Abstract
Study Objectives:
To examine in cognitively intact older men and women the associations between subjective sleep quality and 1-yr incident cognitive impairment.
Design:
Prospective cohort study.
Setting:
General community.
Participants:
1,664 cognitively intact individuals age 65 to 96 years.
Measurements and Results:
Sleep quality at baseline was measured using the Pittsburgh Sleep Quality Index (PSQI). Cognitive functioning was assessed at baseline and 12 months later using the Mini-Mental State Examination (MMSE). Incident general cognitive impairment was defined according to a follow-up MMSE score below the 15th percentile according to normative data and of at least 2 points below baseline. General cognitive impairments were also separated into amnestic and nonamnestic subtypes according to MMSE delayed recall performance. Associations between sleep quality indicators at baseline and incident cognitive impairment were assessed by odds ratio (OR) adjusted for age, education, baseline MMSE score, psychotropic drug use, anxiety, depressive episodes, cardiovascular conditions, and chronic diseases. Results revealed that global PSQI score was significantly linked with incident cognitive impairment (OR 1.17, 95% confidence interval (CI) 1.05-1.30) in men, but not in women. In women, sleep disturbance score (OR 2.62, 95% CI 1.41-4.86) and long sleep duration (≥ 9 hr; OR 3.70, 95% CI 1.49-9.17) were associated with nonamnestic and amnestic incident cognitive impairment, respectively. In men, short sleep duration (≤ 5 hr; OR 4.95, 95% CI 1.72-14.27) and habitual sleep efficiency score (OR 1.94, 95% CI 1.42-2.66) were associated with amnestic and general incident cognitive impairment, respectively.
Conclusions:
Sleep quality in older adults should receive particular attention by clinicians because poor sleep quality can be an early sign of cognitive decline.
Citation:
Potvin O; Lorrain D; Forget H; Dubé M; Grenier S; Préville M; Hudon C. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. SLEEP 2012;35(4):491-499.
Keywords: Sleep quality, elderly, cognition, cognitive decline
INTRODUCTION
Neuropsychiatric symptoms are common manifestations in older adults with cognitive impairment. Approximately 75% of older adults with dementia and up to 75% of older adults with mild cognitive impairment display at least one neuropsychiatric symptom.1,2 Sleep disturbance along with depressed mood and anxiety are among the most frequently encountered symptoms in older adults with mild cognitive impairment.1–4 Because mild cognitive impairment progresses to dementia in many instances,5 late-life neuropsychiatric symptoms could be prodromes of neurodegenerative diseases. Some studies on depressive symptoms support this hypothesis by indicating that depressive symptoms are predictive of dementia6–8 and incident cognitive impairment,9–11 especially of incident cognitive impairment of the amnestic type (including a memory impairment), which is believed to be prodromal or preclinical Alzheimer disease (AD) in many cases.12–15 In addition, previous results suggest sex differences in the association between depressive symptoms and cognitive decline because many studies observed that depressive symptoms predicted cognitive decline9,11,16 or the onset of dementia7,8 in men, but not in women. Some studies on anxiety,10,16–18 but not all,17–19 also showed that this symptom is associated with an increased risk of incident cognitive impairment and dementia.
Unlike depression and anxiety symptoms, poor sleep quality did not receive much attention as a potential predictive symptom of incident cognitive impairment or dementia. One study showed that chronic insomnia was linked 3 yr later to an increase in errors (2 or more at follow-up versus 0 or 1 at baseline) on the Short Portable Mental Status Questionnaire in nondepressed older men, but not older women.20 In this study, insomnia was defined as trouble falling asleep or waking up too early most of the time, but no other subjective sleep variable was measured. These results suggest that, similar to depressive symptoms, poor subjective sleep quality could be a predictor of future cognitive impairment and that this association may be more pronounced or may occur only in older men. In another study in older women using a 2-yr follow-up, no association was found between sleep variables (subjective sleep duration, subjective sleep difficulties, and snoring) and the evolution of cognitive functioning measured by the Telephone Interview for Cognitive Status.21 A third study in older adults, which did not consider sex differences, found that sleep complaints at baseline were linked to poorer Mini-Mental State Examination (MMSE) scores 3 yr later, but this association did not remain significant after controlling for depressive symptoms.22 Together, the results from these studies20–22 are inconsistent, but disparities in these results may be explained by the different measures of subjective sleep quality and by sex differences.
The current study aimed to examine the relationship between multiple subjective sleep quality indicators and 1-yr incident cognitive impairment in older men and women who were cognitively intact at baseline. It was hypothesized that older men would show stronger associations between poor sleep quality indicators and incident cognitive impairment in comparison with older women and that similar to results on depressive symptoms,9,10 incident cognitive impairment of the amnestic subtype (but not nonamnestic subtype) would be predicted by poor subjective sleep quality.
METHODS
ESA Study
Data come from the Enquôete sur la santé des aôinés (ESA study; in English, Survey on Elders' Health), a population-based study that assessed the prevalence and incidence of psychological distress in the province of Quebec, Canada.23 In 2005-2006, a random sample (n = 2,811) of community-dwelling French-speaking adults (95% of the Quebec population speaks French)24 age 65 yr or older and living in the province of Quebec, Canada were recruited. The sampling frame of the study used a random dialing method with a stratification of proportional sample of households according to geographic areas (metropolitan, urban, and rural) and the 16 administrative regions of Quebec's province. A random sampling method was also used to select only one older adult within the household. The response rate was 76.5% at the first interview.
Data were collected through in-home structured interviews conducted by trained research nurses who received 2 days of training by the principal investigator of the ESA study (M.P.) in the administration of a computer-assisted questionnaire (ESA-Q). The ESA-Q comprises many questionnaires, including the French version of the MMSE,25,26 and an adapted version of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) mood and anxiety disorders sections of the Diagnostic Interview Schedule and Composite International Diagnostic Interview.27–29 To ensure the validity of the data in the ESA study, the complete interview was done only with participants who scored 22 or higher on the MMSE (26 participants had a MMSE score < 22 at baseline). The follow-up interview occurred approximately 12 months after the baseline assessment (mean 12.5 months; standard deviation (SD) 1.4). Data from the ESA study were linked with medical records from the Régie de l'assurance maladie du Québec (RAMQ; Quebec's public health insurance plan). All Quebec residents are registered for RAMQ coverage. The respondents were offered $15 in compensation for their participation. Written informed consent was obtained at the beginning of the interview from all participants. The research procedures were previously reviewed and authorized by the ethics committee of the Institut Universitaire de Gériatrie de Sherbrooke (Sherbrooke, Canada).
Study Sample
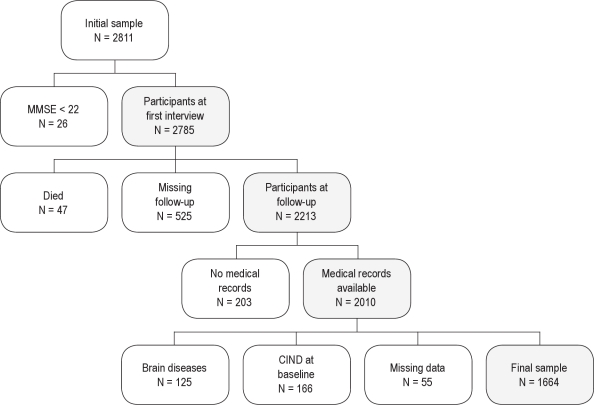
Figure 1 shows the flow chart of study enrollment and exclusion criteria. Among the 2,785 individuals who received the interview at baseline, 47 died and 525 did not participate in the follow-up interview. Medical records from the RAMQ were available for 2,010 individuals and only these participants were included in the current study. The missing data from the RAMQ records were due to refusal of consent to provide medical records, moving outside Quebec, or having additional drug insurance. According to the medical records covering the period of 1 yr before baseline and the period of 1 yr between baseline and follow-up interviews, participants with brain diseases including dementia, cerebrovascular disease, brain trauma/tumor/infections, multiple sclerosis, Parkinson disease, epilepsy, and schizophrenia or other forms of psychosis were excluded from the sample (n = 125). Moreover, participants with baseline MMSE score below the 15th percentile according to normative data for age, sex, and education level30 were considered as having cognitive impairment no dementia (n = 166) and were excluded. Finally, 8 participants were excluded because education level was unknown and 47 had an incomplete interview. Thus, a final sample of 1,664 participants was included in the current study.
Figure 1.
Flow chart of the study sample enrollment with white and gray shapes representing exclusion and inclusion criteria, respectively. CIND, cognitive impairment no dementia.
Sleep Quality
Sleep quality at baseline was assessed using the French version of the Pittsburgh Sleep Quality Index (PSQI),31,32 a questionnaire that measures sleep quality over the previous month using 7 subscales measuring different components of sleep: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. Each component is reflected by a score ranging from 0 to 3, 3 indicating the worse sleep quality.
Incident Cognitive Impairment
The criteria for incident cognitive impairment were meant to identify potentially pathologic cognitive decline and consisted of a follow-up MMSE score below the 15th percentile according to norms for age, education, and sex,30 which is equivalent to a score below 1 SD in a normal distribution. This criterion for cognitive impairment is commonly used.33–35 In addition to this criterion, a loss of at least 2 MMSE points between baseline and follow-up interviews was required to meet incident cognitive impairment criteria, because it was previously established that a reliable change in MMSE score for short intervals corresponds to a loss of at least 2 points.36
Cognitive impairment was labeled as “general cognitive impairment” because the MMSE assesses general cognitive functioning. Moreover, based on previous methods and findings,9,10,37 amnestic and nonamnestic subtypes of cognitive impairment were determined. The 3-word recall task of the MMSE was reported to be an adequate measure of episodic memory in epidemiologic studies.38 Thus, cognitive impairment with a score of 0 or 1 out of 3 on the 3-word recall task of the MMSE was considered as cognitive impairment of the amnestic type.
Covariates
Potential confounders were chosen a priori based on expected risk factors for cognitive decline. These variables included MMSE score at baseline, age, education, sex, psychotropic drug use, depressive episodes, anxiety, cardiovascular conditions, and chronic diseases. Psychotropic drug use was assessed by the RAMQ medical records, and drugs were coded according to the American Hospital Formulary Service.39 Psychotropic drug use was defined as at least 1 prescription during the year between the baseline and the follow-up interviews. Depressive episodes and anxiety were identified for the 12-mo period preceding both interviews according to DSM-IV criteria.40 Depressive episodes included major depressive episode and depressive disorder not otherwise specified (minor depression). Anxiety disorders considered in this study were specific phobia, social phobia, agoraphobia, panic disorder, and generalized anxiety disorder. Because anxiety symptoms not meeting all DSM-IV criteria were previously linked to incident cognitive impairment,9 the anxiety variable included both anxiety disorders and anxiety symptoms. Participants having at least 1 essential symptom of a DSM-IV anxiety disorder without meeting full criteria were considered as having anxiety symptoms. The number of chronic diseases according to the International Classification of Diseases, Tenth Revision, was measured by asking participants at baseline and at follow-up if they had any of the following chronic health problems: arthritis or rheumatism, eye diseases, backache or spinal problems, digestive problems, thyroid disorders, other metabolic disorders, anemia, hypercholesterolemia, asthma or emphysema or chronic bronchial diseases, liver diseases, kidney or urinary problems, skin diseases, and migraine or frequent headaches. A cardiovascular conditions score was computed using self-report of high blood pressure, diabetes, and heart disease at baseline and medical records for the period of 1 yr before baseline and the period of 1 yr between baseline and follow-up interviews. The score was the total of the presence of these 3 health problems.
Statistical Analysis
Differences of characteristics between excluded participants and those included in the study were evaluated using t-tests and chi-squares. Characteristics between men and women were also evaluated by t-tests and chi-squares. The association between sleep quality and incident cognitive impairment was first assessed by crude odds ratio (OR) with 95% confidence intervals (CI). Next, an adjusted OR was computed by a logistic regression with incident cognitive impairment as the predicted variable and PSQI score as predictor, and was adjusted for age, education, baseline MMSE score, anxiety, depressive episodes, psychotropic drug use, cardiovascular conditions score, and chronic diseases. ORs were computed for global PSQI score and each PSQI component (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction) and these measures were used as continuous variables, except sleep duration score. Because sleep longer than 7 hours is considered “normal” in the sleep duration score of the PSQI and that long sleep time can reflect abnormal behavior, sleep duration was categorized as short, average, and long. Extreme sleep duration categories (short: ≤ 5 hr, long: ≥ 9 hr) were based on the categories used by Tworoger et al.21 Short and long sleep duration categories were compared with the average category: > 5 hr and < 9 hr. Age, cardiovascular conditions score, baseline MMSE score, and number of chronic diseases were used as continuous variables whereas other covariables were categorized as shown in Table 1. Interactions between sleep variables and sex were tested. For other covariables, interactions with the global PSQI score were assessed. Because the associations between multiple sleep indicators and incident cognitive impairment were assessed, the alpha level was set at the level of 0.006, which corresponds to the standard P value (0.05) divided by the number of sleep indicators examined (global PSQI score and 7 PSQI subscales). Statistical assumptions were verified and analyses were performed using SPSS software (16.0; Chicago).
Table 1.
Characteristics of the participants (n = 1,664)
| Characteristic | Women n = 1,159 | Men n = 505 | P* |
|---|---|---|---|
| Age, mean (SD) | 73.9 (5.7) | 72.7 (5.0) | < 0.001 |
| Education, n (%) | |||
| Primary or less | 262 (22.6) | 105 (20.8) | < 0.001 |
| Secondary | 540 (46.6) | 172 (34.1) | |
| Postsecondary | 357 (30.8) | 228 (45.1) | |
| Psychotropic drug use, n (%) | 461 (39.8) | 130 (25.7) | < 0.001 |
| Depressive episode, n (%) | 126 (10.9) | 28 (5.5) | < 0.001 |
| Anxiety, n (%) | 241 (20.8) | 49 (9.7) | < 0.001 |
| Cardiovascular condition score, mean (SD) | 1.2 (0.9) | 1.4 (0.9) | < 0.001 |
| Number of chronic diseases, mean (SD) | 3.3 (2.0) | 2.7 (1.8) | < 0.001 |
| Baseline MMSE score, mean (SD) | 29.0 (1.0) | 28.6 (1.2) | < 0.001 |
MMSE, Mini-mental state examination; SD, standard deviation.
P value from t-tests or chi-square tests.
RESULTS
In comparison with the participants included in the current study, excluded participants (Figure 1) were slightly older (74.4 versus 73.5 years; P < 0.001) and somewhat less educated (primary education or less: 27.8% versus 22.1%; secondary education: 40.3% versus 42.8%; postsecondary education: 31.9% versus 35.2%; P = 0.002). However, included and excluded participants were comparable regarding sex (P = 0.294). Finally, compared with those of the study sample, excluded participants without dementia or cognitive impairment at baseline had a negligible, but statistically significant, lower mean MMSE score at baseline (28.8 versus 28.9; P = 0.015).
Table 1 shows the characteristics of the participants. Compared with men, women were older, were less educated, displayed more anxiety and depressive symptoms, and used more psychotropic drugs between the baseline and follow-up interviews. Moreover, men had higher cardiovascular scores, lower numbers of chronic diseases, and lower MMSE scores than women.
Thirty-seven men (7.3%) and 68 women (5.9%) had incident cognitive impairment (22 men and 28 women with amnestic type). The mean MMSE loss between baseline and follow-up interviews in participants with incident cognitive impairment was 3.8 points (SD 0.3) in men and 3.3 points (SD 0.2) in women. Five participants with incident cognitive impairment (3 men, 2 women) were not evaluated for psychiatric symptoms at follow-up because their MMSE score was slightly below 22 at follow-up, which was an exclusion criterion of the ESA study. Because controlling for anxiety and depressive episodes at follow-up had no effect on the results (results not shown), the analyses using all participants with incident cognitive impairment, but controlling for anxiety and depressive episode at baseline, but not at follow-up, are presented.
Statistical analyses for the prediction of general incident cognitive impairment revealed that the interaction of sex with global PSQI score was close to significance (P = 0.082). Sex interactions for habitual sleep efficiency score (P < 0.001) and sleep duration (P = 0.049) were significant and close to significance, respectively. Interactions between global PSQI score and other variables were not significant or close to significance. Because of this sex interaction, ORs were computed separately for men and women for all sleep indicators. Table 2 displays the sleep quality measures for men and women with and without incident cognitive impairment. In women, the sleep disturbances score was significantly associated with incident general cognitive impairment and more particularly with incident nonamnestic cognitive impairment. In men, the global PSQI score was significantly associated with incident general cognitive impairment. Global PSQI score did not appear to be related to a particular type of cognitive impairment because, individually, the associations with amnestic and nonamnestic types were not significant. In men, habitual sleep efficiency score was significantly linked to incident general cognitive impairment and to both amnestic and nonamnestic types.
Table 2.
Subjective sleep quality indicators at baseline and incident cognitive impairment in older men and women (n = 1,664)
| PSQI Scores | Incident Cognitive Impairment |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| General |
Amnestic |
Nonamnestic |
|||||||
| Yes Mean (SD) | No Mean (SD) | Crude OR (95% CI) | Adjusted* OR (95% CI) | P | Adjusted* OR (95% CI) | P | Adjusted* OR (95% CI) | P | |
| Women | n = 68 | n = 1091 | |||||||
| Global sleep quality | 6.3 (4.1) | 5.4 (3.7) | 1.06 (1.00-1.13) | 1.02 (0.96-1.10) | 0.492 | 0.99 (0.89-1.10) | 0.855 | 1.05 (0.96-1.15) | 0.249 |
| Subjective sleep quality | 0.8 (0.7) | 1.0 (0.7) | 1.34 (0.93-1.91) | 1.17 (0.80-1.70) | 0.418 | 1.16 (0.64-2.08) | 0.626 | 1.19 (0.74-1.93) | 0.467 |
| Sleep latency | 1.3 (1.0) | 1.1 (1.0) | 1.24 (0.98-1.57) | 1.12 (0.87-1.42) | 0.377 | 1.17 (0.81-1.70) | 0.390 | 1.09 (0.80-1.49) | 0.591 |
| Habitual sleep efficiency | 0.8 (1.1) | 0.8 (1.0) | 1.05 (0.83-1.32) | 0.96 (0.76-1.22) | 0.765 | 0.84 (0.58-1.24) | 0.382 | 1.06 (0.79-1.42) | 0.707 |
| Sleep disturbances | 1.1 (0.6) | 0.8 (0.5) | 2.26 (1.41-3.63) | 2.00 (1.23-3.27) | 0.005 | 1.40 (0.65-3.01) | 0.396 | 2.62 (1.41-4.86) | 0.002 |
| Use of sleep medication | 0.9 (1.3) | 0.8 (1.2) | 1.07 (0.88-1.29) | 0.92 (0.72-1.17) | 0.491 | 0.74 (0.51-1.07) | 0.112 | 1.08 (0.79-1.48) | 0.613 |
| Daytime dysfunction | 0.5 (0.6) | 0.4 (0.7) | 1.25 (0.89-1.76) | 1.13 (0.79-1.62) | 0.490 | 1.37 (0.84-2.23) | 0.210 | 0.96 (0.59-1.57) | 0.880 |
| Men | n = 37 | n = 468 | |||||||
| Global sleep quality | 5.6 (3.4) | 4.0 (3.1) | 1.15 (1.05-1.26) | 1.17 (1.05-1.30) | 0.004 | 1.15 (1.00-1.33) | 0.051 | 1.18 (1.02-1.37) | 0.027 |
| Subjective sleep quality | 0.7 (0.6) | 0.6 (0.6) | 1.36 (0.80-2.30) | 1.52 (0.86-2.69) | 0.146 | 1.39 (0.65-3.01) | 0.398 | 1.70 (0.77-3.78) | 0.192 |
| Sleep latency | 0.8 (1.0) | 0.7 (0.8) | 1.26 (0.88-1.80) | 1.21 (0.84-1.77) | 0.307 | 1.05 (0.62-1.78) | 0.843 | 1.45 (0.86-2.43) | 0.162 |
| Habitual sleep efficiency | 1.2 (1.2) | 0.5 (0.9) | 1.96 (1.46-2.62) | 1.94 (1.42-2.66) | < 0.001 | 1.94 (1.28-2.93) | 0.002 | 1.92 (1.21-3.02) | 0.005 |
| Sleep disturbances | 0.8 (0.4) | 0.7 (0.5) | 1.19 (0.61-2.30) | 1.30 (0.63-2.66) | 0.474 | 1.08 (0.42-2.75) | 0.879 | 1.63 (0.57-4.72) | 0.364 |
| Use of sleep medication | 0.5 (1.1) | 0.5 (1.1) | 0.99 (0.73-1.35) | 0.95 (0.62-1.45) | 0.815 | 0.80 (0.46-1.41) | 0.443 | 1.23 (0.65-2.34) | 0.523 |
| Daytime dysfunction | 0.4 (0.6) | 0.3 (0.5) | 1.26 (0.72-2.20) | 1.12 (0.61-2.07) | 0.706 | 1.43 (0.67-3.07) | 0.357 | 0.71 (0.24-2.04) | 0.522 |
CI, confidence interval; OR, odds ratio; PSQI, Pittsburgh Sleep Quality Index; SD, standard deviation.
OR and 95% CI were estimated by a logistic regression with incident cognitive impairment as the predicted variable adjusted for age, education, Mini-Mental State Examination score at baseline, depression episodes, anxiety, psychotropic drug use, cardiovascular conditions, and chronic diseases.
P values were obtained by Wald F statistics with df = 1. ORs reflect a 1-point increase of PSQI score (global sleep quality) or PSQI subscale score.
Table 3 shows sleep duration categories for men and women with and without incident cognitive impairment. Incident amnestic cognitive impairment was significantly predicted by long sleep duration in women and short sleep duration in men. No other significant association was observed.
Table 3.
Subjective sleep duration and incident cognitive impairment in older men and women (n = 1,664)
| Sleep Duration | Incident Cognitive Impairment |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| General |
Amnestic |
Nonamnestic |
|||||||
| Yes n (%) | No n (%) | Crude OR (95% CI) | Adjusted* OR (95% CI) | P | Adjusted* OR (95% CI) | P | Adjusted* OR (95% CI) | P | |
| Women | n = 68 | n = 1091 | |||||||
| Long (≥ 9 hr) | 14 (20.6) | 136 (12.5) | 1.97 (1.05-3.70) | 2.10 (1.10-4.00) | 0.024 | 3.70 (1.49-9.17) | 0.005 | 1.34 (0.54-3.34) | 0.530 |
| Short (≤ 5 hr) | 11 (16.2) | 131 (12.0) | 1.61 (0.81-3.20) | 1.31 (0.65-2.65) | 0.454 | 1.60 (0.56-4.61) | 0.382 | 1.18 (0.47-2.95) | 0.729 |
| Average (> 5 hr and < 9 hr) | 43 (63.2) | 824 (75.5) | Ref | Ref | Ref | Ref | |||
| Men | n = 37 | n = 468 | |||||||
| Long (≥ 9 hr) | 1 (2.7) | 50 (10.7) | 0.29 (0.04-2.16) | 0.26 (0.03-2.03) | 0.200 | 0.51 (0.06-4.26) | 0.533 | - | - |
| Short (≤ 5 hr) | 10 (27.0) | 46 (9.8) | 3.11 (1.41-6.86) | 2.91 (1.24-6.82) | 0.014 | 4.95 (1.72-14.27) | 0.003 | 1.19 (0.25-5.64) | 0.830 |
| Average (> 5 hr and < 9 hr) | 26 (70.3) | 372 (79.5) | Ref | Ref | Ref | Ref | |||
CI, confidence interval; OR, odds ratio; Ref, reference category.
OR and 95% CI were estimated by a logistic regression with incident cognitive impairment as the predicted variable adjusted for age, education, Mini-Mental State Examination score at baseline, depressive episodes, anxiety, psychotropic drug use, cardiovascular conditions, and chronic diseases.
P values were obtained by Wald F statistics with df = 1.
DISCUSSION
The current study examined the relationship between sleep quality and 1-yr incident cognitive impairment in a community-dwelling sample of older men and women with intact cognitive functioning at baseline. The results showed that overall sleep quality was linked with incident cognitive impairment in men, but not in women. In men, an increase of 1 point on the global PSQI score increased by 17% the likelihood of developing an incident cognitive impairment in the following year. Moreover, specific sleep quality indicators were differently associated with incident cognitive impairment in men and women. In women, PSQI sleep disturbances score predicted incident cognitive impairment, especially of the nonamnestic type, whereas long sleep duration predicted incident amnestic cognitive impairment. In men, the PSQI habitual sleep efficiency score predicted general incident cognitive impairment whereas short sleep duration predicted incident amnestic cognitive impairment. All significant associations with sleep quality indicators were notable, with the OR ranging from 3.7 to 4.9 for dichotomous variables and from 1.9 to 2.6 for continuous variables. These results suggest that alteration of sleep quality predicts cognitive decline in older men and in women.
Few previous studies assessed whether subjective sleep disturbance predicted cognitive decline in older adults with normal cognitive functioning and they yielded inconsistent results.20–22 Based on the results from Cricco et al.,20 who observed that older men but not older women with insomnia were more likely to show signs of cognitive decline 2 yr later, we expected that men would show stronger association between poor subjective sleep and incident cognitive impairment than women. Our results do not support this hypothesis because 2 sleep quality indicators were significantly linked to incident cognitive impairment in both men and women and that the strength of these associations was similar. The difference of sleep quality indicators used might explain why we observed significant associations in women and Cricco et al. did not. When criteria for insomnia similar to those of Cricco et al. were used on our sample (for example, “cannot get to sleep within 30 minutes” and “wake up in the middle of the night or early morning” 3 or more times a week), no significant association was observed between insomnia and incident cognitive impairment in women (data not shown). This suggests that poor subjective sleep measured by one or two items may not be discriminant enough to identify older women with sleep complaints at risk of incident cognitive impairment. Moreover, our results are inconsistent with the results from Tworoger et al.,21 who observed no link between sleep duration and cognitive decline in older women. The categories of unusual sleep duration in the current study (≤ 5 hr and ≥ 9 hr of sleep) were determined based on Tworoger et al.'s categories. The main difference between the two studies resides in the measures of cognitive decline used. Tworoger et al. conducted analyses on the difference between cognitive score at baseline and at follow-up. Our criteria for incident cognitive impairment were aimed at identifying older adults with significant changes of cognitive functioning, that is, potentially pathologic cognitive decline, and the discrepancies between these 2 procedures may account for the differences of results.
Potential Mechanisms
Although the current findings indicate that subjective sleep indicators may predict cognitive decline, the mechanisms underlying these associations remain unknown. There are many potential mechanisms that could explain these associations. First, poor sleep quality could reflect a consequence of brain neurodegeneration.41 Previous studies showed that sleep quality is poor in older adults with AD and those with the “predementia state” mild cognitive impairment.1,2,4,42,43 In light of our results, it is possible that brain changes due to AD pathology may alter sleep even before the onset of the first cognitive symptoms. Previous results indicated that disrupted sleep-wake patterns increases with AD severity44; recent data showed that older women with altered circadian activity rhythm were more likely to develop mild cognitive impairment or dementia 5 yr later.45 However, it is important to emphasize that the findings of the current study concern 1-yr incident cognitive impairment and this impairment may not necessarily be permanent. It is possible that sleep indicators predictive of short-term cognitive decline differ from those of long-term cognitive decline occurring through several years or decades. Second, incident cognitive impairment could be the consequence of a sleep disorder per se. In previous studies, obstructive sleep apnea was associated with poorer cognitive functioning and higher risk of mild cognitive impairment, in addition to subjective poor sleep quality.46–50 Hence, it is possible that the links between poor sleep quality and incident cognitive impairment observed in the current study were due to obstructive sleep apnea. However, this hypothesis could not be investigated because data on obstructive sleep apnea or body-mass index were not available in the ESA study. The hypothesis of a link between obstructive sleep apnea and AD was also proposed51 and is supported by the fact that apolipoprotein ε4 genotype (APOE4), which increases the risk of AD,52–54 also increases the risk of sleep apnea in many55,56 but not all57 studies. However, the effect of obstructive sleep apnea on cognitive functioning does not appear to increase with age,48 and sleep breathing disorders may have more effect on younger than older adults.48,58,59 Moreover, APOE4 increases the risk of other types of dementia than AD,60,61 rates of sleep apnea between AD patients and control subjects do not greatly diverge,62–64 and treating sleep apnea in AD patients is beneficial for cognitive function.65 Thus, sleep apnea and AD appear to be 2 separate conditions; sleep apnea may aggravate the effect of AD by causing alterations to the cerebrovascular system.66 Moreover, it should be noted that two recent studies using a large cohort of relatively healthy older adults found that after adjusting for potential confounding variables, sleep-related breathing disorders and obstructive sleep apnea had any or few and weak relationships with cognitive functioning.47,67 This suggests that the impact of sleep apnea on cognitive functioning may be observed after reaching a particular threshold of severity rather than a dose-response relationship. Third, sleep disturbances could also be an aggravating factor for cognitive changes related to neurodegenerative disorders. This hypothesis is supported by results showing that chronic sleep deprivation is linked to an increase of amyloid-β plaque formation, a hallmark of AD, in amyloid precursor protein transgenic mice.68 This finding suggests that poor sleep quality in older adults could exacerbate or accelerate the neurodegenerative process of AD. Moreover, the potential effect of sleep deprivation on neurodegenerative processes may not be restricted to AD because data in a small sample of older adults with nonamnestic mild cognitive impairment indicated that wake after sleep onset measured by actigraphy was correlated with cognitive functioning.42
Subjective/Objective Sleep Quality and Sex Differences
Alteration of perceived sleep duration may be due to a problem of self-evaluating sleep rather than an actual objective alteration of sleep duration. Previous studies in older adults indicated that sleep duration measured by self-report and polysomnography or actigraphy diverges.69–71 Subjectively poor sleepers tend to estimate shorter sleep duration, and men appear to show more divergence between subjective and objective sleep duration than women.69 In addition, notable sex differences in sleep parameters are observed in older adults. Women report shorter and poorer sleep than men, whereas men display more objective sleep alterations than women.72,73 Thus, as previously suggested by Cricco et al.,20 sleep complaints could reflect more severe objective sleep alterations in men than in women. This hypothesis may account, at least partially, for the relationship observed in the current study between global PSQI score and incident cognitive impairment in men but not in women. However, it does not explain why men and women had different sleep quality indicators linked to incident cognitive impairment. Further studies on cognitive decline comparing older men and women on both subjective and objective sleep quality measures are needed to clarify these results.
Alternative Hypotheses
Because poor sleep quality and depression are associated,40 it could be argued that poor sleep quality may reflect subclinical depressive symptomatolgy and that incident cognitive impairment was preceded by these subclinical symptoms rather than poor sleep quality per se. Subclinical anxiety symptoms were taken into account because previous results suggested that it can predict cognitive decline,9,74 but for depressive symptoms, only depressive episodes were statistically controlled. It is thus possible that a subclinical depressive symptomatology may have occurred in participants with incident cognitive impairment. We repeated the analyses without participants who experienced one of the two core symptoms of major depressive episode (n = 257), that is, at least 2 weeks of depressed mood or markedly diminished interest or pleasure in daily activities. After these additional analyses, results remained unchanged except for sleep disturbance score, which the association with incident cognitive impairment slightly diminished and was no longer significant in women (P = 0.088; results not shown). Moreover, insomnia increases the risk of depression,75,76 and because cognitive impairment are commonly observed in older adults with depressed mood,77,78 poor sleep quality linked to incident cognitive impairment may simply be due to incident depression. However, controlling for depressive episodes at follow-up had no effect on the results. Thus, the current results suggest that poor sleep quality linked to incident cognitive impairment is an independent phenomenon of the relationship between depressive symptoms and cognitive functioning.
Strengths, Limitations, and Directions for Further Research
The current study has many strengths. First, the sample used was relatively large and was randomly recruited. Second, unlike previous studies assessing the relationship between subjective sleep quality and cognitive decline, the use of the PSQI in the current study allowed the examination of a more complete set of subjective sleep quality indicators, which identified notable associations in addition to important differences between men and women. Third, the current study took into account many potential confounding variables, including baseline cognitive functioning, depressive symptoms, physical health, use of psychotropics, and sociodemographic factors. Unlike previous studies, because anxiety was previously linked to incident cognitive impairment,9,74 anxiety symptoms were also statistically controlled. The current study also has limitations. First, the MMSE was the only cognitive assessment conducted in the ESA study. The MMSE is a screening instrument and is somewhat limited in its ability to detect subtle cognitive impairment. Therefore, it is possible that the use of a more elaborate cognitive assessment could have resulted in stronger or additional significant associations than the one found. Second, the PSQI assesses sleep quality in the preceding month. Therefore, the length of poor sleep quality encountered by participants who showed incident cognitive impairment is unknown. Longitudinal studies using multiple assessment of subjective sleep quality would help to determine if incident cognitive impairment follows acute or chronic poor sleep quality, which could provide valuable information about the potential underlying mechanisms. Third, the current study was restricted to subjective sleep quality. Because objective and subjective sleep quality are not equivalent, further studies measuring objective sleep measures are needed to investigate the biological markers associated with poor subjective sleep quality indicators related to cognitive decline. Fourth, although sex -stratified analyses yielded interesting findings, this procedure reduced the number of participants with incident cognitive impairment in each analysis. This was particularly noticeable in men because of their lower proportion in the sample. It is therefore possible that some effects were not detected in men because of a lack of statistical power and, accordingly, the results regarding sex differences should be interpreted with caution.
In summary, similarly to other neuropsychiatric symptomatology such as depressive symptoms, the results from the current study indicated that poor subjective sleep quality measured by the PSQI was linked to incident cognitive impairment. Sex differences were observed as incident cognitive impairment was predicted by sleep disturbance score and long sleep duration in older women, and habitual sleep efficiency score and short sleep duration in older men. Sleep quality in older adults should receive particular attention by clinicians because poor sleep quality can be an early sign of cognitive decline. Further studies should examine whether poor sleep quality preceding cognitive decline is the consequence of particular sleep disorders and/or an underlying neurodegenerative disorder.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Hudon participates in and receives compensation for studies by Pfizer. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by research grants from the Canadian Institutes of Health Research (CIHR; 200403MOP) and the Fonds de recherche en santé du Québec (FRSQ; 9854). Dr. Potvin is supported by a postdoctoral fellowship award from the CIHR. Dr. Hudon is supported by a FRSQ Chercheur-boursier Junior 2 salary award (22420).
REFERENCES
- 1.Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 2008;25:115–26. doi: 10.1159/000112509. [DOI] [PubMed] [Google Scholar]
- 2.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–83. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 3.Potvin O, Hudon C, Grenier S, Préville M. Non-essential symptoms of depression and cognitive impairment no dementia (CIND) in community-dwelling elders without dysphoria or anhedonia. Int Psychogeriatr. 2010;22:1344–52. doi: 10.1017/S1041610210001419. [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu-Bonneau S, Hudon C. Sleep disturbances in older adults with mild cognitive impairment. Int Psychogeriatr. 2009;21:654–66. doi: 10.1017/S1041610209009120. [DOI] [PubMed] [Google Scholar]
- 5.Palmer K, Backman L, Winblad B, Fratiglioni L. Mild cognitive impairment in the general population: occurrence and progression to Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:603–11. doi: 10.1097/JGP.0b013e3181753a64. [DOI] [PubMed] [Google Scholar]
- 6.Brommelhoff JA, Gatz M, Johansson B, McArdle JJ, Fratiglioni L, Pedersen NL. Depression as a risk factor or prodromal feature for dementia? Findings in a population-based sample of Swedish twins. Psychol Aging. 2009;24:373–84. doi: 10.1037/a0015713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH. Depressive symptoms, sex, and risk for Alzheimer's disease. Ann Neurol. 2005;57:381–7. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 8.Fuhrer R, Dufouil C, Dartigues JF. Exploring sex differences in the relationship between depressive symptoms and dementia incidence: prospective results from the PAQUID Study. J Am Geriatr Soc. 2003;51:1055–63. doi: 10.1046/j.1532-5415.2003.51352.x. [DOI] [PubMed] [Google Scholar]
- 9.Potvin O, Forget H, Grenier S, Préville M, Hudon C. Anxiety, depression and one-year incident cognitive impairment in community-dwelling older adults. J Am Geriatr Soc. 2011;59:1421–8. doi: 10.1111/j.1532-5415.2011.03521.x. [DOI] [PubMed] [Google Scholar]
- 10.Kohler S, van Boxtel MP, van Os J, et al. Depressive symptoms and cognitive decline in community-dwelling older adults. J Am Geriatr Soc. 2010;58:873–9. doi: 10.1111/j.1532-5415.2010.02807.x. [DOI] [PubMed] [Google Scholar]
- 11.Ng TP, Niti M, Zaw MH, Kua EH. Depressive symptoms and incident cognitive impairment in cognitively well-functioning older men and women. J Am Geriatr Soc. 2009;57:1058–63. doi: 10.1111/j.1532-5415.2009.02262.x. [DOI] [PubMed] [Google Scholar]
- 12.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging and Alzheimer's Association workgroup. Alzheimer Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois B, Albert ML. Amnestic MCI or prodromal Alzheimer's disease? Lancet Neurology. 2004;3:246–8. doi: 10.1016/S1474-4422(04)00710-0. [DOI] [PubMed] [Google Scholar]
- 14.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 16.Cervilla JA, Prince M, Mann A. Smoking, drinking, and incident cognitive impairment: a cohort community based study included in the Gospel Oak project. J Neurol Neurosurg Psychiatry. 2000;68:622–6. doi: 10.1136/jnnp.68.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devier DJ, Pelton GH, Tabert MH, et al. The impact of anxiety on conversion from mild cognitive impairment to Alzheimer's disease. Int J Geriatr Psychiatry. 2009;24:1335–42. doi: 10.1002/gps.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bierman EJ, Comijs HC, Rijmen F, Jonker C, Beekman AT. Anxiety symptoms and cognitive performance in later life: results from the longitudinal aging study Amsterdam. Aging Ment Health. 2008;12:517–23. doi: 10.1080/13607860802224276. [DOI] [PubMed] [Google Scholar]
- 19.Liu HC, Wang PN, Wang HC, et al. Conversion to dementia from questionable dementia in an ethnic Chinese population. J Geriatr Psychiatry Neurol. 2007;20:76–83. doi: 10.1177/0891988706298626. [DOI] [PubMed] [Google Scholar]
- 20.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–9. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 21.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–8. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 22.Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline:. report from the Maastricht Ageing Study (MAAS) Int J Geriatr Psychiatry. 2002;17:73–7. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- 23.Préville M, Boyer R, Grenier S, et al. The epidemiology of psychiatric disorders in Quebec's older adult population. Can J Psychiatry. 2008;53:822–32. doi: 10.1177/070674370805301208. [DOI] [PubMed] [Google Scholar]
- 24.Statistics Canada. 2006 Census of Population [Statistics Canada Web site] 2006. [cited November 4, 2009]. Available at http://www40.statcan.gc.ca/l01/cst01/demo15-eng.htm.
- 25.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Blais FC, Gendron L, Mimeault V, Morin CM. [Evaluation of insomnia: validity of 3 questionnaires] L'Encephale. 1997;23:447–53. [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Hébert R, Bravo G, Girouard D. Validation de l'Adaptation Française du Modified Mini-Mental State (3MS) Revue de Gériatrie. 1992;17:443–50. [Google Scholar]
- 29.Erdman HP, Klein MH, Greist JH, et al. A comparison of two computer-administered versions of the NIMH Diagnostic Interview Schedule. J Psychiatr Res. 1992;26:85–95. doi: 10.1016/0022-3956(92)90019-k. [DOI] [PubMed] [Google Scholar]
- 30.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–9. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 31.Wittchen HU, Robins LN, Cottler LB, Sartorius N, Burke JD, Regier D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI): the Multicentre WHO/ADAMHA Field Trials. Br J Psychiatry. 1991;159:645–53. 58. doi: 10.1192/bjp.159.5.645. [DOI] [PubMed] [Google Scholar]
- 32.Hudon C, Potvin O, Turcotte MC, et al. Normalisation du Mini-Mental State Examination (MMSE) chez les Quebecois francophones ages de 65 ans et plus et residant dans la communaute. Can J Aging. 2009;28:347–57. doi: 10.1017/S0714980809990171. [DOI] [PubMed] [Google Scholar]
- 33.Frisoni GB, Fratiglioni L, Fastbom J, Viitanen M, Winblad B. Mortality in nondemented subjects with cognitive impairment: the influence of health-related factors. Am J Epidemiol. 1999;150:1031–44. doi: 10.1093/oxfordjournals.aje.a009927. [DOI] [PubMed] [Google Scholar]
- 34.Potvin O, Hudon C, Dion M, Grenier S, Préville M. Anxiety disorders, depressive episodes and cognitive impairment no dementia in community-dwelling older men and women. Int J Geriatr Psychiatry. 2010 doi: 10.1002/gps.2647. [DOI] [PubMed] [Google Scholar]
- 35.Palmer K, Wang HX, Backman L, Winblad B, Fratiglioni L. Differential evolution of cognitive impairment in nondemented older persons: results from the Kungsholmen Project. Am J Psychiatry. 2002;159:436–42. doi: 10.1176/appi.ajp.159.3.436. [DOI] [PubMed] [Google Scholar]
- 36.Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry. 2007;78:1298–303. doi: 10.1136/jnnp.2006.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62:1160–3. doi: 10.1001/archneur.62.7.1160. discussion 7. [DOI] [PubMed] [Google Scholar]
- 38.Carcaillon L, Amieva H, Auriacombe S, Helmer C, Dartigues JF. A subtest of the MMSE as a valid test of episodic memory? Comparison with the Free and Cued Reminding Test. Dement Geriatr Cogn Disord. 2009;27:429–38. doi: 10.1159/000214632. [DOI] [PubMed] [Google Scholar]
- 39.American Society of Health System Pharmacist. AHFS drug information. 2001.
- 40.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision (DSM-IV-TR) Washington DC: American Psychiatric Publishing, Inc.; 2000. [Google Scholar]
- 41.Meeks TW, Ropacki SA, Jeste DV. The neurobiology of neuropsychiatric syndromes in dementia. Current Opinion in Psychiatry. 2006;19:581–6. doi: 10.1097/01.yco.0000245746.45384.0e. [DOI] [PubMed] [Google Scholar]
- 42.Naismith SL, Rogers NL, Hickie IB, Mackenzie J, Norrie LM, Lewis SJ. Sleep well, think well: sleep-wake disturbance in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23:123–30. doi: 10.1177/0891988710363710. [DOI] [PubMed] [Google Scholar]
- 43.Naismith SL, Rogers NL, Lewis S JG, et al. Sleep disturbance in mild cognitive impairment: differential effects of current and remitted depression. Acta Neuropsychiatrica. doi: 10.1111/j.1601-5215.2011.00555.x. in press. [DOI] [PubMed] [Google Scholar]
- 44.Moe KE, Vitiello MV, Larsen LH, Prinz PN. Symposium: Cognitive processes and sleep disturbances: Sleep/wake patterns in Alzheimer's disease: relationships with cognition and function. J Sleep Res. 1995;4:15–20. doi: 10.1111/j.1365-2869.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 45.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and MCI in older women. Ann Neurol. doi: 10.1002/ana.22468. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sforza E, Roche F, Thomas-Anterion C, et al. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33:515–21. doi: 10.1093/sleep/33.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathieu A, Mazza S, Decary A, et al. Effects of obstructive sleep apnea on cognitive function: a comparison between younger and older OSAS patients. Sleep Med. 2008;9:112–20. doi: 10.1016/j.sleep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Alchanatis M, Zias N, Deligiorgis N, et al. Comparison of cognitive performance among different age groups in patients with obstructive sleep apnea. Sleep Breath. 2008;12:17–24. doi: 10.1007/s11325-007-0133-y. [DOI] [PubMed] [Google Scholar]
- 50.Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS) Sleep. 2000;23(Suppl 4):S102–8. [PubMed] [Google Scholar]
- 51.Abrams B. Add Alzheimer's to the list of sleep apnea consequences. Med Hypotheses. 2005;65:1201–2. doi: 10.1016/j.mehy.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 52.Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993;342:697–9. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 53.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–72. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 54.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–81. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kadotani H, Kadotani T, Young T, et al. Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA. 2001;285:2888–90. doi: 10.1001/jama.285.22.2888. [DOI] [PubMed] [Google Scholar]
- 56.Gottlieb DJ, DeStefano AL, Foley DJ, et al. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology. 2004;63:664–8. doi: 10.1212/01.wnl.0000134671.99649.32. [DOI] [PubMed] [Google Scholar]
- 57.Thakre TP, Mamtani MR, Kulkarni H. Lack of association of the APOE epsilon 4 allele with the risk of obstructive sleep apnea: meta-analysis and meta-regression. Sleep. 2009;32:1507–11. doi: 10.1093/sleep/32.11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young T. Sleep-disordered breathing in older adults: is it a condition distinct from that in middle-aged adults? Sleep. 1996;19:529–30. doi: 10.1093/sleep/19.7.529. [DOI] [PubMed] [Google Scholar]
- 59.Ancoli-Israel S, Coy T. Are breathing disturbances in elderly equivalent to sleep apnea syndrome? Sleep. 1994;17:77–83. doi: 10.1093/sleep/17.1.77. [DOI] [PubMed] [Google Scholar]
- 60.Myers RH, Schaefer EJ, Wilson PW, et al. Apolipoprotein E epsilon4 association with dementia in a population-based study: the Framingham study. Neurology. 1996;46:673–7. doi: 10.1212/wnl.46.3.673. [DOI] [PubMed] [Google Scholar]
- 61.Frisoni GB, Bianchetti A, Govoni S, Trabucchi M, Calabresi L, Franceschini G. Association of apolipoprotein E E4 with vascular dementia. JAMA. 1994;271:1317. [PubMed] [Google Scholar]
- 62.Smallwood RG, Vitiello MV, Giblin EC, Prinz PN. Sleep apnea: relationship to age, sex, and Alzheimer's dementia. Sleep. 1983;6:16–22. doi: 10.1093/sleep/6.1.16. [DOI] [PubMed] [Google Scholar]
- 63.Hoch CC, Reynolds CF, 3rd, Kupfer DJ, Houck PR, Berman SR, Stack JA. Sleep-disordered breathing in normal and pathologic aging. J Clin Psychiatry. 1986;47:499–503. [PubMed] [Google Scholar]
- 64.Bliwise DL, Yesavage JA, Tinklenberg JR, Dement WC. Sleep apnea in Alzheimer's disease. Neurobiol Aging. 1989;10:343–6. doi: 10.1016/0197-4580(89)90046-8. [DOI] [PubMed] [Google Scholar]
- 65.Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076–81. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bliwise DL. Sleep apnea, APOE4 and Alzheimer's disease 20 years and counting? J Psychosom Res. 2002;53:539–46. doi: 10.1016/s0022-3999(02)00436-1. [DOI] [PubMed] [Google Scholar]
- 67.Quan SF, Chan CS, Dement WC, et al. The association between obstructive sleep apnea and neurocognitive performance: the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2011;34:303–14B. doi: 10.1093/sleep/34.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17:295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 70.Unruh ML, Redline S, An MW, et al. Subjective and objective sleep quality and aging in the sleep heart health study. J Am Geriatr Soc. 2008;56:1218–27. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- 71.Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–8. [PubMed] [Google Scholar]
- 72.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 73.van den Berg JF, Miedema HM, Tulen JH, Hofman A, Neven AK, Tiemeier H. Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep. 2009;32:1367–75. doi: 10.1093/sleep/32.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gallacher J, Bayer A, Fish M, et al. Does anxiety affect risk of dementia? Findings from the Caerphilly Prospective Study. Psychosom Med. 2009;71:659–66. doi: 10.1097/PSY.0b013e3181a6177c. [DOI] [PubMed] [Google Scholar]
- 75.Yokoyama E, Kaneita Y, Saito Y, et al. Association between depression and insomnia subtypes: a longitudinal study on the elderly in Japan. Sleep. 2010;33:1693–702. doi: 10.1093/sleep/33.12.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 77.Lockwood KA, Alexopoulos GS, Kakuma T, Van Gorp WG. Subtypes of cognitive impairment in depressed older adults. Am J Geriatr Psychiat. 2000;8:201–8. [PubMed] [Google Scholar]
- 78.Herrmann LL, Goodwin GM, Ebmeier KP. The cognitive neuropsychology of depression in the elderly. Psychol Med. 2007;37:1693–702. doi: 10.1017/S0033291707001134. [DOI] [PubMed] [Google Scholar]