Recently, we discovered chromosome 12q14 duplications in patients with normal tension glaucoma (NTG) (Fingert et al., 2011). Three different but overlapping chromosome 12q14 duplications that all spanned the TBK1, XPOT, and RASSF3 genes were identified. The duplication of TBK1 was judged to be the most likely cause of NTG in these patients because: 1) TBK1 associates with the product of another NTG gene, optineurin (Morton et al., 2008); 2) Duplication of TBK1 leads to increased transcription of TBK1 (Fingert et al., 2011); and 3) TBK1 is specifically expressed in cells affected by glaucoma pathophysiology (retinal ganglion cells and their axons) (Fingert et al., 2011). Finally, the population-based segment of our previous study identified chromosome 12q14 duplications that span TBK1 in 2 (1.3%) of 152 NTG patients from Iowa (Fingert et al., 2011) suggesting that copy number variations of TBK1 may be responsible for a fraction of all NTG cases.
Here we report the first replication study to investigate the role of copy number variations (CNVs) of TBK1 in NTG pathogenesis. The study was approved by local Institutional Review Boards and informed consent was obtained from all study participants. We tested NTG patients and ethnically matched normal control subjects from Japan. NTG patients from New York and North Carolina were also studied. Subjects were tested for duplication of TBK1 using a quantitative PCR assay and microarray analysis of SNPs at chromosome 12q14.
Patients were examined by fellowship-trained glaucoma specialists and received complete ophthalmic examinations including gonioscopy, standardized computerized Humphrey (Zeiss, San Leonardo, Ca.) SITA visual field testing, and stereoscopic optic nerve examination. Subjects were diagnosed with open-angle glaucoma when optic nerve damage and corresponding visual field defects were detected in at least one eye as previously described (Alward et al., 1998) and NTG was diagnosed when the maximum untreated IOP was ≤21 mmHg in both eyes. The study dataset consisted of 252 NTG patients and 202 controls from Japan, 29 NTG patients from North Carolina, and 28 NTG patients from New York.
DNA samples from each subject were tested for duplication of the TBK1 gene using a quantitative PCR assay (TaqMan Copy Number Assay, Applied BioSystems, Carlsbad, CA) as previously described (Fingert et al., 2011). Briefly, a segment of the TBK1 gene was PCR amplified in triplicate for each DNA sample, as was a control amplicon from a different gene on a different chromosome. Experiments were conducted using a 7900HT PCR machine and data was analyzed to detect CNVs using CopyCaller software (Applied BioSystems, Carlsbad, CA) with default settings. Subjects that exhibited a CNV that spanned TBK1 were retested to confirm its presence.
One of 252 (0.40%) unrelated Japanese NTG subjects (patient GGJ-414) was found to carry a TBK1 duplication using quantitative PCR. The duplication in patient GGJ-414 was confirmed using microarray analysis (Affymetrix microarrays, Santa, Clara, CA) as previously described (Fingert et al., 2011). No duplications were detected in the Japanese control subjects or in any of the patients from North Carolina or New York. Further analysis of the genetic data (Partek software package, St. Louis, MO) showed that patient GGJ-414 carried a chromosome 12q14 duplication that extended from 64,802,839 to 65,098,981 bps (human genome build hg19). This 300 kbp duplication encompassed the TBK1 gene as well as XPOT and RASSF3 and is similar in extent to the duplication previously reported in Caucasian NTG subject GGA-1159-1 (Fingert et al., 2011).
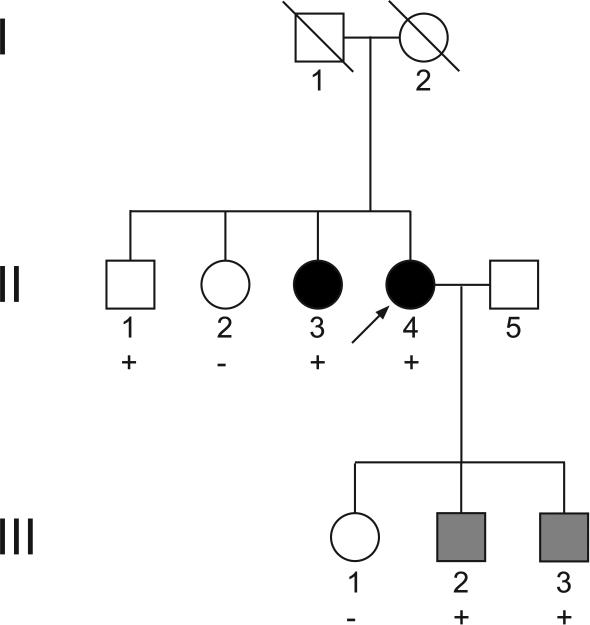
Patient GGJ-414 (subject II-4 in Figure 1) is a Japanese woman who was diagnosed with NTG at age 42 and has a strong family of disease. Representative disc photos from family members are shown in Figure 2. Other members of this family were tested for the presence of the chromosome 12q14 duplication using the quantitative PCR assay described above. Patient GGJ-414 (Figure 1, subject II-4) has a sister with NTG (Figure 1, subject II-3) and two sons that were diagnosed as NTG suspects, based on large cup-to-disc ratios (Figure 1, subjects III-2 and III-3), also carried the TBK1 duplication. The maximum recorded untreated intraocular pressure for patient GGJ-414 was 18 mm Hg OD and 17 mm Hg OS and central corneal thickness was 521 microns OD and 528 microns OS. One family member (subject II-1, Figure 1) that carried the TBK1 duplication had not been diagnosed with glaucoma when he was last examined at 45 years of age. At this examination, subject II-1 had large cup to disc ratios (0.75 OD and 0.70 OS) and had abnormal glaucoma hemifield test OD. Subject II-1 has not been examined in nine years and lacks further information on glaucoma status.
Figure 1. Japanese NTG pedigree.
Family members with NTG are indicated by dark symbols, while those that are NTG suspects are indicated by gray symbols. The proband GGJ-414 (subject II-4) is indicated with the arrow. Deceased family members are indicated with slashes. Those family members that carry the TBK1 duplication are indicated with a “+”
Figure 2. Disc Photos.
The optic disc of the left eye of one family member (Figure 1, II-4) that is affected with NTG and has glaucomatous cupping is shown in panel A, while the optic disc of the left eye of another family member (Figure 1, III-3) that is a glaucoma suspect is shown in panel B.
Over the last 15 years, family-based linkage studies have identified several genes (i.e. myocilin and optineurin) that are capable of causing POAG with little influence from other genes or environmental factors. These single gene or Mendelian forms of glaucoma represent approximately 5% of POAG cases. The vast majority of glaucoma-causing mutations in myocilin and optineurin are missense and nonsense sequence changes. More recently, we reported that duplication of a segment of chromosome 12q14 that encompasses the TBK1 gene is associated with NTG. Our study first detected a chromosome 12q14 duplication in a large African American pedigree with NTG and later identified two different but overlapping chromosome 12q14 duplications in 2 (1.3%) of 152 unrelated Caucasian NTG patients (Fingert et al., 2011).
This is the first confirmation that chromosome 12q14 duplications which encompass the TBK1 gene are associated with NTG. Although it remains possible that other neighboring genes in and around the chromosome 12q14 duplication have a role in the pathogenesis of NTG, there is strong data to suggest that it is duplication of TBK1 that leads to this form of NTG (Fingert et al., 2011). The discovery and confirmation that another gene, TBK1, leads to glaucoma potentially represents a significant advance in glaucoma genetics. TBK1 encodes a kinase that influences gene expression in the NF-κB signaling pathway. Two other NTG genes, optineurin (Rezaie et al., 2002) and toll-like receptor 4 (Zareparsi et al., 2005), also participate in NF-κB signaling. Together these data strongly suggest that this biological pathway has an important role in the pathogenesis of NTG in at least a subset of patients, perhaps via its influence on key cellular processes including apoptosis. Furthermore, duplication of TBK1 is associated with 0.40% to 1.3% of NTG in Caucasians, African Americans (Fingert et al., 2011), and Japanese, suggesting that this defect may have a role in NTG pathogenesis in many ethnicities.
Acknowledgements
This grant was funded in part by NIH RO1EY0018865.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A) N Engl J Med. 1998;9:1022–1027. doi: 10.1056/NEJM199804093381503. [DOI] [PubMed] [Google Scholar]
- Fingert JH, Robin AL, Stone JL, Roos B, Davis LK, Scheetz TA, Bennett SR, Wassink TH, Kwon YH, Alward WL, Mullins RF, Sheffield VC, Stone EM. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Hum Mol Genet. 2011;20:2482–2494. doi: 10.1093/hmg/ddr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton S, Hesson L, Peggie M, Cohen P. Enhanced binding of TBK1 by an optineurin mutant that causes a familial form of primary open angle glaucoma. FEBS Lett. 2008;582:997–1002. doi: 10.1016/j.febslet.2008.02.047. [DOI] [PubMed] [Google Scholar]
- Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- Zareparsi S, Buraczynska M, Branham KE, Shah S, Eng D, Li M, Pawar H, Yashar BM, Moroi SE, Lichter PR, Petty HR, Richards JE, Abecasis GR, Elner VM, Swaroop A. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet. 2005;14:1449–1455. doi: 10.1093/hmg/ddi154. [DOI] [PubMed] [Google Scholar]