Abstract
Background
Studies of season of birth or season of conception can provide clues about etiology. We investigated whether certain months or seasons of conception are associated with increased risk of autism spectrum disorders, for which etiology is particularly obscure.
Methods
The study population comprises 6,604,975 children born from 1990 to 2002 in California. Autism cases (n = 19,238) were identified from 1990 through 2008 in databases of the California Department of Developmental Service, which coordinates services for people with developmental disorders. The outcome in this analysis was autism diagnosed before the child’s sixth birth date. The main independent variables were month of conception and season of conception (winter, spring, summer, and fall). Multivariate logistic regression models were used to estimate odds ratios (ORs) with their 95% confidence intervals (CIs) for autism by month of conception.
Results
Children conceived in December (OR = 1.09 [95% CI = 1.02 – 1.17]), January (1.08 [1.00 –1.17]), February (1.12 [1.04– 1.20]), or March (1.16 [1.08 – 1.24]) had higher risk of developing autism compared with those conceived in July. Conception in the winter season (December, January, and February) was associated with a 6% (OR = 1.06, 95% CI = 1.02 – 1.10) increased risk compared with summer.
Conclusions
Higher risks for autism among those conceived in winter months suggest the presence of environmental causes of autism that vary by season.
An analysis of season of birth or conception can provide clues about causes of some diseases with unknown etiology. An association between season of conception or birth and the occurrence of a health condition suggests periodicity of an environmental etiologic agent acting during the prenatal, perinatal or early postnatal phase of development. Factors that frequently vary by season (and have been suspected to increase risk of neurodevelopmental disorders such as schizophrenia and autism) include viral infections, 1–3 variations in nutrient intake, precipitation rates (rainfall),4 and pesticides (organophosphates and organochlorines). 5,6
Previous studies suggest that children born in the winter have a 10% increased risk of developing schizophrenia compared with those born in other seasons.7,8 However, mixed results have been reported for the association between season or month of birth and risk of autism. The earliest study we were able to identify that examined month of birth in association with autism was published in 1981.9 The investigator compared the birth patterns of 810 children with autism with those of 768 live birth controls and found that children with autism have an excess of March and August births. March birth has also been associated with increased risk of autism in studies conducted in Israel,10 Sweden, 11 and Denmark.12 Some researchers examined season rather than month of birth. Studies from Canada,13 Japan,14 the USA,15 and the United Kingdom16 have reported spring birth as a risk factor for autism. Other researchers have reported an increased risk of autism for summer13,15 and fall15 births. Not all studies have found an association between month or season of birth and autism.17,18 Bolton et al. 19 obtained different results in their analysis depending on how season was defined. When December was included in the winter, they found a difference in the birth pattern of their cases and controls. However when it was included in the fall, there was no difference. Methodological differences among the reviewed studies make it difficult to compare results. For example, source of controls differed across studies. Investigators have used siblings, live-births, or patients with other types of disabilities as controls. If the exposure leading to autism is also a risk factor for other disabilities, then including children with other disabilities in the control group could bias the result towards the null. The use of siblings as controls will also tend to dilute measures of association between environmental factors and diseases because children from the same family will tend to share household or neighborhood exposures, as well as a portion of their genes, increasing the likelihood that their susceptibility to other neurotoxic factors will also be similar.
A major limitation of studies that use time of birth as the exposure is that critical time-windows of development will not be a constant distance (in time) from birth, but will vary according to the length of gestation. However, critical developmental windows are more nearly a constant distance from conception. Thus, analyses based on time of conception will provide more accurate results because they are subject to less exposure misclassification, especially if the window of susceptibility is relatively narrow.
A recent study in the United Kingdom 16 reported that children conceived in summer were at increased risk of developing autism compared with those conceived in autumn (OR = 2.08 [95% CI = 1.18 – 3.70]).16 The study had only 86 cases, thus precluding a reliable estimate for the risk associated with month of conception. Moreover, the results were not adjusted for covariates that could potentially affect the crude findings.
The goal of the present study is to further investigate whether certain months or seasons of conception are associated with increased risk of autism, using a data set far larger than previous studies on seasonality and autism. The study was approved by the Institutional Review Boards for the Protection of Human Subjects of the State of California and of the University of California, Davis.
Methods
Study population
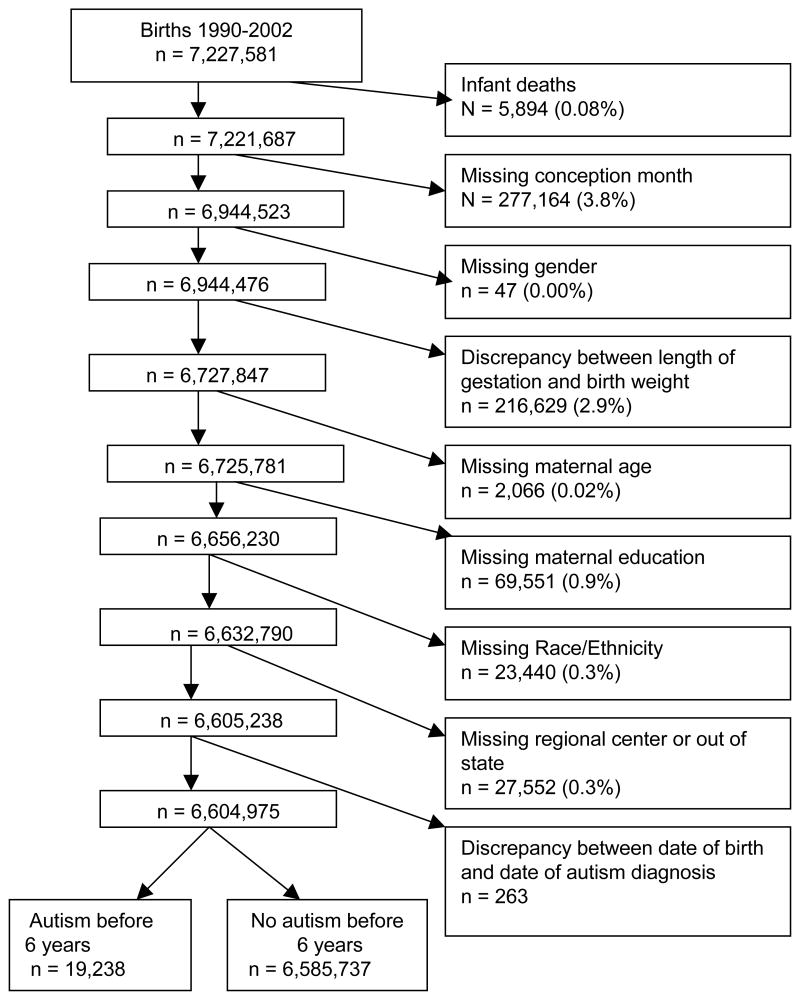
Between January 1990 and December 2002, there were 7,227,581 live births in California. For the purpose of our analysis, we excluded 5894 infants who died before their first birthday because they did not survive to an age when autism is typically diagnosed. An additional 616,449 records (9%) were excluded due to missing information on month of last menstrual period, child’s sex, maternal age, maternal education, race/ethnicity, and mother’s place of residence at time of childbirth, or to records that had a discrepancy between the gestational age and birth weight or had extreme length of gestation. We used the cut-points of the National Center for Health Statistics for implausible birth weight for gestational age reported by Pearl et al.20(gestation <20 weeks, birth weight ≥ 1000 g; 20 – 23 weeks, ≥ 2000 g; 24 – 27 weeks, ≥ 3000g; 28 – 31 weeks, ≥ 4000g; 32 – 47 weeks, ≤ 1000g). Another 263 records were excluded because of discrepancy between date of birth and date of autism diagnosis (e.g., the latter preceded the former, indicating recording errors). The final study cohort included 6,604,975 births (91 %) who were followed to the sixth birthday for a possible diagnosis of autism. Figure 1 provides more details of study population construction.
Figure 1.
Construction of the study population based on California birth file from the period of 1990 – 2002 inclusive, merged with data from the California Department of Developmental Services for the period of 1990 – 2008.
Case identification and matching
The California Department of Developmental Services determines and coordinates services for persons with developmental disabilities through its 21 independent contracting regional centers. The Client Developmental Evaluation Report is the instrument used to periodically evaluate clients age 3 years and older. For younger clients, the Early Start Report is used. We obtained these files from the Department of Developmental Services for the period of 1990 – 2008 and matched them with California birth records of 1990 to 2002 to identify children in the birth file who developed autism. The overall matching rate was above 90%. Details of the matching method and identification of autism cases in the Department of Developmental Services data base have been described elsewhere.21
Autism cases were defined as “full syndrome” autism (status 1) on the Client Developmental Evaluation Report, or International Classification of Disease (ICD-9) code 299.0 in any field in which ICD codes could be noted on either the Client Developmental Evaluation Report or the Early Start Report, or a checkmark for autism under developmental disabilities. Those children who met the criteria and were younger than six years of age at the time of the diagnosis were considered cases.
Statistical analysis
The main exposure variable is month of conception. Month and year of conception were estimated based on the child’s date of birth and the length of gestation. We examined month of conception and season of conception (winter including December, January and February; spring, March, April and May; summer, June, July and August; and fall, September, October and November). Months and seasons of conception were combined across years. The data were reviewed for outliers and missing values using univariate descriptive analyses.
Bivariate analyses were conducted between season of conception and selected covariates previously reported to be associated with increased risk of autism.6,22 We also examined the association between these covariates and the risk of autism. The covariates included preterm birth (gestational length less than 37 weeks), maternal age (age less than 35 years vs. greater than or equal to 35 years), maternal education as an index of socioeconomic status (less than or equal to high school vs. greater than high school graduate), child’s sex, child’s race (white, black, Asian, mixed, and others), ethnicity (non-Hispanic, Hispanic, and others), maternal place of residence at childbirth (grouped into areas defined by the Department of Developmental Services regional centers), and year of conception. We assigned a dummy variable to each calendar year of conception, producing 14 categories (categorical variable for year of conception) with 1999 arbitrarily chosen as the referent year. We also derived a continuous variable for date of conception representing time elapsed (in years, but not rounded to whole numbers) between 1 January 1990 and the estimated date of conception.
First, we fit an unadjusted logistic regression model with autism diagnosis before the sixth birthday (referred to as autism by age 5) as the dependent variable and month or season of conception as the independent variable. We chose July as the reference month because of its association with low influenza activity in California. Following the same logic, summer was chosen as the reference season. A series of multiple logistic regression models were fit to the data to control for potential confounders. We conducted two sets of multivariate logistic regression analyses, controlling for year-of-conception first as a categorical variable and then as continuous calendar time. Covariates associated with both season of conception and autism were considered potential confounders and entered into the full multivariate logistic regression model. Variables were retained in the model only if they changed a beta coefficient of any month or season of conception by at least 10%.
For the purpose of comparison with previous studies, we fitted alternative logistic regression models, with month of birth (rather than conception) as the main independent variable. April was chosen as the reference month because it corresponds to the month of birth for the majority of children conceived in July (the reference month in the analysis of month of conception). We report the odds ratios (ORs) and their 95% confidence intervals (CIs) as measures of association with the corresponding precision between each month or season and autism. The Statistical Analysis Software (SAS®, Cary)23 package was used to carry out all analyses.
Results
In bivariate analyses, maternal age, education, race, ethnicity, place of residence, preterm birth, and year of conception were each associated with season of conception (data not shown). However, only maternal education, child’s ethnicity, and year of conception were confounders; thus, we included these variables in all multivariate models.
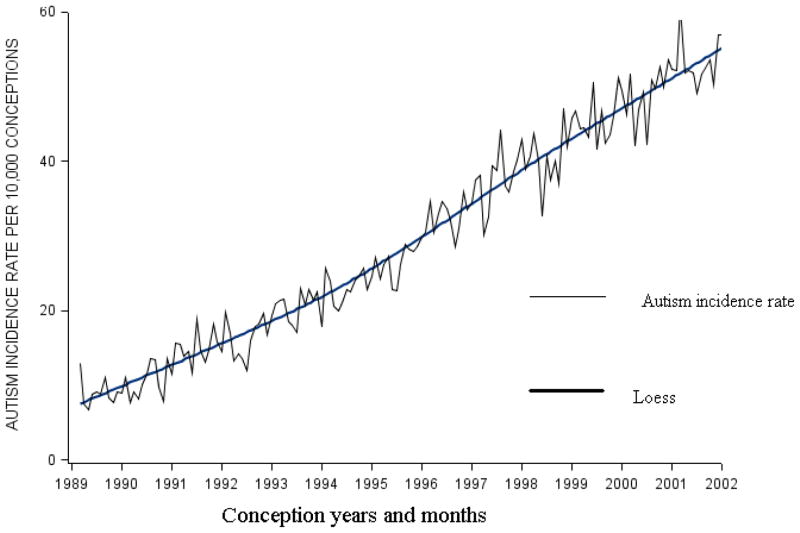
Table 1 summarizes the monthly distributions of conception for all children and for cases, and the unadjusted and multivariate adjusted ORs. Two adjusted models are presented, using the two parameterizations for year of conception. When year of conception was controlled as a continuous variable (model 2), the highest risks of autism occurred in December through March. When year of conception was controlled categorically, the highest risks were in November and December. We used graphical techniques to check the assumption of a linear relationship between the incidence of autism and year of conception (Figure 2); this assumption was adequately met.
Table 1.
Monthly number of conceptions and number of autism cases before 6 years of age associated with those conceptions and the association between month of conception and autism. California births 1990 – 2002.
| Month of conception | No. conceptions | No. autism cases | Unadjusted model OR (95% CI) | Model 1a OR (95% CI) | Model 2b OR (95% CI) |
|---|---|---|---|---|---|
| January | 583,181 | 1775 | 1.13 (1.05 – 1.21) | 1.02 (0.94 – 1.09) | 1.08 (1.00 – 1.16) |
| February | 472,174 | 1507 | 1.19 (1.10 – 1.27) | 1.06 (0.98 – 1.14) | 1.12 (1.04 – 1.20) |
| March | 595,246 | 1944 | 1.22 (1.13 – 1.30) | 1.10 (1.03 – 1.18) | 1.16 (1.08 – 1.24) |
| April | 522,161 | 1467 | 1.04 (0.97 – 1.12) | 1.00 (0.93 – 1.08) | 1.03 (0.96 – 1.11) |
| May | 543,315 | 1453 | 0.99 (0.92 – 1.07) | 1.00 (0.93 – 1.08) | 1.02 (0.95 – 1.09) |
| June | 530,758 | 1440 | 1.01 (0.93 – 1.08) | 1.02 (0.94 – 1.09) | 1.02 (0.95 – 1.09) |
| Julyc | 548,482 | 1473 | 1.00 | 1.00 | 1.00 |
| August | 543,846 | 1572 | 1.08 (1.00 – 1.16) | 1.07 (1.00 – 1.15) | 1.06 (0.99 – 1.14) |
| September | 535,772 | 1518 | 1.05 (0.98 – 1.13) | 1.05 (0.98 – 1.13) | 1.03 (0.96 – 1.11) |
| October | 568,924 | 1609 | 1.05 (0.98 – 1.13) | 1.06 (0.99–1.14) | 1.03 (0.96 – 1.11) |
| November | 563,632 | 1667 | 1.10 (1.02 – 1.18) | 1.11 (1.04 – 1.19) | 1.07 (0.99 – 1.14) |
| December | 597,484 | 1813 | 1.13 (1.05 – 1.212) | 1.14 (1.07 – 1.22) | 1.09 (1.02 – 1.17) |
Model 1 adjusted for maternal education, child ethnicity and year of conception as a categorical variable.
Model 2 adjusted for maternal education, child ethnicity and year of conception as a continuous variable.
Reference category.
Figure 2.
Cumulative incidence of autism to age 5 years by calendar time of conception for children born from 1990 to 2002 in California who survived past their 1st birthday.
In the seasonal analysis, conception in winter was associated with increased risk of autism (OR = 1.06 [95% CI = 1.02 – 1.10]) compared with conception in summer. When seasons were redefined (winter including January – March; spring, April – June; summer, July – September; and fall, October – December) the OR was 1.08 (1.04 – 1.12).
Additional adjustment for child’s sex, preterm status, and maternal age did not alter these results. In addition, restricting the analysis to singleton, full term, or preterm births did not change the pattern of findings (data not shown).
When we reanalyzed data using month of birth rather than month of conception, we found that August, September, October, November, and December births were associated with increased risk of autism. However, after controlling for year of birth, maternal education, and child ethnicity, only November births (OR = 1.12 [95% CI = 1.05 – 1.20]) compared with April births, were associated with increased risk of autism (Table 2).
Table 2.
Association between month of birth and autism before 6 years of age. California births 1990 – 2002.
| Month of birth | Unadjusted model OR (95% CI) | Model 3a OR (95% CI) | Model 4b OR (95% CI) |
|---|---|---|---|
| January | 0.97 (0.90 – 1.04) | 0.98 (0.91 – 1.05) | 1.01 (0.94 – 1.08) |
| February | 0.98 (0.91 – 1.05) | 0.99 (0.92 – 1.06) | 1.01 (0.94 – 1.09) |
| March | 0.97 (0.91 – 1.05) | 0.98 (0.91 – 1.05) | 0.99 (0.92 – 1.06) |
| Aprilc | 1.00 | 1.00 | 1.00 |
| May | 1.03 (0.96 – 1.11) | 1.03 (0.96 – 1.10) | 1.02 (0.95 – 1.09) |
| June | 1.03 (0.96 – 1.11) | 1.03 (0.96 – 1.11) | 1.01 (0.94 – 1.09) |
| July | 1.06 (0.99 – 1.14) | 1.07 (1.00 – 1.15) | 1.04 (0.97 – 1.12) |
| August | 1.07 (1.00 – 1.15) | 1.09 (1.01 – 1.16) | 1.04 (0.97 – 1.12) |
| September | 1.10 (1.02 – 1.17) | 1.11 (1.04 – 1.19) | 1.06 (0.99 – 1.13) |
| October | 1.10 (1.02 – 1.17) | 1.11 (1.04 – 1.20) | 1.04 (0.97 – 1.12) |
| November | 1.18 (1.10 – 1.27) | 1.20 (1.12 – 1.29) | 1.12 (1.04 – 1.20) |
| December | 1.10 (1.03 – 1.18) | 1.12 (1.04 – 1.20) | 1.04 (0.97 – 1.12) |
Model 3 adjusted for maternal education, child ethnicity, and year of birth as a categorical variable.
Model 4 adjusted for maternal education, child ethnicity, and year of birth as a continuous variable.
Reference category.
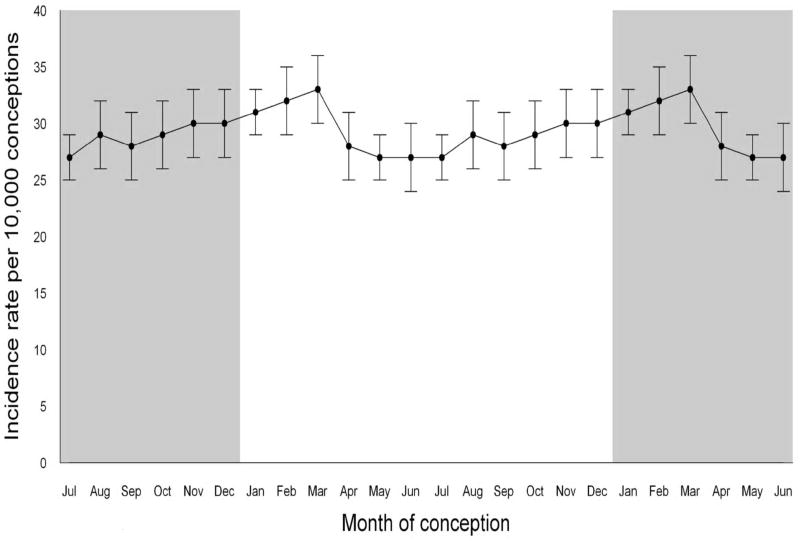
The incidence rate of autism increased steadily beginning with conceptions in August and continuing to March, then decreased from March to April, and stayed flat until July (Figure 3). The rate of autism also rose over time from 9 per 10,000 children conceived in 1989 to 63 per 10,000 children conceived in 2002 (Figure 2), corresponding to results recently published regarding the rise in the incidence of autism in California.21
Figure 3.
Cumulative incidence of autism to age 5 years by month of conception for children born from 1990 to 2002 in California who survived past their 1st birthday. The shaded areas repeat the cycle.
Discussion
In an analysis of a birth cohort of children born in California between January 1990 and December 2002, conception in December, January, February, and March was associated with a modest increased risk of autism, ranging from 8% to 16%, after adjusting for maternal education, child’s year of conception and ethnicity. Conception in March was associated with the highest risk of autism.
Time of conception can provide clues about environmental factors that could be associated with autism. Environmental agents that predominate in California during December – March include virus infections and agricultural applications of certain pesticides.
According to the Centers for Diseases Control and Prevention, February is the month with the highest percentage of influenza cases (nearly 50% of all cases), followed by January (20%), and March and December (about 15% each).24 The California Influenza Surveillance Project data likewise show that influenza activities are at their peak from about December to March.25 These are the specific months in which conception was associated with increased risk of autism in our data.
There has been little epidemiologic research to address a possible association of viral infection with increased risk of autism. In the 1970s, two independent research groups reported an association between prenatal congenital rubella infection and increased risk of autism.2,3 Clinical infection of pregnant women with measles, mumps and chicken pox has also been reported to be associated with increased risk of autism.26 However, the prevalence of those viruses is very low in the US due to effective vaccination programs, and thus unlikely to play a strong role in the etiology of current cases of autism.
Influenza, in contrast, is much more common. One earlier case-control study reported that mothers were more likely to have had influenza or to have been exposed to influenza during their pregnancies with offspring having autism than with their other offspring.26 In contrast, Dassa et al.27 did not find an association between high probability of maternal exposure to influenza during pregnancy and autism. However, the manner in which exposure was characterized in the latter study could have resulted in misclassification, leading to biased results.
Prenatal influenza infection in mice leads to offspring with behavioral deficits that are similar to the deficit in social interaction and restricted interest observed in children with autism. Similar results have been observed in the absence of viral infection by injecting the double stranded RNA poly (I: C) in pregnant dams, which produces an inflammatory response. 28
The heterogeneity of the viruses that have been associated with risk of autism, in addition to the fact that maternal exposure to the double stranded RNA poly (I: C) can produce in animals the same effect as viruses,28 has led researchers to suggest that maternal reaction to infections is a more plausible mechanism of pathology than a direct viral effect on fetuses. 29
Agricultural and other pesticide applications also vary by season. Data from California’s Pesticide Use Report indicate that between 1990 and 2003, about 50% of the total amounts of 13 agricultural organophosphate and organochlorine pesticides were applied in the summer.30 Previous studies have suggested an association between both organophosphate and organochlorine pesticides and neurodevelopmental disorders.5,6,31 In the present analysis, we did not find an association between conception in the summer and risk of autism. However, children conceived in the higher-risk months in our study would have been in their second or third trimester during the summer season. If the excess seasonal risk is related to organophosphate or organochlorine pesticides, this would imply that the sensitive period for a pesticide effect could be in the second or third trimester.
If agricultural pesticides were causes of autism, one might not expect every analysis of seasonality to find the same risk months because agricultural practices (and thus pesticide application) vary by location. Home and garden applications of pesticides also vary by season and deserve consideration.
Other seasonal factors include maternal allergies during pregnancies32 and mild maternal nutritional deficiencies for such factors as vitamin D.33 We know of no epidemiologic studies that have explored the association of such factors with autism.
Our results could be subject to some minor misclassifications of outcome or exposure. We derived months of conception from the date of birth and the length of gestation. The latter was estimated based on the self-reported date of last menstrual period from the birth file. These dates can be subject to recall errors due to uncertainty in the reported dates, bleeding during early pregnancy not associated with menses, menstrual irregularities and variation in the length of the follicular phase of the menstrual cycle. 34 These uncertainties can lead to either differential or nondifferential misclassification of the month of conception. For example, low fertility can be associated with irregularity in menstruation. If low fertility is associated with autism,35 there could be a differential misclassification of exposure (month of conception). In this case, it would be difficult to determine the direction of bias. However, since our findings are clustered in consecutive months, the impact would likely be low.
We relied on diagnoses reported on the Client Developmental Evaluation Report and Early Start Report, which are not systematically confirmed. However, a previous study confirmed that about 98% of cases of autism reported in the Department of Developmental Services database were at least on the autism spectrum, based on either an Autism Diagnostic Interview-Revised or an Autism Diagnostic Observation Schedules-Generic, and 64% were confirmed autistic-disorder cases based on both instruments.36 Thus, we can assume that false-positive cases will be minimal among our cases, if we consider all autism-spectrum disorders as cases.
It is possible that autism or autism spectrum disorders are present among the controls. Not all children with autism in California are registered in the Department of Developmental Services database.21 A previous study reported that about 75–80% of autism cases in California 22 are registered. We do not expect these missed cases to affect our results because they would be a very small fraction of the controls. If we assume that 25% of cases are misclassified as controls, and also assume that this misclassification is independent of month of conception, our ORs and 95% confidence intervals are virtually unchanged (Table 3).
Table 3.
Effect of nondifferential exposure misclassification bias on the estimated ORs for the association between selected months of conception and autism before 6 years of age.
| Month of conception | Observed results | Underlying True Resultsa | Difference between observed OR and true OR (bias) | ||||
|---|---|---|---|---|---|---|---|
| Autism | OR (95% CI) | Autism | OR (95% CI) | ||||
| Yes No. | No No. | Yes No. | No No. | ||||
| January | 1775 | 581,406 | 1.1337 (1.05 – 1.21) | 2367 | 580,814 | 1.1340 (1.06 – 1.20) | −0.0003 |
| February | 1507 | 470,667 | 1.1890 (1.10 – 1.27) | 2009 | 470,165 | 1.1890 (1.11 – 1.26) | −0.0000 |
| March | 1944 | 593,302 | 1.2167 (1.13 – 1.30) | 2592 | 592,654 | 1.2170 (1.14 – 1.29) | −0.0003 |
| Julyb | 1473 | 547,009 | 1.000 | 1964 | 546,518 | 1.000 | |
Assuming 25% of cases are misclassified independently of month of conception.
Reference month.
A recent study in the UK found increased risk of autism associated with conception in summer.16 However, neither age at diagnosis nor the duration or definition of the follow-up period was provided. Moreover, the study had a small number of cases (86 compared with 19,238 cases in our report). Atladotir et al 37 reported no association between season of conception and risk of autism, although, the follow-up time for a chance of a diagnosis of autism differed by birth cohort in their study.
Previous studies on season of birth have reported increased risk of autism in infants born in March or August. We found that November births (corresponding to February conception) had the highest risk after controlling for year of birth, maternal education, and child ethnicity.
Increases in the rate of autism have been reported.21 In order to remove the effect of such increase on the estimated rates, we controlled for time trend.37 Year of conception was a strong confounder of the association between month of conception and risk of autism (and also between month of birth and risk of autism), as evidenced by changes greater than 10% in the magnitude of the beta coefficient for some months or seasons of conception. By adjusting for the time trend on a continuous scale, we minimize residual confounding that can occur when time is controlled on a categorical scale. The relation between the rate of autism and calendar time in our data was approximately linear (Figure 2).
Most previous studies have not controlled for calendar time.9,11–14,18,38 One study controlled for a birth-cohort effect by grouping years of birth into 4-year categories.15 Such analysis could be subject to residual confounding due to wide categories. Kolevzon et al.17 reported controlling their results for year of birth, although they did not state precisely how the variable “year of birth” was parameterized.
We observed modestly higher risks of autism among children born in winter months. Month of conception can represent a surrogate for seasonal causal factors (e.g., potentially, viral infections, pesticides, and so forth). It is possible that by using month of conception, we introduced nondifferential misclassification relative to the actual exposure, for which season is acting as a proxy. Nondifferential misclassification can lead to an underestimate of the true measure of association. Thus, if these findings are reliable, the true underlying causal factor probably has a stronger association with autism than observed here for month of conception.
The importance of small effects has been questioned.39 Odds ratios or relative risks are measures of effect that provide information on the profile of individual cases of diseases but are not informative about the determinants of overall disease incidence in the population. 40 Small changes in the overall population risks can have important effects on population disease burden, particularly when exposures are commonplace.41 Our results suggest the presence of some environmental factors that vary by season are involved in the etiology of autism.
Acknowledgments
Funding: Supported by grants from the National Institute of Environmental Health Sciences: R01-ES015359 and P01-ES11269
References
- 1.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61(8):774–80. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 2.Chess S. Autism in children with congenital rubella. J Autism Child Schizophr. 1971;1(1):33–47. doi: 10.1007/BF01537741. [DOI] [PubMed] [Google Scholar]
- 3.Desmond MM, Montgomery JR, Melnick JL, Cochran GG, Verniaud W. Congenital rubella encephalitis. Effects on growth and early development. Am J Dis Child. 1969;118(1):30–1. [PubMed] [Google Scholar]
- 4.Waldman M, Nicholson S, Adilov N, Williams J. Autism prevalence and precipitation rates in California, Oregon, and Washington counties. Arch Pediatr Adolesc Med. 2008;162(11):1026–34. doi: 10.1001/archpedi.162.11.1026. [DOI] [PubMed] [Google Scholar]
- 5.Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6):e1845–59. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect. 2007;115(10):1482–9. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallagher BJ, 3rd, Jones BJ, McFalls JA, Jr, Pisa AM. Schizophrenic subtype, seasonality of birth and social class: a preliminary analysis. Eur Psychiatry. 2007;22(2):123–8. doi: 10.1016/j.eurpsy.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Torrey EF, Miller J, Rawlings R, Yolken RH. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997;28(1):1–38. doi: 10.1016/s0920-9964(97)00092-3. [DOI] [PubMed] [Google Scholar]
- 9.Bartlik BD. Monthly variation in births of autistic children in North Carolina. J Am Med Womens Assoc. 1981;36(12):363–8. [PubMed] [Google Scholar]
- 10.Barak Y, Ring A, Sulkes J, Gabbay U, Elizur A. Season of birth and autistic disorder in Israel. Am J Psychiatry. 1995;152(5):798–800. doi: 10.1176/ajp.152.5.798. [DOI] [PubMed] [Google Scholar]
- 11.Gillberg C. Do children with autism have March birthdays? Acta Psychiatr Scand. 1990;82(2):152–6. doi: 10.1111/j.1600-0447.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 12.Mouridsen SE, Nielsen S, Rich B, Isager T. Season of birth in infantile autism and other types of childhood psychoses. Child Psychiatry Hum Dev. 1994;25(1):31–43. doi: 10.1007/BF02251098. [DOI] [PubMed] [Google Scholar]
- 13.Konstantareas MM, Hauser P, Lennox C, Homatidis S. Season of birth in infantile autism. Child Psychiatry Hum Dev. 1986;17(1):53–65. doi: 10.1007/BF00707913. [DOI] [PubMed] [Google Scholar]
- 14.Tanoue Y, Oda S, Asano F, Kawashima K. Epidemiology of infantile autism in southern Ibaraki, Japan: differences in prevalence in birth cohorts. J Autism Dev Disord. 1988;18(2):155–66. doi: 10.1007/BF02211943. [DOI] [PubMed] [Google Scholar]
- 15.Lee LC, Newschaffer CJ, Lessler JT, Lee BK, Shah R, Zimmerman AW. Variation in season of birth in singleton and multiple births concordant for autism spectrum disorders. Paediatr Perinat Epidemiol. 2008;22(2):172–9. doi: 10.1111/j.1365-3016.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 16.Hebert KJ, Miller LL, Joinson CJ. Association of autistic spectrum disorder with season of birth and conception in a UK cohort. Autism Research. 2010:3. doi: 10.1002/aur.136. [DOI] [PubMed] [Google Scholar]
- 17.Kolevzon A, Weiser M, Gross R, Lubin G, Knobler HY, Schmeidler J, Silverman JM, Reichenberg A. Effects of season of birth on autism spectrum disorders: fact or fiction? Am J Psychiatry. 2006;163(7):1288–90. doi: 10.1176/ajp.2006.163.7.1288. [DOI] [PubMed] [Google Scholar]
- 18.Landau EC, Cicchetti DV, Klin A, Volkmar FR. Season of birth in autism: a fiction revisited. J Autism Dev Disord. 1999;29(5):385–93. doi: 10.1023/a:1023030911527. [DOI] [PubMed] [Google Scholar]
- 19.Bolton P, Pickles A, Harrington R, Macdonald H, Rutter M. Season of birth: issues, approaches and findings for autism. J Child Psychol Psychiatry. 1992;33(3):509–30. doi: 10.1111/j.1469-7610.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 20.Pearl M, Wier ML, Kharrazi M. Assessing the quality of last menstrual period date on California birth records. Paediatr Perinat Epidemiol. 2007;21 (Suppl 2):50–61. doi: 10.1111/j.1365-3016.2007.00861.x. [DOI] [PubMed] [Google Scholar]
- 21.Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology. 2009;20(1):84–90. doi: 10.1097/EDE.0b013e3181902d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croen LA, Grether JK, Selvin S. Descriptive epidemiology of autism in a California population: who is at risk? J Autism Dev Disord. 2002;32(3):217–24. doi: 10.1023/a:1015405914950. [DOI] [PubMed] [Google Scholar]
- 23.SAS. SAS (Statistical Analysis Software) 9.1.3. Cary, NC: Statistical Analysis Software Institute; 2002. [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) [Accessed February 15, 2010]; http://www.cdc.gov/flu/about/season/flu-season.htm.
- 25.California Influenza Surveillance Project. [Accessed February 15, 2010]; http://www.cdph.ca.gov/data/statistics/Pages/CISPDataArchive.aspx.
- 26.Deykin EY, MacMahon B. Viral exposure and autism. Am J Epidemiol. 1979;109(6):628–38. doi: 10.1093/oxfordjournals.aje.a112726. [DOI] [PubMed] [Google Scholar]
- 27.Dassa D, Takei N, Sham PC, Murray RM. No association between prenatal exposure to influenza and autism. Acta Psychiatr Scand. 1995;92(2):145–9. doi: 10.1111/j.1600-0447.1995.tb09558.x. [DOI] [PubMed] [Google Scholar]
- 28.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23(1):297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.California Department of Pesticide Regulation (PUR) [Accessed January 10, 2010]; http://www.cdpr.ca.gov/docs/pur/purmain.htm.
- 31.Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115(5):792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med. 2005;159(2):151–7. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- 33.Cannell JJ. Autism and vitamin D. Med Hypotheses. 2008;70(4):750–9. doi: 10.1016/j.mehy.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Lynch CD, Zhang J. The research implications of the selection of a gestational age estimation method. Paediatr Perinat Epidemiol. 2007;21 (Suppl 2):86–96. doi: 10.1111/j.1365-3016.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 35.Hvidtjorn D, Schieve L, Schendel D, Jacobsson B, Svaerke C, Thorsen P. Cerebral palsy, autism spectrum disorders, and developmental delay in children born after assisted conception: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2009;163(1):72–83. doi: 10.1001/archpediatrics.2008.507. [DOI] [PubMed] [Google Scholar]
- 36.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114(7):1119–25. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atladottir HO, Parner ET, Schendel D, Dalsgaard S, Thomsen PH, Thorsen P. Variation in incidence of neurodevelopmental disorders with season of birth. Epidemiology. 2007;18(2):240–5. doi: 10.1097/01.ede.0000254064.92806.13. [DOI] [PubMed] [Google Scholar]
- 38.Stevens MC, Fein DH, Waterhouse LH. Season of birth effects in autism. J Clin Exp Neuropsychol. 2000;22(3):399–407. doi: 10.1076/1380-3395(200006)22:3;1-V;FT399. [DOI] [PubMed] [Google Scholar]
- 39.Schoen EJ. Childhood Lead Poisoning: Definitions and Priorities. Pediatrics. 1993;91(2):504–505. [PubMed] [Google Scholar]
- 40.Bellinger DC. Interpretation of small effect sizes in occupational and environmental neurotoxicology: individual versus population risk. Neurotoxicology. 2007;28(2):245–51. doi: 10.1016/j.neuro.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Rockhill B, Kawachi I, Colditz GA. Individual risk prediction and population-wide disease prevention. Epidemiol Rev. 2000;22(1):176–80. doi: 10.1093/oxfordjournals.epirev.a018017. [DOI] [PubMed] [Google Scholar]