Abstract
Seasonal variation of vitamin D status and adequacy of dietary vitamin D and impact of race on maintaining vitamin D sufficiency was assessed in 140 healthy 6–12 year old African American (AA) and Caucasian (C) children residing in Pittsburgh, Pennsylvania during summer and winter. Vitamin D insufficiency was not rare in either group (AA vs. C: summer - 17.2% vs. 14.3%, NS; winter - 34.1% vs. 32.5%, NS) despite a mean dietary intake of vitamin D above the American Academy of Pediatrics (AAP) recommended intake (400 IU/day) (AA vs. C: summer-421 vs. 456 IU/day, NS; winter - 507 vs. - 432 IU/day, NS). Race/season and dietary vitamin D were predictors of serum 25-hydroxyvitamin D [25(OH)D] concentrations. However, dietary vitamin D influenced 25(OH)D only in Caucasians during winter. Current AAP recommended daily intake for vitamin D is inadequate for maintaining vitamin D sufficiency in children.
Keywords: Vitamin D Deficiency, Vitamin D insufficiency, Hypovitaminosis D, Race, Season, Skin Color, Sun exposure, Dietary vitamin D, Serum 25-hydroxyvitamin D, Parathyroid hormone
Introduction
Resurgence of rickets among vulnerable groups of infants and widespread prevalence of hypovitaminosis D in children and adolescents in the US has refocused attention on vitamin D, and suggest that the current American Academy of Pediatrics (AAP) recommended dietary allowance (RDA) for vitamin D (400 IU/day) may be inadequate.1–5 Institute of Medicine (IOM) has recently revised its recommendations for Dietary reference intakes (DRIs) for vitamin D and calcium for US and Canadian populations. The IOM recommended daily intakes of vitamin D were increased from 200 IU to 400 IU in infants (0–12 months) and from 200 IU to 600 IU in children (1–18 years).6 Seasonal variation of sunlight, skin color, and dietary vitamin D are critical factors in the determination of human vitamin D status.7–12 Residents of higher latitudes with excessive skin melanization are most at risk for seasonal hypovitaminosis D, as melanin acts as a natural sunscreen.
The primary function of vitamin D is calcium homeostasis.10 Hypovitaminosis D leads to calcium malabsorption and consequent compensatory hyperparathyroidism resulting in increased bone turnover, bone loss and increased fragility fractures in adults and reduced acquisition of peak bone mass and development of histological rickets in children.13–14 Functions of vitamin D extend beyond calcium homeostasis. Maintenance of higher concentrations of serum 25-hydroxyvitamin D [25(OH)D] may be beneficial for reducing infections, cancer, diabetes, hypertension, and cardiovascular diseases.15–16 Characterizing the racial differences in seasonal variation of vitamin D status and its' determinants in school age children is relevant for addressing their prevailing high rates of vitamin D insufficiency. Therefore, we conducted a short longitudinal study to 1) assess the seasonal variation of vitamin D status and adequacy of dietary vitamin D for maintaining vitamin D sufficiency, and 2) determine the modifying effects of race and effects of other factors on vitamin D status of 6–12 year old pre- and early adolescent children residing at a latitude of 40.4° North, in Pittsburgh, PA.
Methods
Participants
Our study population comprised of 140 healthy 6 to 12 year old pre- and early adolescent African American (94 [67%]) and Caucasian (46 [33%]) children. Participants were recruited from the Primary Care Center of the Children's Hospital of Pittsburgh and from the Greater Pittsburgh area. Children with hepatic or renal disease, metabolic rickets, malabsorptive disorders, cancer, or those on treatment with anticonvulsants or systemic glucocorticoids were excluded. Participants were Tanner staged by physical examination at enrollment by a study investigator (KR or MAH). Racial categorization was parent-identified. The protocol was approved by the University of Pittsburgh Institutional Review Board. Signed parental informed consent and participants' assent was obtained prior to enrollment.
Study design
We conducted a longitudinal study during summer (June through September) and/or winter (December through March) months and assessed the weight, height, dietary intakes of vitamin D and calcium, skin color, sun exposure history, and non-fasting serum calcium, phosphorus, albumin, 25(OH)D, and PTH in participants. Serum for 25(OH)D and PTH measurements were stored at −70° centigrade and batched for analysis. The study was conducted between August 2006 and September 2008.
Study Measurements
Anthropometric data
Body mass index (BMI) was calculated from weight and height at study entry and exit.
Dietary data
We assessed dietary intake of calcium and vitamin D by a vitamin D and calcium questionnaire at study entry and completion. The questionnaire assessed the consumption of multivitamins, calcium supplements, cod liver oil, and vitamin D supplements; average daily intakes of vitamin D fortified milk, other dairy foods, vitamin D fortified orange juice, breakfast bars and cereal; and monthly intakes of fish and dried mushrooms. The questionnaire was analyzed using the Food Processor SQL, version 10.4.0 (2008), ESHA Research, Salem, Oregon to quantify the dietary intake of vitamin D in IU/day.
Sunlight exposure data
We administered a sunlight exposure questionnaire at both study visits to assess the following summertime sun exposure behavior characteristics: i) average duration of summertime sun exposure (≤2 hours or <2 hours); ii) parts of the body that are typically exposed to sunlight (face, hands, arms, and legs); iii) frequency of sunscreen use; and iv) history of travel to a sunny location during summer.
Skin color data
Skin color was estimated by parent reported Fitzpatrick sunreactive skin typing at study entry.17–18 Light skinned individuals were characterized as skin type I (easy burn and no tan) or II (easy burn and slight tan) or III (burn and then tan) and dark skinned individuals were classified as skin type IV (no burn and good tan) or V (never burn and markedly tan).
Biochemical assessments
Serum 25(OH)D was measured using liquid chromatography tandem mass spectrophotometry (LC-MS/MS) assay which measures the contributions of serum 25(OH)D2 and 25(OH)D319. The interassay and intraassay coefficient of variation were 8.1% and 9.4%, respectively. The lowest limit of detection is 2 ng/mL. Serum PTH was measured using Immutopics Human Bioactive PTH 1–84 Elisa kit from Immutopics, Inc., San Clemente, CA. The interassay and intraassay coefficient of variation were 5–8% and 3–5%, respectively.
Statistical Analysis
Demographic characteristics and serum measurements were compared between African American and Caucasian children within each season using two-sample t-tests and Chi-square test (or Fisher's exact tests). Linear mixed models with random intercepts were used to test seasonal differences stratified by race for continuous measures including nutritional measurements and serum measures (calcium, phosphorus, albumin, 25(OH)D, and PTH). We used these models to account for lack of independence between the summer and winter measurements from the same children. We also sought to determine the effects of recognized predictors of vitamin D status on serum 25(OH)D (as a continuous outcome). A four level race/season variable was created from race and season for modeling purposes (Caucasian/summer, Caucasian/winter, AA/summer, and AA/winter) since our primary question was related to seasonality and race modifying effects on serum 25(OH)D. In a linear mixed effects model, we tested for modifying effects of this four level variable on the association between dietary intake of vitamin D and serum 25(OH)D. The additional covariates included in these linear mixed effects models were: age (≤9 yrs old or ≥9 yrs old), sex, skin type (I, II, III or IV, V), BMI (continuous), duration of sun exposure (≤ 2 hrs or > 2 hrs), sunscreen use (no or yes). Unadjusted univariate models followed by a full multivariable model were run. A reduced adjusted multivariable model was derived by including only those predictors with p <0.35 in the full model. In addition, we estimated the proportion of children with vitamin D deficiency defined as serum 25(OH)D <20 ng/mL and insufficiency as 20 - <30 ng/mL.3–4, 20 The association between serum 25(OH)D and PTH was assessed using Spearman correlation stratified by season. The overall significance level for tests was retained as α=.05 and all statistical analyses were done using STATA10 (StataCorp LP, 2008).
Results
Demographic data
We enrolled 140 children (94 [67%] African American and 46 [33%] Caucasian) between August 2006 and September 2008, and assessed them during summer and winter (n=122) or at least during one season (n=18). Mean age for the entire cohort was 9.4±1.7 yrs, 58% were males. African American children when compared with Caucasian children had a higher parent reported Fitzpatrick sunreactive skin type indicative of darker skin color and were more likely to be obese or overweight (Table 1).
Table 1.
Demographic, dietary, laboratory and summertime sun exposure data by race and season
| Summer | Winter | |||
|---|---|---|---|---|
| AA (n=88) | C (n=43) | AA (n=89) | C (n=42) | |
| Age (y) | 9.2±1.7 | 9.7±1.7 | 9.2±1.7 | 9.8±1.8 |
| Male (%) | 58 | 58 | 58 | 57 |
| Tanner Stage (%) | ||||
| Tanner Stage I | 73 | 72 | 71 | 74 |
| Tanner Stage II | 27 | 28 | 29 | 26 |
| Weight (kg) | 35.8±10.5 | 35.2±12.6 | 36.3±11.1 | 35.2±12.0 |
| Height (cm) | 134.6±10.0 | 136.8±12.2 | 134.5±10.1 | 137.3±12.6 |
| BMI (kg/m2) | 19.5±3.9 | 18.2±3.8 | 19.7±4.1 | 18.2±3.6* |
| Weight category (%) | ||||
| Normal (BMI <85tth %tile) | 52 | 76** | 52 | 76** |
| Overweight (BMI 85th – ≤95th %tile) | 22 | 12 | 19 | 14 |
| Obese (BMI >95th %tile) | 26 | 12 | 29 | 10 |
| Skin type (%) | ||||
| TypeI – III | 8 | 79*** | 8 | 79*** |
| TypeIV – V | 92 | 21 | 92 | 21 |
| Dietary data | ||||
| Combined Milk (servings/day) | ||||
| <2 | 38% | 44% | 19% | 40% |
| 2 to 4 | 51% | 47% | 74% | 40% |
| >4 | 11% | 9% | 7% | 20% |
| Combined Milk + OJ (servings/day) | ||||
| <2 | 30% | 40% | 15% | 24% |
| 2 to 4 | 53% | 51% | 67% | 55% |
| >4 | 17% | 9% | 18% | 21% |
| Combined Milk (servings/day) | 2.3±1.6 | 2.5±2.0 | 2.7±1.8 | 2.5±1.9 |
| Combined Milk +OJ (servings/day) | 2.9±1.8 | 2.7±1.9 | 3.3±2.2 | 2.8±1.8 |
| Dietary calcium(mg/day) | 1150±577 | 1039±615 | 1295±894 | 1111±486 |
| Dietary vitamin D (IU/day) | 421±208 | 456±233 | 507±331 | 432±244 |
| Laboratory data | ||||
| Serum calcium (mg/dL) | 9.63 ± 0.28 | 9.63 ± 0.33 | 9.85 ± 0.34† | 9.66 ± 0.29* |
| Serum phosphorus (mg/dL) | 5.00 ± 0.50 | 4.83 ± 0.66 | 4.96 ± 0.48 | 4.81 ± 0.48 |
| Serum albumin (g/dL) | 4.23 ± 0.30 | 4.36 ± 0.27* | 4.28 ± 0.30 | 4.40 ± 0.29* |
| Serum 25(OH)D (ng/mL) | 39.12 ± 11.49 | 45.40 ± 14.69* | 33.79 ± 11.27† | 35.82 ± 10.84‡ |
| Serum PTH (pg/mL) | 38.65 ± 22.51 | 33.03 ± 30.68 | 41.94 ± 23.48 | 31.59 ± 22.80* |
| Summertime Sun Exposure data | ||||
| Duration (> 2 hrs) | 89%* | 80% | 94%* | 83% |
| Sunscreen use (Yes) | 25% | 81%*** | 24% | 81%*** |
| Frequency of sunscreen use (Infrequent) | 91%** | 54% | 67%** | 31% |
| Travel to sunny location (Yes) | 23% | 52%*** | 25% | 50%*** |
Mean ± SD, AA vs. C -
p<.05,
p<.01,
p<.001
p<.001 - Summer vs. Winter - AA;
p <.001 - Summer vs. Winter – C;
OJ – Vitamin D fortified orange juice
Dietary data
There were no differences between racial groups in the usage of multivitamins, vitamin D and/or calcium supplements, or the reported number of servings of vitamin D fortified beverages, or the dietary intake of vitamin D and calcium during summer and winter (Table 1). Mean daily intake of vitamin D was above the current AAP recommended intake for this age group (400 IU daily)5 during summer and winter in both racial groups. However, 42% of Caucasian children during summer and 52% of them during winter, and 55% of African American children during summer and 49% of them during winter did not meet the AAP recommended dietary allowance for vitamin D (data not shown).
Sunlight exposure and sunscreen use
Compared to Caucasian children, a higher proportion of African American children reported > 2 hours of sunlight exposure daily. In contrast, they were less likely to report sunscreen use and travel to a sunny location (Table 1).
Vitamin D status
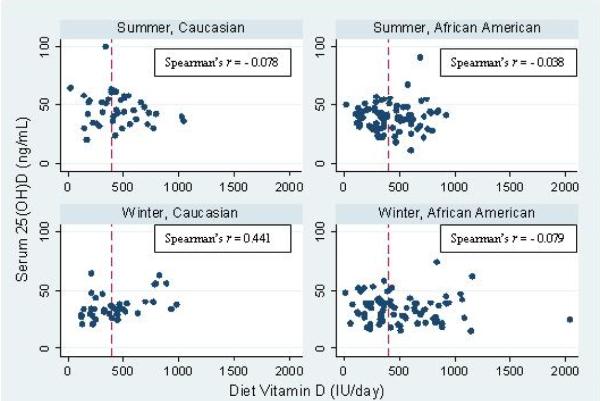
The mean summertime serum 25(OH)D was higher than winter in both African American and Caucasian children (Table 1). The magnitude of change in mean serum 25(OH)D from winter to summer was 45% higher in Caucasian children when compared to African American children (9.8 ± 2.9 vs. 4.4 ± 1.6 ng/mL, p=0.08). African American children when compared to Caucasian children had a lower mean (±SD) serum 25(OH)D during summer and higher PTH during winter. Compared to Caucasian children, African American children had higher serum calcium during winter and lower serum albumin during summer and winter. African American children had lower serum calcium during summer than during winter (Table 1). In simple univariate analyses, dietary intake of vitamin D positively correlated with serum 25(OH)D only in Caucasian children during winter (r=0.44, p=0.004, Figure 1). No differences were apparent in the proportion of children classified as vitamin D deficient (serum 25(OH)D - <20 ng/mL), insufficient (serum 25(OH)D – 20 - <30 ng/mL) or sufficient (serum 25(OH)D - ≥30 ng/mL) during summer and winter between the 2 racial groups (Figure 2).
Figure 1.
Dietary vitamin D and serum 25 (OH) D relationships by race and season
Race/season, dietary D, and other factor effects on serum 25(OH)D concentrations
In each univariate mixed effects model, race/season, skin type, sunscreen use, and dietary vitamin D (only in Caucasians during winter) were significant predictors of circulating concentration of serum 25(OH)D (Table 2). A reduced model showed race/season (Caucasian/winter, African American/summer, and African American/winter) and dietary vitamin D (in Caucasians during winter) to be significant predictors of serum 25(OH)D. For an average dietary intake of vitamin D, compared with Caucasian children during summer (adjusted mean ± SE: 45.27 ± 1.83 ng/mL), both African American and Caucasian children had lower 25(OH)D during winter (AA: 34.07 ± 1.30, Caucasian: 36.07 ± 1.88 ng/mL). Furthermore, African American children had lower serum 25(OH)D than Caucasian children during summer. The association between dietary vitamin D and serum 25(OH)D was not the same across race/season (p=0.0395). There was a positive association among Caucasian children in winter but no association was detected for either race in summer or for African Americans in winter.
Table 2.
Mixed Model Result: Outcome: 25(OH)D
| Unadjusted Model | Adjusted Model | ||
|---|---|---|---|
| Predictor | Coefficient (95% CI) | Full Model | Reduced Model |
| Age | |||
| ≤ 9 yrs old | (Reference) | (Reference) | |
| > 9 yrs old | 1.14(−2.04,4.32) | 0.32(−3.08,3.72) | |
|
| |||
| Sex | |||
| Male | (Reference) | (Reference) | (Reference) |
| Female | −2.10(−5.30,1.10) | −2.01(−5.34,1.31) | −2.05(−5.21,1.11) |
|
| |||
| Race and Season | |||
| Caucasian/Summer | (Reference) | (Reference) | (Reference) |
| Caucasian /Winter | −9.61(−14.45,−4.77) *** | −9.29(−14.31,−4.26) *** | −9.20(−13.97,−4.42) *** |
| AA/Summer | −6.22(−10.60,−1.84) ** | −4.19(−10.17,1.78) | −6.08(−10.47,−1.69) ** |
| AA/Winter | −11.44(−15.82,−7.07) *** | −9.61(−15.67,−3.54) ** | −11.20(−15.60,−6.80) *** |
|
| |||
| Skin Type | |||
| Easily burn(I,II,III) | (Reference) | (Reference) | |
| Not easily burn(IV,V) | −4.71(−8.06,−1.36) ** | −2.38(−7.48,2.72) | |
|
| |||
| BMI (continuous) | −0.07(−0.48,0.34) | 0.03(−0.41,0.48) | |
|
| |||
| Diet Vitamin D | |||
| Caucasian/Summer | −0.01(−0.02,0.01) | −0.01(−0.02,0.01) | −0.01(−0.02,0.01) |
| Caucasian /Winter | 0.02(0.01,0.04) ** | 0.02(0.00,0.04) ** | 0.02(0.01,0.04) ** |
| AA/Summer | −0.00(−0.01,0.01) | 0.00(−0.01,0.01) | 0.00(−0.01,0.01) |
| AA/Winter | −0.00(−0.01,0.00) | −0.00(−0.01,0.01) | −0.00(−0.01,0.00) |
|
| |||
| Sun Exposure | |||
| 2 hrs or less | (Reference) | (Reference) | |
| More than 2 hrs | −2.86(−7.90,2.18) | −1.46(−6.38,3.47) | |
|
| |||
| Sunscreen | |||
| No | (Reference) | (Reference) | |
| Yes | 3.23(0.08,6.38) * | 0.65(−3.21,4.50) | |
.01 <p<.05,
.001<p<.01,
p<.001
Serum 25(OH)D concentrations and PTH
There was a significant negative association between serum 25(OH)D and PTH for the entire cohort during summer (r=−0.22, p=0.019) and winter (r=−0.18, p=0.046). However the magnitude of this association was not significant when assessed by race and season (AA – summer [r=−0.13, p=0.25], winter [r=−0.21, p=0.052]; CC – summer [r=−0.23, p=0.19], winter [r=−0.10, p=0.54]).
Discussion
We found African American and Caucasian children residing in the Northeast to be equally vulnerable for vitamin D insufficiency during summer and winter despite having a mean daily intake of vitamin D above the current AAP recommended intake (400 IU/day). As most children in this study were enrolled from a pediatric clinic, it's likely that dietary advice from pediatric providers may have influenced the parents' to promote greater intakes of vitamin D and calcium. However, our data suggests that the current AAP recommended dietary allowance for vitamin D may be inadequate for optimization of vitamin D status of 6–12 yr old children throughout the year, as 1/3rd of African American and Caucasian children were vitamin D insufficient during winter. The risk of vitamin D insufficiency was higher during winter than summer in both racial groups (AA - 34.1% vs. 17.2%; Caucasian - 32.5% vs. 14.3%).
African American children have a significantly lower concentration of serum 25(OH)D during summer when compared with Caucasian children. In our adjusted multivariable model, race/season and dietary intake of vitamin D stratified by race/season were significant predictors of serum 25(OH)D concentrations. Serum 25(OH)D concentrations are lower in African Americans during summer and winter and in Caucasians during winter when compared with measurements in Caucasians during summer. Race/season was the dominant determinant of vitamin D status. BMI, reported duration of sun exposure, sunscreen use, age, gender, and sunreactive skin type did not influence serum 25(OH)D concentrations.
The influence of dietary vitamin D on serum 25(OH)D varied by race/season – dietary vitamin D positively correlated with serum 25(OH)D only in Caucasian children during winter. As casual sun exposure is the major determinant of vitamin D status – its probable that dietary vitamin D has no significant effect on serum 25(OH)D in either racial group during summer. The observed racial differences in the relationship between dietary vitamin D and serum 25(OH)D during winter suggests that African American children may need higher oral inputs of vitamin D compared to Caucasian children to have a positive impact on serum 25(OH)D.
The magnitude of change in serum 25(OH)D from winter to summer was 45% higher in Caucasian children compared to African American children. This disparity can be explained by the variation in skin pigmentation between the two racial groups. Harris and Dawson-Hughes found similar pattern in the amplitude of seasonal changes in plasma 25(OH)D of 20–40 year old Caucasian and African American women residing in Boston, Massachusetts (latitude: 42°N).7 These findings highlight the relevance of season, skin color, and latitude in the determination of vitamin D status.8–9, 12, 21–22
Residents of higher latitudes (>35°N or S) are at risk for wintertime hypovitaminosis D, as their winter sunlight lacks the UV potency for vitamin D photoproduction.23 Although the capacity for vitamin D photoproduction is similar across all racial groups – dark skinned individuals need 6–10 fold greater duration of sunlight exposure than light-skinned individuals to raise their vitamin D3 to the same concentration.24 Despite the likelihood of higher duration of summertime sunlight exposure and less frequent sunscreen use, African American children had lower 25(OH)D compared to Caucasian children. Although Caucasian children had a higher likelihood of travel to a sunny location below 35° latitude during summer, the effect of such travel on their vitamin D status could have been mitigated by their increased likelihood of sunscreen use.
Many observational and cross-sectional studies of US children have reported higher rates of vitamin D deficiency and insufficiency than what we found.3–4, 25–26 In 2001–2004 National Health and Nutrition Examination Survey (NHANES), among 6–11 yr old children (n=1837) – 18% were vitamin D deficient (serum 25(OH)D <20 ng/mL) and 53% were vitamin D insufficient (20 - <30 ng/mL).4 Furthermore African American children (n=664) were more likely to be classified as vitamin D deficient than Caucasian children (n=510) (51% vs. 9%). Similar to our study, both the racial groups were equally at risk for vitamin D insufficiency (AA [44%] vs. C [51%]). Less consumption of milk and vitamin D supplements and sedentary behavior (surrogate for less sun exposure) were the risk factors for low vitamin D status in the NHANES 2001–2004 survey of 1–21 yr old children.3 Weng et al. found higher prevalence (55%) of hypovitaminosis D (serum 25[OH]D <30 ng/mL) in 6–21 yr old children from Philadelphia, PA, residing in similar latitude as our cohort.27 However, their reported dietary intake of vitamin D was lower. The lower rates of vitamin D deficiency in our cohort may be explained by their relatively higher intakes of dietary vitamin D.
Defining the serum 25(OH)D cutoff values for vitamin D deficiency and insufficiency remains contentious and lacks consensus; and data for defining the threshold levels of serum 25(OH)D for optimal skeletal health in children are limited.28–30 If calcium intake is adequate, a desirable level of serum 25(OH)D will i) suppress PTH to a mid-physiologic range, ii) not decrease PTH during vitamin D supplementation, and iii) maximize calcium absorption, bone accretion, and bone mineral density.13, 31–34 Inverse association between serum 25(OH)D and PTH have been documented in older children and adolescents,35–37 and their threshold levels of 25(OH)D associated with optimal vitamin D status in terms of calcium homeostasis tend to fall between 16–36 ng/mL.35, 38 In adults, calcium absorption is maximal when serum 25(OH)D is ≥32 ng/mL,34 and increasing serum 25(OH)D from 32 to 40 ng/mL is beneficial for bone mineral density.32 However, the recent IOM report, has concluded that in the face of adequate calcium intake, serum 25(OH)D levels ≥20 ng/mL is sufficient for skeletal health, and levels <12 ng/mL and 12-<20 ng/mL are indicative of deficiency and insufficiency respectively.6 The IOM report calls to question the recent reports of high prevalence of vitamin D deficiency and insufficiency based on higher serum 25(OH)D cutoffs for defining vitamin D deficiency (<20 ng/mL) and insufficiency (20-<30 ng/mL).
Serum PTH is a recognized secondary outcome measure in the assessment of vitamin D status.13, 39 Hypovitaminosis D can result in calcium malabsorption and compensatory hyperparathyroidism.10, 3 PTH was inversely associated with 25(OH)D in our entire cohort during summer and winter, suggesting that hypovitaminosis D may have a negative impact on skeletal health of children. Furthermore, the serum PTH was significantly higher in our cohort of African American children when compared with Caucasian children during winter. The observed differences in serum calcium and serum albumin between the two racial groups were clinically non-relevant.
Our study has several limitations. Sampling timeframe would influence the seasonal differences in vitamin D status. Therefore, our findings would have been exaggerated if we had sampled only during the peak (end of August) and nadir (end of March) time-points for seasonal change in serum 25(OH)D status, instead of throughout summer and winter. Assessment of degree of melanization using a skin spectrophotometer would have been more objective than the parent reported sunreactive skin typing. Although our vitamin D intake questionnaire was detailed and thorough, we would like to caution the potential limitation in the reliability of questionnaires for assessment of dietary intake of vitamin D. Our observed effect size for differences between the racial groups could be limited by the smaller number of Caucasian children. Collection of blood samples for PTH assessment throughout the day instead of a specified time could potentially confound those results, as PTH has an endogenous circadian rhythm with a high peak in the early hours of morning and a nadir in the late morning and a small peak in the late afternoon.40–41
However, our study has several strengths. We enrolled significant proportion of African American children (67%), and had good retention rate (87%). We assessed serum 25(OH)D with the state of the art LC-MS/MS assay which reduces the potential confounding associated with variability in measurements of serum 25(OH)D among various assays and laboratories.20, 42 Moreover, our cohort had an adequate dietary intake of vitamin D, which enabled us to discern the effect of season, latitude, and skin color on vitamin D status of young school age children.
Conclusion
We conclude that African American and Caucasian children are vulnerable for seasonal variation in their vitamin D status, and are more likely to have hypovitaminosis D during winter than summer. Compared with Caucasian children, African American children are at a higher risk for hypovitaminosis D. Higher melanin content in the skin precludes African American children from sustaining a significant summertime increase in their vitamin D status. The relatively higher mean intakes of vitamin D in our cohort may explain their lower rates of vitamin D deficiency.
However, as significant proportion of children in our cohort were vitamin D insufficient despite having a mean dietary intake of vitamin D above 400 IU/day – we believe that the current AAP recommended intake of vitamin D for children (400 IU/day) is inadequate for maintaining optimal vitamin D status in 6–12 yr old children. The recently revised IOM DRI recommendation for daily vitamin D intake (increased from 200 IU to 400 IU in infants [0–12 months] and from 200 IU to 600 IU in children [1–18 years]) is a step in the right direction.6 Effect of dietary vitamin D on serum 25(OH)D varies by race and season. African American children may need a higher intake of vitamin D during winter to increase their serum 25(OH)D concentrations than Caucasian children. Randomized controlled dose-response trials are warranted to understand the racial differences in the seasonal variation in the responsive changes in serum 25(OH)D during vitamin D supplementation. Future studies are also warranted for refining the threshold levels of serum 25(OH)D for optimal skeletal health in children. Benefits of optimization of vitamin D status of young school age children need further exploration.
Figure 2. Vitamin D status by race and season.
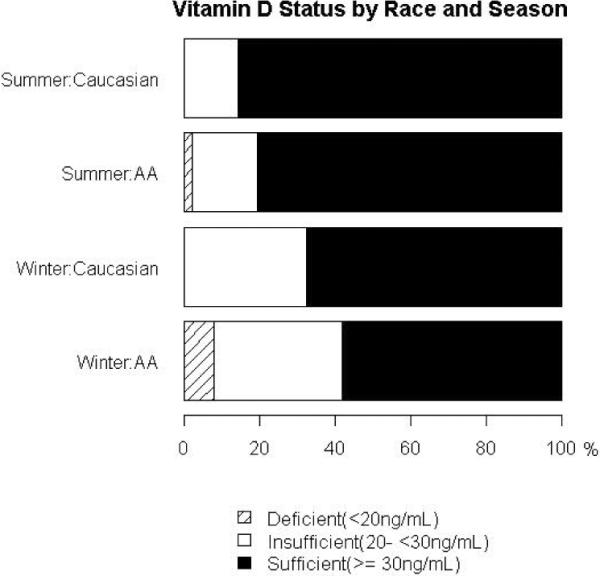
Vitamin D deficient (summer – AA [2.3%], C [0%]; winter – AA [8%], C [0%]) Vitamin D Insufficient (summer – AA [17.2%], C [14.3%]; winter – AA [34.1%], C [32.5%])
Acknowledgement
This project was supported by the following National Institutes of Health grants: R03HD053479 (Kumaravel Rajakumar) and K23HD052550 (Kumaravel Rajakumar) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD); 2P60MD00020707 (Stephen B Thomas) from the National Center on Minority Health and Health Disparities (NCMHD); K24DK062895 (Susan L Greenspan) from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); UL1 RR024153-01 (Pediatric Clinical Translational Research Center at Children's Hospital of Pittsburgh of UPMC); and 5UL1 RR024153-04 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official view of NICHD, NCMHD, NIDDK, NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Grant Support: This project was supported by National Institutes Health grant R03HD053479 (KR), K23HD052550 (KR), 2P60MD000207-07 (SBT), K24DK062895 (SLG), UL1 RR024153-01, and 5UL1 RR024153-04
Abbreviations
- AA
African American
- C
Caucasian
- 25(OH)D
25-hydroxyvitamin D
- PTH
Parathyroid hormone
- RDA
recommended dietary allowance
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
References
- 1.Ginde AA, Liu MC, Camargo CA., Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreiter SR, Schwartz RP, Kirkman HN, Jr., Charlton PA, Calikoglu AS, Davenport ML. Nutritional rickets in African American breast-fed infants. J Pediatr. 2000;137(2):153–157. doi: 10.1067/mpd.2000.109009. [DOI] [PubMed] [Google Scholar]
- 3.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and Associations of 25-Hydroxyvitamin D Deficiency in US Children: NHANES 2001–2004. Pediatrics. 2009;124:e362–e370. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansbach JM, Ginde AA, Camargo CA., Jr. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124(5):1404–1410. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 6.IOM (Institute of Medicine) Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 7.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67(6):1232–1236. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF, MacLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210(4466):203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin M, Raggatt PR, Fairney A, Brown DJ, Lester E, Wills MR. Seasonal variations in serum 25-hydroxycholecalciferol in healthy people. Lancet. 1974;1(7857):536–538. doi: 10.1016/s0140-6736(74)92717-2. [DOI] [PubMed] [Google Scholar]
- 10.IOM (Insititue of Medicine) Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press; Washington, DC: 1997. [PubMed] [Google Scholar]
- 11.Norman AW. Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. Am J Clin Nutr. 1998;67(6):1108–1110. doi: 10.1093/ajcn/67.6.1108. [DOI] [PubMed] [Google Scholar]
- 12.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 13.McKenna MJ, Freaney R. Secondary hyperparathyroidism in the elderly: means to defining hypovitaminosis D. Osteoporos Int. 1998;8(Suppl 2):S3–6. doi: 10.1007/pl00022725. [DOI] [PubMed] [Google Scholar]
- 14.Slemenda CW, Peacock M, Hui S, Zhou L, Johnston CC. Reduced rates of skeletal remodeling are associated with increased bone mineral density during the development of peak skeletal mass. J Bone Miner Res. 1997;12(4):676–682. doi: 10.1359/jbmr.1997.12.4.676. [DOI] [PubMed] [Google Scholar]
- 15.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E. Expanding roles of vitamin D. J Clin Endocrinol Metab. 2009;94(2):418–420. doi: 10.1210/jc.2008-2695. [DOI] [PubMed] [Google Scholar]
- 17.Pathak M, Jimbow K, Szabo G, et al. Sunlight and melanin pigmentation. In: Smith K, editor. Photochemical and photobiological reviews. Vol 1. Plenum Press; New York: 1976. pp. 211–239. [Google Scholar]
- 18.Jimbow K, Fitzpatrick TB, Wick WM. Biochemistry and physiology of melanin pigmentation. In: LA G, editor. Physiology, biochemistry, and molecular biology of the skin. Vol 2. Oxford University Press; New York: 1991. pp. 873–909. [Google Scholar]
- 19.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamp TC, Round JM. Seasonal changes in human plasma levels of 25-hydroxyvitamin D. Nature. 1974;247(442):563–565. doi: 10.1038/247563a0. [DOI] [PubMed] [Google Scholar]
- 22.Webb AR. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol. 2006;92(1):17–25. doi: 10.1016/j.pbiomolbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 24.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 25.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 26.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(2):558S–564S. doi: 10.1093/ajcn/88.2.558S. [DOI] [PubMed] [Google Scholar]
- 27.Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007;86(1):150–158. doi: 10.1093/ajcn/86.1.150. [DOI] [PubMed] [Google Scholar]
- 28.Greer FR. Defining vitamin D deficiency in children: beyond 25-OH vitamin D serum concentrations. Pediatrics. 2009;124(5):1471–1473. doi: 10.1542/peds.2009-2307. [DOI] [PubMed] [Google Scholar]
- 29.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 30.Rovner AJ, O'Brien KO. Hypovitaminosis D among healthy children in the United States: a review of the current evidence. Arch Pediatr Adolesc Med. 2008;162(6):513–519. doi: 10.1001/archpedi.162.6.513. [DOI] [PubMed] [Google Scholar]
- 31.Abrams SA, Hicks PD, Hawthorne KM. Higher serum 25-hydroxyvitamin D levels in school-age children are inconsistently associated with increased calcium absorption. J Clin Endocrinol Metab. 2009;94(7):2421–2427. doi: 10.1210/jc.2008-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 33.Guillemant J, Le HT, Maria A, Allemandou A, Peres G, Guillemant S. Wintertime vitamin D deficiency in male adolescents: effect on parathyroid function and response to vitamin D3 supplements. Osteoporos Int. 2001;12(10):875–879. doi: 10.1007/s001980170040. [DOI] [PubMed] [Google Scholar]
- 34.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 35.Harkness L, Cromer B. Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos Int. 2005;16(1):109–113. doi: 10.1007/s00198-004-1656-8. [DOI] [PubMed] [Google Scholar]
- 36.Rajakumar K, Fernstrom JD, Janosky JE, Greenspan SL. Vitamin D insufficiency in preadolescent African-American children. Clin Pediatr (Phila) 2005;44(8):683–692. doi: 10.1177/000992280504400806. [DOI] [PubMed] [Google Scholar]
- 37.Willett AM. Vitamin D status and its relationship with parathyroid hormone and bone mineral status in older adolescents. Proc Nutr Soc. 2005;64(2):193–203. doi: 10.1079/pns2005420. [DOI] [PubMed] [Google Scholar]
- 38.Outila TA, Karkkainen MU, Lamberg-Allardt CJ. Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: associations with forearm bone mineral density. Am J Clin Nutr. 2001;74(2):206–210. doi: 10.1093/ajcn/74.2.206. [DOI] [PubMed] [Google Scholar]
- 39.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351(9105):805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 40.el-Hajj Fuleihan G, Klerman EB, Brown EN, Choe Y, Brown EM, Czeisler CA. The parathyroid hormone circadian rhythm is truly endogenous--a general clinical research center study. J Clin Endocrinol Metab. 1997;82(1):281–286. doi: 10.1210/jcem.82.1.3683. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt CP, Homme M, Schaefer F. Structural organization and biological relevance of oscillatory parathyroid hormone secretion. Pediatr Nephrol. 2005;20(3):346–351. doi: 10.1007/s00467-004-1767-7. [DOI] [PubMed] [Google Scholar]
- 42.Binkley N, Krueger D, Cowgill CS, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89(7):3152–3157. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]