Abstract
Sphingosylphosphorylcholine (SPC) induces differentiation of human adipose tissue-derived mesenchymal stem cells (hASCs) into smooth muscle-like cells expressing α-smooth muscle actin (α-SMA) via transforming growth factor-β1/Smad2- and RhoA/Rho kinase-dependent mechanisms. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) have been known to have beneficial effects in the treatment of cardiovascular diseases. In the present study, we examined the effects of simvastatin on the SPC-induced α-SMA expression and Smad2 phosphorylation in hASCs. Simvastatin inhibited the SPC-induced α-SMA expression and sustained phosphorylation of Smad2 in hASCs. SPC treatment caused RhoA activation via a simvastatin-sensitive mechanism. The SPC-induced α-SMA expression and Smad2 phosphorylation were abrogated by pretreatment of the cells with the Rho kinase inhibitor Y27632 or overexpression of a dominant negative RhoA mutant. Furthermore, SPC induced secretion of TGF-β1 and pretreatment with either Y27632 or simvastatin inhibited the SPC-induced TGF-β1 secretion. These results suggest that simvastatin inhibits SPC-induced differentiation of hASCs into smooth muscle cells by attenuating the RhoA/Rho kinase-dependent activation of autocrine TGF-β1/Smad2 signaling pathway.
Keywords: cell differentiation; mesenchymal stem cells; myocytes, smooth muscle; rhoA GTP-binding protein; simvastatin; sphingosine phosphorylcholine; transforming growth factor β1
Introduction
Smooth muscle cells (SMCs) play an important role in angiogenesis, vessel maintenance, and regulation of blood pressure. SMCs exhibit a contractile phenotype characterized by high expression of specific contractile proteins, including α-SMA, calponin-1, SM22α, smoothelin, h-caldesmon, and smooth muscle myosin heavy chain (Shanahan et al., 1993; Owens et al., 2004). The phenotypic expression of SMCs is implicated in vascular development as well as in a variety of cardiovascular diseases, including hypertension and atherosclerosis (Liu et al., 2004; Owens et al., 2004).
Mesenchymal stem cells (MSCs) have a self-renewal capacity, long-term viability, and potential to differentiate into cells of diverse lineages, such as adipogenic, osteogenic, chondrogenic, and myogenic lineages (Pittenger and Martin, 2004; Caplan, 2007; Chamberlain et al., 2007; Prockop et al., 2010). Bone marrow-derived MSCs have been shown to differentiate to smooth muscle cells (SMCs) in response to transforming growth factor-β (TGF-β) (Kinner et al., 2002; Wang et al., 2004), mechanical stress (Kobayashi et al., 2004), and direct contact with vascular endothelial cells in vitro (Ball et al., 2004). Moreover, injected bone marrow-derived MSCs have been reported to have differentiated into SMCs and to have contributed to the remodeling of vasculature in vivo (Davani et al., 2003; Gojo et al., 2003; Yoon et al., 2005). In a previous study, we showed that sphingosylphosphorylcholine (SPC) increased the expression levels of α-SMA and other smooth muscle-specific proteins in human adipose tissue-derived mesenchymal stem cells (hASCs) via an autocrine TGF-β/Smad2-dependent mechanism (Jeon et al., 2006). In addition, we have previously reported that SPC stimulated the small GTPase RhoA and that the RhoA-Rho kinase pathway played a key role in SPC-induced differentiation of hASCs to SMCs. RhoA-Rho kinase pathway plays a key role in SMC differentiation by regulating the integrity of the actin cytoskeleton and MRTF-dependent gene transcription (Cen et al., 2004; Miano et al., 2007). Therefore, SPC-induced SMC differentiation of MSCs would be an ideal model for the study of vascular diseases-associated SMC differentiation.
3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) reportedly exert beneficial effects in patients with cardiovascular diseases via pleiotropic functions, including reduction of plaque inflammation and platelet aggregation, enhanced plaque stability and endothelial function, and inhibition of SMC proliferation and increased apoptosis (Calabro and Yeh, 2005; Liao, 2005). Accumulating evidence suggests that statins attenuate neointimal formation and vascular remodeling by blocking the activation of the Rho family of small G proteins (Rolfe et al., 2005). Statins inhibit the activity of HMG-CoA reductase which catalyses the conversion of HMG-CoA into mevalonate during cholesterol biosynthesis. Mevalonate can be converted into farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP), 2 isoprenoid residues that can be anchored onto several intracellular proteins through farnesylation or geranylgeranylation (Wong et al., 2002; Graaf et al., 2004). Simvastatin has been reported to inhibit the relocalization of RhoA to cell membranes and the resulting activation of RhoA by blocking geranylgeranylation (Laufs et al., 1999). However, whether statins can affect the SPC-induced differentiation of MSCs to SMCs has not been studied. In the present study, we show for the first time that simvastatin inhibits the differentiation of hASCs into SMCs by blocking RhoA-Rho kinase-dependent activation of autocrine TGF-β/Smad2 signaling pathway.
Results
Simvastatin inhibits SPC-induced differentiation of hASCs to SMCs
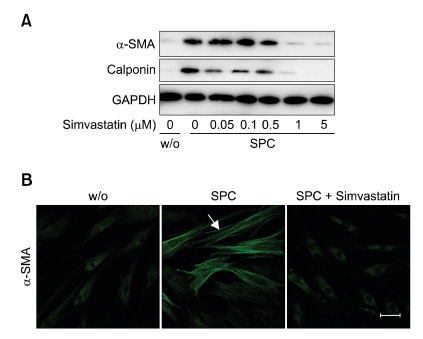
To explore whether statin can affect SPC-induced differentiation of hASCs to SMCs, we examined the effect of simvastatin on the SPC-induced expression of smooth muscle-specific markers, including α-SMA and calponin. As shown in Figure 1, SPC treatment increased the expression of α-SMA and calponin in hASCs, and simvastatin dose-dependently attenuated SPC-induced expression of α-SMA and calponin with a complete inhibition at a 1 µM concentration, suggesting simvastatin has an inhibitory effect on the SPC-induced differentiation of hASCs to SMCs.
Figure 1.
Effect of simvastatin on SPC-induced expression of smooth muscle markers in hASCs. (A) hASCs were treated with serum-free medium containing 2 µM SPC or vehicles (0.1% DMSO, w/o) in the presence of indicated concentrations of simvastatin for 4 days. Expression levels of α-SMA, calponin, and GAPDH were determined by Western blotting. (B) Inhibitory effects of simvastatin on SPC-induced α-SMA expression in hASCs were further determined by immunostaining with anti-α-SMA antibody. Scale bar = 50 µm. Representative data from three independent experiments are shown.
To confirm these results, we determined the effects of simvastatin on α-SMA expression and actin filament formation using immunocytochemistry. As shown in Figure 1B, treatment of hASCs with 2 µM SPC for 4 days increased α-SMA expression levels, and pretreatment of the cells with simvastatin completely abrogated SPC-induced expression of α-SMA in hASCs.
Simvastatin inhibits SPC-induced sustained phosphorylation of Smad2
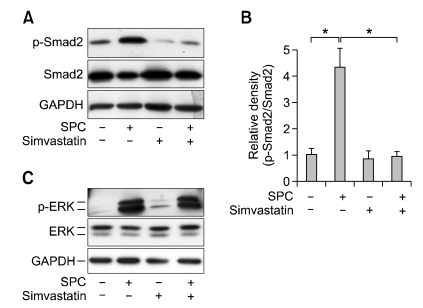
We previously reported that SPC treatment elicited phosphorylation of Smad2 on day 1 that was sustained until day 4, and that the sustained phosphorylation of Smad2 was responsible for the increased expression of α-SMA (Jeon et al., 2006). Therefore, we sought to determine the effect of simvastatin on SPC-induced Smad2 phosphorylation on day 4. As shown in Figures 2A and 2B, treatment of hASCs with SPC for 4 days induced phosphorylation of Smad2 and pretreatment of the cells with simvastatin markedly attenuated Smad2 phosphorylation.
Figure 2.
Effects of simvastatin on SPC-induced phosphorylation of Smad2 and ERK. (A) hASCs were treated with serum-free medium containing 2 µM SPC or vehicles (0.1% DMSO) in the absence or presence of 1 µM simvastatin for 4 days. Phosphorylation levels of Smad2 and expression levels of Smad2 and GADPH were determined by Western blotting. (B) The densities of p-Smad2 were quantified and normalized to those of Smad2. Data are expressed as mean ± SD (n = 4; *P < 0.05). (C) hASCs were pretreated with serum-free medium containing 1 µM simvastatin or vehicles (0.1% DMSO) for 1 day and then treated with 2 µM SPC or vehicles for 10 min. Phosphorylation levels of ERK and expression levels of ERK and GADPH were determined by Western blotting. Representative data from three independent experiments are shown.
We have shown that SPC elicited acute ERK phosphorylation in 10 min and ERK phosphorylation played a key role in SPC-induced sustained phosphorylation of Smad2 (Jeon et al., 2006). Therefore, we examined the effect of simvastatin in SPC-induced ERK phosphorylation. As shown in Figure 2C, treatment of hASCs with SPC for 10 min induced phosphorylation of ERK, whereas SPC-induced ERK phosphorylation was not attenuated by pretreatment with simvastatin. This suggests that ERK is not a target of simvastatin-induced inhibition of Smad2 phosphorylation and α-SMA expression.
Simvastatin inhibits SPC-induced RhoA activation
Increasing body of evidence suggests that RhoA-mediated rearrangement of the actin cytoskeleton plays a pivotal role in SMC differentiation (Cen et al., 2004). It has been reported that activated RhoA translocated from the cytosol to the plasma membrane through geranylgeranylation, and that simvastatin inhibited this translocation (Laufs et al., 1999).
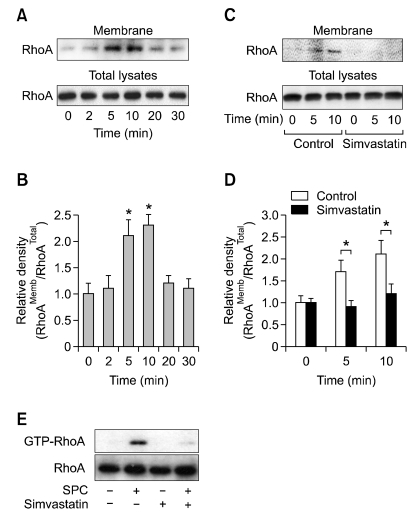
To explore whether SPC can induce RhoA activation, hASCs were treated with SPC for the indicated periods, and the amounts of membrane-associated RhoA were determined. SPC treatment increased the amounts of RhoA associated with cell membrane in a time-dependent manner, with a maximal increase at 5 min (Figures 3A and 3B). The SPC-induced translocation of RhoA was completely abrogated by pretreatment of the cells with simvastatin (Figures 3C and D). To confirm these results, we determined the amounts of GTP-loaded RhoA after treatment with SPC and/or simvastatin. As shown in Figure 3E, SPC treatment increased GTP-RhoA levels and pretreatment with simvastatin abrogated SPC-induced increase of GTP-RhoA.
Figure 3.
Effect of simvastatin on SPC-induced RhoA activation. (A) hASCs were treated with 2 µM SPC for the indicated time periods. (C) hASCs were pretreated with serum-free medium containing 1 µM simvastatin or vehicles (0.1% DMSO) for 1 day and then treated with 2 µM SPC or vehicles for the indicated time periods. The amounts of RhoA in total lysates and membrane fractions were determined by Western blotting. (B, D) The densities of RhoA in membrane fractions were quantified and normalized to those of RhoA in total lysates. Data are expressed as mean ± SD (n = 4; *P < 0.05). (E) hASCs were pretreated with 1 µM simvastatin (0.1% DMSO) or vehicles for 1 day, followed by treatment with 2 µM SPC for 5 min. The amounts of RhoA in the whole cell lysates (Total RhoA) or the GTP-bound RhoA precipitated from the lysates were revealed by Western blotting. Representative data from three independent experiments are shown.
RhoA-Rho kinase is responsible for the SPC-induced α-SMA expression and Smad2 phosphorylation
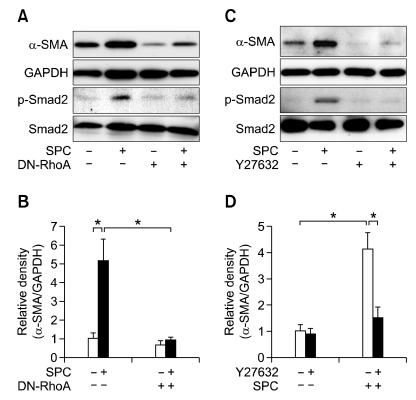
In order to delineate whether RhoA is involved in the SPC-induced α-SMA expression and Smad2 phosphorylation, we examined the effect of a dominant-negative mutant of RhoA (RhoAN19) on SPC-induced α-SMA expression and Smad2 phosphorylation. As shown in Figures 4A and 4B, overexpression of RhoAN19 attenuated the SPC-induced α-SMA expression and Smad2 phosphorylation, suggesting that RhoA plays a key role in SPC-induced α-SMA expression through a Smad2-dependent mechanism.
Figure 4.
Role of RhoA-Rho kinase pathway in the SPC-induced phosphorylation of Smad2. (A) hASCs were transfected with the dominant-negative mutant of RhoA (DN-RhoA) or control vector and then treated with serum-free medium containing 2 µM SPC or vehicles for 4 days. (C) hASCs were treated with serum-free medium containing 2 µM SPC or vehicles in the absence or presence of 10 µM Y27632 for 4 days. Phosphorylation levels of Smad2 and expression levels of Smad2, α-SMA, and GAPDH were determined by Western blotting. (B, D) The densities of α-SMA were quantified and normalized to those of GAPDH. Data are expressed as mean ± SD (n = 4; *P < 0.05).
To explore whether Rho kinase, a major downstream target of RhoA, is involved in SPC-induced α-SMA expression and Smad2 phosphorylation in hASCs, we examined the effect of Y27632, a Rho kinase-specific inhibitor, on α-SMA expression and Smad2 phosphorylation induced by SPC. As shown in Figures 4C and 4D, SPC-induced α-SMA expression and Smad2 phosphorylation were abrogated by pretreatment of the cells with Y27632, suggesting that Rho kinase is involved in SPC-induced α-SMA expression and Smad2 phosphorylation. Moreover, basal expression levels of α-SMA in the absence of SPC treatment were slightly inhibited by either DN-RhoA overexpression or pretreatment with Y27632, suggesting a potential role of RhoA in basal expression of α-SMA as well as in the SPC-induced α-SMA expression.
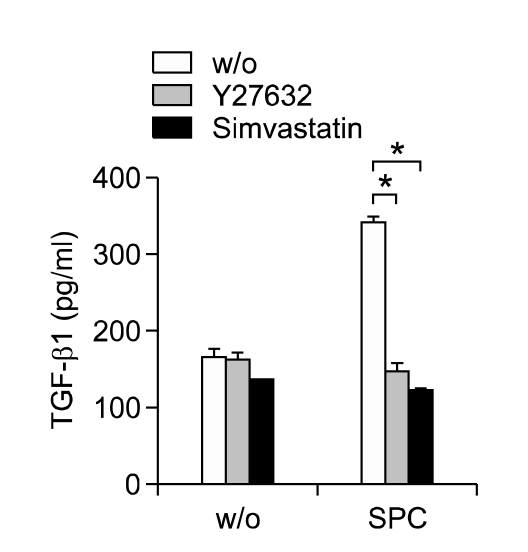
Simvastatin inhibits SPC-induced secretion of TGF-β1 through Rho kinase-dependent mechanism
We previously reported that autocrine secretion of TGF-β1 is responsible for SPC-induced Smad2 phosphorylation and α-SMA expression (Jeon et al., 2006). To explore whether the inhibitory effects of simvastatin on SPC-induced Smad2 phosphorylation and α-SMA expression are due to the decreased secretion of TGF-β1, we measured the effect of simvastatin on SPC-induced TGF-β1 secretion. As shown in Figure 5, SPC stimulated secretion of TGF-β1 from hASCs, and pretreatment of the cells with simvastatin abrogated SPC-induced secretion of TGF-β1. Furthermore, pretreatment of the cells with Y27632 inhibited SPC-induced TGF-β1 secretion. These results suggest that simvastatin inhibits the SPC-induced autocrine TGF-β1/Smad2 signaling pathway and α-SMA expression by inhibiting Rho kinase-dependent TGF-β1 secretion.
Figure 5.
Effect of simvastatin on SPC-induced secretion of TGF-β1. hASCs were treated with serum-free medium containing 2 µM SPC or vehicles (w/o) in the absence or presence of 10 µM Y27632 or 1 µM simvastatin for 2 days. The conditioned medium was subjected to ELISA for determination of TGF-β1 protein levels. Data represent mean ± SD (n = 4). *indicates P < 0.05.
Discussion
The RhoA-Rho kinase signaling pathway plays a key role in the SPC-induced differentiation of hASCs to SMCs. Inhibition of the RhoA-Rho kinase pathway by over-expression of DN-RhoA or pre-treatment with Y27632 completely abrogated SPC-induced α-SMA expression (Jeon et al., 2008). It has been documented that the RhoA/Rho kinase-dependent pathway regulates transcription of SMC-specific genes (Mack et al., 2001; Wamhoff et al., 2004). Sphingosine-1-phosphate increased RhoA activity and expression level of α-SMA in smooth muscle cells, and that inhibition of Rho kinase by Y27632 attenuated the sphingosine-1-phosphate-stimulated α-SMA expression (Lockman et al., 2004). In the present study, we demonstrated that simvastatin treatment blocked SPC-induced translocation of RhoA to membrane fractions and abrogated SPC-induced α-SMA expression in hASCs. Inactive GDP-bound RhoA becomes activated by membrane translocation and subsequent exchange of GDP and GTP in the presence of guanine nucleotide exchange factor (Bokoch et al., 1994). Statins lead to the deprivation of mevalonate and downstream metabolites such as mevalonate, GGPP and FPP by blocking HMG-CoA reductase (Nakagami et al., 2003). GGPP and FPP are transferred to proteins such as small GTPases by GGPP and FPP, respectively (Wong et al., 2002; Graaf et al., 2004). Statins has been reported to inhibit membrane localization and GTP binding activity of RhoA (Kusama et al., 2001; Yoshida et al., 2001). Taken together, these results support the notion that ablation of RhoA-Rho kinase signaling cascade is responsible for simvastatin-induced inhibition of SPC-induced differentiation of hASCs to SMCs.
We have previously showed that SPC-induced activation of the autocrine TGF-β1 signaling loop is responsible for the sustained phosphorylation of Smad2 on day 4 (Jeon et al., 2006). In the present study, we demonstrated for the first time that inhibition of Rho kinase by treatment with Y27632 or simvastatin blocked SPC-induced secretion of TGF-β1 and sustained Smad2 phosphorylation. Although the molecular mechanisms by which activation of Rho kinase regulates expression of TGF-β1 are largely elusive, a possible role of Rho kinase in the regulation of TGF-β1 is supported by a report that mechanical stretch of human airway SMCs augmented TGF-β1 expression through a RhoA-Rho kinase-dependent mechanism (Mohamed and Boriek, 2010). Inhibition of Rho kinase by treatment with Y27632 has been reported to abrogate renal fibrosis-associated increase of α-SMA expression and TGF-β1-induced myofibroblastic differentiation of gingival fibroblasts (Nagatoya et al., 2002; Smith et al., 2006). Consistently, inhibition of RhoA by C3 exoenzyme or DN-RhoA and pharmacological inhibition of Rho kinase by pretreatment with Y27632 blocked TGF-β1-induced expression of α-SMA in Monc-1 neural crest stem cells (Chen et al., 2006). Taken together with these results, the present study suggests that Rho kinase is involved in the SPC-induced autocrine secretion of TGF-β1 and subsequent activation of TGF-β receptor and Smad2 phosphorylation.
Differentiation and phenotypic modulation of vascular smooth muscle cells (VSMCs) are implicated in the development of vascular diseases including atherosclerosis (Rzucidlo et al., 2007). Accumulating evidence suggests that MSCs from various tissues reside in a perivascular location and can be identified as pericytes that play a key role in development of microvessels (Covas et al., 2008; Feng et al., 2010). It has been reported that hASCs also originate from perivascular cells (Cai et al., 2011). Moreover, several studies suggest that pericytes surrounding capillaries and microvessels but also adventitial cells located around larger arteries and veins natively express MSC markers and multipotent differentiation potentials similar to MSCs (Chen et al., 2009; Corselli et al., 2010). Therefore, these results suggest that MSCs may play a role in the vascular development and vascular diseases. SPC has been reported to exist in high-density lipoprotein (HDL) particles (Tolle et al., 2008). Increasing body of evidence demonstrate that HDL exerts anti-atherogenic and anti-inflammatory effects (Nofer et al., 2004; Scanu and Edelstein, 2008). HDL induced expression of TGF-β2, an anti-inflammatory cytokine, in human umbilical vein endothelial cells and SPC mimicked the effect of HDL (Norata et al., 2005). These results suggest that SPC included in HDL may promote differentiation of tissue-resident MSCs or pericytes to SMCs, and that statins can interfere the differentiation of MSCs to SMCs. Similar to the results of the present study, it has been reported that TGF-β1-induced α-SMA expression in human tenon fibroblasts was attenuated by treatment with lovastatin, a member of the statin family (Meyer-Ter-Vehn et al., 2008). However, lovastatin treatment has been shown to stimulate α-SMA expression in VSMCs and prevent phenotypic dedifferentiation of VSMCs (Wada et al., 2008; Wagner et al., 2010). These results raise a possibility that statins differentially affect expression of SMC markers in different cell types. To clarify functional role of statins on MSC differentiation, it is necessary to determine further whether statins can affect differentiation of MSCs to SMCs using in vivo vascular disease animal models such as atherosclerosis and vascular injury that are associated with phenotypic modulation of SMCs.
Methods
Materials
Trypsin, α-minimum essential medium, fetal bovine serum, and Lipofectamine 2000 reagent were purchased from Invitrogen (Carlsbad, CA). Y27632 and simvastatin were purchased from Calbiochem (La Jolla, CA). D-erythro-SPC was purchased from Matreya (Pleasant Gap, PA). Anti-α-SMA antibody was purchased from Sigma-Aldrich (St. Louis, MO). Anti-phospho-Smad2 (Ser465/467) and anti-Smad2 antibodies were obtained from Cell Signaling Technology (Beverly, MA). Anti-RhoA antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was purchased from Millipore (Temecula, CA). Peroxidase-labeled secondary antibodies were obtained from Amersham Biosciences (Franklin Lakes, NJ).
Cell culture
Subcutaneous adipose tissue was obtained from elective surgeries with the patient's consent as approved by the Institution Review Board of Pusan National University Hospital. To isolate hASCs, adipose tissues were washed at least three times with sterile PBS and treated with an equal volume of collagenase type I suspension [1 g/liter of Hank's balanced salt solution (HBSS) buffer with 1% bovine serum albumin] for 60 min at 37℃ with intermittent shaking. The floating adipocytes were separated from the stromal-vascular fraction by centrifugation at 300 × g for 5 min. The cell pellet was resuspended in α-minimum essential medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin, and cells were plated in tissue culture dishes at 3500 cells/cm2. Primary hASCs were cultured for 4-5 days until they reached confluence and were defined as passage "0." The passage number of hASCs used in these experiments was 3-10. Adipose tissues were collected from six different patients, and six different hASC clones exhibited similar differentiation potentials toward osteogenic and adipogenic lineages as well as SMCs. Multipotent differentiation and proliferation capacities of hASCs remained consistent up until 10th passage. These six hASC clones exhibited SPC-induced α-SMA expression which could be inhibited by simvastatin treatment. They were positive for CD29, CD44, CD73, CD90, and CD105, whereas CD31 and CD45 were not expressed in hASCs (Supplemental Data Figure S1).
Western blotting
Confluent, serum-starved hASCs were treated with the appropriate conditions, washed with ice-cold PBS, and then lysed in lysis buffer (20 mM Tris-HCl, 1 mM EGTA, 1 mM EDTA, 10 mM NaCl, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 30 mM sodium pyrophosphate, 25 mM β-glycerol phosphate, and 1% Triton X-100, pH 7.4). Lysates were resolved by SDS-PAGE, transferred onto a nitrocellulose membrane, and then stained with 0.1% Ponceau S solution (Sigma-Aldrich). After blocking with 5% nonfat milk, the membranes were immunoblotted with various antibodies, and the bound antibodies were visualized with horseradish peroxidase-conjugated secondary antibodies using the enhanced chemiluminescence Western blotting system (ECL, Amersham Biosciences). Images were obtained using a LAS3000 image capture system (FUJIFILM, Japan). The immunoblots were quantified by measuring the intensity of the protein bands with LAS3000 software. Immunoblot images were obtained at different exposure times, and images whose band intensities were within the linear range between the intensity increase and exposure time were selected for quantification of images.
Measurement of membrane translocation of RhoA
To determine the amounts of membrane-associated RhoA, membrane fractions were isolated as previously described (Negre-Aminou et al., 2001). Cells were made quiescent and stimulated as described in the figure legends. They were then washed in ice-cold PBS, lysed by incubation in hypotonic buffer (5 mM Tris-HCl, 5 mM NaCl, 1 mM CaCl2, 2 mM EGTA, 1 mM MgCl2, and 2 mM dithiothreitol, pH 7.0) containing a mixture of protease inhibitors. Membrane fractions were separated from cytosolic fractions by centrifugation (100,000 × g, 1 h at 4℃) of the lysates. Membrane fractions were prepared by resuspending the pellets in the hypotonic buffer. The lysates and membrane fractions were diluted in SDS-PAGE buffer and the protein levels of RhoA were determined by Western blotting.
RhoA activation assay
A commercial pull-down assay (Rho activation assay kit, Upstate) was used to measure the effect of SPC on RhoA activity in hASCs. The cells were treated washed twice with α-MEM and incubated for 24 h in fresh modified α-MEM without serum, SPC was added, and the cell suspension was centrifuged at the indicated time after SPC addition. After lysis of the cell pellet (lysis buffer, Upstate) and the activated RhoA was precipitated using a fusion protein consisting of GST and the Rho binding domain of Rhotekin, according to the manufacturer's protocol. Protein concentrations were equalized between treatment groups prior to pull-down assay.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde in PBS for 15 min. For immunostaining, specimens were incubated with anti-α-SMA antibody for 2 h and Alexa Fluor 488-conjugated anti-mouse secondary antibody (Invitrogen) for 1 h. The specimens were finally washed and mounted in Vectashield medium (Vector Laboratories) with 4',6-diamidino-2-phenylindole (DAPI) for visualization of nuclei. Fluorescence images were collected using a Leica TCL-SP2 confocal microscope system (Leica Microsystems Heidelberg GmbH, Heidelberg, Germany).
ELISA
ELISA was carried out according to the manufacturer's instructions. In brief, cells were seeded onto 24-well culture plates at a density of 1 × 104 cells/well and cultured in growth medium for 48 h to confluence. After treatment of the confluent cells under appropriate conditions, conditioned media were collected and centrifuged at 15000 × g for 30 min to remove particulates. A commercially available sandwich ELISA kit (R&D Systems Inc., Minneapolis, MN) was used to evaluate the secretion of TGF-β1 in the conditioned medium. The absorbance (450 nm) for each sample was analyzed by an ELISA reader and was interpolated with a standard curve.
Supplemental data
Supplemental data include a figure and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-44-2-11.pdf.
Acknowledgements
This work was supported by the Bio Research Grant funded by the Pusan National University (PNU, Bio Research Fund; PNU-2008-059-7000).
Abbreviations
- FPP
farnesylpyrophosphate
- GGPP
geranylgeranylpyrophosphate
- hASCs
human adipose tissue-derived mesenchymal stem cells
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- MSCs
mesenchymal stem cells
- SMCs
smooth muscle cells
- SPC
sphingosylphosphorylcholine
- statins
3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors
Supplementary Material
Supplemental Data
References
- 1.Ball SG, Shuttleworth AC, Kielty CM. Direct cell contact influences bone marrow mesenchymal stem cell fate. Int J Biochem Cell Biol. 2004;36:714–727. doi: 10.1016/j.biocel.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Bokoch GM, Bohl BP, Chuang TH. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J Biol Chem. 1994;269:31674–31679. [PubMed] [Google Scholar]
- 3.Cai X, Lin Y, Hauschka PV, Grottkau BE. Adipose stem cells originate from perivascular cells. Biol Cell. 2011;103:435–447. doi: 10.1042/BC20110033. [DOI] [PubMed] [Google Scholar]
- 4.Calabro P, Yeh ET. The pleiotropic effects of statins. Curr Opin Cardiol. 2005;20:541–546. doi: 10.1097/01.hco.0000181482.99067.bf. [DOI] [PubMed] [Google Scholar]
- 5.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 6.Cen B, Selvaraj A, Prywes R. Myocardin/MKL family of SRF coactivators: key regulators of immediate early and muscle specific gene expression. J Cell Biochem. 2004;93:74–82. doi: 10.1002/jcb.20199. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 8.Chen CW, Montelatici E, Crisan M, Corselli M, Huard J, Lazzari L, Peault B. Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine Growth Factor Rev. 2009;20:429–434. doi: 10.1016/j.cytogfr.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Crawford M, Day RM, Briones VR, Leader JE, Jose PA, Lechleider RJ. RhoA modulates Smad signaling during transforming growth factor-beta-induced smooth muscle differentiation. J Biol Chem. 2006;281:1765–1770. doi: 10.1074/jbc.M507771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corselli M, Chen CW, Crisan M, Lazzari L, Peault B. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–1109. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- 11.Covas DT, Panepucci RA, Fontes AM, Silva WA, Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, Zago MA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Herve P, Etievent JP, Kantelip JP. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108(Suppl 1):II253–II258. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- 13.Feng J, Mantesso A, Sharpe PT. Perivascular cells as mesenchymal stem cells. Expert Opin Biol Ther. 2010;10:1441–1451. doi: 10.1517/14712598.2010.517191. [DOI] [PubMed] [Google Scholar]
- 14.Gojo S, Gojo N, Takeda Y, Mori T, Abe H, Kyo S, Hata J, Umezawa A. In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp Cell Res. 2003;288:51–59. doi: 10.1016/s0014-4827(03)00132-0. [DOI] [PubMed] [Google Scholar]
- 15.Graaf MR, Richel DJ, van Noorden CJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30:609–641. doi: 10.1016/j.ctrv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM, Bae YC, Jung JS, Kim JH. Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-β-dependent mechanism. J Cell Sci. 2006;119:4994–5005. doi: 10.1242/jcs.03281. [DOI] [PubMed] [Google Scholar]
- 17.Jeon ES, Park WS, Lee MJ, Kim YM, Han J, Kim JH. A Rho kinase/myocardin-related transcription factor-A-dependent mechanism underlies the sphingosylphosphorylcholine-induced differentiation of mesenchymal stem cells into contractile smooth muscle cells. Circ Res. 2008;103:635–642. doi: 10.1161/CIRCRESAHA.108.180885. [DOI] [PubMed] [Google Scholar]
- 18.Kinner B, Zaleskas JM, Spector M. Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res. 2002;278:72–83. doi: 10.1006/excr.2002.5561. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi N, Yasu T, Ueba H, Sata M, Hashimoto S, Kuroki M, Saito M, Kawakami M. Mechanical stress promotes the expression of smooth muscle-like properties in marrow stromal cells. Exp Hematol. 2004;32:1238–1245. doi: 10.1016/j.exphem.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, Akedo H, Nakamura H. Inhibition of epidermal growth factor-induced RhoA translocation and invasion of human pancreatic cancer cells by 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors. Cancer Res. 2001;61:4885–4891. [PubMed] [Google Scholar]
- 21.Laufs U, Marra D, Node K, Liao JK. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1) J Biol Chem. 1999;274:21926–21931. doi: 10.1074/jbc.274.31.21926. [DOI] [PubMed] [Google Scholar]
- 22.Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol. 2005;96:24F–33F. doi: 10.1016/j.amjcard.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C, Nath KA, Katusic ZS, Caplice NM. Smooth muscle progenitor cells in vascular disease. Trends Cardiovasc Med. 2004;14:288–293. doi: 10.1016/j.tcm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- 25.Mack CP, Somlyo AV, Hautmann M, Somlyo AP, Owens GK. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J Biol Chem. 2001;276:341–347. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- 26.Meyer-Ter-Vehn T, Katzenberger B, Han H, Grehn F, Schlunck G. Lovastatin inhibits TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci. 2008;49:3955–3960. doi: 10.1167/iovs.07-1610. [DOI] [PubMed] [Google Scholar]
- 27.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292:C70–C81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 28.Mohamed JS, Boriek AM. Stretch augments TGF-beta1 expression through RhoA/ROCK1/2, PTK, and PI3K in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2010;299:L413–L424. doi: 10.1152/ajplung.90628.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagatoya K, Moriyama T, Kawada N, Takeji M, Oseto S, Murozono T, Ando A, Imai E, Hori M. Y-27632 prevents tubulointerstitial fibrosis in mouse kidneys with unilateral ureteral obstruction. Kidney Int. 2002;61:1684–1695. doi: 10.1046/j.1523-1755.2002.00328.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakagami H, Jensen KS, Liao JK. A novel pleiotropic effect of statins: prevention of cardiac hypertrophy by cholesterol-independent mechanisms. Ann Med. 2003;35:398–403. doi: 10.1080/07853890310001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negre-Aminou P, van Erck M, van Leeuwen RE, Collard JG, Cohen LH. Differential effect of simvastatin on various signal transduction intermediates in cultured human smooth muscle cells. Biochem Pharmacol. 2001;61:991–998. doi: 10.1016/s0006-2952(01)00566-4. [DOI] [PubMed] [Google Scholar]
- 32.Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck LK, Baba HA, Tietge UJ, Godecke A, Ishii I, Kleuser B, Schafers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norata GD, Callegari E, Marchesi M, Chiesa G, Eriksson P, Catapano AL. High-density lipoproteins induce transforming growth factor-beta2 expression in endothelial cells. Circulation. 2005;111:2805–2811. doi: 10.1161/CIRCULATIONAHA.104.472886. [DOI] [PubMed] [Google Scholar]
- 34.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 35.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 36.Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14:2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolfe BE, Worth NF, World CJ, Campbell JH, Campbell GR. Rho and vascular disease. Atherosclerosis. 2005;183:1–16. doi: 10.1016/j.atherosclerosis.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg. 2007;45(Suppl A):A25–A32. doi: 10.1016/j.jvs.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Scanu AM, Edelstein C. HDL: bridging past and present with a look at the future. FASEB J. 2008;22:4044–4054. doi: 10.1096/fj.08-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shanahan CM, Weissberg PL, Metcalfe JC. Isolation of gene markers of differentiated and proliferating vascular smooth muscle cells. Circ Res. 1993;73:193–204. doi: 10.1161/01.res.73.1.193. [DOI] [PubMed] [Google Scholar]
- 41.Smith PC, Caceres M, Martinez J. Induction of the myofibroblastic phenotype in human gingival fibroblasts by transforming growth factor-beta1: role of RhoA-ROCK and c-Jun N-terminal kinase signaling pathways. J Periodontal Res. 2006;41:418–425. doi: 10.1111/j.1600-0765.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 42.Tolle M, Pawlak A, Schuchardt M, Kawamura A, Tietge UJ, Lorkowski S, Keul P, Assmann G, Chun J, Levkau B, van der GM, Nofer JR. HDL-associated lysosphingolipids inhibit NAD(P)H oxidase-dependent monocyte chemoattractant protein-1 production. Arterioscler Thromb Vasc Biol. 2008;28:1542–1548. doi: 10.1161/ATVBAHA.107.161042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wada H, Abe M, Ono K, Morimoto T, Kawamura T, Takaya T, Satoh N, Fujita M, Kita T, Shimatsu A, Hasegawa K. Statins activate GATA-6 and induce differentiated vascular smooth muscle cells. Biochem Biophys Res Commun. 2008;374:731–736. doi: 10.1016/j.bbrc.2008.07.098. [DOI] [PubMed] [Google Scholar]
- 44.Wagner RJ, Martin KA, Powell RJ, Rzucidlo EM. Lovastatin induces VSMC differentiation through inhibition of Rheb and mTOR. Am J Physiol Cell Physiol. 2010;299:C119–C127. doi: 10.1152/ajpcell.00429.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV, Owens GK. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a rho kinase/myocardin/SRF-dependent mechanism. Circ Res. 2004;95:406–414. doi: 10.1161/01.RES.0000138582.36921.9e. [DOI] [PubMed] [Google Scholar]
- 46.Wang D, Park JS, Chu JS, Krakowski A, Luo K, Chen DJ, Li S. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor beta1 stimulation. J Biol Chem. 2004;279:43725–43734. doi: 10.1074/jbc.M407368200. [DOI] [PubMed] [Google Scholar]
- 47.Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–519. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 48.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin G, Cha DH, Johnson KL, Aikawa R, Asahara T, Losordo DW. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida M, Sawada T, Ishii H, Gerszten RE, Rosenzweig A, Gimbrone MA, Jr, Yasukochi Y, Numano F. Hmg-CoA reductase inhibitor modulates monocyte-endothelial cell interaction under physiological flow conditions in vitro: involvement of Rho GTPase-dependent mechanism. Arterioscler Thromb Vasc Biol. 2001;21:1165–1171. doi: 10.1161/hq0701.092143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data