Abstract
Study Objectives:
To compare the components of the extracellular matrix in the lateral pharyngeal muscular wall in patients with and without obstructive sleep apnea (OSA). This may help to explain the origin of the increased collapsibility of the pharynx in patients with OSA.
Design:
Specimens from the superior pharyngeal constrictor muscle, obtained during pharyngeal surgeries, were evaluated using histochemical and immunohistochemical analyses to determine the fractional area of collagen types I and III, elastic fibers, versican, fibronectin, and matrix metalloproteinases 1 and 2 in the endomysium.
Setting:
Academic tertiary center.
Patiens:
A total of 51 nonobese adult patients, divided into 38 patients with OSA and 13 nonsnoring control subjects without OSA.
Interventions:
Postintervention study performed on tissues from patients after elective surgery.
Measurements and Results:
Pharyngeal muscles of patients with OSA had significantly more collagen type I than pharyngeal muscles in control subjects. Collagen type I was correlated positively and independently with age. The other tested components of the extracellular matrix did not differ significantly between groups. In a logistic regression, an additive effect of both the increase of collagen type I and the increase in age with the presence of OSA was observed (odds ratio (OR), 2.06; 95% confidence interval (CI), 1.17-3.63), when compared with the effect of increased age alone (OR, 1.11; 95% CI, 1.03-1.20).
Conclusion:
Collagen type I in the superior pharyngeal constrictor muscle was more prevalent in patients with OSA and also increased with age. It was hypothesized that this increase could delay contractile-relaxant responses in the superior pharyngeal constrictor muscle at the expiratory-inspiratory phase transition, thus increasing pharyngeal collapsibility.
Citation:
Dantas DAS; Mauad T; Silva LFF; Lorenzi-Filho G; Formigoni GGS; Cahali MB. The extracellular matrix of the lateral pharyngeal wall in obstructive sleep apnea. SLEEP 2012;35(4):483-490.
Keywords: Extracellular matrix, obstructive sleep apnea, pharynx, collagen type I, pharyngeal constrictor muscle, inflammation
INTRODUCTION
There is mounting evidence that the lateral pharyngeal muscular wall plays a major role in the genesis of upper airway collapses in patients with obstructive sleep apnea (OSA). These patients have an exceedingly collapsible lateral pharyngeal wall during respiration, which is associated with a muscle thickening at this region that narrows the pharynx in the lateral dimension.1 Moreover, surgeries that act on the musculature of the lateral wall, such as lateral pharyngoplasty2 and maxillomandibular advancement,3 are more effective in reducing OSA severity than surgeries that excise and reconstruct tissues of the pharyngeal surface, such as uvulopalatopharyngoplasty.4 The superior pharyngeal constrictor is a skeletal muscle that forms the deep lateral and posterior layer of the pharyngeal airway. Its activation is similar to that of the pharyngeal dilator muscles at the end of obstructive apneas.5 Paradoxically, superior pharyngeal constrictor muscle may help dilate and stiffen the pharynx in response to increased airflow resistance, stabilizing the airway during expiration.6 Hence, the actual action of a pharyngeal muscle depends on both the respiratory phase and the concurrent activation of other muscles in the region.
The composition of the extracellular matrix (ECM) of a given tissue has an important influence on its mechanical and biologic properties. The most important biologic role of the ECM is the integration between cells and tissues, including cell morphogenesis and the development and regulation of cell proliferation. In addition, the ECM provides mechanical support, along with resistance and elasticity to tissues.7 Collagen and elastic fibers form the scaffolding of the connective tissue, and proteoglycans and structural glycoproteins (fibronectin) have important roles in hydration, resiliency, and the adhesive properties of tissue.8 The ECM of skeletal muscle is organized in levels, with the endomysial ECM having the most intimate contact with the muscle cells.7,9 Tissue inflammation may significantly alter ECM composition due to the expression of proteases. The matrix metalloproteinases (MMPs) are involved in ECM degradation in several tissues, including the skeletal muscles.8 Modifications of the ECM composition or of its regulators, either by inflammatory or genetic factors, may lead to the onset of pathologic processes.8
Chronic vibration from snoring and prolonged pharyngeal collapse from apneas, which is associated with increased negative intrapharyngeal pressure in OSA, place the pharyngeal muscles under increased mechanical load.10 Beyond a certain threshold, the mechanical forces applied to a muscle may alter the tissue structure.11 The myofibrils may bear most of the resting tension in human skeletal muscle,12 and overstretched sarcomeres may lead to an immediate drop in the tension-generating capacity,11 which could aggravate pharyngeal collapsibility. Presumably, changes in the ECM of pharyngeal muscles could be regarded either as a contributor to pharyngeal instability (e.g., a decrease in elastic fibers) or a consequence of mechanical load (e.g., an increase in collagen type I to make the muscle more load-resistant).13 In addition, an increase in collagen type I might lead to an incomplete relaxation of the superior pharyngeal constrictor after the end of the expiratory phase,6,14 which could also be a contributor to pharyngeal collapsibility.
The objective of this study is to determine and compare the proportional (fractional) area of collagen fibers, elastic fibers, proteoglycans (versican), fibronectin, and MMP-1 and MMP-2 in the endomysium of the musculature of the lateral pharyngeal wall in control subjects and patients with OSA. We hypothesized that alterations in the densities of specific ECM elements could be associated with OSA.
METHODS
We evaluated 51 adult patients (older than 18 yr) who underwent blunt dissection palatine tonsillectomy in our institution between 2005 and 2006. The reason for the surgeries was either chronic caseous or repeated acute tonsillitis, without OSA or snoring (control group, n = 13) or palatopharyngoplasty plus palatine tonsillectomy due to OSA (OSA group, n = 38). Obese patients (body mass index (BMI) > 30 kg/m2) were excluded, as were patients with neuromuscular diseases, retrolingual obstructions, noncontrolled hypothyroidism or controlled hypothyroidism present for less than 1 yr, Down syndrome, apparent syndromic or acquired craniofacial deformities, a past history of phlegmon or peritonsillar abscesses, or those who had undergone previous oropharyngeal surgeries. Patients with OSA were selected for surgical treatment because they had bulky lateral pharyngeal walls and had refused clinical treatments for OSA.
This study was approved by the Ethics Committee of our institution, and signed informed consent was obtained from all patients enrolled in the study. All patients underwent standard clinical evaluations, including a subjective daytime sleepiness assessment, as evaluated by the Epworth Sleepiness Scale (ESS), and patients with scores greater than 10 on this scale were considered to have excessive daytime sleepiness.
Patients were divided into a control or OSA group according to clinical characteristics and supervised type I polysomnography findings, which were performed within the 3 mo prior to surgery. All polysomnograms were performed in the absence of acute upper airway inflammation. The control subjects (13 patients) had no history of snoring, with an apnea-hypopnea index (AHI) of less than 5 events/hr. OSA (38 patients) was considered to be present when the AHI was greater than 5 events/hr, there was a history of chronic loud snoring, and there was excessive sleepiness (ESS > 10). There were 14 other patients who were excluded from our study: 9 had an AHI less than 5 events/hr and presented with loud snoring (either as a complaint or during the polysomnography), 4 patients with OSA had a BMI > 30 kg/m2 at the time of their surgeries, and 1 control subject had insufficient muscle samples for the analysis.
We analyzed both the ECM composition and the presence of inflammation within the muscular fibers adhered to the tonsils, which are predominantly within the superior pharyngeal constrictor muscle and are typically present in a blunt dissection tonsillectomy. No additional muscle resection was performed in any case.
Tissue Preparation
The removed tonsils were fixed in 10% buffered formaldehyde for 24 hours. Cross-sectional samples, 0.2 cm thick, of the palatine tonsils were made, creating multiple sections. After fixation, sections were dehydrated in progressively increasing (70%-95%) alcohol concentrations (ethanol and xylol), diaphanized, and paraffin-embedded. Blocks were subsequently cut into 4 μm-thick sections and stained with hematoxylin and eosin (H&E) to evaluate the adequacy of the specimen for the study. Adequate specimens had sufficient musculature for analysis and an absence of artifacts such as bleeding and cauterization effects. We analyzed the two best sections per case for each stain.
Histochemical Analysis
For identification of elastic fibers, the Weigert resorcin-fuchsin stain technique with oxidation was used as described previously.15
Immunohistochemical Analysis
Collagen type I, collagen type III, MMP-1 and MMP-2, versican, and fibronectin were characterized by immunohistochemical reactions.
The sections were incubated with the following primary antibodies: a rabbit polyclonal collagen type I antibody at a 1:50 dilution (Biodesign International, Saco, ME), a monoclonal collagen type III antibody at a 1:500 dilution (CP19 clone, Calbiochem, Darmstadt, Germany), a monoclonal versican antibody at a 1:100 dilution (2-b-1 clone, Seikagaku CO, Tokyo, Japan), a monoclonal MMP-1 antibody at a 1:2000 dilution (41-1E5 clone, Calbiochem, Darmstadt, Germany), a monoclonal MMP-2 antibody at a 1:100 dilution (A-Gel Vc2 clone, Labvision, Fremont, CA), and a rabbit polyclonal fibronectin antibody at a 1:8000 dilution (Dako Cytomation, Glostrup, Denmark). Envision System secondary antibodies with rabbit and mouse Dual Link polymers (Dako Cytomation, Glostrup, Denmark) were used for collagen type I. The LSAB kit, with a biotinylated antibody and the rabbit and mouse HRP complex (Dako Cytomation; Glostrup, Denmark) was used for all other markers. The antibodies were visualized with the chromogen 3.3'- Diaminobenzidine (Sigma-Aldrich, St. Louis, MO). All sections were stained during the same staining session using antibodies coming from the same batch. For negative control sections, the primary antibody was replaced by phosphate buffer solution during the staining process.
Morphometric Analysis
The fractional areas of the different components of the ECM present in the endomysium of the lateral pharyngeal wall were quantified through image analysis using the Image-Pro® Plus 4.1 for Windows® (Media Cybernetics, Silver Spring, MD) software connected to a digital camera (JVC TK-C1380 Color Video Camera, Victor Company of Japan Limited, Japan) and to an optical microscope (Leica DMR, Leica Microsystems, Wetzlar GmbH, Germany). We analyzed only the fragments that contained sufficient transversally cut muscle tissue for histologic analysis (15 fields at a 400× magnification). The area of positive staining for each antibody within the superior pharyngeal constrictor was determined by color thresholding. For this purpose, we used different sections of a given antibody (6-8 cases per group) and negative controls to achieve the most adequate range of positive labeling in each case, and this was always verified by two experienced pathologists (L.F.F.S and T.M.) These procedures generated a file containing all color selection data, which was then applied to all cases stained with the same antibody. Results were expressed as the area of the specific antibody staining divided by the total muscle area of the analyzed region (μm2/μm2) × 100 (%) (fractional area). The measurements were made by an examiner blinded to the study groups. The data were used to calculate the mean value of 15 fields for each marker per patient.
In the H&E stained slides, two examiners, blinded to the study groups, assessed the presence of inflammatory cell infiltrations in a semi-quantitative analysis based on scores from 0 (absent) to 4 (intense).
Statistical Analysis
Data were expressed as medians and interquartile ranges. Statistical analysis was performed with the SPSS software version 15.0 (SPSS Inc.©, Chicago, IL). The distribution of the study variables was nonparametric, according to the Kolmogorov-Smirnov normality. For comparisons between groups, a Mann-Whitney U test was used. To correlate the ECM components with the clinical variables, a Spearman correlation coefficient was calculated. To study the interactions between the variables, whenever an ECM component and a clinical variable were found to be significantly different between groups and were also significantly correlated between them, they were included as covariates in a logistic regression of the presence of OSA. The level of significance was set at 5% (P < 0.05).
RESULTS
The demographic, clinical, and polysomnographic characteristics of the patients are shown in Table 1. The patients in the OSA group were significantly older than those in the control group (P = 0.002) and had a higher BMI (P = 0.024). Detailed data on the age range of the groups are displayed in Table 2.
Table 1.
Demographic, anthropometric and polysomnographic data of the patients studied (data presented as median and interquartile range)
| Control subjects (n = 13) | OSA (n = 38) | P value | |
|---|---|---|---|
| Male/female | 6 / 7 | 25 / 13 | – |
| Age, years | 25.0 (12.0) | 37.0 (18.0) | 0.002* |
| BMI, kg/m2 | 23.1 (4.8) | 27.0 (5.2) | 0.024* |
| AHI, events/hr | 0.8 (2.3) | 16.4 (20.3) | < 0.0001* |
| Minimum SaO2 % | 89.0 (6.0) | 86.5 (9.5) | 0.064 |
AHI, apnea-hypopnea index; BMI, body mass index; minimum SaO2, minimum oxyhemoglobin saturation; OSA, obstructive sleep apnea.
Level of significance P < 0.05.
Table 2.
Detailed age ranges of the control and OSA groups
| Age group, yr | Control subjects (n = 13) | OSA (n = 38) |
|---|---|---|
| 18-20r | 4 | 2 |
| 21-30 | 5 | 10 |
| 31-40 | 3 | 11 |
| 41-50 | 1 | 8 |
| 51-60 | 0 | 7 |
OSA, obstructive sleep apnea.
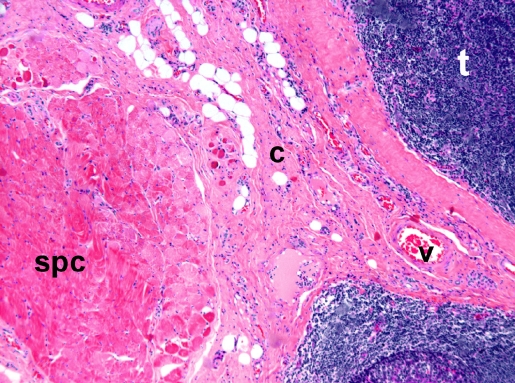
H&E staining showed that the skeletal muscle bundles of the superior pharyngeal constrictor were localized adjacent to the tonsillar tissue and separated by a rim of connective tissue of variable thickness with small vessels. Semiquantitative analysis demonstrated the absence of inflammatory cell infiltrations within the muscle tissue in all control subjects and patients with OSA (Figure 1). The median inflammation score for the control subjects was 0 and the interquartile range was 1, scores similar to those of the patients in the OSA group (P = 0.59). Tonsillar tissue presented hyperplastic follicles in most instances, and there was no suppurative inflammation.
Figure 1.
Hematoxylin and eosin section. Skeletal muscle bundles of the superior pharyngeal constrictor were localized adjacent to the tonsillar tissue and separated by a rim of connective tissue of variable thickness with small vessels. Note the absence of inflammatory cell infiltrations. t, tonsil; c, connective tissue; v, vessel; spc, superior pharyngeal constrictor muscle.
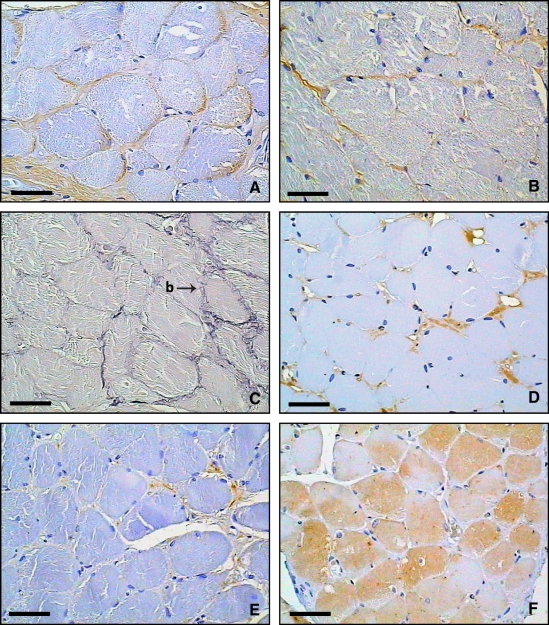
All the components of the ECM were sparsely present within the endomysium. Collagen was the most abundant component, specifically collagen type I, followed by collagen type III (Figure 2A and B). The collagen fibers presented as thin fibers involving each muscle cell and as small bridges between cells or between a cell and the ECM. Histochemical analysis revealed that the elastic fibers were organized in slender fibers within individual muscle cells and existed as small bridges that interconnected the cells and connected them to the ECM (Figure 2C). Versican (Figure 2D) and fibronectin (Figure 2E) were the most sparse ECM components in the endomysium of the superior pharyngeal constrictor and were present in thin, continuous pericellular distributions. MMP-1 expression in the muscle cells was variable, both within the same muscle fiber (Figure 2F) and between patients. MMP-2 was not seen in the skeletal muscle and therefore could not be quantified.
Figure 2.
Transverse histologic sections of endomysial extracellular matrix components in the superior pharyngeal constrictor muscle. (A) Representative immunohistochemical section (typical brown coloration) of collagen type I. (B) Immunohistochemical section of collagen type III. (C) Oxidized Weigert resorcin-fuchsin staining showing the endomysial distribution of elastic fibers (slender fibers within individual muscle cells and small bridges [b] that interconnect the cells and connect them to the extracellular matrix). (D) Immunohistochemical section of versican. (E) Immunohistochemical section of fibronectin. (F) Immunohistochemical section of matrix metalloproteinase 1 (note the variability of expression in muscle cells). Scale bar = 50 μm.
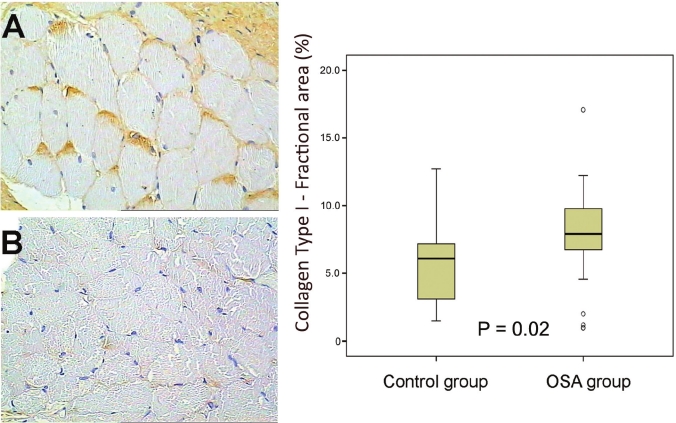
Histochemistry and immunohistochemistry data on the fractional area of each component of the ECM are depicted in Table 3. We observed a significant increase in collagen type I in the OSA group in comparison with control subjects (P = 0.02, Figure 3), whereas no significant difference was seen between groups in the remaining ECM components. There were no significant correlations between the fractional area of the ECM proteins with sex or polysomnographic data (AHI and minimum oxyhemoglobin saturation).
Table 3.
Fractional area of the endomysial ECM in the lateral pharyngeal wall in control and OSA patients (data presented as median and interquartile range)
| ECM components | Fractional area (%) |
||
|---|---|---|---|
| Control subjects (n = 13) | OSA (n = 38) | P value | |
| Collagen type I | 6.1 (4.1) | 7.9 (3.2) | 0.02* |
| Collagen type III | 4.1 (4.2) | 3.7 (3.3) | 0.46 |
| Elastic fibers | 0.6 (0.2) | 0.6 (1.4) | 0.86 |
| MMP – 1 | 5.0 (7.3) | 3.9 (6.7) | 0.85 |
| Versican | 0.3 (1.1) | 0.2 (0.1) | 0.18 |
| Fibronectin | 0.6 (0.9) | 0.8 (0.7) | 0.55 |
ECM, extracellular matrix; MMP-1, matrix metalloproteinase 1.
Level of significance P < 0.05.
Figure 3.
Representative immunohistochemical sections of collagen type I in the superior pharyngeal constrictor muscle (brown coloration). (A) OSA patient. (B) Control patient. There was a significantly higher expression of collagen type I in the OSA group than the control group (P = 0.02).
The amount of collagen type I correlated significantly with age (r = 0.432, P = 0.001, Figure 4) but not with the BMI. After controlling for the presence of OSA, collagen type I still correlated significantly with age (r = 0.327, P = 0.02). Likewise, after controlling for AHI, this correlation remained significant (r = 0.425, P = 0.001). Both collagen type I and age were included as covariates in a logistic regression of the presence of OSA. We noted an additive effect of the increase of collagen type I and age with the presence of OSA (odds ratio (OR), 2.06; 95% confidence interval (CI), 1.17-3.63, P = 0.012), when compared with the effect of age alone (OR, 1.11; 95% CI, 1.03-1.20, P = 0.007) or the increase of collagen type I alone (OR, 1.02; 95% CI, 0.81-1.43, P = 0.052).
Figure 4.
Correlation between collagen type I expression in the superior pharyngeal constrictor muscle and patient age. There was a significant positive correlation between age and the amount of collagen type I.
We performed an age-stratified analysis that compared the fractional area of collagen type I in the younger median split of our entire casuistic (35 yr or younger). This younger age group included 11 control subjects with a median (interquartile range) age of 24 (8) yr (range, 18-32 yr, 54.5% males) and 20 OSA patients with a median age of 28 (9) yr (range, 19-35 yr, 80% males). There was also a significant increase in collagen type I in this younger OSA age group (median fractional area, 7.6%; interquartile range, 3.0%) compared with the younger control age group (median fractional area, 5.4%; interquartile range, 4.0%, P = 0.04).
DISCUSSION
The ECM composition of the muscle walls may play an important role in determining cell activity and function.8 In the current study, we analyzed the composition of the ECM and its regulators in the endomysial region of the superior pharyngeal constrictor muscle in non-OSA and nonsnoring control subjects and in patients with OSA. Our data showed that the levels of collagen type I, the major component of skeletal muscle ECM,16 were significantly higher in the OSA group than in control subjects and increased with age. In the 18- to 35-yr age group (the younger median split of our data), the levels of collagen type I were already significantly higher in patients with OSA than in control subjects. The power of this study to detect collagen type I differences between control subjects (13 patients) and patients with OSA (38 patients) using the measured data was 93.8%. For the same measured data, this study has an 80% power to detect collagen type I differences of at least 18% between groups. To the best of our knowledge, this is the first study describing the ECM composition in the pharyngeal musculature.
Changes in collagen in the aging human skeletal muscles are not well known. There is no change with age in the endomysial collagen of the vastus lateralis muscle.17 In contrast, studies of the cardiac skeletal muscle have shown an increase in collagen type I18 and a decrease in collagen type III19 that is associated with increasing age. These changes, which lead to an increase in stiffness, might have functional consequences by impairing the ability of the muscle to contract (typically incomplete relaxation during early diastolic filling). Our study showed that collagen type I levels in the pharyngeal muscle increased with age and increased the risk of OSA.
The absence of inflammation in the superior pharyngeal constrictor muscle, located deep in the pharynx, suggested that the inflammatory effect of habitual snoring20 could primarily affect the tissues that line the upper airway, with minor consequences to the deeper layers. All of our surgical procedures were done during the morning period; even after a night of apnea episodes, we could not find significant inflammatory cell infiltrations at the superior pharyngeal constrictor level. Boyd and colleagues21 observed an increase in inflammatory cells in the mucosal and muscle cells of the soft palate and of the tonsillar pillars in patients with OSA. In that study, the degree of inflammation at those more superficial muscle layers was only one fifth of the total inflammation within the mucosa. Oropharyngeal muscles with inflammatory characteristics in patients with OSA have been described by other authors21–23; these studies included tissue layers that were more superficial than the superior constrictor, such as the uvula, soft palate, and tonsillar pillars.
Therefore, our data indicated that pharyngeal muscle inflammation could not play a role in the increase of collagen type I in the superior constrictor seen in patients with OSA. There are other possible mechanisms that could explain this type of muscle remodeling in the upper airway in patients with OSA. Rats submitted to skeletal muscle denervation showed an accumulation of collagen type I and type III within muscular fibers,24,25 and signs of denervation have already been demonstrated in the upper airway in patients with OSA.20 In addition, similar to what was observed in the lower airway,26,27 repeated stretching and contracting of the pharyngeal muscles might have induced muscle remodeling in the absence of any inflammation. This could represent an attempt to prevent overstretching of the muscle fiber bundles.13
Elastic fibers are important tissue components that contain elastometric properties, which conserve the potential energy of deformed muscles (contracted or extended) and return that energy to conserve the muscle position.28 One qualitative study reported a disorganization of elastic fibers around the musculus uvulae, which was more pronounced within the uvula mucosa, in patients with OSA. This finding did not have significant, functional correlations with OSA.23 Although we have not detected quantitative alterations in elastic fibers, we could not exclude the possibility that alterations at the ultrastructural level could occur in OSA. Sullivan and colleagues described a high prevalence of OSA in patients with Marfan syndrome, a genetic disease that causes marked muscle flaccidity and increased collapsibility of pharyngeal muscles due to abnormal elastic fibers.29
Fibronectin and versican were present in small proportions in the endomysium of the superior pharyngeal constrictor of the groups in this study. MMP-1 in superior pharyngeal constrictor muscle cells showed a variable expression between patients and within muscular fibers and did not differ between groups. Changes in the amounts of these proteins in the skeletal muscles seemed to be related to pathologic conditions such as inflammatory and dystrophic myopathies and muscle denervation.30–32 Our results suggested that these processes were not taking place at the pharyngeal constrictor muscle in patients with OSA.
Methodologic errors were kept to a minimum. To our knowledge, this was the first study to include quantitative analyses of ECM components in the pharyngeal muscles of well-characterized control and OSA patient groups. We have used image analysis to determine the components of the ECM in histologic sections, thus avoiding the subjectivity of a semiquantitative analysis. We have analyzed muscle fibers that adhered to the deep inner surface of surgically removed palatine tonsils.33,34 The superior pharyngeal constrictor muscle is the major component of the tonsillar fossa35; part of the palatopharyngeus muscle may have been present in a few samples, as this muscle may form part of the tonsillar fossa in some individuals.35 However, we took care to preserve this muscle in our surgical technique. We have not examined other muscular compartments, such as the perimysium and epimysium, of the superior pharyngeal constrictor. We believe that the endomysium, having the most intimate contact with the muscle cells, would have more influence on the functional and biologic properties of muscle cells. We excluded obese patients from this study to reduce the interference of obesity on the pharyngeal structure. There are reports suggesting that obesity modifies the turnover of collagen in cardiac skeletal muscles and may alter muscle function.36 Our patients with OSA were relatively young (median age = 37 yr), which may decrease their acceptance or tolerance of a lifetime of required clinical treatments for OSA. In addition, only 4 patients from the OSA group and 1 from the control group were smokers; this reduces the possible influence of tobacco on the ECM composition due to upper airway inflammation.
We have analyzed only transverse sections of our muscle samples. This provides a good basis for quantitative measures, because the relationship of the ECM and the muscle would be the most uniformly maintained. In contrast, longitudinally cut sections would have better shown the organization of the ECM components within the muscle fibers. However, in this type of section, an adequate orientation of the muscle fiber within the bundles could not be guaranteed, which would have introduced variations into our quantitative measurement. In general, collagen fibers presented as thin fibers in each muscle cell and as small bridges between cells or between a cell and the ECM.
What are the clinical implications of our findings? For instance, surgical procedures such as the uvulopalatopharyngoplasty aim not only to increase the airway space but also to produce fibrosis to reduce pharyngeal collapsibility, with variable but usually poor results.37 Fibrosis due to uvulopalatopharyngoplasty may be an unorganized deposition of collagen types, in contrast to the kind of organization that we found. Our study showed that patients with OSA already had increased amounts of collagen type I at the lateral pharyngeal wall and that collagen type I also increased with age. Thus, the concept of increasing collagen type I at the upper airway to treat OSA could not be supported by our histologic findings.
The reasons for the increased passive collapsibility of the pharyngeal structure in OSA are not known. It is possible that OSA pathogenesis involves a genetic trait that affects muscle proteins that are related to contractility.38 One explanation could be alterations in the myofibrils, which may bear the passive muscle tension in human skeletal muscle.12 Contrary to what might be expected, we found a stiffening protein to be increased at that structure in OSA. We hypothesize that the increase in collagen type I could play a role in turning OSA into a progressive disease; in an attempt to make the muscle more load-resistant,13 increased amounts of collagen type I could impair or delay the relaxation of the superior pharyngeal constrictor muscle, similar to what happens at the myocardium.18 Because of the expiratory activation of the constrictor muscle when faced with increased airflow resistance,6,14 this incomplete relaxation could occur at the expiratory-inspiratory phase transition, when the pharyngeal lumen is the narrowest. These actions could further reduce the airway diameter during a particularly collapsing ventilatory phase, which could aggravate OSA.
In summary, histologic analysis of the ECM composition of the lateral muscular pharyngeal wall of a large group of patients revealed that collagen type I was the major component of the muscle ECM and was increased with age and with the presence of OSA. Further studies describing the composition of the skeletal muscle contractile proteins and studies of the viscoelastic properties of soft tissues in vivo, using new techniques such as magnetic resonance elastography, could help to explain the pharyngeal muscle biomechanics and complement our histological findings.39
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors wish to thank Ângela Santos and Maria Cristina Medeiros for preparing the immunohistochemical reactions on tissue sections. Fundação de Amparo èa Pesquisa do Estado de São Paulo (grant# 06/50630-7). Conselho Nacional de Desenvolvimento Cientíifico e Tecnolíogico.
Footnotes
A commentary on this article appears in this issue on page 449.
REFERENCES
- 1.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing: significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;125:1673–89. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 2.Cahali MB. Lateral pharyngoplasty: a new treatment for obstructive sleep apnea hypopnea syndrome. Laryngoscope. 2003;113:1961–8. doi: 10.1097/00005537-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Li KK, Guilleminault C, Riley RW, Powell NB. Obstructive sleep apnea and maxillomandibular advancement: an assessment of airway changes using radiographic and nasopharyngoscopic examinations. J Oral Maxillofac Surg. 2002;60:526–30. doi: 10.1053/joms.2002.31849. [DOI] [PubMed] [Google Scholar]
- 4.Cahali MB, Formigoni GGS, Gebrim EMMS, Miziara ID. Lateral pharyngoplasty versus uvulopalatopharyngoplasty: a clinical, polysomnographic and computed tomography mensurement comparison. Sleep. 2004;27:942–50. doi: 10.1093/sleep/27.5.942. [DOI] [PubMed] [Google Scholar]
- 5.Kuna ST, Smickley JS. Superior faringeal constrictor activation in obstructive sleep apnea. Am J Respir Crit Care Med. 1997;156:874–80. doi: 10.1164/ajrccm.156.3.9702053. [DOI] [PubMed] [Google Scholar]
- 6.Yaman Z, Kogo M, Senoo H, Iida S, Ishii S, Matsuya T. Role of the superior pharygeal constrictor muscle in forced breathing in dogs. Cleft Palate Craniofac J. 2000;37:197–204. doi: 10.1597/1545-1569_2000_037_0197_rotspc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 7.Kovanen V. Intramuscular extracellular matrix: complex environment of muscle cells. Exerc Sport Sci Rev. 2002;30:20–5. doi: 10.1097/00003677-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Labat-Robert J, Bihari-Varga M, Robert L. Extracellular matrix. FEBS Lett. 1990;268:386–93. doi: 10.1016/0014-5793(90)81291-u. [DOI] [PubMed] [Google Scholar]
- 9.Järvinen TAH, Jíozsa L, Kannus P, Järvinen TL, Järvinen M. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscle: an immunohistochemical, polarization and scanning electron microscopic study. J Muscle Res Cell Motil. 2002;23:245–54. doi: 10.1023/a:1020904518336. [DOI] [PubMed] [Google Scholar]
- 10.Steier J, Jolley CJ, Seymour J, et al. Increased load on the respiratory muscles in obstructive sleep apnea. Respir Physiol Neurobiol. 2010;171:54–60. doi: 10.1016/j.resp.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Morgan DL, Allen DG. Early events in stretch-induced muscle damage. J Appl Physiol. 1999;87:2007–15. doi: 10.1152/jappl.1999.87.6.2007. [DOI] [PubMed] [Google Scholar]
- 12.Magid A, Law DJ. Myofibrils bear most of the resting tension in frog skeletal muscle. Science. 1985;230:1280–2. doi: 10.1126/science.4071053. [DOI] [PubMed] [Google Scholar]
- 13.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–98. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 14.Kuna ST, Smickley JS, Vanoye CR. Respiratory-related pharyngeal constrictor muscle activity in normal human adults. Am J Respir Crit Care Med. 1997;155:1991–9. doi: 10.1164/ajrccm.155.6.9196107. [DOI] [PubMed] [Google Scholar]
- 15.Montes GS. Structural biology of the fibers of the collagenous and elastic systems. Cell Biol Int. 1996;20:15–27. doi: 10.1006/cbir.1996.0004. [DOI] [PubMed] [Google Scholar]
- 16.Kurosu H. Biochemical studies on collagen and connectin from human skeletal muscle: age-related changes in the properties of elasticity. Nippon Seikeigeka Gakkai Zasshi. 1979;53:1641–52. [PubMed] [Google Scholar]
- 17.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol. 2007;103:2068–76. doi: 10.1152/japplphysiol.00670.2007. [DOI] [PubMed] [Google Scholar]
- 18.Souza RR. Aging of myocardial collagen. Biogerontology. 2002;3:325–35. doi: 10.1023/a:1021312027486. [DOI] [PubMed] [Google Scholar]
- 19.Mays PK, Bishop JE, Laurent GJ. Age related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45:203–12. doi: 10.1016/0047-6374(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 20.Friberg D, Ansved T, Borg K, Carlsson-Norlander B, Larsson H, Svanborg E. Histological indications of a progressive snorers disease in an upper airway muscle. Am J Respir Crit Care Med. 1998;157:586–93. doi: 10.1164/ajrccm.157.2.96-06049. [DOI] [PubMed] [Google Scholar]
- 21.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:541–6. doi: 10.1164/rccm.200308-1100OC. [DOI] [PubMed] [Google Scholar]
- 22.Woodson BT, Garancis JC, Toohill RJ. Histopathologic changes in snoring and obstructive sleep apnea syndrome. Laryngoscope. 1991;101:1318–22. doi: 10.1002/lary.5541011211. [DOI] [PubMed] [Google Scholar]
- 23.Sérièes F, Chakir J, Boivin D. Influence of weight and sleep apnea status on the immunological and structural features of the uvula. Am J Respir Crit Care Med. 2004;170:1114–19. doi: 10.1164/rccm.200404-458OC. [DOI] [PubMed] [Google Scholar]
- 24.Sawai H. Collagen metabolism of skeletal muscles following injuries of the peripheral nerves. Nippon Seikeigeka Gakkai Zassh. 1982;56:753–64. [PubMed] [Google Scholar]
- 25.Tsuji M. Alterations in collagen synthesis after transection of peripheral nerves. Nippon Seikeigeka Gakkai Zasshi. 1989;63:860–70. [PubMed] [Google Scholar]
- 26.Grainge CL, Lau LC, Ward JA, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364:2006–15. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 27.Mackey AL, Donnelly AE, Turpeenniemi-Hujanen T, Roper HP. Skeletal muscle collagen content in humans after high-force eccentric contractions. J Appl Physiol. 2004;97:197–203. doi: 10.1152/japplphysiol.01174.2003. [DOI] [PubMed] [Google Scholar]
- 28.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–28. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 29.Cistulli PA, Sullivan CE. Sleep apnea in Marfan's syndrome: increased upper airway collapsibility during sleep. Chest. 1995;108:631–5. doi: 10.1378/chest.108.3.631. [DOI] [PubMed] [Google Scholar]
- 30.Kieseier BC, Schneider C, Clements JM, et al. Expression of specific matrix metalloproteinases in inflammatory myopathies. Brain. 2001;124:341–51. doi: 10.1093/brain/124.2.341. [DOI] [PubMed] [Google Scholar]
- 31.Zanotti S, Saredi S, Ruggieri A, et al. Altered extracellular matrix transcript expression and protein modulation in primary Duchenne muscular dystrophy myotubes. Matrix Biol. 2007;26:615–24. doi: 10.1016/j.matbio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Salonen V, Lehto M, Kalimo H, Penttinen R, Aro H. Changes in intramuscular collagen and fibronectin in denervation atrophy. Muscle Nerve. 1985;8:125–31. doi: 10.1002/mus.880080208. [DOI] [PubMed] [Google Scholar]
- 33.Erkiliç S, Aydin A, Koçer NE. Histological features in routine tonsillectomy specimens: the presence and the proportion of mesenchymal tissues and seromucinous glands. J Laryngol Otol. 2002;116:911–3. doi: 10.1258/00222150260369435. [DOI] [PubMed] [Google Scholar]
- 34.Gnepp DR, Souther J. Skeletal muscle in routine tonsillectomy specimens: a common finding. Hum Pathol. 2000;31:813–6. doi: 10.1053/hupa.2000.8439. [DOI] [PubMed] [Google Scholar]
- 35.Ohtsuka K, Tomita H, Murakami G. Anatomy of the tonsillar bed: topographical relationship between the palatine tonsil and the lingual branch of the glossopharyngeal nerve. Acta Otolaryngol Suppl. 2002;546:99–109. doi: 10.1080/00016480260046472. [DOI] [PubMed] [Google Scholar]
- 36.Quilliot D, Alla F, Böhme P, et al. Myocardial collagen turnover in normotensive obese patients: relation to insulin resistance. Int J Obes (Lond) 2005;29:1321–8. doi: 10.1038/sj.ijo.0803022. [DOI] [PubMed] [Google Scholar]
- 37.American Sleep Disorders Association. Practice parameters for the treatment of obstructive sleep apnea in adults: the efficacy of surgical modifications of the upper airway. Sleep. 1996;19:152–5. [PubMed] [Google Scholar]
- 38.Schwab RJ, Pasirstein M, Kaplan L, et al. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med. 2006;173:453–63. doi: 10.1164/rccm.200412-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng S, Gandevia SC, Green M, Sinkus R, Bilston LE. Viscoelastic properties of the tongue and soft palate using MR elastography. J Biomech. 2011;44:450–4. doi: 10.1016/j.jbiomech.2010.09.027. [DOI] [PubMed] [Google Scholar]