PL00000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Sun Y. Insulin-like growth factor receptor-1 as an anti-cancer target: Blocking transformation and inducing apoptosis. Curr Cancer Drug Targets. 2002;2:191–207. doi: 10.2174/1568009023333863. [DOI] [PubMed] [Google Scholar]
- 3.Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: From basic to clinical studies and clinical applications. Oncology. 2002;63:317–332. doi: 10.1159/000066230. [DOI] [PubMed] [Google Scholar]
- 4.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 5.Burtrum D, Zhu Z, Lu D, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–8921. [PubMed] [Google Scholar]
- 6.Wu J, Odman A, Higgins L, et al. In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clin Cancer Res. 2005;11:3065–3074. doi: 10.1158/1078-0432.CCR-04-1586. [DOI] [PubMed] [Google Scholar]
- 7.Morgillo F, Kim WY, Kim ES, et al. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007;13:2795–2803. doi: 10.1158/1078-0432.CCR-06-2077. [DOI] [PubMed] [Google Scholar]
- 8.Barnes CJ, Ohshiro K, Rayala SK, et al. Insulin-like growth factor receptor as a therapeutic target in head and neck cancer. Clin Cancer Res. 2007;13:4291–4299. doi: 10.1158/1078-0432.CCR-06-2040. [DOI] [PubMed] [Google Scholar]
- 9.Slomiany MG, Black LA, Kibbey MM, et al. Insulin-like growth factor-1 receptor and ligand targeting in head and neck squamous cell carcinoma. Cancer Lett. 2007;248:269–279. doi: 10.1016/j.canlet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Brentjen R, Saltz L. Epidermal growth factor receptor blockade and treatment of solid tumor malignancies. In: DeVita J, Helman S, Rosenber S, editors. Progress in Oncology. Boston, MA: Jones and Bartlett; 2002. pp. 113–128. [Google Scholar]
- 11.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 12.Saltz L, Rubin M, Hochster H, et al. Cetuximab plus irinotecan is active in CPT-11–refractory colorectal cancer that expresses epidermal growth factor receptor. Proc Am Soc Clin Oncol. 2001;20:3a. abstr 7. [Google Scholar]
- 13.Saltz LB, Meropol NJ, Loehrer PJ, Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 14.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 15.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 16.Thomas RK, Baker AC, Debiasi RM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 17.Fumagalli D, Gavin PG, Taniyama Y, et al. A rapid, sensitive, reproducible and cost-effective method for mutation profiling of colon cancer and metastatic lymph nodes. BMC Cancer. 2010;10:101. doi: 10.1186/1471-2407-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 19.Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–2648. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 20.Freeman DJ, Juan T, Reiner M, et al. Association of K-ras mutational status and clinical outcomes in patients with metastatic colorectal cancer receiving panitumumab alone. Clin Colorectal Cancer. 2008;7:184–190. doi: 10.3816/CCC.2008.n.024. [DOI] [PubMed] [Google Scholar]
- 21.Peeters M, Siena S, Van Cutsem E, et al. Association of progression-free survival, overall survival, and patient-reported outcomes by skin toxicity and KRAS status in patients receiving panitumumab monotherapy. Cancer. 2009;115:1544–1554. doi: 10.1002/cncr.24088. [DOI] [PubMed] [Google Scholar]
- 22.Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361:98–99. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- 23.Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]