Abstract
Sensitization involving the CNS has been studied in many conditions but has received little attention in investigation of the pathogenesis of hypertension. Our experiments were initiated to determine if angiotensin II (ANGII)-induced hypertension can be sensitized by prior ANGII treatment and the role of the brain renin-angiotensin-aldosterone system (RAAS) in this process. To demonstrate ANGII-induced sensitization, we employed an experimental design of Induction-Delay-Expression (I-D-E). Male rats were implanted for telemetered blood pressure (BP) recording. During I, low doses of sc or icv ANGII were delivered for 1 week, and then the rats were rested for 1 week (D) to assure that any exogenous ANGII was metabolized. After this, a second higher dose of ANGII was given sc for 2 weeks (E).
During I and D the low doses of ANGII had no sustained effects on BP. However, during E the ANGII-induced BP increase was greater in the groups that had received low doses of ANGII during I in comparison to the group receiving saline during I. Central ANG-type 1 receptor antagonist delivery blocked this sensitization. Brain tissue collected at the end of D and E showed increased mRNA expression of several RAAS components in key forebrain regions of sensitized rats. Fra-like immunoreactivity was also increased at the end of E in the sensitized forebrain. These results indicate that sub-pressor doses of ANGII act on the brain to sensitize the hypertensive response to subsequent ANGII, and that sensitization is associated with altered expression of RAAS components in forebrain cardiovascular control structures.
Keywords: Angiotensin II, Blood Pressure, Sensitization, Neuronal Activation, RAAS Component Expression
Introduction
It has been nearly 50 years since Dickenson and colleagues first described the effect of infusing non-pressor doses of angiotensin II (ANGII) into rabbits to gradually produce hypertension over the course of several days.1,2 This slow ANGII-induced pressor phenomenon, defined as autopotentiation,3 has been demonstrated in several species including rats and humans.1,2,4 Recently, autopotentiation to ANGII in the induction of hypertension resembles several models of neuroplasticitity and behavior that are investigated in the context of sensitization. For example, the sensitization of sodium appetite (i.e., the ingestion of salty substances) and thirst (water drinking) has been associated with the central actions of ANGII and aldosterone (Aldo).5,6 Although such behavioral studies indicate that increased activity of the brain renin-angiotensin-aldosterone system (RAAS) is involved in fluid-related behavioral and neurohormonal sensitization, the involvement of brain RAAS components in similar processes has received little attention in the context of the pathogenesis of hypertension.
RAAS plays critical roles in the regulation of blood pressure (BP) and body fluid homeostasis. The metabolic cascade of the classic (aka the circulating) RAAS involving renin, angiotensinogen (AGT), ANGI, angiotensin converting enzyme (ACE1), ANGII, as well as the key role of ANGII controlling Aldo release, has been appreciated for more than 50 years. More recently it has been established that ANG II acts on AT1-R and ANG type 2 receptors (AT2-R). Although AT1-R mediate the majority of ANGII physiological and pathophysiological effects, ANGII binding to the AT2-R appears to activate mechanisms that counteract many AT1-R-mediated effects.7 Angiotensin-converting enzyme (ACE) 2, a homologue of ACE1, is a newly identified component of RAAS, which cleaves ANGII to generate ANG(1–7) which binds to the Mas receptor to activate mechanisms that generally exert effects opposite to those produced by ANGII.8
It has been shown that components of the classic RAAS are also synthesized de novo in various tissues including heart, kidney and brain.9 In experimental animals or hypertensive patients, infusions of ANGII induced an increased expression of renin, AGT, ACE and AT1-R in the kidney, brain, or T-lymphocyte, which resulted in generating higher ANGII levels and hypertension.10–13 These results suggest that ANGII can act in a feed-forward manner to enhance the activity and expression of several RAAS components, and that such increases in RAAS activity can potentially contribute to an augmented rise in BP induced by ANGII. The present experiments were initiated to determine if ANGII has the capacity to sensitize the brain in the process of the generation of ANGII-induced hypertension. The studies also investigated which components of the brain RAAS are likely to play a role in such an ANGII initiated sensitization process.
Methods
What follows is a brief summary of the experimental protocols. A detailed description of some methods can be found in the expanded Methods section in the online Data Supplement (available at http:/hyper.ahajournals.org).
Animals
Sprague-Dawley rats (10–12 weeks old) were obtained from Harlan (Indianapolis, IN). They were housed in temperature- and light-controlled animal quarters and were provided with rat chow (7013 NIH-31 modified rat diet, 0.25% NaCl) ad libitum. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by The University of Iowa Animal Care and Use Committee.
Experimental Protocol
Operationally the process of sensitization can be viewed as consisting of a period of induction (I) followed by a period of expression (E). During E, the consequences of I can be evaluated. Because it is possible to vary the amount of time between I and E, in other words, introduce a period of delay (D), there is an opportunity to observe the response elicited at E in the absence of the treatment (e.g., a low dose of ANGII) presented during I. Therefore, the present studies followed an I-D-E experimental design. During I, a low sub-pressor dose of ANGII or vehicle was delivered subcutaneously (sc, 10 ng/kg/min) or centrally (icv, 1 ng/kg/min) by osmotic minipump (model 2001, Alzet) for 1 week. The rats then rested for 1 week (D), after which time, a second pump (model 2002, Alzet) was implanted to deliver a higher sc dose of ANGII (120 ng/kg/min) for 2 weeks (E). Rats were randomly assigned to one of six groups: 1) I with ANGII plus E with saline, 2) I with saline plus E with ANGII, 3) I with ANGII plus E with ANGII, 4) I with ANGII and icv AT1R antagonist (irbesartan; 125 µg/d, Sigma) plus E with ANGII, and 5) I with icv saline plus E with ANGII, and (6) I with icv ANGII plus E with ANGII. Brains were collected at the end of E for regional (the lamina terminalis, LT and the paraventricular hypothalamic nucleus, PVN) analysis for mRNA expression or Fos-related antigen immunoreactivity (Fra-IR, indicating neuronal excitation). Two additional control (saline) and experimental (low dose of ANGII) groups received identical I and D procedures but had their brains collected at the end of D for mRNA expression analysis.
Physiological Studies
Under Ketamine-xylazine mixture, rats were chronically instrumented with telemetry probes (TA11PA-C40; DSI) placed in the femoral artery for continuous monitoring of mean arterial pressure (MAP) and heart rate (HR), as described previously.14 Beginning seven days after recovery from surgery, MAP and HR data collection was initiated.
Immunohistochemistry
Free-floating sections (40 µm) were incubated in a primary antibody, rabbit polyclonal anti-Fra (K-25, 1:1000, Santa Cruz) at room temperature overnight, followed by incubation in biotinylated goat anti-rabbit immunoglobins (1:200, Vector laboratories) for 1h. Then the immunoreaction was detected with an ABC kit (Vector Laboratories) and metal enhanced DAB (Sigma).
Measurement of mRNA Expression in the LT and PVN
Total RNA was isolated from LT and PVN using Trizol method (Invitrogen). Total RNA was reverse transcribed using random hexamers following the manufacturer’s instructions (Applied Biosystems). cDNA was amplified and analyzed using a C1000 thermocycler system (Bio-Rad). Changes in mRNA expression levels were normalized to GAPDH levels and calculated using the ΔΔCt method. Results are expressed as relative fold change, mean of fold change ± SE.
Data Analysis
MAP and HR data were collected for 5 baseline days and then for 28 consecutive days throughout I, D and E. MAP and HR are presented as mean daily values averaged from daytime and nighttime measurements. Difference scores for MAP and HR were calculated for each animal based on the mean of the 5-day baseline subtracted from the mean of the final 5 days of treatment. One-way ANOVAs for the experimental groups were then conducted on the means of calculated difference scores. After establishing a significant ANOVA, post hoc analysis was performed with Fisher's least significant difference multiple comparison tests between pairs of mean change scores. To test for differences in the means of the 5 baseline days vs the means of the final 5 days of treatments, t tests were performed.
The atlas of Paxinos and Watson was used to define regions of interest to evaluate Fra-IR in the subfornical organ (SFO) and PVN. An experimenter who was “blind” to the experimental group counted cells showing Fra-IR in 2 or 3 histological sections from each structure for each animal. Statistical evaluation of Fra-IR counts was performed by one-way ANOVA and student t tests. The same statistical methods were used to analyze the differences in mRNA expression of brain RAAS components in the groups that previously had received the low dose of ANGII during I vs. the vehicle treated group.
Results
Effect of ANGII-induced Sensitization during I on MAP and HR Induced by Subsequent ANGII during E
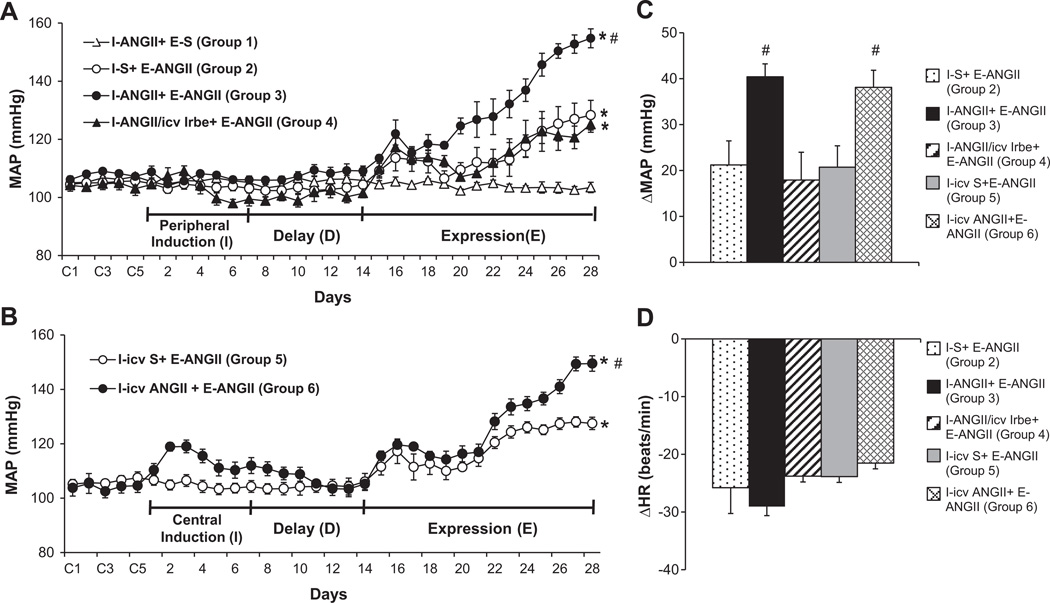
During I and D, the low sc dose ANGII had no effect on MAP. However, over the course of E, the high dose of ANGII induced a greater increase in MAP in the rats that received the low dose of ANGII (Δ40.4±2.8 mmHg, n=6, p<0.05, Fig. 1A, 1C) during I as compared to the rats treated with saline (Δ21.2±5.3 mmHg, n=6) during I. This augmentation of the pressor effect induced by the high dose of ANGII was attenuated by concurrent icv infusions of the AT1-R antagonist irbesartan along with the low sc dose of ANGII during I (Δ17.9±6.0 mmHg, n=5, p<0.05).
Figure 1.
Augmented pressor effects induced by ANGII (120 ng/kg/min) during the expression (E) period in male rats after peripheral (Fig 1A, 10 ng/kg/min) or central (Fig 1B, 1 ng/kg/min) treatment with a low dose of ANGII during the induction (I) period. This effect was attenuated by central blockade of AT1-R. Figure 1C and 1D show the changes in MAP and HR after infusion of ANGII during E in all groups. I-S = peripheral treatment with saline during I; I-ANGII = peripheral treatment with a low dose of ANGII during I; I-ANGII/icv Irbe = peripheral treatment with a low dose of ANGII plus central treatment with irbesartan (Irbe) during I; I-icv S = central treatment with saline during I; I-icv ANGII = central treatment with a low dose of ANGII during I; E-S = peripheral treatment with saline during E; E-ANGII = peripheral treatment with a high dose of ANGII during E. (* p<0.05 vs. baseline or I–ANGII+E-S, # p<0.05 vs. I-S+E-ANGII or I-ANGII/icv Irbe+E-ANGII).
To confirm that the effect of ANGII-induced sensitization was through its actions on the central nervous system (CNS), a low dose of ANG II was infused icv for 1 week during I. This resulted in a mild but significant increase in MAP during the first three days which gradually returned to the basal level by the end of D. The icv infusion of the low dose of ANGII also enhanced the pressor effects induced by a subsequent high dose of ANGII (Δ38.1±3.7 mmHg, n=5, p<0.05) during E as compared to that of icv saline treated rats (Δ20.7±4.7 mmHg, n=4, Fig 1B, 1C).
ANGII infusion during E produced a significant decrease in HR in all groups (p<0.05). However, the fall in HR during ANGII treatment was similar in all groups (Fig 1D).
Effect of ANGII Infusion on the mRNA Expression of RAAS Components in the LT
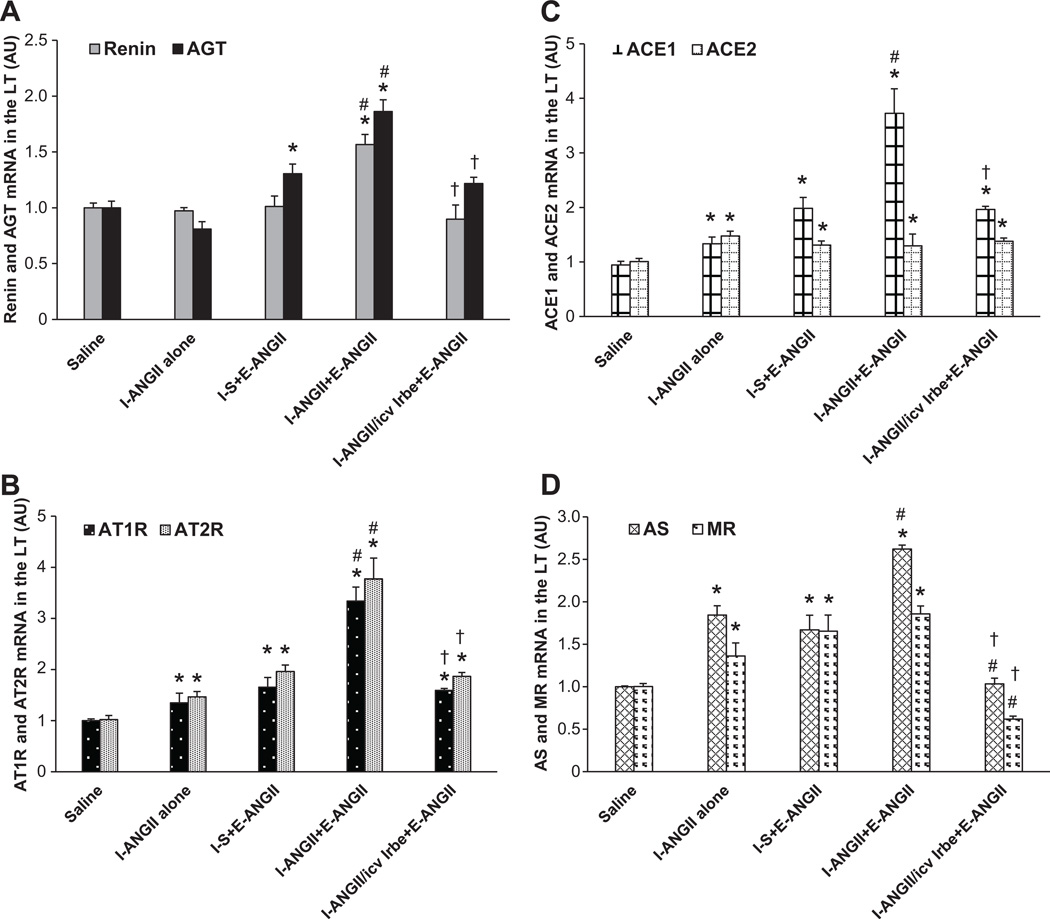
In LT tissue collected at the end of D, a low dose of ANGII during I induced a significant increase in the mRNA expression of AT1-R (1.35±0.08 fold, p<0.05, Fig 2B), AT2-R (1.46±0.09 fold, p<0.05, Fig 2B), ACE1(1.34±0.11 fold, p<0.05, Fig 2C), ACE2 (1.48±0.09 fold, p<0.05, Fig 2C), aldosterone synthase (AS,1.84±0.11 fold, p<0.05, Fig 2D), and mineralocorticoid receptor (MR, 1.36±0.15 fold, p<0.05, Fig 2D) in the LT when compared with controls (1.00±0.04). The expression of renin and AGT in the LT was not higher (p>0.05, Fig 2A) after D.
Figure 2.
ANGII infusion induced greater increases in mRNA expression of renin, AGT (Fig 2A), AT1-R, AT2-R (Fig 2B), ACE1 (Fig 2C), and AS (Fig 2D) in the lamina terminalis (LT) of rats receiving a low dose ANGII as compared with those receiving saline during induction (I). Central infusion of AT1-R antagonist blocked these effects. I-S = peripheral treatment with saline during I; I-ANGII = peripheral treatment with a low dose of ANGII during I; I-ANGII/icv Irbe = peripheral treatment with a low dose of ANGII plus central treatment with irbesartan (Irbe) during I; E-ANGII = peripheral treatment with a high dose of ANGII during E. (* p<0.05 vs. saline, # p<0.05 vs. I-ANGII alone or I-S+E-ANGII, † p<0.05 vs. I-ANGII+E-ANGII).
At the end of E, the higher dose of ANGII induced a similar increase in the mRNA expression of the RAAS components in the rats treated with saline during I. However, the ANGII infusion during E resulted in greater increases in mRNA expression of renin (1.57±0.09 fold, p<0.05), AGT (1.86±0.10 fold, p<0.05), AT1-R (3.34±0.27 fold, p<0.05), AT2-R (3.76±0.22 fold, p<0.05), ACE1(3.73±0.24 fold, p<0.05), and AS (2.62±0.05 fold, p<0.05) in the LT of the group that received a low dose of ANGII during I. Central infusion of the AT1-R antagonist during I blocked these augmented effects on mRNA expression.
Effect of ANGII Infusion on mRNA Expression of RAAS Components in the PVN
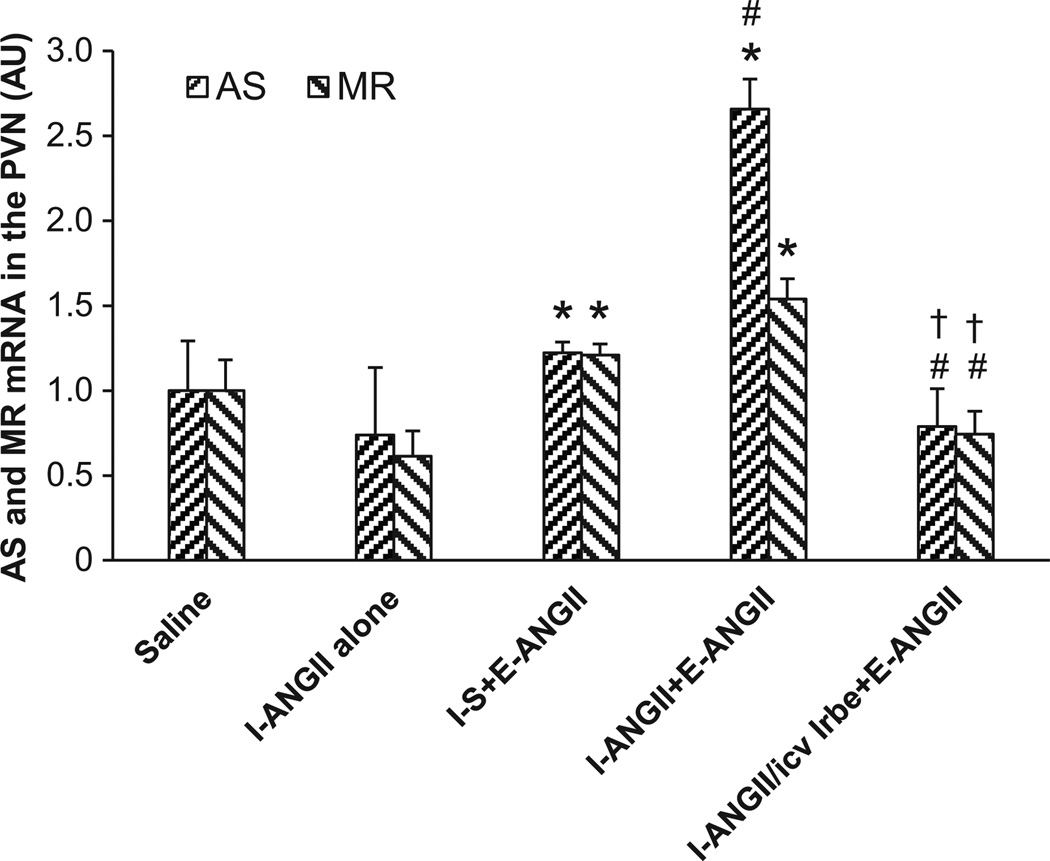
The low dose of ANGII administered during I had no effect on mRNA expression of any of the RAAS components studied in the PVN at the end of D. At the end of E, the higher dose of ANGII induced a mild, but significant, increase in mRNA expression of AS (1.22±0.06 fold, p<0.05) and MR (1.21±0.66 fold, p<0.05) in the PVN of the animals that received saline during I. The mRNA expression of other components of RAAS in the PVN of rats receiving saline during I was not increased. However, a low dose of ANGII delivered during I enhanced ANGII-induced mRNA expression only of AS during E (2.66±0.18 fold, p<0.05), while the mRNA expression of MR remained elevated above the control condition, but not significantly higher than the animals that received saline during I followed by ANGII during E (1.54±0.12, Fig 3). Central infusion of the AT1-R antagonist during I blocked the effects produced by the infusion of the higher ANG II dose.
Figure 3.
ANGII infusion only induced significant increases in mRNA expression of AS and MR, but not mRNA expression of other components of the RAAS in the PVN of rats receiving either saline or a low dose of ANGII during I. Central infusion of the AT1-R antagonist blocked these effects. I-S = peripheral treatment with saline during I; I-ANGII = peripheral treatment with a low dose of ANGII during I; I-ANGII/icv Irbe = peripheral treatment with a low dose of ANGII plus central treatment with irbesartan (Irbe) during I; E-ANGII = peripheral treatment with a high dose of ANGII during E. (* p<0.05 vs. saline, # p<0.05 vs. I-S+E-ANGII, † p<0.05 vs. I-ANGII+E-ANGII).
Effects of ANGII Infusion on Neuronal Activation in the SFO and PVN of Rats Treated with Saline or a Low Dose of ANGII during I
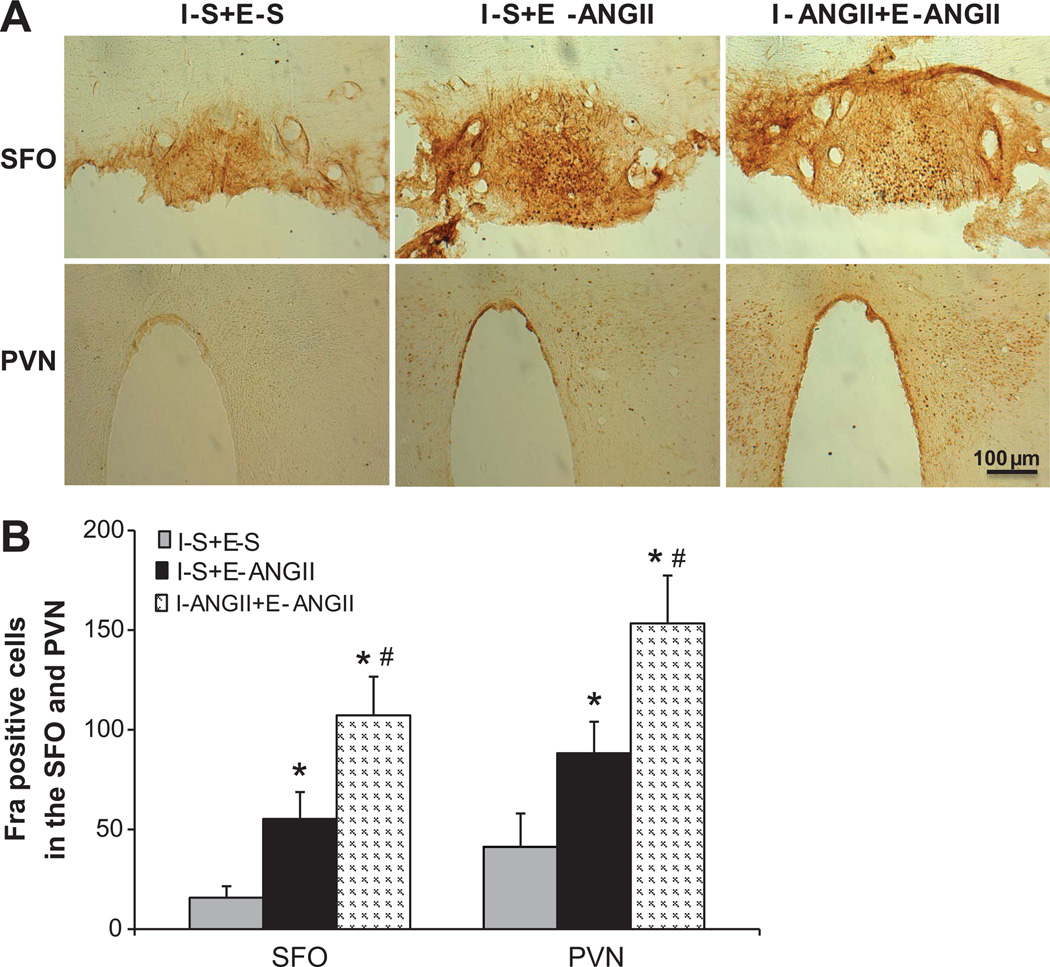
In the rats that received saline during I, the higher dose of ANGII infused during E produced significant increases in Fra-IR in both the SFO (55.3±13.5 cells, p<0.05) and PVN (88.3±15.7 cells, p <0.05) compared with sparse labeling seen in these structures in the animals receiving saline during I and E (SFO 15.7±5.8, PVN 41.3±16.7 cells). A low dose of ANGII given during I augmented Fra-IR in the SFO (107.3±19.3 cells, p<0.05) and PVN (153.3±2.4 cells, p<0.05, Figs 4A,4B) after the higher dose of ANGII infusion during E.
Figure 4.
Treatment with a low dose of ANGII during I enhanced subsequent ANGII-induced Fra expression (indicating neuronal excitation) in the SFO and PVN as compared to those treated with saline during I. Representative fluorescence microscopic images of section from all groups of rats (Fig 4A). Fig 4B. Quantification of Fra positive neurons in the SFO and PVN of each group. I-S+E-S means I with saline plus E with saline; I-S+E-ANGII = I with saline plus E with ANGII; I-ANGII+E-ANGII = I with low dose ANGII plus E with ANGII. (* p<0.05 vs. I-S+E-S, # p<0.05 vs. I-S+E-ANGII)
Discussion
The present study investigated the capacity of ANGII to sensitize the brain and thereby enhance the onset of hypertension to a subsequent treatment with ANGII. The activation of forebrain areas and the expression of mRNA for components of the brain RAAS was also studied to gain insight into the role of the brain and neurohumoral mediators in this process. The most important finding of these studies demonstrates that a low dose of ANGII can sensitize the brain to produce an enhanced hypertensive response to ANGII. The amplified pressor effects produced by sensitizing the brain are associated with increased neuronal activation and elevated mRNA expression of RAAS components in two key forebrain cardiovascular control regions. Both the functional sensitization and enhanced mRNA expression were blocked by central AT1-R antagonist administration. These results suggest that sub-pressor levels of ANGII can induce sustained CNS changes that increase the vulnerability to hypertension.
The initiation and development of hypertension involves the interactions of multiple organs and systems. Targeting experimental ablations to specific organs (c.f. ablation of the organum vasculosum of the lamina terminalis, OVLT)15 or particular cellular components with those organs (c.f. renal AT1-R)16 can interrupt the course of development or maintenance of high BP produced by the systemic infusion of ANGII. Current evidence indicates that there are at least two distinct phases of ANGII-induced hypertension. First, an initial elevation in pressure associated with an ANGII-mediated systemic vasoconstriction which is then replaced by a second, long-term, neurogenic component.17,18 In the present studies the initial increase in BP response to systemic ANGII administration (i.e., within 4–7 day) was comparable for both groups with or without the low dose of ANGII as a pretreatment. After day 4–7, the increase in BP accelerated in the low dose of ANGII pretreated group. This suggests that the sensitization occurs during the time when neurogenic mechanisms are contributing to the progression of increased BP.
Forebrain structures along with the LT (i.e., the SFO, median preoptic nucleus and OVLT), and the hypothalamus (particularly the PVN) play important roles in the long-term regulation of BP and body fluid homeostasis. LT structures are involved in both sensing and processing input derived from humoral factors (e.g., ANGII and extracellular osmolality) and transmitting this information to the PVN, which in turn projects to hindbrain and spinal cord cardiovascular control structures.19 Recent studies demonstrate that chronic 14-day infusions of pressor doses of ANGII into rabbits and rats result in sustained SFO and PVN activation (i.e., increased Fra-IR) and increased BP.20,21 The present results are consistent with these studies and extend them by showing that a low, sensitizing dose of ANGII during I augments both hypertension and neuronal SFO and PVN activation produced by 14 days of ANGII infusion.
In hypertensive patients and experimental animals, it has been shown that ANGII can induce increases in both local AT1-R expression and ANGII levels, which are likely in turn to contribute to increased BP.10,11 In the present studies, the low dose of ANGII administered during I may have facilitated the generation of brain ANGII and increased the number of AT1-R. The failure to find an enhancement in BP response during E in animals receiving low doses of ANGII in conjunction with central infusion of an AT1-R antagonist during I, indicates that activation of AT1-R in the brain is necessary during the I phase in order to sensitize the hypertensive response.
ANGII up-regulates AGT and renin expression in the kidney, and increases intrarenal ANGII levels along with systolic BP, thereby suggesting a direct link between AGT or renin gene expression and BP.11 In the brain AGT is widely distributed in astroglia, but has a more restricted regional distribution in neurons.22 Renin has been identified in neurons,23 and renin and AGT are located in close proximity to AT-R-containing neurons, suggesting a model for the local production and action of ANGII. Double-transgenic mice, which overexpress human renin (hRen) and human AGT (hAGT, SRA mice) in the brain, exhibit a marked increase in ANGII–like immunoreactivity in the SFO and elevated water and salt intakes, which are significantly reduced after chronic icv delivery of an AT1-R antagonist. This provides evidence that increased renin and AGT expression results in an increase in ANGII production in the brain.24 In the present study, although the low dose of ANGII alone did not increase renin and AGT expression in the LT after D, it did increase expression of message at the end of E. Although it cannot be ruled out that this increase in expression was a result of the elevated level of BP, it may be more parsimonious to propose that it was a progressive increase in brain ANGII through a positive feedback mechanism during systemic ANGII infusion that contributed to the elevated hypertension.
Of the AT-R, the AT1-R is known to mediate most actions of ANGII. Abnormal regulation and function of the AT1-R have been proposed to contribute to development and maintenance of various forms of hypertension. In contrast, AT2-R have been suggested to act as functional antagonists of AT1-R.25 Gao et al. demonstrated AT2-R protein expression in the brain.26 Either overexpression or selective activation of central AT2-R decreases BP and norepinephrine levels partially by reducing sympathetic outflow.27,28 The up-regulation of AT1-R and down-regulation of AT2-R expression creates a functional imbalance that may be associated with the pathogenesis of hypertension and heart failure.29,30 However, in the present study, ANGII induced a significant increase in expression of both AT1-R and AT2-R, suggesting that a low dose of ANGII sensitizes the expression of message for both receptors. This enhanced central AT2-R expression may reflect the activation of inhibitory mechanisms that attempt to buffer against the actions of ANGII, which collectively act to increase BP. This is in agreement with a recent study conducted in obese Zucker rats.31
High ACE has been associated with increased susceptibility to hypertension and end organ damage. ANGII can increase ACE expression and cause renal pathology in rats.10,11 A recent study showed that ANGII can interact with ACE through a calcium signaling mechanism to activate ACE and promote increased reactive oxygen species. This result suggests that ACE is not only an ANGII generating enzyme but that it is also a signaling receptor for this peptide.32 Inhibition of ACE alone prevents increased ACE protein expression, enhanced formation of ANG II and increased BP during systemic ANGII infusion.11
The ACE2 has a high substrate specificity for ANGII to convert it into ANG(1–7). Infusion of ANG(1–7), or overexpression of ACE2, has been shown to counteract most typical actions of ANGII and to reduce BP.33–35 In the present study, we found that infusion of a high dose of ANGII during E did not increase ACE2 expression beyond the significant increase produced by a low dose of ANGII given during I. This result is consistent with a recent study from Xia and colleagues showing that AT1-R in the brain of chronically hypertensive mice overexpressing hRen and hAGT have reduced ACE2 activity, but not ACE2 expression.34 Incontrast, in the present study, a low dose of ANGII was found to sensitize ACE1 expression in the LT with ACE1 showing higher expression in response to the ANGII administered during E in the rats that received the low dose of ANGII during I. This high ACE1 expression and unchanged ACE2 expression may reflect a shift of homeostatic equilibrium from a normotensive to a prohypertensive state.
In recent years, Aldo has come to be recognized as a key mediator of RAAS-related effects. Like ANGII, Aldo is not only a major regulator of extracellular fluid volume and electrolytes, but is also linked to the pathogenesis of hypertension and heart failure.36 Synthesis of Aldo from 11-deoxycorticosterone (DOC) in the adrenal cortex is catalyzed by AS, the product of the CYP11B2 gene. The brain has been identified as a major extra-adrenal site of CYP11B2 expression, and ANGII is a stimulus for CYP11B2-related Aldo synthesis.37 Recent studies highlight the interactions between ANGII and Aldo. Fiebeler et al. reported that systemic administration of an AS inhibitor reduced circulating Aldo levels and ameliorated ANGII-induced organ damage in transgenic rats overexpressing both hRen and hAGT.38 Furthermore, we and others have demonstrated that central infusion of MR blocker or AS inhibitor attenuates hypertension induced by systemic ANGII administration.14,21 These data suggest that in the CNS, activation of a local Aldo system acts in a neuromodulatory mode to induce the pressor response to circulating ANGII, and that a CNS Aldo-dependent neurogenic component contributes to hypertension induced by circulating ANGII.
In the present study, we performed microdissections of the PVN and LT to collect tissue to quantitate mRNA expression, and found that the low dose of ANGII during I had no effect on mRNA expression of most RAAS components in the PVN, but did produce a marked enhancement of AS mRNA in response to ANGII administered during E in the group that received ANGII during I. In the PVN, mRNA expression of MR was high both after D and E. In contrast to the PVN, the low dose of ANGII during I was associated with increased expression of all the RAAS components in the LT. It is also notable that the responses to ANGII in LT tissues were greater than in the PVN. These results suggest that there is likely to be differential regulation among different cardiovascular-related brain nuclei involved in the ANGII-induced sensitization process.
Perspectives
The present studies demonstrate that exposure to a non-pressor infusion of ANGII has a sensitizing effect which acts to facilitate an increase in BP produced by a subsequent slow pressor treatment with ANGII. The findings also indicate that this ANGII-induced sensitization involves the CNS and components of the brain RAAS. Through a brain mediated mechanism, ANGII acts to increase the gain of its own action. One important implication of these observations is that in the course of producing hypertension, ANGII has the capacity for a feed-forward action so that low levels of circulating octapeptide progressively exert a greater and greater pressor action. This observation may account for why some hypertensive patients with plasma ANGII concentrations deemed to be within the normal range respond with lower BP after ACE inhibition or AT1-R blockade.
The sensitization process is likely to involve a cascade of cellular and molecular events in the coupling between I and E in the CNS. The I-D-E experimental paradigm we have developed and employed in the present studies is likely to be a valuable model for future exploration of the cellular and molecular pathways involved in sensitization processes associated with ANGII-induced hypertension.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the NIH grants HL-14388, HL-98207, and MH-80241.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Dickinson DM, Lawrence JR, Adelaide MB. A slowly developing pressor response to small concentrations of angiotensin its bearing on the pathogenesis of chronic renal hypertension. Lancet. 1963;281:1354–1356. doi: 10.1016/s0140-6736(63)91929-9. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson CJ, Yu R. The progressive pressor response to angiotensin in the rabbit. J Physiol. 1967;190:91–99. doi: 10.1113/jphysiol.1967.sp008195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohr DF. Angiotensin on vascular smooth muscle. In: Page IH, Bumpus FM, editors. Angiotensin. NY, Heidelberg, Berlin: Springer-Verlag; 1965. pp. 424–440. [Google Scholar]
- 4.Simon G, Abraham G, Cserep G. Pressor and subpressor angiotensin II administration: two experimental models of hypertension. Am J Hypertens. 1995;8:645–650. doi: 10.1016/0895-7061(95)00047-S. [DOI] [PubMed] [Google Scholar]
- 5.Sakai RR, Fine WB, Epstein AN, Frankmann SP. Salt appetite is enhanced by one prior episode of sodium depletion in the rat. Behav Neurosci. 1987;101:724–731. doi: 10.1037//0735-7044.101.5.724. [DOI] [PubMed] [Google Scholar]
- 6.Moellenhoff E, Blume A, Culman J, Chatterjee B, Herdegen T, Lebrun C, Unger T. Effect of repetitive icv injections of ANG II on c-Fos and AT(1)-receptor expression in the rat brain. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1095–R1104. doi: 10.1152/ajpregu.2001.280.4.R1095. [DOI] [PubMed] [Google Scholar]
- 7.Gao L, Zucker IH. AT2 receptor signaling and sympathetic regulation. Curr Opin Pharmacol. 2011;11:124–130. doi: 10.1016/j.coph.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol. 2011;300:R804–R817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system- an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 10.Coppo M, Bandinelli M, Berni A, Galastri S, Abbate R, Poggesi L, Marra F, Gensini GF, Boddi M. Ang II Upregulation of the T-lymphocyte renin-angiotensin system is amplified by low-grade inflammation in human hypertension. Am J Hypertens. 2011;24:716–723. doi: 10.1038/ajh.2011.32. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010;298:F150–F157. doi: 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter JP. Chronic intracerebroventricular infusion of angiotensin II increases brain AT1 receptor expression in young rats. Devopmental Brain Res. 1999;112:293–295. doi: 10.1016/s0165-3806(98)00182-5. [DOI] [PubMed] [Google Scholar]
- 13.Wei SG, Yu Y, Zhang ZH, Felder RB. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J physiol Heart Circ Physiol. 2008;296:H1425–H1433. doi: 10.1152/ajpheart.00942.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue B, Beltz TG, Yu Y, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Central interactions of aldosterone and angiotensin II in aldosterone- and angiotensin-induced hypertension. Am J Physiol Heart Circ Physiol. 2011;300:H555–H564. doi: 10.1152/ajpheart.00847.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vieira AA, Nahey DB, Collister JP. Role of the organum vasculosum of the lamina terminalis for the chronic cardiovascular effects produced by endogenous and exogenous ANG II in conscious rats. Am J phsiol Regul Integr Comp Physiol. 2010;299:R1564–R1571. doi: 10.1152/ajpregu.00034.2010. [DOI] [PubMed] [Google Scholar]
- 16.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorbea-Oppliger VJ, Melaragno MG, Potter GS, Petit RL, Fink GD. Time course of losartan blockade of angiotensin II hypertension versus blockade of angiotensin II fast pressor effects. J Pharmacol Exp Ther. 1994;271:804–810. [PubMed] [Google Scholar]
- 18.Cox BF, Bishop VS. Neural and humoral mechanisms of angiotensin-dependent hypertension. Am J Physiol. 1991;261:H1284–H1291. doi: 10.1152/ajpheart.1991.261.4.H1284. [DOI] [PubMed] [Google Scholar]
- 19.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 20.Davern PJ, Head GA. Fos-related antigen immunoreactivity after acute and chronic angiotensin II-induced hypertension in the rabbit brain. Hypertension. 2007;49:1170–1177. doi: 10.1161/HYPERTENSIONAHA.106.086322. [DOI] [PubMed] [Google Scholar]
- 21.Huang BS, Ahmadi S, Ahmad M, White RA, Leenen FH. Central neuronal activation and pressor responses induced by circulating ANG II: role of the brain aldosterone-"ouabain" pathway. Am J Physiol Heart Circ Physiol. 2010;299:H422–H430. doi: 10.1152/ajpheart.00256.2010. [DOI] [PubMed] [Google Scholar]
- 22.Imboden H, Harding JW, Hilgenfeldt U, Celio MR, Felix D. Localization of angiotensinogen in multiple cell types of rat brain. Brain Res. 1987;410:74–77. doi: 10.1016/s0006-8993(87)80022-7. [DOI] [PubMed] [Google Scholar]
- 23.Fuxe K, Ganten D, Hökfelt T, Locatelli V, Poulsen K, Stock G, Rix E, Taugner R. Renin-like immunocytochemical activity in the rat and mouse brain. Neurosc. Lett. 1980;18:245–250. doi: 10.1016/0304-3940(80)90292-x. [DOI] [PubMed] [Google Scholar]
- 24.Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest. 2007;117:1088–1095. doi: 10.1172/JCI31242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masaki H, Kurihara T, Yamaki A, Inomata N, Nozawa Y, Mori Y, Murasawa S, Kizima K, Maruyama K, Horiuchi M, Dzau VJ, Takahashi H, Iwasaka T, Inada M, Matsubara H. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J Clin Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu L, Zheng M, Wang W, Rozanski GJ, Zucker IH, Gao L. Developmental changes in AT1 and AT2 receptor-protein expression in rats. J Renin Angiotensin Aldosterone Syst. 2011;11:214–221. doi: 10.1177/1470320310379065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J, Zhang H, Le KD, Chao J, Gao L. Activation of central angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am J Hypertens. 2011;24:724–730. doi: 10.1038/ajh.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao L, Wang W, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal Rats. Hypertension. 2008;51:521–527. doi: 10.1161/HYPERTENSIONAHA.107.101717. [DOI] [PubMed] [Google Scholar]
- 29.Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension. 2008;52:708–714. doi: 10.1161/HYPERTENSIONAHA.108.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng JF, Phillips MI. Opposite regulation of brain angiotensin type 1 and type 2 receptors in cold-induced hypertension. Regul Pept. 2001;97:91–102. doi: 10.1016/s0167-0115(00)00218-4. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui AH, Ali Q, Hussain T. Protective role of angiotensin II subtype 2 receptor in blood pressure increase in obese Zucker rats. Hypertension. 2009;53:256–261. doi: 10.1161/HYPERTENSIONAHA.108.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guimarães PB, Alvarenga ÉC, Siqueira PD, Paredes-Gamero EJ, Martin RP, Shimuta SI, Carmona AK, Nakaie CR, Jasiulionis MG, Ferreira AT, Pesquero JL, Oliveira SM, Bader M, Costa-Neto CM, Pesquero JB. Angiotensin II binding to angiotensin I-converting enzyme triggers calcium signaling. Hypertension. 2011;57:965–972. doi: 10.1161/HYPERTENSIONAHA.110.167171. [DOI] [PubMed] [Google Scholar]
- 33.Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1–7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- 34.Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension. 2009;53:210–216. doi: 10.1161/HYPERTENSIONAHA.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD, Lazartigues E. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106:373–382. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funder JW, Mihailidou AS. Aldosterone and mineralocorticoid receptors: Clinical studies and basic biology. Mol Cell Endocrinol. 2009;301:2–6. doi: 10.1016/j.mce.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 37.Gomez-Sanchez CE, Zhou MY, Cozza EN, Morita H, Foecking MF, Gomez-Sanchez EP. Aldosterone biosynthesis in the rat brain. Endocrinology. 1997;138:3369–3373. doi: 10.1210/endo.138.8.5326. [DOI] [PubMed] [Google Scholar]
- 38.Fiebeler A, Nussberger J, Shagdarsuren E, Rong S, Hilfenhaus G, Al-Saadi N, Dechend R, Wellner M, Meiners S, Maser-Gluth C, Jeng AY, Webb RL, Luft FC, Muller DN. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation. 2005;111:3087–3094. doi: 10.1161/CIRCULATIONAHA.104.521625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.