Abstract
Study Objectives:
Disturbances of sleep are hypothesized to contribute to pain. However, experimental data are limited to healthy pain-free individuals. This study evaluated the effect of sleep loss during part of the night on daytime mood symptoms and pain perceptions in patients with rheumatoid arthritis in comparison with control subjects.
Design:
A between-groups laboratory study with assessment of mood symptoms and pain perception before and after partial night sleep deprivation (PSD; awake 23:00 hr to 03:00 hr).
Setting:
General clinical research center.
Participants:
Patients with rheumatoid arthritis (n = 27) and volunteer comparison control subjects (n = 27).
Measurements:
Subjective reports of sleep, mood symptoms and pain, polysomnographic assessment of sleep continuity, and subjective and objective assessment of rheumatoid arthritis-specific joint pain.
Results:
PSD induced differential increases in self-reported fatigue (P < 0.09), depression (P < 0.04), anxiety (P < 0.04), and pain (P < 0.01) in patients with rheumatoid arthritis compared with responses in control subjects, in whom differential increases of self-reported pain were independent of changes in mood symptoms, subjective sleep quality, and objective measures of sleep fragmentation. In the patients with rheumatoid arthritis, PSD also induced increases in disease-specific activity as indexed by self-reported pain severity (P < 0.01) and number of painful joints (P < 0.02) as well as clinician-rated joint counts (P < 0.03).
Conclusion:
This study provides the first evidence of an exaggerated increase in symptoms of mood and pain in patients with rheumatoid arthritis after sleep loss, along with an activation of rheumatoid arthritis-related joint pain. Given the reciprocal relationship between sleep disturbances and pain, clinical management of pain in patients with rheumatoid arthritis should include an increased focus on the prevention and treatment of sleep disturbance in this clinical population.
Citation:
Irwin MR; Olmstead R; Carrillo C; Sadeghi N; FitzGerald JD; Ranganath VK; Nicassio PM. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. SLEEP 2012;35(4):537-543.
Keywords: Sleep, sleep deprivation, fatigue, depression, anxiety, pain, joint pain, rheumatoid arthritis
INTRODUCTION
Rheumatoid arthritis (RA) is an inflammatory autoimmune disorder that is characterized by joint pain and swelling.1 In addition, more than half of patients with RA report sleep disturbance (e.g., sleep fragmentation),2,3 a rate of prevalence that is 2 to 3 times greater than that found in the general population. Cross-sectional studies have found that sleep disturbance correlates with greater pain and disease activity,4,5 and it is often thought that difficulties with sleep are due to RA-related pain. Indeed, polysomnographic studies have confirmed that chronic pain is associated with poor sleep continuity and reduced total sleep time in other populations.6 However, sleep disturbance and pain may be bidirectionally related, and it is also hypothesized that sleep disturbance might drive RA-related pain. No study has experimentally evaluated whether sleep loss alters joint pain in RA, a cardinal symptom that is key to the evaluation of clinical improvement and/or disability in this clinical population.7
In healthy volunteers, there is limited evidence that sleep loss influences pain reporting,8,9,10,11,12–13 with studies either constrained by small sizes or the lack of control groups. Furthermore, most have relied on selective sleep stage deprivation or on total sleep deprivation, which do not mimic the kind of sleep disturbance found in clinical populations such as patients with RA who typically show loss of sleep during part of the night or sleep fragmentation.3 For example, 2 studies found that uncontrolled selective slow-wave sleep deprivation decreased mechanical pain thresholds,9,10 whereas rapid eye movement (REM) sleep deprivation was found to increase thermal pain sensitivity,12 although another study failed to find an effect of REM sleep deprivation on pain threshold responses.13 In contrast, 3 studies reported that total sleep deprivation led to hyperalgesia or enhanced responsivity to painful stimuli, with evidence of a dose-response effect when total sleep deprivation occurs over 2 nights.8,11,13 Recently, Smith et al.14 found that disturbances of sleep continuity, as opposed to total sleep restriction, increase spontaneous pain in healthy females.
In this study, an experimental model of partial night sleep deprivation (PSD), a ubiquitous sleep disruption pattern, was used to activate self-reported symptoms of pain and to determine whether sleep loss induces a differential increase in symptoms of self-reported pain in patients with RA in comparison with healthy control patients, taking into account subjective sleep quality and objective measures of sleep fragmentation. Additionally, sleep loss is hypothesized to contribute to disease activity and functional impairments in this clinical population; hence, joint pain was evaluated in patients with RA using guidelines created by the American College of Rheumatology with assessment of both clinician-rated joint counts as well as subjective reports of pain using visual analog scales before and after PSD.15 Finally, given evidence that mood symptoms such as fatigue, depression, and anxiety often co-occur with sleep disturbance and pain in RA patients,16 and that changes in mood symptoms might contribute to self-reported pain after sleep disturbance, the effect of sleep disturbance on mood symptoms and the contribution of these symptoms to pain perceptions in patients with RA in comparison with control subjects was also examined.
MATERIALS AND METHODS
Design Overview
This experimental study evaluated the effects of early night PSD (awake 23:00 to 03:00 hr) on self-reported measures of mood symptoms and pain between groups of patients with RA and control subjects, and on self-reported arthritis-specific and clinician-rated measures of joint pain in patients with RA. Participants were recruited through newspaper advertisements that stated the aim of the study as evaluating “sleep in rheumatoid arthritis patients [or healthy volunteers].” No additional information about the study hypothesis or about evaluation of pain perception was provided. Hence, participants were blinded to the study objectives and outcomes for self-rated measures of mood symptoms and pain perception.
Setting and Participants
Following approval by the UCLA Institutional Review Board, the study was conducted at the UCLA General Clinical Research Center (GCRC). The subjects included 27 patients with RA and 27 volunteer comparison control subjects. After a telephone screening, prospective patients with RA were referred to the UCLA Division of Rheumatology where study rheumatologists (JDF, VKR) confirmed the diagnosis of RA by conducting diagnostic evaluations that included review of medical records, completion of a medical history, and assessment of tender and swollen joints and disease activity.15 A structured comprehensive joint count assessment was completed with scoring of the number of joints that were tender and/or swollen. Sixty joints were evaluated on a scale from 0 (none) to 3 (severe) to indicate the extent of pain and swelling.
Eligible patients with RA were age 18 yr or older; met the 1987 American College of Rheumatology revised criteria for RA; were stable on a disease-modifying drug regimen for 3 mo prior to study entry; had a stable disease course for 3 mo; and did not have serious co-morbid medical conditions such as diabetes, congestive heart failure, renal failure, or cancer. Use of the following types of medication in the previous 2 mo was obtained for each patient with RA: analgesics/nonsteroidal anti-inflammatory drugs (NSAIDs); biologic agents; and disease-modifying antirheumatic drugs (DMARDs). Comparison control subjects also underwent a medical interview and physical examination by a staff physician; none had a history of an inflammatory disorder, cancer, or chronic or active infections. All participants had reference range results from screening laboratory tests (complete white blood cell count, metabolic panel) and for women a negative pregnancy test. Two-week sleep diaries confirmed that participants regularly slept between 22:30 and 07:30 prior to study entry; none were using sedative-hypnotic medications for sleep.
Procedures
After completion of screening and eligibility assessments, subjects spent 4 nights in the GCRC: adaptation, baseline, PSD, and recovery. The adaptation night served to acclimate subjects to the recording environment and to screen for sleep apnea (> 15 desaturation events per hour) and/or nocturnal myoclonus (> 10 movement-related, 3-sec arousals per hour);17 subjects with sleep apnea and/or nocturnal myoclonus were excluded. During baseline and recovery nights, uninterrupted sleep occurred between 23:00 and 07:00 hr, whereas during early- night PSD, subjects were awake between 23:00 and 03:00 hr, with sleep occurring between 03:00 and 07:00 hr. Throughout the night, polysomnography was performed with ambient light less than 50 lux. Objective measures of sleep continuity at baseline and during the sleep interval (i.e., after PSD and prior to the morning assessment) were obtained by polysomonography; a separate report will more comprehensively examine RA-specific clinical factors associated with polysomnographic sleep at baseline and in response to PSD in patients with RA compared with control subjects. During the days after baseline and PSD nights, measures of mood symptoms and pain perception were repeatedly obtained. In the patients with RA only, clinician-rated joint counts were measured at 08:00 hr after baseline, PSD, and recovery nights. Patients were monitored by GCRC nursing staff with routine checks every 15 min to prevent napping behavior.
Measures
Severity of sleep disturbance at baseline was evaluated by the Pittsburgh Sleep Quality Index (PSQI), a widely used, self-report questionnaire that measures sleep disturbances.18,19 Objective measures of sleep continuity (i.e., total sleep time, sleep latency, wake after sleep onset, sleep efficiency) were obtained by all-night polysomnography using methods previously reported.20 The Profile of Mood States21 evaluated changes in fatigue, depression, and anxiety. To evaluate changes in subjective pain perception, the McGill Pain Questionnaire (MPQ) was used22; the MPQ provides a sum of the ranked values associated with adjectives depicting the severity of pain22 and shows good reliability (0.78) and also correlates moderately with physician assessment of disease activity (r = 0.44) and patient assessment of disease activity (r = 0.68) in patients with RA. In these patients, a 5-point visual analog scale was also used to estimate RA- specific disease activity (i.e., number of painful joints, overall pain severity), and the structured 60-joint count was also completed before and after PSD by the RA clinicians.
Data Analyses
Data were analyzed using SAS version 9.13 for Windows (SAS Institute, Inc., Cary, NC). Differences between the patients with RA and the control patients in demographic and clinical variables were tested by t-tests and chi-square tests. To determine the effects of PSD on mood symptoms and self-reported measures of pain in the patients with RA compared with control subjects, repeated- measures mixed-model analyses of variance (ANOVAs) were performed using a 2 (group: RA, control) × condition (baseline, PSD) × 5 (time: 08:00, 12:00, 16:00, 20:00, 23:00 hr) design. To determine the effects of PSD on RA-related joint pain, repeated-measures mixed-model ANOVAs were performed using a condition × time design. Mixed models allows for all available data to be used during estimation without the deletion of valid data; the amount of missing data, however, was minimal (< 4%).
RESULTS
Demographic and Clinical Characteristics
Table 1 displays the demographic and clinical characteristics of the RA and control groups. The 2 groups were similar in terms of age, sex, education, income, employment status, marital status, and body mass index. There was a trend for the RA group to have more non-white subjects, although this difference was not significant. Patients with RA reported significantly greater sleep disturbance in comparison with control subjects, with mean PSQI scores indicating clinical sleep impairment. Patients with RA reported treatment with a combination of biologic agents, DMARDs, and NSAIDs to manage their symptoms. DMARDs were the most commonly used medication (55.6%), with the use of biologic agents (48.1%), steroids (14.8%), and NSAIDs (3.7%) also reported. Among the patients with RA, covarying for the use of these various medications as a block on all disease-specific outcomes did not alter the results.
Table 1.
Demographic and clinical characteristics of comparison control patients and those with rheumatoid arthritis
| Variables | Comparison control (n = 27) |
Rheumatoid arthritis (n = 27) |
t | P | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age (yr) | 60.4 | 10.2 | 59.9 | 11.1 | 0.19 | 0.85 |
| Educational level (yr) | 16.1 | 3.1 | 15.1 | 5.1 | 1.17 | 0.25 |
| Income ($k/yr) | 66.1 | 51.6 | 64.5 | 48.9 | 0.13 | 0.90 |
| BMI | 25.4 | 5.0 | 27.2 | 4.6 | 1.31 | 0.20 |
| PSQI | 2.4 | 2.0 | 7.6 | 3.8 | 6.18 | < 0.001 |
| N | % | N | % | χ2 | P | |
| Sex (female) | 21 | 79 | 24 | 89 | 1.2 | 0.28 |
| Minority (non-Euro-American) | 9 | 33 | 16 | 59 | 3.7 | 0.06 |
| Marital status (married/partner) | 13 | 48 | 12 | 44 | 0.1 | 0.79 |
| Employment status (employed) | 13 | 48 | 8 | 30 | 1.9 | 0.16 |
BMI, body mass index; PSQI, Pittsburgh Sleep Quality Index; SD, standard deviation.
Mood responses to PSD
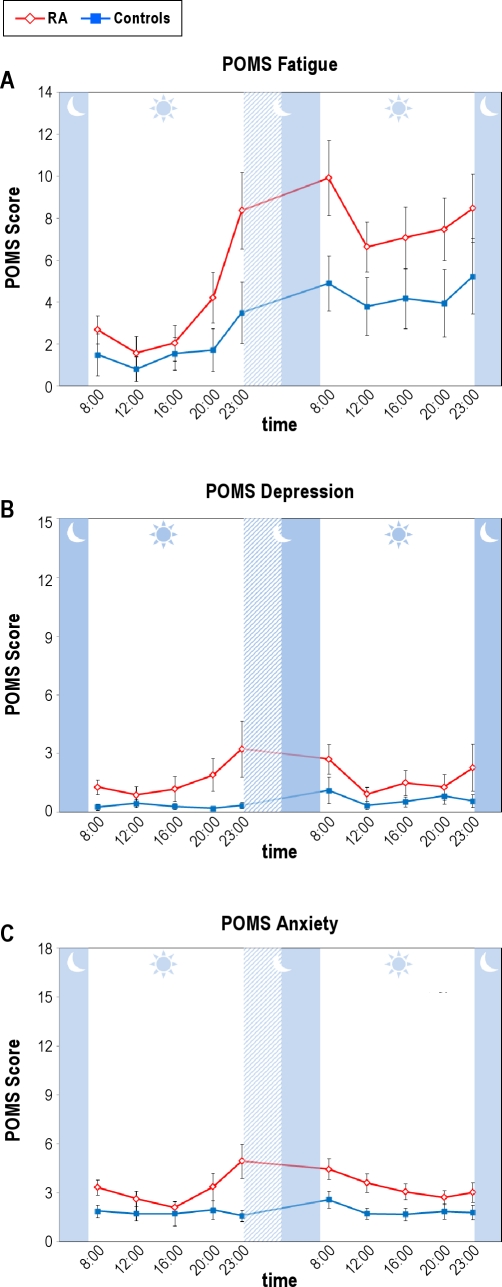
PSD induced differential increases in self-reported fatigue, depression, and anxiety in the patients with RA in comparison with the control subjects. For fatigue, there were significant main effects for group (F(1,51.15) = 9.05, P < 0.005), condition (F(1,440.81) = 73.25, P < 0.001), and time (F(4,440.44) = 7.01, P < 0.001), with a trend for a group × condition interaction (F(1,440.81) = 2.96, P < 0.09) in which patients with RA reporting greater increases of fatigue after PSD than control subjects (Figure 1A). Likewise for self-reported depression, there were significant main effects for group (F(1,51.23) = 3.93, P = 0.05) and time (F(4,440.55) = 3.51, P < 0.01), but not condition, with a group × time interaction (F(4,440.55) = 2.65, P < 0.04) in which patients with RA showed higher levels of depression immediately before (23:00 hr) and after PSD (08:00 hr) as compared with control subjects (P < 0.05) (Figure 1B). Finally, for self-reported anxiety, there were main effects for group (F(1,51.43) = 7.45, P < 0.01) and time (F(4,439.88) = 3.32, P < 0.02), with a group × condition × time interaction (F(4,439.87) = 2.62, P < 0.04), in which patients with RA showed higher levels of anxiety immediately before (23:00 hr) and after PSD (8:00, 12:00, 16:00) as compared with control subjects (P < 0.05) (Figure 1C).
Figure 1.
Effects of partial night sleep deprivation on self-reported fatigue (A), depressed mood (B), and anxiety (C) as indexed by the Profile of Mood States (POMS) in patients with rheumatoid arthritis and control patients. The shaded area represents the sleep interval during the night, whereas the hatched area represents the sleep deprivation interval. Mean ± SEM (error bars).
Self-reported pain responses to PSD
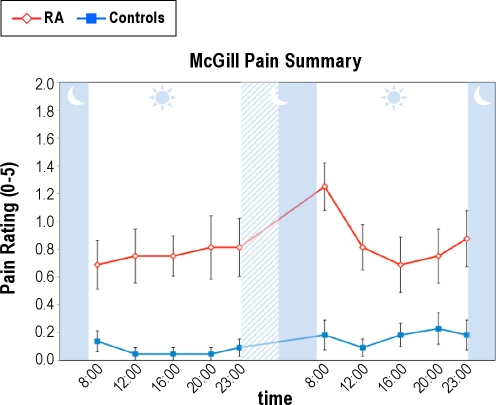
For self-reported pain as indexed by the MPQ, patients with RA also showed an exaggerated response as compared with control subjects (Figure 2). There was an overall significant group effect (F(1,50.0) = 37.95, P < 0.001) with the RA group reporting more pain than the control group. There was an overall condition effect (F(1,426.6) = 13.66, P < 0.001), a marginal time effect (F(4,426.36) = 2.11, P = 0.06), and a significant group × condition × time interaction (F(4,426.4) = 3.04, P < 0.02). Specific comparisons demonstrated significant (P < 0.01) time effects for both groups at 08:00 immediately after PSD, with patients with RA having greater (P < 0.01) self-reported pain at 08:00 immediately after PSD in comparison with control subjects.
Figure 2.
Effects of partial night sleep deprivation on self-reported pain as indexed by the McGill Pain Summary in patients with rheumatoid arthritis and control patients. The shaded area represents the sleep interval during the night, whereas the hatched area represents the sleep deprivation interval. Mean ± SEM (error bars).
Given the differential changes of fatigue, depression, and anxiety in response to PSD between the RA and control groups, as well as evidence that these mood symptoms are associated with RA pain,16 we examined whether variations in self-reported mood were explanatory to changes in pain between the 2 groups. Using time-varying covariates, there were main effects for fatigue (F(1,464.78) = 10.07, P < 0.005) and depression (F(1,466.61) = 18.00, P < 0.001), but not anxiety. When all 3 covariates were used, the results for self-reported pain were largely maintained with a group × condition × time interaction (F(4,417.58) = 2.26, P < 0.06) compared with the original result (F(4,426.4) = 3.04, P < 0.02). Additional exploratory analyses tested whether these mood measures predicted pain values using within-time and lagged correlations; none of these correlations were significant (P > 0.1). Partial correlation analyses also failed to support the exploratory hypothesis that antecedent mood measures predicted subsequent pain.
Baseline differences in PSQI scores were found between the 2 groups. Hence, differential change in self-reported pain was examined with PSQI scores included as a main effect covariate and as a component of a competing higher-order interaction term with condition and time. Whereas there was a main covariate effect of PSQI scores on self-reported pain (F(1,47.95) = 6.39, P < 0.02), inclusion of PSQI did not eliminate the main group effect (F(1,47.91) = 12.98, P < 0.005) nor the group × condition × time interaction (F(1,430.84) = 2.65, P < 0.04) for self-reported pain.
Given our findings that sleep loss increased pain perceptions, and that pain is associated with sleep disturbance (e.g., sleep fragmentation), we explored whether sleep fragmentation occurred in patients with RA versus control patients by obtaining polysomnographic measures of sleep continuity at baseline and during the sleep interval after PSD immediately prior to the morning assessment of pain responses. At baseline, patients with RA showed greater amounts of wake after sleep onset and poorer sleep efficiency at baseline in comparison with control patients (Table 2). However, in the immediate sleep interval after PSD, these 2 objective markers of sleep fragmentation were similar in the 2 groups. Measures of total sleep time and sleep latency were similar in the 2 groups before and after PSD (Table 2).
Table 2.
Polysomnographic measures of sleep continuity at baseline and during the sleep interval after early night partial sleep deprivation in patients with rheumatoid arthritis and control patients
| Baseline night |
PSD sleep interval |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison control |
Rheumatoid arthritis |
t | P | Comparison control |
Rheumatoid arthritis |
t | P | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Total sleep time(min) | 392 | 33 | 378 | 34 | 1.50 | 0.14 | 202 | 35 | 199 | 28 | 0.35 | 0.73 |
| Sleep latency (min) | 25.2 | 22.9 | 25.1 | 18 | 0.01 | 0.99 | 8.6 | 8.4 | 16.8 | 24.3 | −1.65 | 0.11 |
| Wake after sleep onset (min) | 53.7 | 30.1 | 73.3 | 32.3 | −2.24 | 0.03 | 26.4 | 33.3 | 24.4 | 18.7 | 0.26 | 0.80 |
| Sleep efficiency (%) | 88.0 | 6.6 | 83.8 | 7.0 | 2.20 | 0.04 | 88.4 | 14.4 | 89.1 | 7.9 | −0.22 | 0.83 |
PSD, partial night sleep deprivation; SD, standard deviation.
RA-specific self-reported and objective pain responses to PSD
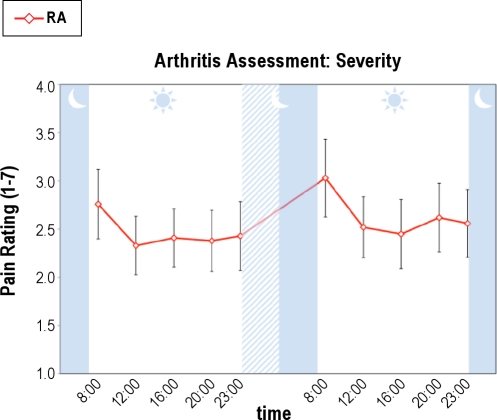
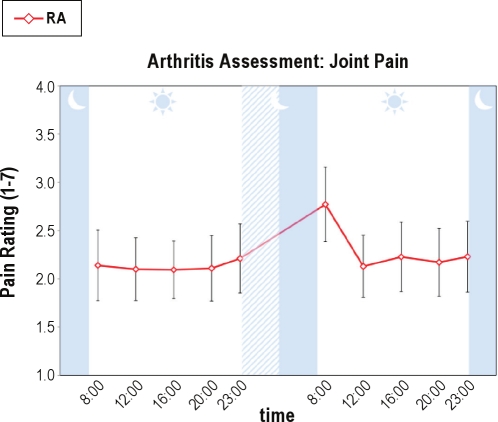
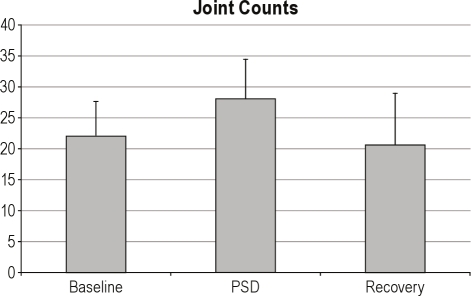
PSD also induced increases in RA-specific disease activity as indexed by joint pain severity and self-reported number of painful joints as measured in the patients with RA only. For overall RA-related joint pain severity (Figure 3), there was both a significant effect for condition (F(1,214.03) = 8.84, P < 0.005) and a significant overall time effect (F(4,214.04) = 7.69, P < 0.001), but no condition × time interaction. Specific comparisons demonstrated that pain severity at 08:00 immediately after PSD was significantly greater than all other time points (F(1,17) = 5.63, P < 0.03). Likewise, for self-reported number of painful joints, there was a significant time effect (F(2.5,58.3) = 4.09, P < 0.02) and a nonsignificant trend for a condition × time interaction (F(3.4,58.3) = 2.50, P < 0.07), in which joint pain at 08:00 immediately after PSD was significantly greater than all other time points (F(1,17) = 4.79, P < 0.05) (Figure 4). Finally, for clinician-rated joint counts (Figure 5), planned comparisons showed increases in the morning after PSD as compared to baseline (t(15) = 2.07, P < 0.03) and recovery mornings (t(9) = 1.96, P < 0.04).
Figure 3.
Effects of partial night sleep deprivation on self-reported joint pain severity in patients with rheumatoid arthritis. The shaded area represents the sleep interval during the night, whereas the hatched area represents the sleep deprivation interval. Mean ± SEM (error bars).
Figure 4.
Effects of partial night sleep deprivation on self-reported number of painful joints in patients with rheumatoid arthritis. The shaded area represents the sleep interval during the night, whereas the hatched area represents the sleep deprivation interval. Mean ± SEM (error bars).
Figure 5.
Effects of partial night sleep deprivation (PSD) on clinician-rated joint counts in patients with rheumatoid arthritis. The shaded area represents the sleep interval during the night, whereas the hatched area represents the sleep deprivation interval. Mean ± SEM (error bars).
DISCUSSION
This study provides the first evidence of an exaggerated increase in symptoms of pain in patients with RA compared with control subjects after sleep loss. In the morning after PSD, self-reported pain symptoms were elevated in RA patients in comparison with control subjects. In addition, sleep loss activated RA-related joint pain as indicated by increases in the number of painful joints and the severity of associated joint pain. Moreover, clinician-rated painful and tender joints increased after this modest sleep loss in patients with RA. Finally, given that objective measures of sleep fragmentation were similar in the 2 groups during the sleep interval immediately after PSD, it appears that sleep loss, as opposed to sleep fragmentation, has a unique role in the differential induction of pain symptoms in patients with RA versus control subjects.
Prior studies have found that mood symptoms including fatigue, depression, and anxiety are prominent in patients with RA.16 The current study confirms these observations and further demonstrates that sleep loss is associated with exaggerated increases of these mood symptoms, which is due either to the direct effects of sleep loss (e.g., fatigue) or to indirect effects related to the anticipation of sleep loss (e.g., anxiety) in patients with RA in comparison with control subjects. Importantly, whereas cross-sectional and longitudinal naturalistic studies report associations between symptoms of mood, sleep disturbance, and pain in patients with RA,16,23 the current findings indicate that the effect of sleep loss on pain symptoms is independent of changes in mood symptoms. Increases of mood symptoms in response to sleep loss do not appear to mediate increases in self-reported pain responses.
Although increases of self-reported pain were short-lasting, transient increases in symptoms of pain after sleep loss have implications of increased disease activity in patients with RA, as cross-sectional and prospective data suggest that sleep disturbance is a risk factor for the exacerbation of pain and functional decline in these patients.2,23,24,25–26 In addition, sleep disturbance is associated with an overnight increase in tenderness in the peripheral joints in patients with RA who are experiencing an acute flareup of symptoms.27 Hence, in the midst of recurrent nights of sleep loss, it is possible that repeated, transient elevations of RA-specific joint pain might lead to an exacerbation of RA symptomatology. Together these data support future efforts to prevent and treat sleep disturbance (e.g., improving sleep duration) in patients with RA as a means of potentially reducing pain. Indeed, cognitive behavioral treatment (CBT) for insomnia has been found to be as effective as sedative hypnotic medications in the short- and long-term management of clinical insomnia,28,29 and there is some evidence that either CBT or pharmacotherapy for insomnia improves sleep and pain symptoms in various chronic pain populations.24,30,31,32–33 One trial reported improvements in sleep and morning stiffness with pharmacotherapy for insomnia (i.e., triazolam) in patients with RA.33
Consistent with prior experimental findings in healthy volunteers, PSD also led to a modest increase in self-reported pain. One small study had previously found that PSD induced hyperalgesia in healthy volunteers.12 However, Smith et al.14 reported that only sleep fragmentation, not restriction of sleep duration, was associated with increases in spontaneous pain in healthy women, a finding that contrasts with our observations that differential increases in pain perceptions in the patients with RA versus control subjects are associated with sleep loss but not sleep fragmentation. Yet, when PSD is repeated for several nights (i.e, 12 nights at 4 hr of sleep per night) or the duration of sleep loss is prolonged (i.e., 1 or 2 nights), there is evidence of hyperalgesia,8,11,13 although not all studies have found that total sleep deprivation alters pain sensitivity.34 These varying findings, taken together with our observations in patients with RA, suggest that activation of pain can occur after sleep loss but this response is heterogeneous and/or more likely in certain populations, possibly due, for example, to a heightened signaling of the neurobiologic mechanisms (e.g., inflammation) that contribute to pain.
RA is characterized as an inflammatory disorder with evidence of activation of inflammatory signaling (i.e., activation of nuclear factor-κB, NF-κB) as well as increases in the localized, cellular, and systemic expression of inflammatory cytokines such as interleukin-6 (IL-6).35 Additionally, we have found that PSD induces increases in NF-κB signaling,36 which leads to the cellular and genomic expression of inflammatory markers,37 which is exaggerated in women.36,37–38 Other data show sex differences in sympathetic nervous system upregulation of IL-6 production,39 and we have found that PSD induces increases in sympathetic activity.40 Hence, it is possible that sleep loss-induced increases in proinflammatory cytokine activity contribute to pain responses after sleep loss, as animal studies have shown that exogenous doses of IL-6 or inflammatory challenges (e.g., endotoxin) induce states of hyperalgesia, in which increases in inflammation are thought to be linked to the development of chronic pain.41 Future studies are under way to understand whether differences in inflammatory signaling drive the exaggerated pain profile in patients with RA versus controls patients after following sleep loss. Haack et al.42 have found that prolonged partial sleep restriction elevated circulating levels of IL-6 in association with pain ratings in healthy volunteers.42
Interpretation of these findings requires consideration of several limitations. First, the experimental sleep deprivation used in this study, like all other sleep deprivation studies that target sleep continuity or duration, was single blinded. Hence, it is possible that the observed effects might be partially attributable to subject expectancies. However, it is unlikely that expectancy effects contributed to differences in response between the 2 groups, who were given similar information about the study. Moreover, such bias would not likely have contributed to the changes in objective, clinician-rated joint counts; the clinician(s) were blinded to the experimental condition. Second, the study relied on reports of spontaneous pain and/or RA-related joint pain by self-report and clinician ratings. Laboratory assessments of pain such as mechanical or thermal pain sensitivity are not used in studies of RA. Rather, the assessments used here followed clinical guidelines for the evaluation of joint pain in patients with RA, with implications for disease activity in this patient population. Third, the sample population was composed mainly of female subjects consistent with the increased prevalence of RA in females. Hence, conclusions about the generalizability of these findings to males cannot be made. Finally, it should also be noted that although an attempt was made to control for baseline differences in PSQI scores, the combination of modest sample size, and limited power for testing interactions with continuous variables, this variable may be more important than the current results suggest. Indeed, additional analyses are under way to examine the associations between subjective and objective measures sleep disturbance and pain in patients with RA, and to test whether differences in homeostatic regulation of sleep, as we found in alcohol-dependent populations,42 contribute to exaggerated pain responses after PSD in patients with RA.
This study addresses two major clinical issues in RA patients: sleep disturbance and joint pain. Although it is widely thought that sleep disturbance is a consequence of joint pain in patients with RA, these experimental observations support an alternative hypothesis that sleep loss leads to increases in joint pain in patients with RA. This profile of sleep loss during part of the night, ubiquitous in those with RA as well as other patients with chronic pain, may result in a vicious cycle in which sleep disturbance activates clinical symptoms of pain, which then contributes to further sleep loss. Importantly, these findings have the potential to redirect the clinical management of pain in patients with RA to include an increased focus on sleep and the prevention and treatment of sleep disturbance in this clinical population.
DISCLOSURE STATEMENT
This was not an industry supported study. Drs. Irwin, Olmstead, FitzGerald, Ranganath, and Nicassio have received research support from the National Institutes of Health. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by a National Institute of Health grant # R01 HL 079955 and in part by grants R01-AG 18367; T32-MH18399, R01- AG 026364, R01-AG 034588, R01-CA 10014152, M01-RR00865, P30-AG028748, General Clinical Research Centers Program, the UCLA Cousins Center at the Semel Institute for Neurosciences, and the UCLA Older Americans Independence Center Inflammatory Biology Core.
REFERENCES
- 1.Verbrugge LM, Juarez L. Profile of arthritis disability: II. Arth Rheum. 2006;55:102–13. doi: 10.1002/art.21694. [DOI] [PubMed] [Google Scholar]
- 2.Drewes AM. Pain and sleep disturbances with special reference to fibromyalgia and rheumatoid arthritis. Rheumatology (Oxford) 1999;38:1035–38. doi: 10.1093/rheumatology/38.11.1035. [DOI] [PubMed] [Google Scholar]
- 3.Taylor-Gjevre RM, Gjevre JA, Nair B, Skomro R, Lim HJ. Components of sleep quality and sleep fragmentation in rheumatoid arthritis and osteoarthritis. Musculoskel Care. 2011 doi: 10.1002/msc.208. http://dx.doi.org/10.1002/msc.208. [DOI] [PubMed] [Google Scholar]
- 4.Murphy S, Creed F, Jayson MI. Psychiatric disorder and illness behaviour in rheumatoid arthritis. Br J Rheumatol. 1988;27:357–63. doi: 10.1093/rheumatology/27.5.357. [DOI] [PubMed] [Google Scholar]
- 5.Frank RG, Beck NC, Parker JC, et al. Depression in rheumatoid arthritis. J Rheumatol. 1988;15:920–5. [PubMed] [Google Scholar]
- 6.Wittig RM, Zorick FJ, Blumer D, Heilbronn M, Roth T. Disturbed sleep in patients complaining of chronic pain. J Nerv Mental Dis. 1982;170:429–31. doi: 10.1097/00005053-198207000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 8.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 9.Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–92. [PubMed] [Google Scholar]
- 11.Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66:932–37. doi: 10.1097/01.psy.0000145912.24553.c0. [DOI] [PubMed] [Google Scholar]
- 12.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–51. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 13.Azevedo E, Manzano GM, Silva A, Martins R, Andersen ML, Tufik S. The effects of total and REM sleep deprivation on laser-evoked potential threshold and pain perception. Pain. 2011;152:2052–58. doi: 10.1016/j.pain.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 14.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 15.Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36:729–40. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 16.Nicassio PM, Kay MA, Custodio MK, Irwin MR, Olmstead R, Weisman MH. An evaluation of a biopsychosocial framework for health-related quality of life and disability in rheumatoid arthritis. J Psychosom Res. 2011;71:79–85. doi: 10.1016/j.jpsychores.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vgontzas AN, Kales A. Sleep and its disorders. Ann Rev Med. 1999;50:387–400. doi: 10.1146/annurev.med.50.1.387. [DOI] [PubMed] [Google Scholar]
- 18.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 19.Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29:112–6. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- 20.Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry. 2002;51:632–41. doi: 10.1016/s0006-3223(01)01304-x. [DOI] [PubMed] [Google Scholar]
- 21.McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states. San Diego: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 22.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 23.Nicassio PM, Wallston KA. Longitudinal relationships among pain, sleep problems, and depression in rheumatoid arthritis. J Abnorm Psychol. 1992;101:514–520. doi: 10.1037//0021-843x.101.3.514. [DOI] [PubMed] [Google Scholar]
- 24.Drewes AM, Svendsen L, Taagholt SJ, Bjerregard K, Nielsen KD, Hansen B. Sleep in rheumatoid arthritis: A comparison with healthy subjects and studies of sleep/wake interactions. Br J Rheumatol. 1998;37:71–81. doi: 10.1093/rheumatology/37.1.71. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch M, Carlander B, Verge M, et al. Objective and subjective sleep disturbances in patients with rheumatoid arthritis. A reappraisal. Arthritis Rheum. 1994;37:41–9. doi: 10.1002/art.1780370107. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe F, Michaud K, Li T. Sleep disturbance in patients with rheumatoid arthritis: evaluation by medical outcomes study and visual analog sleep scales. J Rheumatol. 2006;33:1942–51. [PubMed] [Google Scholar]
- 27.Moldofsky H, Lue FA, Smythe HA. Alpha EEG sleep and morning symptoms in rheumatoid arthritis. J Rheumatol. 1983;10:373–9. [PubMed] [Google Scholar]
- 28.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 29.Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331:1169. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moldofsky H, Lue FA, Mously C, Roth-Schechter B, Reynolds WJ. The effect of zolpidem in patients with fibromyalgia: a dose ranging, double blind, placebo controlled, modified crossover study. J Rheumatol. 1996;23:529–33. [PubMed] [Google Scholar]
- 31.Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch Intern Med. 2005;165:2527–35. doi: 10.1001/archinte.165.21.2527. [DOI] [PubMed] [Google Scholar]
- 32.Scharf MB, Baumann M, Berkowitz DV. The effects of sodium oxybate on clinical symptoms and sleep patterns in patients with fibromyalgia. J Rheumatol. 2003;30:1070–74. [PubMed] [Google Scholar]
- 33.Walsh JK, Muehlbach MJ, Lauter SA, Hilliker NA, Schweitzer PK. Effects of triazolam on sleep, daytime sleepiness, and morning stiffness in patients with rheumatoid arthritis. J Rheumatol. 1996;23:245–52. [PubMed] [Google Scholar]
- 34.Drewes AM, Rossel P, Arendt-Nielsen L, et al. Sleepiness does not modulate experimental joint pain in healthy volunteers. Scand J Rheumatol. 1997;26:399–400. doi: 10.3109/03009749709065709. [DOI] [PubMed] [Google Scholar]
- 35.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–96. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 36.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 38.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24:54–7. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Connor MF, Motivala SJ, Valladares EM, Olmstead R, Irwin MR. Sex differences in monocyte expression of IL-6: role of autonomic mechanisms. Am J Physiol Reg Integc Comp Physiol. 2007;293:R145–51. doi: 10.1152/ajpregu.00752.2006. [DOI] [PubMed] [Google Scholar]
- 40.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999;84:1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 41.Watkins LR, Maier SF. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- 42.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]