Abstract
Objectives
Meniscal tears have been linked to knee osteoarthritis progression, presumably via impaired load attenuation. How meniscal tears affect OA is unclear; subregional examination may help to elucidate whether the impact is local. We examined the association between a tear within a specific meniscal segment and subsequent 2-year cartilage loss in subregions that the torn segment overlies.
Methods
Participants with knee OA underwent bilateral knee MRI at baseline and two years. Mean cartilage thickness within each subregion was quantified. We used logistic regression with GEE to analyze the relationship between baseline meniscal tear in each segment and baseline-to-2-year cartilage loss in each subregion, adjusting for age, gender, BMI, tear in the other two segments, and extrusion.
Results
We studied 261 knees in 159 persons. Medial meniscal body tear was associated with cartilage loss in external subregions and in central and anterior tibial subregions, and posterior horn tear specifically with posterior tibial subregion loss; these relationships were independent of tears in the other segments and persisted in tibial subregions after adjustment for extrusion. Lateral meniscal body and posterior horn tear were also associated with cartilage loss in underlying subregions but not after adjustment for extrusion. Cartilage loss in the internal subregions, not covered by the menisci, was not associated with meniscal tear in any segment.
Conclusion
These results suggest that the detrimental effect of meniscal tears is not spatially uniform across the tibial and femoral cartilage surfaces and that some of the effect is experienced locally.
INTRODUCTION
The primary functions of the meniscus are load distribution and shock absorption (1–4). The meniscus provides stability by improving surface congruency and may aid in proprioception and lubrication (5–8). Meniscal damage or resection impairs these functions and subjects articular cartilage to focal axial and aberrant shear stresses; there is evidence of focal stress increase even after partial meniscectomy (9, 10). Detrimental effects of meniscal lesions on cartilage may not be uniform across the joint surfaces, especially since some of the surface is not covered by the semicircular menisci.
In theory, the tibiofemoral cartilage subregions that the meniscus overlies may be exposed to greater load when that meniscal segment is damaged. It is not clear if meniscal lesions have a local impact, i.e., specifically, whether a lesion within a meniscal segment (anterior horn, body, or posterior horn) is associated with greater loss in the underlying cartilage subregion. Meniscal extrusion, a condition in which partial or full meniscal displacement uncovers the cartilage, represents another meniscal pathology that may coexist with meniscal tears in knee osteoarthritis (OA) (11–13). It is important to examine if any specific meniscal tear effect on subregional cartilage loss is explained by concomitant meniscal extrusion.
Previous studies have demonstrated a relationship between meniscal lesions and development (14) and progression (15–18) of knee OA. Early reports describe some effort to explore the meniscal lesion/cartilage loss relationship at a subregional level (19–22). A meniscal lesion effect at the articular cartilage subregion(s) that the lesion overlies will add support to a local, protective role of the meniscus in cartilage integrity and to continued effort on strategies to preserve meniscal tissue in osteoarthritic knees.
The purpose of this study was to examine the role of meniscal tears at baseline in subsequent cartilage loss in tibial and femoral subregions in persons with knee OA. We tested two hypotheses: 1) A tear in each medial meniscal segment at baseline is associated with articular cartilage thickness loss in the subsequent two years within the same medial subregions as the torn segment. 2) A tear in each lateral meniscal segment at baseline is associated with cartilage loss in the subsequent two years within the same lateral subregions as the torn segment.
METHODS
Sample
Study participants are from a knee OA natural history study, the MAK-2 Study (Mechanical Factors in Arthritis of the Knee-Study 2) and were community-recruited through advertising, neighborhood organizations, the Buehler Center on Aging, Health and Society registry at Northwestern University, and medical center referrals.
Inclusion criteria were: definite tibiofemoral osteophytes [Kellgren/Lawrence (K/L) (23) ≥ 2] in one or both knees; and ≥ “a little difficulty” for ≥ 2 items in the WOMAC physical function scale (24). Exclusion criteria were: corticosteroid injection within three months; avascular necrosis, rheumatoid or other inflammatory arthritis, periarticular fracture, Paget’s disease, villonodular synovitis, joint infection, ochronosis, neuropathic arthropathy, acromegaly, hemachromatosis, gout, pseudogout, osteopetrosis, meniscectomy; or exclusion criteria for magnetic resonance imaging (MRI), e.g. pacemaker, artificial heart valve, aneurysm clip or shunt, metallic stent, implanted device, or orbital metallic fragment.
Approval was obtained from the Office for the Protection of Research Subjects of Northwestern University and Evanston Northwestern Healthcare; all participants gave written consent.
MRI Acquisition
All participants had bilateral knee MRIs at baseline and two years later using a commercial knee coil and 1.5T or 3.0T (only 15 participants) whole-body scanners (GE Healthcare, Waukesha, WI); previous work revealed no systematic offset and very high measurement correlation between these field strengths (25). Each knee was scanned and re-scanned on the same machine with the same protocol at the two time points.
Quantitative tibial and femoral cartilage thickness measurement was obtained from double-oblique coronal T1-weighted 3-dimensional spoiled gradient-echo images with water excitation. Meniscal parameter grading utilized coronal T1-weighted spin-echo and sagittal fat-suppressed dual-echo turbo spin-echo sequences.
The 1.5T parameters were:
| Sequence | TR(ms)/TE (ms)/FA(°) | FOV(cm) | Matrix size | Slice Thk/gap(mm) | Acquisition time(min) |
|---|---|---|---|---|---|
| Cor T1 SE | 574/11/90 | 12 | 256×256 | 3.0/3.0 | 4:54 |
|
| |||||
| Sag dual echo FSE | 3800/19,65/90 | 14 | 256×256 | 3.0/3.0 | 7:06 |
|
| |||||
| Axial+Cor T1 3D SPGR WE |
17.2/7.85/10 | 16 | 512×512 | 1.5/0.0 | 8:51 |
The 3.0T parameters were:
| Sequence | TR(ms)/TE (ms)/FA(°) | FOV(cm) | Matrix size | Slice Thk/gap(mm) | Acquisition time(min) |
|---|---|---|---|---|---|
| Cor T1 SE | 800/11/90 | 12 | 288×224 | 3.0/0.5 | 6:08 |
|
| |||||
| Sag dual echo FSE | 3000/16,65/90 | 14 | 224×224 | 3.0/1.0 | 5:42 |
|
| |||||
| Axial+Cor T1 3D SPGR WE |
18.2/5.7/15 | 16 | 512×512 | 1.5/0.0 | 9:00 |
Assessment of Meniscal Tears and Extrusion
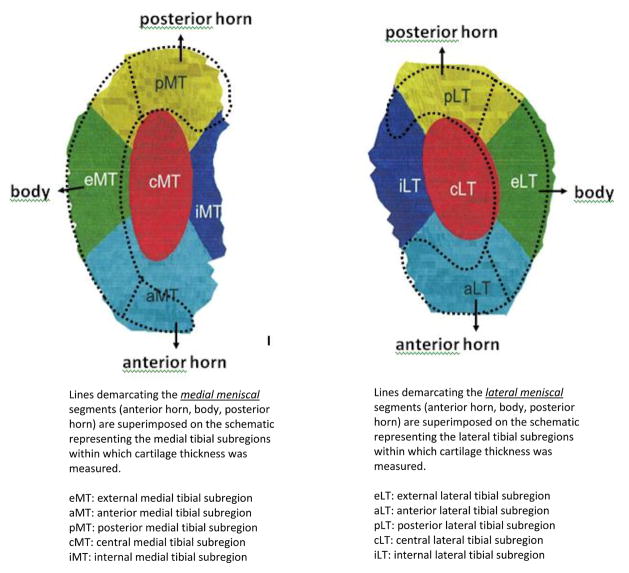
Each segment (anterior horn, body, posterior horn) of each meniscus was graded separately for tears, using the WORMS system (26), (0 = intact, 1 = minor radial or parrot-beak tear, 2 = non-displaced tear, 3 = displaced tear or partial maceration, and 4 = complete maceration and destruction), with tear defined as grade ≥2 to maximize test specificity. Figure 1 shows the meniscal segments superimposed over tibial subregions of cartilage thickness measurement. Extrusion of each meniscus was graded 0–2 (0 = none, 1 = less than half of the meniscus, and 2 = more than half of the meniscus) using coronal images at the collateral ligament level, with extrusion defined as grade > 0. One of three experienced musculoskeletal radiologist readers (including AG) graded meniscal parameters. Their inter-reader reliability (weighted kappa) was 0.80 for tears and 0.65 for extrusion. The readers were blinded to all other data.
Figure 1.
Quantification of Subregional Cartilage Thickness Loss on MR Images
Segmentation involved manual tracing of the total subchondral bone area (tAB) and cartilage surface area (AC) of the medial and lateral tibia and weightbearing femoral condyles on paired (baseline and follow-up) images by ten operators with standardized training and expertise in knee cartilage segmentation, using dedicated software (Chondrometrics GmbH, Ainring, Germany) (27). Quality control of all segmentations was performed by one expert (FE). The operators and quality evaluator were blinded to image acquisition order and all other data. Cartilage thickness was computed over the tAB and in five tibial (central, internal, external, anterior, posterior) and three weightbearing femoral (central, internal, external) subregions (27). The central (elliptical) subregion occupied 20% of the tAB around its center of gravity; test-retest precision error for cartilage thickness measurement was 2.4% (RMS CV%) and 1.6% for the medial and lateral central tibial subregions (27). Planes running through the tAB center at a 45° angle with the plane connecting the center of gravity of the medial and lateral tibia were used to define tibial anterior, posterior, internal, and external subregions. Precision error ranged from 1.5% in the external medial tibial subregion to 4.7% in the posterior lateral tibial subregion (27). Each weightbearing femoral condylar subregion occupied 33.3% of the tAB. Precision error was 3.3% and 2.4% in the central medial and lateral femoral subregions, and ranged from 2.6% in the internal medial femoral subregion to 4.3% in the external lateral femoral subregion (27). For each subregion, cartilage thickness loss was defined as ≥ 5% decrease in cartilage thickness over two years, a threshold exceeding the precision errors.
Knee Radiographs
Bilateral, anteroposterior, weightbearing knee radiographs were acquired in all participants at baseline in the semi-flexed position with fluoroscopic confirmation of tibial plateau line superimposition and tibial spine centering (28), and read for K/L grade (0 = normal; 1 = possible osteophytes; 2 = definite osteophytes without joint space narrowing; 3 = definite joint space narrowing, some sclerosis, possible attrition; and 4 = large osteophytes, marked narrowing, severe sclerosis, definite attrition). Intra-observer reliability for the single x-ray reader was high (Kappa coefficient 0.86).
Statistical Analysis
All analyses were knee based. We used logistic regression with generalized estimating equations (GEE) to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between baseline meniscal tear in the anterior horn, body, and posterior horn and subsequent within-subregion cartilage thickness loss from baseline to two years. Cartilage thickness loss in each subregion was defined as a decrease ≥ 5% in thickness at the two year measurement compared to baseline thickness in the subregion. Analyses were adjusted for age, gender, body mass index (BMI), and meniscal tear in the other two meniscal segments. Presence of a tear in a meniscal segment was coded as a 0/1 (no tear/tear) indicator variable separately for each segment; all three indicators (one per segment) were included concurrently in each of the logistic models. We first present results adjusting for age, gender, BMI and tears in the other meniscal segments. Additional logistic regression models were used to further adjust for meniscal extrusion to examine whether extrusion explained a subregional tear association with cartilage loss. Since meniscal tear and extrusion contribute to the joint space component of the K/L grade, we did not further adjust for K/L grade. Results from logistic regression models are reported as ORs and associated 95% CIs. Statistical significance is defined at the 5% level, which corresponds to a 95% CI that excludes the value 1.0.
RESULTS
Of 202 participants with knee OA in one or both knees who completed the baseline evaluation, 20 did not return at two years due to the following reasons: deceased, bilateral total knee replacement, moved away, or new MRI contraindications. Among the 302 knees of 182 participants, 14 knees were excluded due to missing cartilage data at baseline or at follow-up, 11 for having baseline cartilage thickness of 0 in a subregion, 16 for missing meniscal data, leaving 261 knees from 159 persons as the analysis sample. The 159 participants included 75% women, had a mean age of 66.1 years (± 11.1, SD) and a mean BMI of 30.1 kg/m2 (± 5.9). Persons without longitudinal data did not differ in age (66.6 ± 11.5) or gender (77% women) but had a higher BMI (31.9 ± 6.2). Among the 261 knees, K/L grade was 0 in 39 (15%), 1 in 49 (19%), 2 in 100 (38%), 3 in 54 (21%), and 4 in 19 knees (7%). Ninety-two knees (35%) had a medial meniscal tear, 91 (35%) medial meniscal extrusion, 64 (25%) a lateral meniscal tear, 48 (18%) lateral meniscal extrusion, 60 (23%) medial meniscal tear and extrusion, and 33 (13%) lateral meniscal tear and extrusion. Within the medial meniscus, 13 knees (5%) had a tear in the anterior horn, 62 (24%) in the body, and 77 (30%) in the posterior horn. Within the lateral meniscus, 50 (19%) had a tear in the anterior horn, 50 (19%) in the body, and 37 (14%) in the posterior horn. As in Table 1, medial meniscal tears were most common in the posterior horn and body concurrently and lateral tears in all three segments concurrently.
Table 1. Number (%) of knees with meniscal tear in specific meniscal segment(s) (n = 261 knees).
The table shows the number (%) of knees, of the total of 261 knees, with evidence of meniscal tear in the given segment or combinations of segments of the medial and lateral menisci.
| Anterior + body + posterior | Anterior + body | Anterior + posterior | Body + posterior | Anterior only | Body only | Posterior only | No tear in any of the three segments | |
|---|---|---|---|---|---|---|---|---|
| Medial meniscal tear | 8 (3%) | 4 (2%) | 0 (0%) | 40 (15%) | 1 (0.4%) | 10 (4%) | 29 (11%) | 169 (65%) |
| Lateral meniscal tear | 26 (10%) | 13 (5%) | 0 (0%) | 8 (3%) | 11 (4%) | 3 (1%) | 3 (1%) | 197 (75%) |
Medial Meniscal Segment Tears and Medial Subregional Cartilage Thickness Loss
At the medial tibia, in the 261 knees, 86 (33%) lost cartilage in the central, 95 (36%) in the external, 53 (20%) in the internal, 85 (33%) in the anterior, and 72 (28%) in the posterior subregion. At the medial weightbearing femur, 94 knees (36%) lost cartilage in the central, 86 (33%) in the external, and 80 (31%) in the internal subregion.
The independent effects of a tear within each medial meniscal segment on subsequent subregional cartilage thickness loss, adjusted for age, gender, BMI, and tears in the other meniscal segments are shown in Tables 2A and 2B. There was no association between baseline anterior horn tear and cartilage loss in any subregion. A body tear at baseline was significantly associated with cartilage loss in the central, external, and anterior subregions of the medial tibia and in the external subregion of the medial weightbearing femur. A posterior horn tear at baseline was significantly associated with cartilage loss in the medial tibial posterior subregion. Further adjustment for meniscal extrusion affected only the external femoral result (Tables 2A and 2B).
Table 2A. Medial Tibial Subregions: Adjusted Odds Ratios (95% CI) for Baseline-to-2-year Cartilage Thickness Loss.
The tables show the odds ratios and associated 95% confidence intervals (CIs) for baseline-to-2-year cartilage thickness loss in the medial tibial (Table 2A) and medial weightbearing femoral (Table 2B) subregions, adjusted for age, gender, BMI, and tears in the other meniscal segments (covariate set A) and then adjusted for these factors and for meniscal extrusion (covariate set B).
| Medial meniscal tears at baseline, present vs. absent (independent variable) | Central subregion | External subregion | Internal subregion | Anterior subregion | Posterior subregion | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| adjusted for covariate set A | adjusted for covariate set B | adjusted for covariate set A | adjusted for covariate set B | adjusted for covariate set A | adjusted for covariate set B | adjusted for covariate set A | adjusted for covariate set B | adjusted for covariate set A | adjusted for covariate set B | |
| Anterior horn tear | 0.76 (0.23, 2.47) | 0.73 (0.22, 2.43) | 1.39 (0.45, 4.31) | 1.53 (0.49, 4.74) | 1.07 (0.25, 4.54) | 1.03 (0.24, 4.38) | 0.86 (0.25, 3.00) | 0.82 (0.23, 2.90) | 2.99 (0.83, 10.74) | 3.08 (0.84, 11.25) |
| Body tear | 4.18 (1.80, 9.72)c | 3.80 (1.47, 9.78)b | 5.39 (2.44, 11.88)d | 8.04 (2.99, 21.66)d | 1.19 (0.50, 2.82) | 1.07 (0.40, 2.90) | 3.22 (1.49, 6.96)b | 2.76 (1.23, 6.21)a | 0.95 (0.42, 2.11) | 1.04 (0.44, 2.48) |
| Posterior horn tear | 1.02 (0.50, 2.08) | 1.01 (0.49, 2.05) | 0.97 (0.44, 2.13) | 0.99 (0.46, 2.17) | 1.11 (0.47, 2.59) | 1.10 (0.47, 2.55) | 0.57 (0.26, 1.27) | 0.57 (0.25, 1.26) | 2.63 (1.23, 5.62)a | 2.65 (1.23, 5.71)a |
p < .05
p < .01
p < .001
p < .0001
Table 2B. Medial Weightbearing Femoral Subregions: Adjusted Odds Ratios (95% CI) for Baseline-to-2-Year Cartilage Thickness Loss.
The tables show the odds ratios and associated 95% confidence intervals (CIs) for baseline-to-2-year cartilage thickness loss in the medial tibial (Table 2A) and medial weightbearing femoral (Table 2B) subregions, adjusted for age, gender, BMI, and tears in the other meniscal segments (covariate set A) and then adjusted for these factors and for meniscal extrusion (covariate set B).
| Medial meniscal tears at baseline, present vs. absent (independent variable) | Central subregion | External subregion | Internal subregion | |||
|---|---|---|---|---|---|---|
| adjusted for covariate set A | adjusted for covariate set B | adjusted for covariate set A | adjusted for covariate set B | adjusted for covariate set A | adjusted for covariate set B | |
| Anterior horn tear | 0.48 (0.14, 1.73) | 0.47 (0.13, 1.69) | 1.34 (0.39, 4.57) | 1.21 (0.35, 4.15) | 1.55 (0.45, 5.30) | 1.49 (0.44, 5.09) |
| Body tear | 1.79 (0.89, 3.61) | 1.58 (0.71, 3.51) | 2.61 (1.20, 5.66)a | 1.87 (0.77, 4.53) | 1.10 (0.46, 2.62) | 0.95 (0.37, 2.48) |
| Posterior horn tear | 1.58 (0.82, 3.04) | 1.56 (0.82, 2.99) | 1.30 (0.63, 2.69) | 1.26 (0.62, 2.57) | 0.54 (0.26, 1.16) | 0.54 (0.25, 1.14) |
p < .05
p < .01
p < .001
p < .0001
Lateral Meniscal Segment Tears and Lateral Subregional Cartilage Thickness Loss
At the lateral tibia, in the 261 knees, 106 (41%) lost cartilage in the central, 69 (26%) in the external, 105 (40%) in the internal, 86 (33%) in the anterior, and 85 (33%) in the posterior subregion. At the medial weightbearing femur, 84 knees (32%) lost cartilage thickness in the central, 87 (33%) in the external, and 72 (28%) in the internal subregion.
Tables 3A and 3B show the independent effects of a tear within each lateral meniscal segment on subsequent subregional cartilage thickness loss after adjustment for age, gender, BMI, and tears in the other meniscal segments. There was no association between baseline anterior horn tear and cartilage loss in any subregion. A body tear at baseline was significantly associated with cartilage loss in the central, external, anterior, and posterior subregions of the lateral tibia. In addition, a posterior horn tear at baseline was significantly associated with cartilage loss in the lateral tibial external subregion. After further adjustment for meniscal extrusion, there was no evidence of an independent association between body or posterior horn tear and cartilage loss in any subregion (Tables 3A and 3B). All analyses were repeated using another definition of meniscal tear, grade ≥ 1, with minimal impact on results.
Table 3A. Lateral Tibial Subregions: Adjusted Odds Ratio (95% CI) for Baseline-to-2-Year Cartilage Thickness Loss.
The tables show the odds ratios and associated 95% confidence intervals (CIs) for baseline-to-2-year cartilage thickness loss in the lateral tibial (Table 3A) and lateral weightbearing femoral (Table 3B) subregions, adjusted for age, gender, BMI, and tears in the other meniscal segments (covariate set A) and then adjusted for these factors and for meniscal extrusion (covariate set B).
| Lateral meniscal tears at baseline, present vs. absent (independent variable) | Central subregion | External subregion | Internal subregion | Anterior subregion | Posterior subregion | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| adjusted for covariate set A | adjusted for covariate set B | adjusted for covariate set A | adjusted for covariate set B | adjusted for covariate set A | adjusted for covariate set B | adjusted for covariate set A | adjusted for covariate set B | adjusted for covariate set A | adjusted for covariate set B | |
| Anterior horn tear | 1.07 (0.38, 3.02) | 0.94 (0.22, 3.94) | 0.91 (0.27, 3.09) | 0.47 (0.08, 2.67) | 0.99 (0.32, 3.07) | 1.65 (0.47, 5.76) | 0.93 (0.35, 2.48) | 1.63 (0.42, 6.29) | 0.77 (0.27, 2.20) | 1.95 (0.56, 6.80) |
| Body tear | 3.81 (1.12, 13.00)a | 0.88 (0.39, 1.98) | 3.99 (1.01, 15.85)a | 0.84 (0.33, 2.16) | 1.93 (0.54, 6.93) | 1.23 (0.60, 2.50) | 3.24 (1.02, 10.35)a | 1.94 (0.88, 4.29) | 6.47 (1.74, 24.05)b | 0.70 (0.28, 1.75) |
| Posterior horn tear | 2.58 (0.80, 8.32) | 1.11 (0.53, 2.31) | 3.94 (1.26, 12.38)a | 1.05 (0.48, 2.32) | 2.77 (0.93, 8.26) | 0.99 (0.51, 1.95) | 0.66 (0.20, 2.21) | 0.65 (0.33, 1.28) | 0.63 (0.18, 2.17) | 0.76 (0.34, 1.69) |
p < .05
p < .01
Table 3B. Lateral Weightbearing Femoral Subregions: Adjusted Odds Ratio (95% CI) for Baseline-to-2-Year Cartilage Thickness Loss.
The tables show the odds ratios and associated 95% confidence intervals (CIs) for baseline-to-2-year cartilage thickness loss in the lateral tibial (Table 3A) and lateral weightbearing femoral (Table 3B) subregions, adjusted for age, gender, BMI, and tears in the other meniscal segments (covariate set A) and then adjusted for these factors and for meniscal extrusion (covariate set B).
| Lateral meniscal tears at baseline, present vs. absent (independent variable) | Central subregion | External subregion | Internal subregion | |||
|---|---|---|---|---|---|---|
| adjusted for covariate set A | adjusted for covariate set B | adjusted for covariate set A | adjusted for covariate set B | adjusted for covariate set A | adjusted for covariate set B | |
| Anterior horn tear | 0.95 (0.33, 2.73) | 1.77 (0.51, 6.17) | 1.26 (0.42, 3.78) | 1.14 (0.25, 5.28) | 1.30 (0.46, 3.70) | 0.57 (0.13, 2.56) |
| Body tear | 2.26 (0.55, 9.30) | 0.40 (0.14, 1.13) | 2.46 (0.66, 9.14) | 0.48 (0.18, 1.28) | 0.93 (0.26, 3.41) | 1.20 (0.50, 2.89) |
| Posterior horn tear | 1.90 (0.51, 7.13) | 1.08 (0.51, 2.31) | 2.24 (0.66, 7.68) | 0.47 (0.21, 1.05) | 3.10 (0.93, 10.30) | 1.16 (0.53, 2.54) |
p < .05
p < .01
DISCUSSION
The deleterious effect of a medial meniscal tear on cartilage thickness loss was not uniform across the medial compartment. Meniscal body tear was associated with cartilage loss in external tibial and femoral subregions and in adjacent central and anterior tibial subregions. Meniscal posterior horn tear was associated specifically with cartilage loss in the posterior tibial and no other subregion. These relationships were independent of age, gender, BMI, and tears in the other two meniscal segments and persisted in the tibial subregions after further adjustment for meniscal extrusion. In the lateral compartment, meniscal body tear was associated with cartilage loss in the external and adjacent central, anterior, and posterior tibial subregions and meniscal posterior horn tear with cartilage loss in the external tibial subregion. These lateral compartment relationships were attenuated by further adjustment for meniscal extrusion. Cartilage loss in the internal subregions, which are not covered by the meniscus, was not associated with any meniscal segment tear. These findings demonstrate the significance of meniscal damage for subsequent subregional cartilage thickness loss and suggest that at least some of the meniscal tear effect is experienced locally.
Our findings are in keeping with previous reports in which meniscal damage was associated with knee OA progression at the larger joint surface (15–18) and some early subregional findings. Lynch et al found that, in knees with or at higher risk of developing knee OA, medial cartilage score worsening associated with medial meniscal tear appeared to be more frequent in the central subregions (21). In the BOKS study, Niu et al used an M:N matched case-control design including knees with 6 tibial subregions eligible for cartilage score worsening and with worsening in one subregion at follow-up (20). In the 37 knees examined, compared to tibial subregions without meniscal damage in identical locations, the OR of cartilage worsening in sites with such damage was significantly increased.
In contrast, we examined quantitative cartilage thickness loss within tibial and femoral subregions and were thereby not vulnerable to potential bias associated with grading menisci and cartilage subregions together in the same session. Crema et al also measured cartilage thickness, but studied women with or without knee OA (22). As their goals and analytic methods differed from ours, it is difficult to derive answers to the questions we posed directly from their report. In contrast to all of these previous studies, we adjusted not only for extrusion, but addressed potential confounding from tears in the other two meniscal segments, a key step in the examination of a local effect.
In our study, isolated meniscal tear occurred most frequently in the medial meniscal posterior horn, in agreement with previous reports (29–31). The reduced mobility from soft tissue attachments and greatest load transmitted through this region during knee flexion makes this meniscal segment particularly vulnerable (30–32). The most common medial pattern, however, was concurrent body and posterior horn tear. The most common lateral meniscal tear pattern (involving all three segments) may relate to its greater mobility and more even between-segment sharing of load (31,32).
The finding that baseline medial meniscal body tear had the strongest association with subsequent cartilage loss in the external tibial subregion where the body segment overlies supports some local impact of meniscal damage on cartilage health. Baseline posterior horn tear had an isolated effect in the posterior tibial subregion. The small number of knees with anterior horn tear may have limited our ability to detect relationships involving tears in this segment. The relationship between body tear and anterior tibial cartilage loss may relate to the proximity of anterior and external subregions (Figure 1).
The effects of baseline lateral meniscal body tear were less confined to the anatomical mapping proposed in our hypotheses. During knee flexion, the lateral meniscus experiences nearly twice the magnitude of antero-posterior translation as the medial meniscus (32). With this movement, the lateral meniscal body segment may overlie the anterior, external, central, and posterior tibial cartilage subregions and the posterior horn may overlie the posterior and external tibial subregions. The associations between lateral meniscal tear and cartilage loss were attenuated by adjustment for meniscal extrusion. In theory, extrusion of the more mobile lateral meniscus could itself contribute to a more diffuse tear pattern, and less matching between tear site and subregion of cartilage loss.
It is important to acknowledge that some knees without longitudinal data came from persons whose BMI was greater than the persons we analyzed; it is uncertain how this may have affected results. While the WORMS system is commonly applied to assess meniscal tears in knee OA, it does not include tear type or location in relation to vascular supply. Further, efforts to match meniscal segment to cartilage subregion are inherently imperfect: optimal meniscal grading and cartilage segmentation require different sets of MR images; knees vary in meniscal and articular cartilage shape; and the match may be altered by meniscal movement and/or extrusion during activity.
In summary, the detrimental effect of meniscal tears is not spatially uniform across the tibial and femoral cartilage surfaces, and at least some of it is experienced locally. These results support a focal protective role of meniscal tissue on articular cartilage integrity in knee OA.
Acknowledgments
Support: NIH/NIAMS R01 AR48216, R01 AR48748, P60 AR48098
Footnotes
Neither the corresponding author nor the co-authors have any competing interests with regard to the manuscript.
The corresponding author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in ARD and any other BMJPGL products and sublicenses such use and exploit all subsidiary rights, as set out in the license (http://ARD.bmjjournals.com/ifora/licence.pdf).
References
- 1.Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980;149:283–90. [PubMed] [Google Scholar]
- 2.Fukubayashi T, Kurosawa H. The contact area and pressure distribution pattern of the knee. A study of normal and osteoarthrotic knee joints. Acta Orthop Scand. 1980;51:871–9. doi: 10.3109/17453678008990887. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed AM, Burke DL. In-vitro measurement of static pressure distribution in synovial joints--Part I: Tibial surface of the knee. J Biomech Eng. 1983;105:216–25. doi: 10.1115/1.3138409. [DOI] [PubMed] [Google Scholar]
- 4.Paletta GA, Manning T, Snell E, et al. The effect of allograft meniscal replacement on intraarticular contact area and pressures in the human knee. A biomechanical study. Am J Sports Med. 1997;25:692–8. doi: 10.1177/036354659702500519. [DOI] [PubMed] [Google Scholar]
- 5.Shoemaker SC, Markolf KL. The role of the meniscus in the anterior-posterior stability of the loaded anterior cruciate-deficient knee. Effects of partial versus total excision. J Bone Joint Surg Am. 1986;68:71–9. [PubMed] [Google Scholar]
- 6.Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64:883–8. [PubMed] [Google Scholar]
- 7.Jerosch J, Prymka M, Castro WH. Proprioception of knee joints with a lesion of the medial meniscus. Acta Orthop Belg. 1996;62:41–5. [PubMed] [Google Scholar]
- 8.Messner K, Gao J. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J Anat. 1998;193:161–78. doi: 10.1046/j.1469-7580.1998.19320161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vadher SP, Nayeb-Hashemi H, Canavan PK, Warner GM. Finite element modeling following partial meniscectomy: effect of various size of resection. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2098–101. doi: 10.1109/IEMBS.2006.259378. [DOI] [PubMed] [Google Scholar]
- 10.Zielinska B, Donahue TLH. 3D finite element model of meniscectomy: changes in joint contact behavior. J Biomech Eng. 2006;128:115–23. doi: 10.1115/1.2132370. [DOI] [PubMed] [Google Scholar]
- 11.Gale DR, Chaisson CE, Totterman SM, et al. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage. 1999;7:526–32. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- 12.Adams JG, McAlindon T, Dimasi M, et al. Contribution of meniscal extrusion and cartilage loss to joint space narrowing in osteoarthritis. Clin Radiol. 1999;54:502–6. doi: 10.1016/s0009-9260(99)90846-2. [DOI] [PubMed] [Google Scholar]
- 13.Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: is extent associated with severity of degeneration or type of tear? Am J Roentgenol. 2004;183:17–23. doi: 10.2214/ajr.183.1.1830017. [DOI] [PubMed] [Google Scholar]
- 14.Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–9. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswal S, Hastie T, Andriacchi TP, et al. Risk factors for progressive cartilage loss in the knee: a longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheum. 2002;46:2884–92. doi: 10.1002/art.10573. [DOI] [PubMed] [Google Scholar]
- 16.Berthiaume M, Raynauld J, Martel-Pelletier J, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556–63. doi: 10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter DJ, Zhang YQ, Niu JB, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 18.Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58:1716–26. doi: 10.1002/art.23462. [DOI] [PubMed] [Google Scholar]
- 19.Lynch JA, Guermazi A, Roemer FW, et al. Baseline meniscal lesions are associated with frequency and anatomical site of cartilage loss on the femoral condyles over 15 months (abstract) Arthritis Rheum. 2006;54(9, supplement):S137. [Google Scholar]
- 20.Niu J, Hunter D, Felson D, et al. Evaluating the proximity of meniscal damage on risk of local cartilage loss using a matched case-control design (abstract) Arthritis Rheum. 2006;54(9, supplement):S158. [Google Scholar]
- 21.Lynch JA, Javaid MK, Roemer F, et al. Associations of medial meniscal tear and extrusion with the sites of cartilage loss in the knee – results from the MOST study (abstract) Arthritis Rheum. 2008;58(9, supplement):S235. [Google Scholar]
- 22.Crema MD, Guermazi A, Li L, et al. The association of prevalent medial meniscal pathology with cartilage loss in the medial tibiofemoral compartment over a 2-year period. Osteoarthritis Cartilage. 2010;18:336–43. doi: 10.1016/j.joca.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 25.Eckstein F, Charles HC, Buck RJ, et al. Accuracy and precision of quantitative assessment of cartilage morphology by magnetic resonance imaging at 3. 0T. Arthritis Rheum. 2005;52:3132–6. doi: 10.1002/art.21348. [DOI] [PubMed] [Google Scholar]
- 26.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27:737–44. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 28.Buckland-Wright C. Protocols for precise radio-anatomical positioning of the tibiofemoral and patellofemoral compartments of the knee. Osteoarthritis Cartilage. 1995;3(Suppl A):71–80. [PubMed] [Google Scholar]
- 29.Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones AO, Houang MTW, Low RS, et al. Medial meniscus posterior root attachment injury and degeneration: MRI findings. Australas Radiol. 2006;50:306–13. doi: 10.1111/j.1440-1673.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 31.Vedi V, Williams A, Tennant SJ, et al. Meniscal movement. An in-vivo study using dynamic MRI. J Bone Joint Surg Br. 1999;81:37–41. doi: 10.1302/0301-620x.81b1.8928. [DOI] [PubMed] [Google Scholar]
- 32.Thompson WO, Thaete FL, Fu FH, et al. Tibial meniscal dynamics using three-dimensional reconstruction of magnetic resonance images. Am J Sports Med. 1991;19:210–5. doi: 10.1177/036354659101900302. [DOI] [PubMed] [Google Scholar]