Abstract
Introduction:
Cognitive and brain hyperactivation have been associated with trouble falling asleep and sleep misperception in patients with primary insomnia (PI). Activation and synchronization/temporal coupling in frontal and frontoparietal regions involved in executive control and endogenous attention might be implicated in these symptoms.
Methods:
Standard polysomnography (PSG) and electroencephalogram (EEG) were recorded in 10 unmedicated young patients (age 19-34 yr) with PI with no other sleep/medical condition, and in 10 matched control subjects. Absolute power, temporal coupling, and topographic source distribution (variable resolution electromagnetic tomography or VARETA) were obtained for all time spent in waking, Stage 1 and Stage 2 of the wake-sleep transition period (WSTP), and the first 3 consecutive min of N3. Subjective sleep quality and continuity were evaluated.
Results:
In comparison with control subjects, patients with PI exhibited significantly higher frontal beta power and current density, and beta and gamma frontoparietal temporal coupling during waking and Stage 1.
Conclusion:
These findings suggest that frontal deactivation and disengagement of brain regions involved in executive control, attention, and self-awareness are impaired in patients with PI. The persistence of this activated and coherent network during the wake-sleep transition period (WSTP) may contribute to a better understanding of underlying mechanisms involved in difficulty in falling asleep, in sleep misperception, and in the lighter, poorer, and nonrefreshing sleep experienced by some patients with PI.
Citation:
Corsi-Cabrera M; Figueredo-Roríguez P; del Río-Portilla Y; Sánchez-Romero J; Galán L; Bosch-Bayard J. Enhanced frontoparietal synchronized activation during the wake-sleep transition in patients with primary insomnia. SLEEP 2012;35(4):501-511.
Keywords: Primary insomnia, spectral EEG, EEG coherence, EEG temporal coupling, EEG topography, VARETA
INTRODUCTION
Trouble falling asleep and misperceptions of one's sleep state are core symptoms of primary insomnia (PI). Such misperceptions have been found to occur in morning judgments of sleep continuity when compared with the results of polysomnographic (PSG) assessments of sleep latency (SL), wakefulness after sleep onset, and total sleep time.1,2 The coexistence of PSG signs of sleep with the feeling of not being asleep suggests an alteration of the individual's conscious awareness in cases of PI.
Among healthy subjects without insomnia, the transition from wakefulness to sleep recorded in electroencephalograms (EEGs) occurs as a gradual progression from wake-related frequency activity (gamma, beta, and alpha frequencies) to sleep-related, low-frequency activity (theta and delta frequencies), and is marked by phasic events (K complexes and spindles) that appear in anterior scalp locations earlier than in posterior areas.3,4 In patients with insomnia, particularly those with sleep initiation problems, this transition is not only prolonged but has also been characterized as incomplete, due to microarousals and sleep instability,5,6 also based on quantitative EEG analysis; that is, the awake state-related frequencies, gamma, beta, and alpha, are higher during wakefulness and tend to persist into non-rapid eye movement (NREM) sleep intermixed with the dominant theta and delta frequencies.1,2,7,8,9,10,11,12,13–14 Although delta activity may decrease, the K complexes15 and sleep spindles16 are not altered. These findings suggest a higher cortical activation that may interfere with sleep and is consistent with the hyperarousal theory of insomnia.17
The wake-sleep transition period (WSTP) involves two different mechanisms: the thalamocortical oscillator mode, which is involved in promoting sleep EEG activity, and other mechanisms participating in regulating wakefulness that act to reduce activating influences in the brainstem, the basal forebrain, and the hypothalamus, all of which participate in regulating wakefulness.18,19 An imbalance between these two processes can provoke a mixed state of conscious awareness that results in the person experiencing the feeling of not being asleep despite PSG signs that indicate the presence of this state.20,21
Experiences of control over one's behavior, awareness of inner thoughts, representations of the mental self, and conscious perceptions of the external world are all central aspects of the conscious state.22 All of the most frequent complaints of insomnia patients related to sleep onset, including the tendency to worry about being able to fall asleep,23 an inability to stop thinking or having intrusive thoughts,24 distractions by external stimuli,25 processing information, forming long-term memories,26 and, in particular, attempts to control sleep directly by focusing attention and actively striving to fall asleep,27 are compatible with enhanced conscious awareness.
As is well known, conscious experience28 and representations of the states of self29 have been attributed to the executive regions of the prefrontal cortex and cingulate gyrus, with the dorsolateral prefrontal cortex playing a crucial role in tasks that demand executive control, such as the need to hold information in one's mind or plan future actions.30 The anterior cingulate, meanwhile, participates in voluntary actions31 including attention, self-regulation, and consciousness.32 This evidence and the nature of the psychologic complaints suggest that the frontal cortex is the region most likely to show high beta and gamma activity in patients with insomnia. Glucose metabolism in the prefrontal cortex and anterior cingulate is correlated with the amount of waking time after sleep onset in patients with chronic insomnia33 and is higher in those subjects than in good sleepers.34 However, the topographic distribution of fast activity during the WSTP has not yet been explored in patients with PI.
EEG and neuroimaging studies have shown that the interaction among, and coordinated activation of, brain regions are both involved in conscious awareness of external stimuli and in self-awareness during wakeful states. Specifically, reports indicate that frontoparietal interaction is involved in focusing attention35 and selecting sensory stimuli derived from internal goals,36 as well as (with the precuneus and angular gyrus) the conscious perception of visual verbal stimuli37 and reflective self-awareness.22 It is important to point out that the associations among specific brain regions over both short and long distances are facilitated by beta and gamma activity38,39; for example, enhanced gamma synchronization has been shown to underlie conscious visual perception.40 Sleep, on the other hand, is characterized by the loss of executive control and the self-awareness associated with decreased synchronization or temporal coupling in the theta and gamma frequencies between the frontal and posterior association regions.41,42 Thus, enhanced temporal coupling in the beta and gamma activity that links the frontal region with the parietal and posterior midline regions could be expected in patients with PI.
All of this evidence suggests that the feeling of being awake despite objective signs of sleep during the WSTP in PI may be supported by increased frontal activation and temporal coupling in the regions involved in self-awareness, inner attention, and executive control, such as the prefrontal, cingulate, and parietal cortices. The aim of this study was to explore beta and gamma temporal coupling and its topographic distribution as an index of cortical activation during the WSTP in a group of young, nondepressed, unmedicated patients with PI. We anticipated that in comparison with control subjects having regular sleep patterns, the patients with PI would show higher beta and gamma activity and greater temporal coupling among the prefrontal, cingulate, and parietal regions during wakefulness, and that synchronized activation would persist during the sleep stages of the WSTP.
METHOD
Ten young, right-handed patients (4 women, 6 men) participated in the study. To avoid possible confounding effects of age and hormonal status on EEG and sleep, age was restricted to a range of 19-32 yr. All subjects met the criteria for PI: difficulty with sleep onset 3 or more nights per wk for at least 6 mo, and impaired daytime functioning.43 None had any medical, psychiatric, or neurologic conditions, nor had they been medicated for insomnia or suffered other sleep disorders. All subjects in the control group (CG) (n = 10; 5 women and 5 men) were right-handed and matched for age and education, but had no complaints of insomnia. They reported their sleep as restorative and satisfactory and had regular sleep habits. Women were recorded between days 3 and 5 of their menstrual cycle.
Participants were recruited from the Sleep Clinic at the Faculty of Medicine of the Universidad Nacional Autónoma de México and through announcements posted around the campus. Each subject underwent a 4-step screening that included several instruments: first, after initial contact, potential participants were invited for a session at the sleep laboratory where their clinical histories were recorded and a psychiatric interview conducted. They were given a 15-day log to assess subjective sleep continuity and normal sleep habits. Second, they were asked to return to the laboratory once their sleep logs had been completed to verify the insomnia symptoms of the subjects in the PI group, the absence of sleep complaints in the CG, and regular bedtime hours (from 22:00-24:00 h to 06:00-08:00 h) in both groups, based on their sleep logs. Potential participants with phase-delay insomnia were excluded. At that time, additional tests were administered: the Pittsburgh Sleep Quality Index44 and the Athens Insomnia Scale45 to further confirm sleep complaints, and the Beck Depression Inventory46 and Hamilton Depression Scale47 to verify the absence of clinical depression. All participants scored below the cutoff point for moderate depression on both scales. Third, those individuals who satisfied the screening criteria were scheduled for 1 night of PSG at the laboratory. Fourth, the absence of medications and subjects' drug-free condition were corroborated before PSG using the Multi Drug 6 Panel Urine Test (MEDIMPEX United Inc, Bensalem, PA). Also, the absence of respiratory sleep disorders and periodic limb movements was further corroborated by PSG. This resulted in the exclusion of 3 candidates from the final study groups; 2 because of clinically asymptomatic periodic limb movements and 1 because of clinically asymptomatic sleep apnea. They were replaced by other subjects.
All participants gave their informed, written consent and were offered treatment if they so desired. The protocol was approved by the Ethics Committee of the Faculty of Medicine at the Universidad Nacional Autónoma de México.
Polysomnography
Standard PSG on the first night was conducted at the laboratory with EEGs (C4-A1), electrooculogram (EOG), and electromyograms (EMGs) of the mentalis muscle. Oral-nasal airflow and anterior tibialis EMGs were also recorded to further ensure that no participant had signs of sleep apnea or periodic limb movements.
The first-night PSG at the sleep laboratory was recorded to take advantage of the so-called first night effect that has been proposed as a model of transient insomnia,48 and the so-called reverse first night effect that some patients with PI exhibit; that is, while regular sleepers tend to sleep more poorly under these conditions compared with their normal patterns, some patients with PI sleep much better49 during the first night at the laboratory. Thus, when comparing regular sleepers and those with PI using both objective and subjective measures of sleep, the results tend to differ very little and, accordingly, any observed difference in brain activity would therefore be expected to correspond to factors other than sleep continuity and to represent trait versus state differences between groups.
Additionally, EEG activity was recorded at 19 locations of the 10-20 International System (Fp1, Fp2, F3, F4, F7, F8, Fz, C3, C4, Cz, T3, T4, T5, T6, P3, P4, Pz, O1, O2), and referred to the earlobes with filter settings of 0.03-70 Hz. All- night PSG data were digitized and stored at 1,024 Hz with a 12-bit A/D converter using the acquisition program GRASS-GAMMA version 4.4.
PSG was scored in 30-sec epochs by 2 experts who were blind to the subject group, as per standard criteria,50 and sleep variables were obtained for both groups for both the entire night and the WSTP, from lights out after saying good night to the participant until steady slow-wave sleep was attained. For the purposes of this study, steady slow-wave sleep was defined as 3 consecutive min of NREM sleep stages 3+4 (SN3). The EEGs taken during the WSTP were analyzed.
EEG Analysis
The EEGs from WSTP were segmented into nonoverlapping 2-sec epochs and carefully inspected for artifacts. Each artifact-free, 2-sec epoch from the total time spent in wakefulness (W), Stage 1 (N1) and Stage 2 (N2) WSTP, and the 3 consecutive min of SN3 (isolated epochs from N3 were not analyzed) were then fast Fourier- transformed (0.5-50 Hz) using the POTENCOR program.51 The absolute power (AP) values for each 0.5-Hz bin were averaged to obtain two broadbands: beta (17-30 Hz) and gamma (31-45 Hz) for each subject over the same electrode and stage. The lower range for the beta frequencies was defined considering that those frequencies from 13 to 16 Hz overlap the sigma frequency band and thus correspond to either beta during wakefulness or sleep spindle frequencies during sleep. These frequency bands are comparable to those used in other sleep studies.2,12 To achieve gaussianity, logarithmic transformations were applied to the AP values.52
Temporal coupling between electrode pairs was assessed by cross-correlation functions with zero time delay calculated in the time domain by the same POTENCOR program after filtering the signals in the frequency domain for frequencies corresponding to the beta and gamma bands. Cross-correlation values were transformed into Fisher Z scores to approximate them to a normal distribution before conducting statistical comparisons.53
Source Localization
A source localization analysis was performed for the full frequency range (1-50 Hz) and for all the sources at the brain (number of sources, 3,244) to determine if there were significant differences in current density between patients with PI and the CG. The variable resolution electromagnetic tomography (VARETA) method54 was used in the source localization analysis. VARETA is a technique for estimating the distribution of the primary current in the source generators of EEG data. Like low resolution electromagnetic tomography (LORETA),55 VARETA is a discrete spline distributed solution. Spline estimates are the spatially smoothest solutions compatible with the observed data. However, although LORETA imposes maximal spatial smoothness, VARETA imposes different amounts of spatial smoothness for different types of generators, the actual degree of smoothness in each voxel being determined by the data itself, hence the use of the term variable resolution. VARETA allows spatially adaptive nonlinear estimates of current sources and eliminates ghost solutions (artifactual interference patterns), which are often present in linear distributed inverse solutions. Due to this procedure, VARETA produces focal solutions for point sources, as well as distributed solutions for diffuse sources. In addition, anatomic constraints are placed on the allowable solutions by introducing a gray matter weight for each voxel. The effect of these weights on the inverse solution is to prohibit sources where the mask is zero (for example, cerebrospinal fluid or white matter).
EEG activity was re-referenced to the average of all electrodes at each time point for this analysis, as required by VARETA and other inverse solution methods. To render the inverse solution in different subjects comparable for statistics at the sources, VARETA uses a regularization parameter: after transformation to the average reference, the geometric power is standardized by a global scale factor to control for individual differences in power values due to skull thickness, hair volume, electrode impedance, and other factors of variance that can affect the EEG amplitude but are not related to electrophysiologic matters. This procedure is described in detail elsewhere.54 VARETA has been used satisfactorily for source localization in psychiatric patients.56,57
Self-Reported Measures
Before going to sleep, participants' daytime alertness was evaluated with the Hyperarousal Scale.58 Subjective estimations of sleep quality and continuity were assessed the morning after spontaneous awakening, and the degree of insomnia was re-evaluated using the Insomnia Severity Index.59
Statistical Analysis
PSG variables and self-reported measures were compared using independent Student t-tests. AP and temporal coupling were compared by mixed analyses of variance (ANOVAs) with groups (PI and CG) as the between-subjects variable, and stages (W, N1, N2, and SN3) and derivations as the within-subjects variables. For AP, two ANOVAs were performed: one for beta activity and another for gamma activity on the electrodes of interest (Fp1, F7, F3, Fp2, F4, F8, Fz, P3, P4, and Pz). Because we were interested in the differences between PI and CG in temporal coupling among frontal regions, and among the frontal, parietal, and posterior midline regions, four ANOVAs were performed: two for the frontal electrode pairs, one each for the left (Fp1-F3, Fp1-F7, F3-F7, Fp1-Fz, F3-Fz, F7-Fz) and right hemispheres (Fp2-F4, Fp2-F8, F4-F8, Fp2-Fz, F4-Fz, F8-Fz), and two that included frontal and parietal electrode pairs, also one each for the left (Fp1-P3, Fp1-Pz, F3-P3, F3-Pz, F7-P3, F7-Pz) and right hemispheres (Fp2-P4, Fp2-Pz, F4-P4, F4-Pz, F8-P4, F8-Pz). The significance level was set at 0.05. Only significant results of group main effects and group-by-stage-by-derivation interactions were considered and are described. Post-hoc planned comparisons between the two groups for W, N1, N2, and SN3 for significant interactions were made with Tukey Student t-tests to control for multiple comparisons. To evaluate differences in EEG activity at the sources, ANOVA tests of group-by-stage were conducted for the full frequency range (1-50 Hz). To account for multiple comparisons, the permutation test was used (10,000 permutations were conducted). The maximum F statistics for the main effects and interactions, as well as the maximum t statistics for the post-hoc contrasts between the PI group and the CG, were calculated for each frequency and for all sources in the brain, and 95th percentiles were obtained.
The permutation test has the following advantages: it is distribution-free, no assumption of any underlying correlation structure is required, and it provides exact P values for any number of subjects, frequencies and sources. The distribution estimated by the permutation techniques for maximum F and maximum t can then be used to set significance levels that control the experiment-wise error for the simultaneous univariate comparisons. Thus, one can test the differences for all points in a sequence of frequencies simultaneously (for all sources) and avoid the inflation of type I errors.60 Only those results that exceeded the significance level for cortical gray matter sources in the frequencies of interest (beta and gamma) were taken into account and are reported, on the assumption that EEGs recorded on the scalp are generated primarily by highly synchronized cortical clusters of neurons.
RESULTS
The characteristics of the subjects by group are shown in Table 1. There were no significant differences in terms of age, sex distribution, or education. As expected, the PI group had significantly longer sleep latency, shorter sleep duration, and less time in bed as reported in their 15-day sleep logs, and higher scores than the CG on the Pittsburgh Sleep Quality Index44 and the Athens Insomnia Scale45; these findings confirm the insomnia diagnoses in the patients with PI and the absence of sleep complaints in the CG. Both groups scored below the cutoff point for clinical depression on the Beck Depression Inventory (< 13) and the Hamilton Depression Scale (< 12); however, statistical analysis of both instruments showed that patients with PI had higher subclinical signs of depression than the CG.
Table 1.
Demographic and subject characteristics by group
| PI (n = 10) |
CG (n = 10) |
P | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (yr) | 25.9 | 4.3 | 25.6 | 4.6 | ns |
| Education (yr) | 14.6 | 2.4 | 16.2 | 1.39 | ns |
| Illness duration (mo) | 6.3 | 4.08 | – | – | – |
| Sleep log | |||||
| Subjective sleep latency (min) | 59 | 33.23 | 12-9 | 5.25 | 0.001 |
| Subjective sleep duration (hr) | 5.1 | 0.78 | 7.8 | 0.78 | 0.001 |
| Scales | |||||
| Pittsburgh Sleep Quality Index | 12.5 | 2.7 | 4 | 1.4 | 0.0001 |
| Athens Insomnia Scale | 12.3 | 3.5 | 2.2 | 1.8 | 0.0001 |
| Beck Depression Inventory | 6.9 | 2.6 | 2.5 | 1.3 | 0.003 |
| Hamilton Depression Scale | 8.3 | 2.4 | 4.1 | 1.1 | 0.003 |
Between-group analysis P < 0.05. CG, control group; ns, nonsignificant; PI, primary insomnia group.
Polysomnography
There were no significant differences (Table 2) between the groups with respect to any PSG variable for either the entire night or the WSTP.
Table 2.
Objective sleep measures for the whole night and for the wake-sleep transition period
| PI |
CG |
P | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Whole night | |||||
| Total sleep time (min) | 363.22 | 30.63 | 368.5 | 23.53 | ns |
| Sleep efficiency (%) | 86.16 | 6.38 | 88.22 | 4.51 | ns |
| N1/TST | 7.38 | 2.26 | 5.98 | 3.33 | ns |
| N2/TST | 53.64 | 6.08 | 54.65 | 11.78 | ns |
| N3/TST | 21.54 | 6.12 | 20.74 | 7.02 | ns |
| REM Sleep/TST | 17.48 | 6.60 | 18.62 | 8.73 | ns |
| WASO/TST | 6.67 | 3.60 | 4.85 | 3.65 | ns |
| Latency to N1 (min) | 5.44 | 3.60 | 7.61 | 3.90 | ns |
| Latency to N2 (min) | 8.11 | 4.62 | 9.50 | 4.20 | ns |
| Latency to REM (min) | 102.38 | 33.24 | 120.05 | 76.11 | ns |
| Latency to SN3 (min) | 25.33 | 7 | 24.22 | 6.66 | ns |
| Wake-sleep transition period | |||||
| N1/WSTP | 16.71 | 11.31 | 12.09 | 5.60 | ns |
| N2/WSTP | 47.07 | 17.95 | 48.53 | 13.51 | ns |
| WASO/WSTP | 1.44 | 1.44 | 2.33 | 3.69 | ns |
Between-group analysis P < 0.05. CG, control group; N3 refers to Stage 3 + Stage 4; PI, primary insomnia group; REM, rapid eye movement sleep; SN3, steady N3; TST, total sleep time; WASO, wake after sleep onset; WSTP, wake-sleep transition period from lights out to steady N3.
Absolute Power
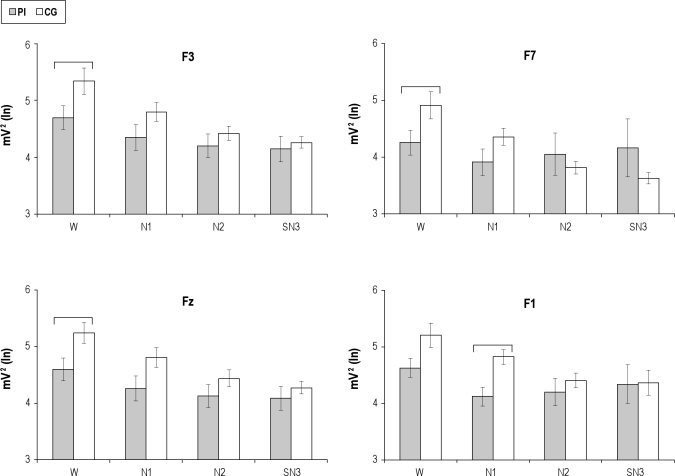
Group main effects were not significant for either beta or gamma power; however, group-by-stage-by-derivation interaction was significant for beta AP (Table 3). Post-hoc planned comparisons between groups showed that beta AP at F3, F7, and Fz during waking, and at Fp1 during N1, was higher among patients with insomnia than in the CG (Figure 1). The differences between the groups at F3, F7, and Fz disappeared in N1, and the difference between them at Fp1 in N1 disappeared in N2. The groups did not differ in N2 and SN3.
Table 3.
Significant (P < 0.05) main effects of group, and group by condition by derivation interactions for absolute power and temporal coupling.
| Absolute power | Group DF = 1,19 |
Group × Condition × Derivation DF = 27,486 |
||
|---|---|---|---|---|
| F | P | F | P | |
| Beta | 1.5 | 0.23 | 1.70 | 0.01 |
| Gamma | 1.57 | 0.24 | 1.16 | 0.27 |
| Frontoparietal temporal coupling | Group DF = 1,19 |
Group × Condition × Derivation DF = 27,486 |
||
|---|---|---|---|---|
| F | P | F | P | |
| Beta | ||||
| Left hemisphere | 6.88 | 0.01 | 2.62 | 0.001 |
| Right hemisphere | 2.24 | 0.15 | 1.22 | 0.26 |
| Gamma | ||||
| Left hemisphere | 6.65 | 0.01 | 2.22 | 0.006 |
| Right hemisphere | 4.55 | 0.04 | 1.37 | 0.16 |
DF, degrees of freedom.
Figure 1.
Frontal Beta power from four electrode locations (F3, F7, Fz, F1) as a function of group and stage. Mean and standard error of beta absolute power, log transformed, in the primary insomnia (PI) and control groups (CG) during waking (W), stage 1 (N1), stage 2 (N2) and the first 3 min of stage 3+4 (SN3). Electrodes showing post-hoc significant differences for group-by-stage-by-derivation interaction are illustrated. Horizontal brackets indicate significant differences between groups.
Temporal Coupling
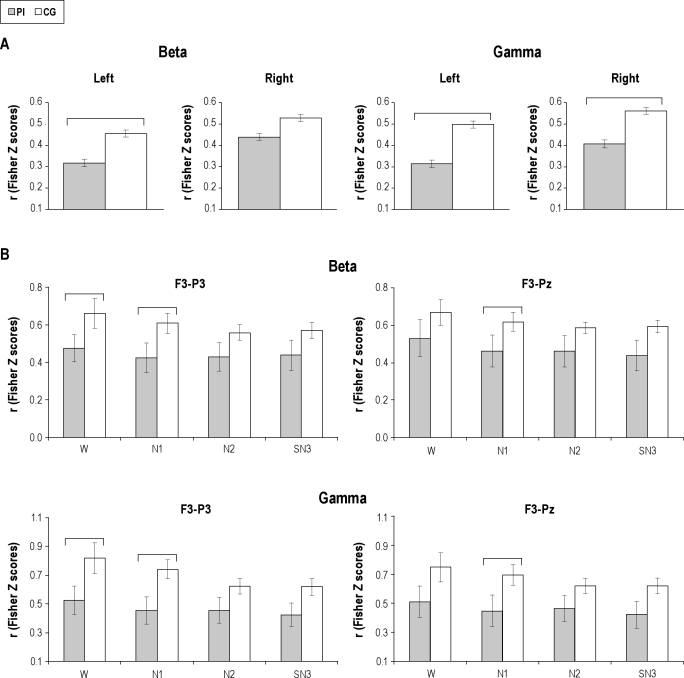
The group main effects for the intrahemispheric cross-correlation between frontoparietal regions (Table 3) showed significantly higher levels in PI than CG in the left hemisphere in the beta and gamma bands, and in the right hemisphere in the gamma band (Figure 2A). The increased temporal coupling in the right hemisphere was independent of sleep stages and pairs of electrodes, because group-by-stage-by-derivation interaction was not significant; whereas in the left hemisphere there were significant group-by-stage interactions for beta and gamma activity. As can be seen in Figure 2B, post-hoc comparisons showed that beta temporal coupling was significantly higher between the F3-P3 electrodes during wakefulness and N1, and that the groups no longer showed differences in N2 and SN3. During N1, beta temporal coupling was higher in the PI group than in the CG between F3-Pz. In the gamma band, temporal coupling was significantly higher in the PI group than in the CG during wakefulness between Fp1-P3, Fp1-Pz (not shown) and F3-P3, and remained higher during N1. F3-Pz gamma temporal coupling was also higher in N1. The difference between the groups disappeared from N2. Although the differences between groups were not significant in the other combinations of electrodes, the same tendency toward a higher cross-correlation in PI than CG was observed. There were no significant main effects or group-by-stage-by-derivation interactions for beta and gamma cross-correlations among frontal derivations.
Figure 2.
Frontoparietal temporal coupling as a function of group and stage. Mean and standard error of beta and gamma cross-correlations, transformed to Fisher Z scores, in the primary insomnia (PI) and control groups (CG) for significant group main effects (A), and for electrodes showing post-hoc significant differences for group-by-stage-by-derivation interactions (B). Horizontal brackets indicate significant differences between groups.
Current Density Topography
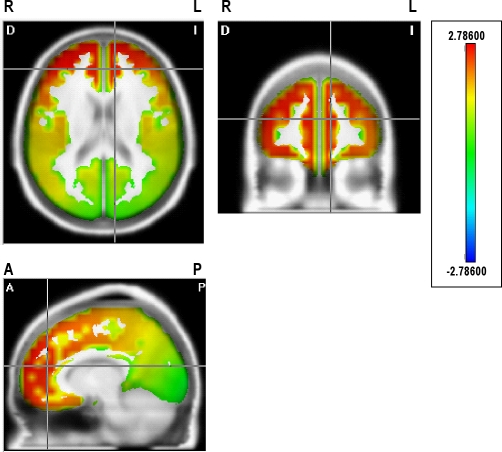
The statistical comparisons of the cortical sources between groups revealed significant interactions by stages for fast beta frequencies from 23 to 30 Hz (shown in Tables 4 and 5), with significantly higher current density in the group with PI than in the CG in wakefulness and N1. Current sources for these beta frequencies, shown for waking in Figure 3 for 29 Hz, were widely distributed bilaterally in the superior, middle, and medial frontal gyri, in the medial and lateral orbital frontal gyri, in the anterior cingulate region, and in the right inferior frontal gyrus. Higher current density in the PI group was also observed in the right insula, the superior temporal gyrus, the superior parietal lobule, and the angular gyrus for some beta frequencies.
Table 4.
Regions showing significantly higher current density in beta frequencies (*) in the patients with primary insomnia than in the control group in wake condition.
| 23 Hz |
24 Hz |
25 Hz |
26 Hz |
27 Hz |
28 Hz |
29 Hz |
30 Hz |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | |
| Superior frontal g | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Middle frontal g | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Inferior frontal g | * | * | * | * | * | * | * | * | * | * | ||||||
| Medial frontal g | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Lateral orbital g | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Medial orbital g | * | * | * | * | * | * | * | * | * | * | * | * | * | * | ||
| Cingulate region | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Insula | * | * | * | * | * | |||||||||||
| Precentral cortex | * | * | * | * | * | * | * | |||||||||
| Postcentral cortex | * | * | * | |||||||||||||
| Superior temporal g | * | * | * | * | * | |||||||||||
| Superior parietal l | * | * | * | * | ||||||||||||
| Angular g | * | * | * | * | ||||||||||||
All of the regions presented are thresholded at P < 0.05 corrected. L, left hemisphere; R, right hemisphere; g, gyrus; l, lobule.
Table 5.
Regions showing significantly higher current density in beta frequencies (*) in the patients with primary insomnia than in the control group in stage 1 (N1)
| 23 Hz |
24 Hz |
25 Hz |
26 Hz |
27 Hz |
28 Hz |
29 Hz |
30 Hz |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | |
| Superior frontal g | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Middle frontal g | * | * | * | * | * | * | * | * | * | * | * | * | * | * | ||
| Inferior frontal g | * | * | * | * | * | * | * | * | ||||||||
| Medial frontal g | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Lateral orbital g | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |
| Medial orbital g | * | * | * | * | * | * | * | * | * | * | * | * | * | * | ||
| Cingulate region | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| Insula | * | * | * | * | ||||||||||||
| Precentral cortex | * | * | * | * | ||||||||||||
| Postcentral cortex | * | * | * | * | * | |||||||||||
| Superior temporal g | * | * | * | * | * | * | * | |||||||||
| Middle temporal g | * | * | * | * | * | * | ||||||||||
| Inferior temporal g | * | * | * | * | * | * | * | |||||||||
| Supramarginal g | * | * | * | * | * | |||||||||||
| Superior parietal l | * | * | * | |||||||||||||
| Angular g | * | * | * | * | * | |||||||||||
| Medial occipitotemporal g | * | * | ||||||||||||||
| Lateral occipitotemporal g | * | * | * | * | * | |||||||||||
| Superior occipital g | * | |||||||||||||||
| Middle occipital g | * | * | ||||||||||||||
| Inferior occipital g | * | |||||||||||||||
| Precuneus | * | |||||||||||||||
All of the regions presented are thresholded at P < 0.05 corrected. L, left hemisphere; R, right hemisphere; g, gyrus; l, lobule.
Figure 3.
Statistical map showing the significant distributed current source density in 29 Hz for the contrast between primary insomnia and control groups during waking. The scale represents significance level of current density differences between groups. Red represents a significantly higher current density in the primary insomnia group compared to the control group (P < 0.05 threshold). R, right; L, left; A, anterior; P, posterior. See Table 4 for all significant differences.
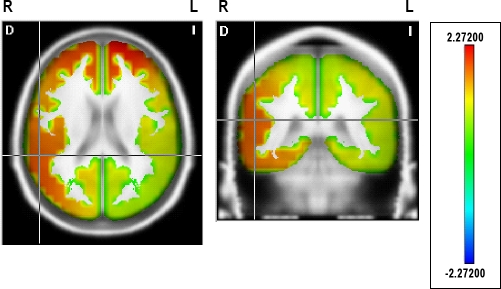
During N1 (Table 5, Figure 4), the higher current density for fast beta frequencies (23-30 Hz) persisted bilaterally in the superior, middle (except for 26 Hz), and medial frontal gyri, in the medial and lateral orbital frontal gyri, in the anterior cingulate region, and in the right inferior frontal gyrus (except for 29 and 30 Hz). Current source distribution expanded to the right posterior regions in some beta frequencies, mostly in the right hemisphere (see Table 5 for significant locations and frequencies), including the inferior, middle, and superior temporal gyri, the angular gyrus, the supramarginal gyrus, the superior parietal lobule, and the posterior cingulate; as well as in isolated frequencies in the precentral, postcentral, occipitotemporal, and occipital regions.
Figure 4.
Statistical map showing the significant distributed current source density in 29 Hz for the contrast between primary insomnia and control groups during Stage 1. The scale represents significance level of current density differences between groups. Red represents a significantly higher current density in the primary insomnia group compared to the control group (P < 0.05 threshold). R, right; L, left. See Table 5 for all significant differences.
Self-Reported Measures
Self-reported measures after morning awakening are shown in Table 6. Patients in the PI group rated their sleep as shorter and of poorer quality, less refreshing and lighter than the CG, and expressed the desire for more than 2 hr of additional sleep. The Insomnia Severity Index59 confirmed those sleep difficulties. The groups did not differ in their subjective estimations of sleep latency, number of intrasleep awakenings, mood upon awakening in the morning, bed comfort, or levels of hyperarousal.
Table 6.
Self-reported postsleep measures
| PI |
CG |
P | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Hyperarousal scale | 39.86 | 10.57 | 38.83 | 11.25 | ns |
| Insomnia Severity Index | 15.3 | 3.77 | 2.3 | 1.63 | 0.0001 |
| Subjective sleep quality and continuity | |||||
| Latency to sleep (min) | 23.5 | 14.7 | 20.5 | 8.6 | ns |
| Number of awakenings | 3.2 | 1.6 | 2.4 | 1.2 | ns |
| Duration of awakenings (min) | 16.6 | 10 | 13.4 | 8.2 | ns |
| Sleep duration (hr) | 5.7 | 1.4 | 6.8 | 0.7 | 0.05 |
| Additional hr of sleep desired | 2.3 | 1.7 | 0.6 | 0.4 | 0.01 |
| Sleep (poor = 0, good = 10) | 5.3 | 1.4 | 8.0 | 1.3 | 0.0004 |
| Sleep (refreshing less = 0, more = 10) | 5.4 | 2.1 | 8.1 | 1.9 | 0.008 |
| Sleep (light = 0, deep = 10) | 5 | 1.9 | 7.4 | 1.6 | 0.008 |
| Mood after awakening (terrible = 0, excellent = 10) | 6.9 | 1.1 | 8.4 | 2.3 | ns |
| Bed (uncomfortable = 0, comfortable = 10) | 8.1 | 1.6 | 7.1 | 2.3 | ns |
Between-group analysis P < 0.05. CG, control group; PI, primary insomnia group; SD, standard deviation.
DISCUSSION
The aim of this study was to explore beta and gamma EEG activity and temporal coupling as an index of cortical activation and its probable topographic source distribution during the WSTP in a group of young, nondepressed, unmedicated patients with PI suffering from sleep-onset insomnia. The main finding of this exploratory study is that it demonstrated higher frontal and frontal midline activation, and increased temporal coupling linking the frontal, parietal, and posterior midline regions during wakefulness and N1 in the left hemisphere and throughout the initial period of sleep in the right hemisphere in PI patients compared with the CG.
Increased beta activity was prominent in the left frontal and frontal midline regions during W and N1. The source localization analysis suggested the frontal gyri and the anterior cingulate bilaterally as possible generators of beta activity during waking and N1, which is consistent with the higher metabolic activation in the frontal and anterior cingulate reported in depressed patients with insomnia,33,34 and expands those findings to include PI without clinical depression.
The higher beta activity during waking and stage 1 (N1) of NREM sleep is consistent with enhanced beta activity before sleep onset7,8–9,11 and initial stage 1 sleep.7,8 Also, it concords with the notion that PI patients are hyperaroused, though no significant differences appeared on the Hyperarousal Scale.
The present results do not agree with studies reporting increased beta activity during NREM sleep.1,2,10,12 However, a direct, full comparison of these data and findings from other studies is not possible because of several methodologic variations that may account for some of the differences, such as the use of relative power,1 and the composition of the groups being compared: i.e., patients with both idiopathic and psychophysiologic insomnia,10 those with exclusively psychophysiologic insomnia,1 or insomnia patients with subjective complaints2; and different recording sites, mostly central derivations only. Also, other studies analyzed the EEGs of several cycles of NREM sleep without separating Stage 2 and Stages 3 and 4, whereas in this study Stage 2 was analyzed separately and only the first 3 min of consecutive N3 were examined. Finally, this study included only young and treatment- naïve patients, whereas others have incorporated larger age spans and, in some instances, patients who had been or were using benzodiazepines.
The current results extend the findings of higher brain activation during the WSTP in PI to enhanced beta temporal coupling that links the frontal, parietal, and posterior midline regions, which are important cortical nodes for the endogenous attention control.30,32,35,36 The enhanced synchronized beta activity in the regions involved in attention and self-awareness is consistent with increased temporal coupling during a visual sustained-attention reaction time task61 and with the joint activation of the frontal and posterior regions during conscious self-awareness.22
The increased activation and temporal coupling in PI in the regions involved in executive control and willed action,30–31 inner attention,32,35 the retrieval of past experience, planning future actions,62 and conscious awareness22,28,29,37 are consistent with the main complaints of patients with PI; namely, actively trying to control sleep,27 having uncontrollable, intrusive thoughts,24 attention to and awareness of their surroundings,63,64 sensory information processing, and long-term memory formation.65 This suggests that in addition to heightened activation, patients with PI also have specific difficulties in deactivating frontal executive regions and in disengaging the frontal-posterior-medial attentional network during the WSTP and initial sleep.
It has been proposed that an increase in the default-mode network activity that has been associated with internal processing, monitoring of the body, and maintenance of consciousness, parallel to a decrease in goal-directed cognition and attentional network, are all important at sleep onset.66 During N1, the topographic distribution of the inferred current density for beta activity spread out toward the right posterior and midline association areas. The cortical sources of higher beta activity in patients with PI in the superior and medial frontal gyrus, the angular gyrus and the posterior cingulate, correspond to some regions of the default-mode network; however, the frontoparietal attentional network maintained higher temporal coupling, which suggests a dissociation between these two networks in PI during the WSTP.
Diagnoses of insomnia were confirmed by the Pittsburgh Sleep Quality Index,44 the Athens Insomnia Scale,45 and the Insomnia Severity Index.59 Although the groups did not differ in the sleep variables derived from PSG, the insomnia group did evaluate their sleep as shorter, poorer, lighter, and less refreshing than that of regular sleepers. Moreover, they expressed the desire to have slept more additional hours, thus confirming the mismatch between objective PSG signs of sleep and subjective estimations of sleep reported in some patients with insomnia in the literature.1–2
The groups showed a mild first-night effect characterized by shorter total sleep time and slightly lower sleep efficiency and REM sleep percentages, compared with the expected values for patient age. The similar effect of the first night at the laboratory on objective signs of sleep and subjective sleep latency in both groups is consistent with the expected first-night effect in the CG and a reverse night effect in the PI group. The existence of variations in brain activation without differences in objective signs between the groups under leveled arousing circumstances suggests that the differences in EEG activity between patients suffering from sleep-onset insomnia and regular sleepers during the WSTP are not dependent on transient circumstances created by the sleep laboratory environment or by the discomfort produced by the PSG procedure. This, in turn, indicates that a similar disrupting environment has a differential effect on those with PI and regular sleepers, and may imply that the differences in brain activity between the groups probably represent trait differences.
Overestimation of sleep latency and underestimation of sleep time, as well as higher fast EEG activity, distinguish insomnia patients with subjective complaints from those with objective signs.2 The discrepancy between objective signs of sleep and subjective sleep evaluation in the PI group may suggest a predominant paradoxical insomnia type in the particular PI group studied. However, because the main focus of the study was to look for EEG signs of activation and temporal coupling in insomnia patients with difficulties initiating sleep, and because the overlapping of objective and subjective symptoms in psychophysiologic as well as paradoxical insomnia renders the subclassification of PI into two different entities somewhat controversial,12,67 the inclusion criteria for the current cohort of patients were based on general criteria for PI as defined in the International Classification of Sleep Disorders,43 and no attempt was made to distinguish between psychophysiologic or paradoxical insomnia. Thus, no conclusive remarks on the type of PI can be made for this group of patients.
The differences between the two groups were found during the WSTP of the first night of PSG. Although some studies have not found noticeable differences between first and second nights,48 or have even reported an increased brain activation on the second night compared with the first night in patients with insomnia,14 additional research designed to analyze consecutive nights using shorter epochs to score the different sleep stages5 is necessary to determine whether EEG activation and temporal coupling during the WSTP constitute a stable pattern that is repeated every night.
Although the focus of this study was on the WSTP and future studies that analyze temporal coupling for the entire night are certainly needed, the coexistence of EEG signs of sleep and synchronized brain activation during the first minutes of sleep may help us reach a better understanding of the difficulties involved in falling asleep and in subjective underestimations of sleep quality.
These results should be taken as exploratory. The small number of participants limits the possibility of generalizing the findings to a larger population. However, we preferred to focus on a homogeneous group of young adults that met the clinical diagnosis of PI and thereby avoid confounding factors such as age, depression, and previous treatment history (medications). Future studies with larger samples of patients and high electrode density recordings may well provide further evidence and more precise localizations of neurophysiologic disturbances in PI. Nonetheless, our findings are consistent with recent models of PI and, moreover, suggest new insights related to enhanced self-awareness and inner attention in patients with insomnia.
The results of this basic, exploratory research on the topographic distribution of brain activation and temporal coupling of scalp-recorded EEGs suggest that frontal deactivation and the disengagement of the brain regions involved in executive control and willed action, inner attention, planning future actions, retrieval of past experience, and self-awareness are impaired during the WSTP in stages of PI. The persistence of this synchronously activated network simultaneously with EEG signs of sleep may contribute to achieving a better understanding of the underlying mechanism involved in difficulties in falling asleep, in subjective feelings of not being asleep, and in the lighter, poorer, and nonrefreshing nature of sleep experienced by some patients with PI.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was partially financed by DGAPA, project IN202406-2 y CONACyT. Pedro Figueredo Rodríguez was granted by DGEP (UNAM). Paul Kersey corrected the English version of the manuscript. Alfonso Pérez Ortiz participated during the sleep recordings and was granted by DGAPA, project IN228409.
Footnotes
A commentary on this article appears in this issue on page 451.
REFERENCES
- 1.Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–17. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 2.Krystal AD, Edinger JD, Wohlgemuth WK, et al. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–40. [PubMed] [Google Scholar]
- 3.De Gennaro L, Ferrara M, Curcio G, Cristiani R. Antero-posterior EEG changes during wakefulness-sleep transition. Clin Neurophysiol. 2001;112:1901–11. doi: 10.1016/s1388-2457(01)00649-6. [DOI] [PubMed] [Google Scholar]
- 4.Corsi-Cabrera M, Muñoz-Torres Z, del Río-Portilla Y, Guevara MA. Power and coherent oscillations distinguish REM sleep, stage 1 and wakefulness. Int J Psychophysiology. 2006;60:59–66. doi: 10.1016/j.ijpsycho.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Moul DE, Germain A, Cahmere JD, Quigley M, Miewald JM, Buysse DJ. Examining initial sleep onset in primary insomnia: a case-control study using 4-second epochs. J Clin Sleep Med. 2007;3:479–88. [PMC free article] [PubMed] [Google Scholar]
- 6.Parrino L, Milioli G, De Paolis F, Grassi A, Terzano MG. Paradoxical insomnia: the role of CAP and arousals in sleep misperception. Sleep Med. 2009;10:1139–45. doi: 10.1016/j.sleep.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Freedman RR. EEG power spectra in sleep-onset insomnia. Electroenceph Clin Neurophysiol. 1986;63:408–13. doi: 10.1016/0013-4694(86)90122-7. [DOI] [PubMed] [Google Scholar]
- 8.Merica H, Gaillard JM. The EEG of the sleep onset period in insomnia: a discriminant analysis. Physiol Behav. 1992;52:199–204. doi: 10.1016/0031-9384(92)90258-4. [DOI] [PubMed] [Google Scholar]
- 9.Lamarche CH, Ogilvie RD. Electrophysiological changes during the sleep onset period of psychophysiological insomniacs, psychiatric insomniacs and normal sleepers. Sleep. 1997;20:724–33. [PubMed] [Google Scholar]
- 10.Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–34. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 11.Staner L, Cornette F, Maurice D, et al. Sleep microstructure around sleep onset differentiates major depressive insomnia from primary insomnia. J Sleep Res. 2003;12:319–30. doi: 10.1046/j.0962-1105.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- 12.Buysse DJ, Germain A, Hall ML, et al. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31:1673–82. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueredo-Rodríguez P, del Río-Portilla Y, Sánchez-Romero JI, Pérez-Ortiz A, Corsi-Cabrera M. Frontal alpha activity in primary insomniacs with sleep onset difficulties. Salud Mental. 2009;32:59–67. [Google Scholar]
- 14.Marzano C, Ferrara M, Sforza E, De Gennaro L. Quantitative electroencephalogram (EEG) in insomnia: a new window on pathophysiological mechanisms. Curr Pharmaceutical Des. 2008;14:3446–55. doi: 10.2174/138161208786549326. [DOI] [PubMed] [Google Scholar]
- 15.Bastien CH, St-Jean G, Turcotte I, et al. Spontaneous K-complexes in chronic psychophysiological insomnia. Psychosom Res. 2009;67:117–25. doi: 10.1016/j.jpsychores.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Bastien CH, St-Jean G, Turcotte I, Morin ChM, Lavallée M. Sleep spindles in chronic psychophysiologial insomnia. J Psychosom Res. 2009;66:59–65. doi: 10.1016/j.jpsychores.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Bonet MH, Arand DL. 24-hours metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–8. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 18.Steriade M. Cellular substrates of brain rhythms. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: basic principles, clinical applications, and related fields. Baltimore: Lippincott Williams and Wilkins; 1998. pp. 28–75. [Google Scholar]
- 19.Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol. 2005;493:92–8. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- 20.Merica H, Fortune RD. A neuronal transition probability model for the evolution of power in the sigma and delta frequency bands of sleep EEG. Physiol Behav. 1997;62:585–9. doi: 10.1016/s0031-9384(97)00165-0. [DOI] [PubMed] [Google Scholar]
- 21.Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. J Neurosci. 2008;28:10167–84. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kjaer TE, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImag. 2002;17:1080–6. [PubMed] [Google Scholar]
- 23.Morin CM. New York: Guilford Press; 1993. Insomnia. Psychological assessment and management. [Google Scholar]
- 24.Lichstein KL, Rosenthal TL. Insomniacs' perceptions of cognitive versus somatic determinants of sleep disturbance. J Abnorm Psychol. 1980;89:105–7. doi: 10.1037//0021-843x.89.1.105. [DOI] [PubMed] [Google Scholar]
- 25.Tang NKY, Schmidt DA, Harvey AG. Sleeping with the enemy: clock monitoring in the maintenance of insomnia. J Behav Ther Exp Psychiatr. 2007;38:40–55. doi: 10.1016/j.jbtep.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioral and neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 27.Espie CA, Broomfield NM, MacMahon KMA, Macphee LM, Taylor LM. The attention-intention-effort pathway in the development of psychophysiological insomnia: a theoretical review. Sleep Med Rev. 2006;10:215–45. doi: 10.1016/j.smrv.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Mazoyer B, Zago L, Mellet E, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–98. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 29.Frith CD, Frith U. Interacting minds: a biological basis. Science. 1999;286:1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- 30.Fuster J. Cortex and mind: unifying cognition. New York: Oxford University Press; 2003. [Google Scholar]
- 31.Frith CD, Friston KJ, Liddle PF, Frackowiack RSJ. Willed action and the prefrontal cortex in man: a study with PET. Proc R Soc London B. 1991;244:241–6. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- 32.Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos Trans Soc London B Biol Sci. 1998;353:1915–27. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nofzinger EA, Nissen C, Germain A, et al. Regional cerebral metabolic correlates of WASO during NREM sleep in insomnia. J Clin Sleep Med. 2006;2:316–22. [PubMed] [Google Scholar]
- 34.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–29. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 35.Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent and overlapping neural systems? Proc Natl Acad Scie USA. 1998;95:831–8. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–7. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- 37.Kjaer TW, Nowak M, Kjaer KW, Lou AR, Lou HC. Precunus-prefrontal activity during awareness of visual verbal stimuli. Conscious Cogn. 2001;10:356–65. doi: 10.1006/ccog.2001.0509. [DOI] [PubMed] [Google Scholar]
- 38.Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Scie. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- 39.Varela F, Lachaux JP, Rodríguez E, Martinerie J. The brainwebb: phase synchronization and large scale integration. Nature Rev Neuroscie. 2001;2:229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan R, Russell DP, Edelman GM, Tononi G. Increased synchronization of neuromagnetic responses during conscious experience. J Neuroscie. 1999;19:5435–48. doi: 10.1523/JNEUROSCI.19-13-05435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pérez-Garci E, del Río-Portilla Y, Guevara MA, Arce C, Corsi-Cabrera M. Paradoxical sleep is characterized by uncoupled gamma activity between frontal and perceptual cortical regions. Sleep. 2001;24:118–26. doi: 10.1093/sleep/24.1.118. [DOI] [PubMed] [Google Scholar]
- 42.Corsi-Cabrera M, Miró E, del Río-Portilla Y, Pérez-Garci E, Villanueva Y, Guevara MA. Rapid eye movement sleep dreaming is characterized by uncoupled EEG activity between frontal and perceptual cortical regions. Brain Cognition. 2003;51:337–45. doi: 10.1016/s0278-2626(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 43.American Academy of Sleep Medicine. American Academy of Sleep Medicine; 2005. ICSD-2-International classification of sleep disorders, 2nd edition: Diagnostic and coding manual. [Google Scholar]
- 44.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 45.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosomatic Res. 2000;48:555–60. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 46.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 48.Curcio G, Ferrara M, Piergianni A, Fratello F, De Gennaro L. Paradoxes of the first night effect: a quantitative analysis of antero-posterior EEG topography. Clin Neurophysiol. 2004;115:1178–88. doi: 10.1016/j.clinph.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 49.Hauri PJ. Reverse first night effect in insomnia. Sleep. 1989;12:97–105. doi: 10.1093/sleep/12.2.97. [DOI] [PubMed] [Google Scholar]
- 50.Rechtschaffen A, Kales A, editors. Los Angeles: Brain Information Service, Brain Research Institute, University of California; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 51.Guevara MA, Ramos J, Hernández-González M, Zarabozo D, Corsi-Cabrera M. POTENCOR: a program to calculate power and correlation spectra of EEG signals. Comput Methods Programs Biomed. 2002;72:241–50. doi: 10.1016/s0169-2607(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 52.Gasser T, Bacher P, Mocks J. Transformations toward the normal distribution of broad band spectral parameters of the EEG. Electroenceph Clin Neurophysiol. 1982;53:119–24. doi: 10.1016/0013-4694(82)90112-2. [DOI] [PubMed] [Google Scholar]
- 53.John ER, Ahn H, Prichep L, Treptin M, Brown D, Kaye H. Developmental equations for the electroencephalogram. Science. 1980;210:1255–58. doi: 10.1126/science.7434026. [DOI] [PubMed] [Google Scholar]
- 54.Bosch-Bayard J, Valdés-Sosa P, Virués-Alba T, et al. 3D statistical parametric mapping of EEG source spectra by means of variable resolution electromagnetic tomography (VARETA) Clin Electroencephalogr. 2001;32:47–61. doi: 10.1177/155005940103200203. [DOI] [PubMed] [Google Scholar]
- 55.Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- 56.Bolwig TG, Hansen ES, Hansen A, Merkin H, Prichep LS. Toward a better understanding of the pathophysiology of OCD SSRI responders: QEEG source localization. Acta Pyschiatr Scand. 2007;115:237–42. doi: 10.1111/j.1600-0447.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 57.Ricardo-Garcell J, González-Olvera JJ, Miranda E, et al. EEG sources in a group of patients with major depressive disorders. Int J Psychophysiol. 2009;71:70–74. doi: 10.1016/j.ijpsycho.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 58.Pavlova M, Berg O, Gleason R, Walker F, Roberts S, Regenstein Q. Self-reported hyperarousal traits among insomnia patients. J Psychosom Res. 2001;51:435–41. doi: 10.1016/s0022-3999(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 59.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;4:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 60.Westfall PH, Young SS. Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment. New York: Wiley; 1993. [Google Scholar]
- 61.Corsi-Cabrera M, Pérez-Ortiz A, Sánchez-Romero J, et al. Electroencephalographic activity during visual sustained attention task in primary insomnia. Clin Neurophysiol. 2008;119:e118. [Google Scholar]
- 62.Andreasen NC, O'Leary DS, Cizadlo T, et al. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiat. 1995;152:1576–85. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- 63.Bastien CH, St-Jean G, Morin ChM, Turcotte I, Carrier J. Chronic psychophysiological insomnia: hyperarousal and/or inhibition deficits? An ERPs investigation. Sleep. 2008;31:887–98. doi: 10.1093/sleep/31.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang ChM, Lo HS. ERP evidence of enhanced excitatory and reduced inhibitory processes of auditory stimuli during sleep in patients with primary insomnia. Sleep. 2007;30:585–92. doi: 10.1093/sleep/30.5.585. [DOI] [PubMed] [Google Scholar]
- 65.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 66.Picchioni D, Kukunaga M, Carr WS, et al. fMRI differences between early and late stage-1 sleep. Neurosci Lett. 2008;441:81–5. doi: 10.1016/j.neulet.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edinger JD, Fins AI, Goecke JM, et al. The empirical identification of insomnia subtypes: a cluster analytic approach. Sleep. 1996;19:398–411. [PubMed] [Google Scholar]