Abstract
Benzodiazepines negatively affect motor coordination and balance and produce myorelaxation. The aim of the present study was to examine the extent to which populations of GABAA receptors containing α1 and α5 subunit contribute to these motor-impairing effects in rats. We used the nonselective agonist diazepam and the α1-selective agonist zolpidem, as well as nonselective, α1- and α5-subunit-selective antagonists flumazenil, βCCt and XLi093, respectively. Ataxia and muscle relaxation were assessed by rotarod and grip strength tests performed 20 minutes after i.p. treatment. Diazepam (2 mg/kg) induced significant ataxia and muscle relaxation which were completely prevented by pretreatment with flumazenil (10 mg/kg) and βCCt (20 mg/kg). XLi093 antagonized the myorelaxant, but not ataxic actions of diazepam. All three doses of zolpidem (1, 2 and 5 mg/kg) produced ataxia, but only the highest dose (5mg/kg) significantly decreased the grip strength. These effects of zolpidem were reversed by ßCCt at doses of 5 and 10 mg/kg, respectively. The present study demonstrates that α1 GABAA receptors mediate ataxia and indirectly contribute to myorelaxation in rats, while α5 GABAA receptors contribute significantly, although not dominantly, to muscle relaxation but not ataxia.
Keywords: ataxia, muscle relaxation, rotarod, grip strength, rat
INTRODUCTION
Benzodiazepines (BZs) were introduced into clinical practice at the beginning of the 1960s and since then have been widely prescribed as anxiolytic, hypnotic, anticonvulsant and myorelaxant drugs. During the 1990s, it became clear that pharmacological effects of BZs are mediated via positive modulation of four different subtypes of GABAA receptors, namely those containing the α1-, α2-, α3-, or α5-subunit, in addition to the γ2 subunit (Sieghart 2006). Genetic and pharmacological studies, by the means of the generation of mutant mouse lines [α1(H101R), α2(H101R), α3(H126R) and α5(H105R) knock-ins] (Rudolph and Mohler 2004) and synthesis of novel, subtype-selective ligands, have helped in linking particular behavioral responsse to specific GABAA receptor subtypes. Sedative effects of BZs were principally attributed to the α1-GABAA receptor subtype, anxiolytic actions to α2-/α3- containing receptors, anterograde amnesic effects to α1/α5 subtypes and anticonvulsant activity partially to α1-GABAA receptors (McKernan et al. 2000; Low et al. 2000; Collinson et al. 2002; Savić et al. 2009).
Benzodiazepines negatively affect motor coordination and balance, i.e. they induce ataxia, which is together with myorelaxation often referred to as motor impairment (Verster et al. 2002; Licata et al. 2009). In contrast to ataxia, myorelaxation can be therapeutically desirable, and disentangling the molecular substrates of these two effects would benefit the development of compounds with an improved pharmacological profile. Like sedation, the impaired coordination and balance were also ascribed to potentiation at α1-GABAA receptors and these results were consistent with experiments in both rodents and non-human primates (Mc Kernan et al. 2000; Platt et al. 2002; Licata et al. 2009). Ligands that lack or have substantially decreased activity at α1-GABAA receptors, compared to conventional nonselective benzodiazepines, did not engender ataxia over the wide dose range tested (Licata et al. 2005; Mirza et al. 2008; Savić et al. 2008; Atack 2010). The experiments on genetically-modified mice have excluded the role of the α1 subunit as a molecular substrate of myorelaxation (Rudolph et al., 1999; McKernan et al. 2000) and found that the myorelaxant properties of diazepam are mainly mediated by α2-GABAA receptors; at very high doses of diazepam, the α3- and α5-GABAA receptor subtypes may also become implicated (Crestani et al. 2001). However, a number of pharmacological studies have shown that muscle relaxation induced by nonselective BZ site agonists could be reversed by the use of the α1-GABAA selective antagonist ß-CCt, demonstrating ambiguity in this area (Griebel et al. 1999; Licata et al. 2009).
The overall aim of the present study was to examine, by pharmacological means, the extent to which α1- and α5-GABAA receptor subtypes contribute to BZ-induced ataxia and musle relaxation in Wistar rats, and to provide further information on the molecular substrates of these two effects. Benzodiazepine-induced ataxia in rodents is usually measured using the rotarod test (Mirza et al. 2008; Savić et al. 2008), while the myorelaxant effects of BZs are often assessed using the grip strength test (Maurissen et al. 2003). In the present study we used diazepam, a ligand with high efficacy and no selectivity for GABAA receptor subtypes, and the α1-GABAA receptor-selective agonist zolpidem, which possesses intermediate and no affinity for α2/α3 and α5-GABAA receptor subtypes, respectively (Sanna et al. 2002). By the use of the GABAA nonselective antagonist flumazenil, the α1-subunit affinity-selective antagonist βCCt (Shannon et al. 1984) and the α5-subunit affinity- and efficacy-selective antagonist XLi093 (Li et al. 2003), we examined the degree to which zolpidem- and diazepam-induced ataxia and myorelaxation could be antagonized.
METHODS
Subjects
Male Wistar rats, weighing 200–230g, were supplied by Military Farm, Belgrade, Serbia. Rats were housed in groups of six and were maintained under standard laboratory conditions (21 ± 2°C, relative humidity 40–45%) with free access to pellet food and tap water. They were kept on 12:12 h light/dark cycle with lights on at 07.00 h. All handling and testing took place during the light phase of the diurnal cycle. Experiments were carried out in accordance with the EEC Directive 86/609 and were approved by the Ethical Committee on Animal Experimentation of the Faculty of Pharmacy in Belgrade.
Rotarod test
Motor performance was assessed using an automated rotarod (Ugo Basile, Italy). Before testing, rats were trained for three days until they could remain on a revolving rod for 120 s with acceleration from 15 rpm to 25 rpm. During the training days, all animals were given three training sessions of 2 min each, with a 30 min inter-session interval. On the fourth day, rats that fit the given criteria were selected for inclusion in the experiment. Groups of 6–8 animals received one of the following treatments: diazepam (0 and 2 mg/kg) in combination with ßCCt (0, 1, 5, 20 and 30 mg/kg), flumazenil (0, 10 and 20 mg/kg), or XLi093 (0, 10 and 20 mg/kg), as well as zolpidem (0, 1, 2 and 5 mg/kg) and zolpidem (0 and 2 mg/kg) combined with ßCCt (0, 5 and 20 mg/kg) or flumazenil (0, 10 and 20 mg/kg). Latency to falling off the rod was recorded automatically for each animal.
Grip strength test
This test was used to examine the myorelaxant properties of agonists, antagonists and their combinations. Two experiments were performed: in the first, animals received diazepam (0 and 2 mg/kg) in combination with three levels of flumazenil (0, 10 and 20 mg/kg), ßCCt (0, 20 and 30 mg/kg) and XLi093 (0, 10 and 20 mg/kg); in the second experiment, animals received zolpidem (0, 1, 2 and 5 mg/kg) and zolpidem (0 and 5 mg/kg) in combination with ßCCt (0 and 10 mg/kg). After administration of the appropriate treatment, rats were allowed to grip with their front paws a metal trapezoid wire attached to a grip-strength meter (Ugo Basile, Italy). Grip strength was tested by dragging the rat gently by the tail. The apparatus measured the pull force (expressed in grams) necessary to overcome the animal's forelimbs grip-strength to the bar connected to a force transducer. Each animal was given three consecutive trials and the maximum value was taken.
Drugs
The compounds used were diazepam (Galenika, Serbia), zolpidem (Toronto Chemical Research, Canada), flumazenil (Feicheng BoYuan Fine Chemicals Co., Ltd, China), XLi093 (4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid, 8-ethynyl-5,6-dihydro-5-methyl-6-oxo-, 1,3-propanediyl ester), the α5-subunit affinity- and efficacy-selective antagonist and ßCCt (t-butyl-ß-carboline-3-carboxylate), the α1-subunit affinity-selective antagonist; the latter two agents were synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin–Milwaukee, USA. The ligands were suspended in a solvent containing 85% distilled water, 14% propylene glycol and 1% Tween-80. All animals received two i.p. injections consisting of the appropriate ligand(s) and/or solvent (in a total volume of 2 ml/kg), twenty minutes before the testing. When a combination of two compounds was administered, the first compound was injected into the lower right and the second into the lower left quadrant of the peritoneum.
Statistics
All numerical data presented in the figures are shown as the mean ± S.E.M. The dose response of zolpidem was assessed using one-way ANOVA, with post- hoc Student-Newman-Keuls test (SNK). The effects of combined treatments were assessed using two-way ANOVA with post-hoc SNK test, where applicable.
RESULTS
Rotarod
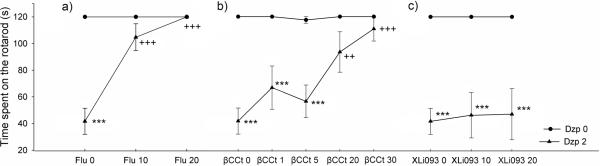
Animals that received 2 mg/kg diazepam spent significantly less time on the rotarod than the control group of rats (Fig. 1; p<0.001). When diazepam was injected immediately after flumazenil, a significant main effect of flumazenil [F (2, 40) = 18.07, p<0.001] and diazepam × flumazenil interaction [F (2, 45) = 18.07, p<0.001] were found. Both 10 mg/kg and 20 mg/kg of flumazenil antagonized the motor incoordination induced by diazepam (Fig. 1a; both p<0.001 compared to 2 mg/kg diazepam). Similarly, co-administration of ßCCt resulted in a significant treatment effect [F(4,68)= 4.05, p<0.005] and a significant diazepam × ßCCt interaction [F(4,77)=3.83, p<0.01]. While the two lower doses of ßCCt (1 and 5 mg/kg) failed to antagonize the diazepam-induced motor impairment, co-administration of the two higher doses of ßCCt (20 and 30 mg/kg) significantly increased the time spent on the rotarod (Fig. 1b; both p<0.001), when compared to diazepam dosed at 2 mg/kg. XLi093, an α5-selective antagonist, did not antagonize the diazepam-induced motor incoordination (Fig. 1c).
Figure 1.
The influence of pretreatment with antagonists flumazenil (Flu), ß-CCt and XLi093 on diazepam-induced (Dzp) ataxia on the rotarod. Data are mean ± S.E.M. from n=8 rats per group. ***p<0.001 versus vehicle; ++p<0.01 versus 2 mg/kg diazepam; +++p<0.001 versus 2 mg/kg diazepam.
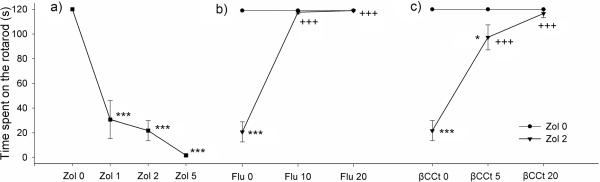
All three doses of zolpidem (1, 2 and 5 mg/kg) impaired motor coordination (Fig. 2a; p<0.001 in all three cases). Pretreatment with flumazenil significantly influenced the zolpidem induced-ataxia [zolpidem: F(1,37)=114.02, p<0.001; zolpidem × flumazenil interaction: F(2,42)=108.54, p<0.001]. When compared with animals that received only 2 mg/kg of zolpidem, animals treated with the combination of zolpidem 2mg/kg + flumazenil (10 or 20 mg/kg) spent significantly more time on the rotarod (Fig. 2b; p<0.001 and p<0.001, respectively). The effect on motor coordination of ßCCt [F(1,34)=73.94, p<0.001] and the zolpidem × ßCCt interaction [F(2,39)=40.61, p<0.001] were also significant. The subsequent post hoc test showed that both 5 and 20 mg/kg of ßCCt antagonized the zolpidem induced ataxia (Fig. 2c; both p<0.001, compared to 2 mg/kg zolpidem). There was also a significant difference in the time spent on the rotarod between animals that received 2 mg/kg zolpidem + 5 mg/kg ßCCt and animals that received only 5 mg/kg ßCCt (p<0.025). None of the antagonists (flumazenil, ßCCt and XLi093) itself impaired the motor performance on the rotarod.
Figure 2.
Effects of zolpidem (Zol) on rotarod performance and the influence of pretreatment with flumazenil (Flu) and ß-CCt on ataxia induced by zolpidem (2 mg/kg). Data are mean ± S.E.M. from n=6 rats per group. *p<0.05 versus vehicle; ***p<0.001 versus vehicle; +++p<0.001 versus 2 mg/kg zolpidem.
Grip strength
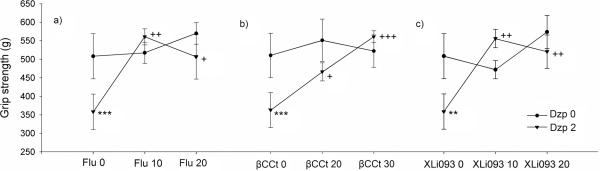
Application of 2 mg/kg diazepam produced significant muscle relaxation (Fig. 3; p<0.01, relative to control). For the combination of diazepam + flumazenil, two-way ANOVA showed significant effects of diazepam [F (1, 31) =6.09, p<0.02] and the flumazenil × diazepam interaction [F (2, 36) =5.94, p<0.01); co-administration of flumazenil (10 and 20 mg/kg) reversed the diazepam-induced myorelaxation (Fig. 3a; p<0.001 and p<0.01, compared to diazepam 2 mg/kg, respectively). As with flumazenil, the effect of ßCCt did not reach statistical significance while the effect of diazepam [F (1, 28) =7.82, p<0.01] as well as the interaction [F (2, 33) =5.83, p<0.01] were significant. There were significant differences between the group that received 2 mg/kg diazepam and groups that received 2 mg/kg diazepam with either 20 mg/kg or 30 mg/kg of ßCCt (Fig. 3b; p<0.05 and p<0.001, respectively). The assessment of the results obtained with the α5-selective antagonist showed no significant effect of XLi093 on grip strength [F (2, 30) =2.46, NS] but a significant diazepam × XLi093 interaction [F (2, 35) =6.18, p<0.01]; the differences between groups that received diazepam + XLi093 (10 and 20 mg/kg) and the group that received diazepam were statistically significant (Fig. 3c; p<0.002 and p<0.005, respectively).
Figure 3.
The influence of pretreatment with the antagonists flumazenil (Flu), ß-CCt and XLi093 on the diazepam-induced (Dzp) muscle relaxation measured in the grip strength test. Data are mean ± S.E.M. from n=8 rats per group. **p<0.05 versus vehicle; ***p<0.001 versus vehicle; +p<0.05 versus 2 mg/kg diazepam; ++p<0.01 versus 2 mg/kg diazepam; +++p<0.001 versus 2 mg/kg diazepam.
Zolpidem significantly decreased grip strength [F (3, 20) =10.34, p<0.001]. Muscle relaxation was significant with 5 mg/kg zolpidem (p<0.001) while the two lower doses (1 mg/kg and 2 mg/kg) were at the control level (Fig. 4a). When the combination 5 mg/kg zolpidem + 10 mg/kg ßCCt was assessed, significant effects of zolpidem [F(1,15)=19.74, p<0.001], ßCCt F(1,15)=16.11, p<0.001] and their interaction [F(1,18)=27.53, p<0.001] were found. While ßCCt itself did not alter grip strength, its addition to zolpidem reversed the zolpidem-induced muscle relaxation (Fig. 4b; p<0.001, compared to 5 mg/kg zolpidem).
Figure 4.
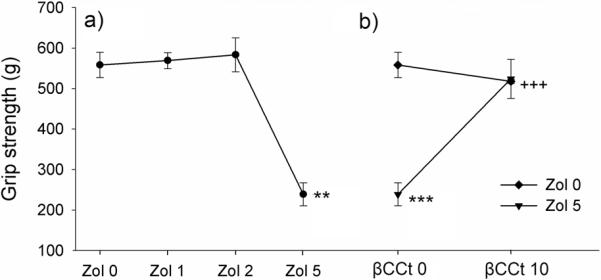
(a) Muscle relaxant effect of zolpidem (Zol) and (b) the influence of pretreatment with ßCCt Data are mean ± S.E.M. from n=6 rats per group. **p<0.05 versus vehicle; ***p<0.001 versus vehicle; +++p<0.001 versus 5mg/kg zolpidem.
DISCUSSION
Studies on genetically modified mice, in which a distinct α subunit of GABAA receptors is rendered insensitive to diazepam, represent valuable tools in revealing which receptor subtype is necessary for the expression of a specific behavioral response. These experiments pointed toward α1-GABAA receptors as the main subtype in eliciting ataxia in mice (McKernan et al. 2000). In the present study, diazepam- and zolpidem-induced ataxia on the rotarod in rats were successfully antagonized with the α1-selective antagonist ßCCt. Because of its 20-fold selectivity for α1-GABAA receptors compared with α2-GABAA and α3-GABAA receptors, ßCCt is one of the most selective BZ-site ligands identified to date (Cox et al. 1995; Huang et al. 2000). In many behavioral studies, ßCCt successfully reversed effects of BZs related to the α1-GABAA receptor subtype, such as ataxia, sedation and anticonvulsant activity (Griebel et al. 1999; Platt et al. 2002; Savić et al. 2009). However, not all experiments using ßCCt as the α1-selective ligand have reported antagonism of the diazepam-induced ataxia in mice or rats. Such discrepancies may have resulted from differences in experimental design. Shannon and colleagues (1984) reported that administration of 30 mg/kg ßCCt did not attenuate the diazepam-induced ataxia in mice. The degree of motor impairment was assessed using an inverted-screen test, where the concomitant myorelaxation was likely to influence the performance of the test. Another study found that motor incoordination engendered by diazepam, triazolam and zolpidem in mouse pups was not sensitive to ßCCt (Rowlett et al. 2001). However, motor impairment was related to rolling motions, as opposed to normal locomotor activity of mouse pups, and probably involved a predominantly spinal mechanism and engagement of α2- and α3-GABAA receptor subtypes (McKernan and Whiting 1996). In the present study, the dose of ßCCt needed to antagonize zolpidem-induced ataxia was substantially lower than the dose that antagonized the effect of diazepam (5 mg/kg vs. 20 mg/kg). This implies that an effect of diazepam, possibly myorelaxation, mediated by receptors other than the α1-GABAA receptor, may have contributed to the influence of diazepam, but not zolpidem, on rotarod test performance. In this scenario, the dose of 20 mg/kg of ßCCt may have either blocked the α1-GABAA receptor population more completely or started to prevent binding of diazepam to non α1-GABAA receptors.
The possibility that the α5-GABAA receptor subtype exhibits a modulatory role on behavioral effects predominantly conferred via the α1 subunit, such as sedation, tolerance development and memory impairment, has been previously proposed (van Rijnsoever et al. 2004; Savić et al. 2008; Savić et al. 2009). Hence, we tested the ability of the α5 selective antagonist XLi093 to influence the diazepam-induced ataxia. At the dose of 20 mg/kg, which was previously shown to intensify diazepam-induced sedation (Savić et al. 2009), XLi093 did not significantly affect the motor-impairing effect of diazepam. This means that ataxia, as assessed in the rotarod test in rats, is not dependent on activation of α5-GABAA receptors.
While genetic studies did not detect any role of the α1 subunit in mediating muscle relaxation (Rudolph et al. 1999; McKernan et al. 2000), the data from experiments with subtype-selective ligands varied from study to study depending on the species used and the dose of agonist or antagonist applied (Griebel et al. 1999; Elliot and White, 2001; Licata et al. 2009). In a radiotelemetric study in rats, zolpidem at the dose of 5 mg/kg, but not 2.5 mg/kg, induced a significant decrease in electromyographic activity, a parameter aimed to assess muscle relaxation (Elliot and White, 2001). In the present study, significant myorelaxation observed after both diazepam and zolpidem administration was prevented by pretreatment with ßCCt. As the dose of zolpidem producing myorelaxation (5 mg/kg) was substantially higher than the minimal dose that induced ataxia (1 mg/kg), the possibility that zolpidem-induced myorelaxation is not mediated via α1-GABAA receptors needs to be discussed. Despite its binding preference for α1-GABAA receptors, zolpidem also binds to and potentiates effects at α2-GABAA and α3-GABAA receptors (Sanna et al. 2002). The in vivo selectivity of zolpidem for the α1-enriched cerebellum, in contrast to α2/α3-enriched spinal cord, assessed through the reduction in flumazenil binding, is generally less than the α1 selectivity of this compound in vitro (Atack et al. 1999). However, the displacement curve for zolpidem in the spinal cord of rats (Benaviders et al., 1992) and mice (Atack et al., 1999) is relatively flat, and very high doses of zolpidem (>30 mg/kg in mice; Atack et al., 1999) are needed for half-inhibition of radio-labeled flumazenil binding in this region predominantly implicated in GABA-mediated myorelaxation (Bohlhalter et al., 1996). Thus, one can conclude that muscle-relaxant effect of zolpidem at the dose of 5 mg/kg may not be exclusively mediated by α2-GABAA receptors, the subtype largely responsible for the muscle-relaxant effect of diazepam (Crestani et al. 2001). On the other hand, ßCCt (30 mg/kg) reversed diazepam-induced muscle relaxation in mice (Griebel et al. 1999) and at the dose of 3 mg/kg it attenuated myorelaxant properties of several nonselective benzodiazepine agonists in squirrel monkeys (Licata et al. 2009). The propensity of ßCCt to antagonize some of the principally non-α1 mediated effects of diazepam was also shown in the elevated plus-maze and light-dark test of anxiety (Griebel et al. 1999; Belzung et al. 2000). Nonetheless, a potentiating effect of 30 mg/kg ßCCt on the anxiolytic actions of BZs in rats has also been repeatedly reported (Savić et al. 2004; 2005), which cannot be a consequence of putative antagonism on α2-GABAA receptors. Assessment of the ability of 10 mg/kg ßCCt (i.p.) to displace the radio-labeled flumazenil in mice indicates that ßCCt at the given dose level preferentially targets the cerebellum, while it binds to less than 40% of GABAA receptors, mainly of the α2-subtype, in the spinal cord (Rowlett et al. 2005). Given the doses of zolpidem and ßCCt that we used, we hypothesize that under our experimental conditions the actions of these ligands may, to a small extent, have involved the α2-, in addition to the predominantly affected α1-GABAA receptor subtype. In the presence of intense activation of α1-GABAA receptors by a large dose of zolpidem, the presumed small involvement of α2-GABAA receptors may have been large enough to trigger muscle relaxation.
The contribution of the α5 subunit in mediating the muscle-relaxant effect of diazepam was observed in α5 (H105R) mutant mice (Crestani et al. 2002). Here we report on antagonism of the muscle-relaxant effect of diazepam with the α5 selective ligand XLi093 in rats. Nonetheless, muscle relaxation can be achieved without apparent activation of α5-GABAA receptors, as demonstrated in experiments with zolpidem (Elliot and White, 2001; Licata et al. 2009). Furthermore, an α2/α3 selective compound devoid of agonistic activity at the α5 subunit exerted muscle relaxation in monkeys (Licata et al. 2005). These results suggest that the role of the α5 subunit in the BZ-induced myorelaxation could be described as non-dominant, but still significant, and prompt further investigation.
The present study demonstrates that α1- and α5-GABAA receptor subtypes differentially contribute to motor impairing effects of BZs in rats. While activation of α1-GABAA receptors is a prerequisite for eliciting ataxia, these receptors are probably not directly involved in mediating muscle relaxation but still may contribute to manifestation of this effect triggered by a small fraction of activated α2-GABAA receptors. On the other hand, activation of α5-GABAA receptors contributes significantly, although not dominantly, to muscle relaxation, but not ataxia. Thus, in the quest for ligands with an improved pharmacological profile, it could be of importance to avoid substantial potentiation through α1 subunits, if ataxia is to be prevented, whereas a certain level of activation at both α1 and α5 subunits could be advantageous when muscle relaxation is required.
ACKNOWLEDGEMENTS
The authors acknowledge the support by The Ministry of Science, R. Serbia - Grant No. 175076 (MMS) and by NIMH grant MH-046851 (JMC).
Source of Funding: The work has been funded by The Ministry of Science, R. Serbia – Grant No. 175076 (MMS) and by NIMH grant MH-046851 (JMC)
Footnotes
Conflicts of Interest: We have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Atack JR. GABAA receptor alpha2/alpha3 subtype-selective modulators as potential nonsedating anxiolytics. Curr Top Behav Neurosci. 2010;2:331–60. doi: 10.1007/7854_2009_30. [DOI] [PubMed] [Google Scholar]
- Atack JR, Smith AJ, Emms F, McKernan RM. Regional differences in the inhibition of mouse in vivo [3H]Ro 15-1788 binding reflect selectivity for alpha 1 versus alpha 2 and alpha 3 subunit-containing GABAA receptors. Neuropsychopharmacology. 1999;20:255–62. doi: 10.1016/S0893-133X(98)00052-9. [DOI] [PubMed] [Google Scholar]
- Belzung C, Le Guisquet AM, Griebel G. Beta-CCT, a selective BZ-omega1 receptor antagonist, blocks the anti-anxiety but not the amnesic action of chlordiazepoxide in mice. Behav Pharmacol. 2000;11:125–31. doi: 10.1097/00008877-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Benavides J, Peny B, Durand A, Arbilla S, Scatton B. Comparative in vivo and in vitro regional selectivity of central omega (benzodiazepine) site ligands in inhibiting [3H]flumazenil binding in the rat central nervous system. J Pharmacol Exp Ther. 1992;263:884–96. [PubMed] [Google Scholar]
- Bohlhalter S, Weinmann O, Mohler H, Fritschy JM. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci. 1996;16:283–97. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox ED, Hagen TJ, McKernan RM, Cook JM. BZ1 receptor subtype specific ligands. Syntesis and biological properties of beta-CCt, a BZ1 receptor subtype specific antagonist. Med Chem Res. 1995;5:710–718. [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–80. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Löw K, Keist R, Mandelli M, Möhler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001;59:442–5. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, et al. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci U S A. 2002;99:8980–5. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot EE, White JM. The acute effects of zolpidem compared to diazepam and lorazepam using radiotelemetry. Neuropharmacology. 2001;40:717–21. doi: 10.1016/s0028-3908(00)00196-9. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Letang V, Granger P, Avenet P, Schoemaker H, Sanger DJ. New evidence that the pharmacological effects of benzodiazepine receptor ligands can be associated with activities at different BZ (omega) receptor subtypes. Psychopharmacology (Berl) 1999;146:205–13. doi: 10.1007/s002130051108. [DOI] [PubMed] [Google Scholar]
- Huang Q, He X, Ma C, Liu R, Yu S, Dayer CA, Wenger GR, et al. Pharmacophore/receptor models for GABA(A)/BzR subtypes (alpha1beta3gamma2, alpha5beta3gamma2, and alpha6beta3gamma2) via a comprehensive ligand-mapping approach. J Med Chem. 2000;13:71–95. doi: 10.1021/jm990341r. [DOI] [PubMed] [Google Scholar]
- Li X, Cao H, Zhang C, Furtmueller R, Fuchs K, Huck S, et al. Synthesis, in vitro affinity, and efficacy of a bis 8-ethynyl-4H-imidazo[1,5a]-[1,4]benzodiazepine analogue, the first bivalent alpha5 subtype selective BzR/GABA(A) antagonist. J Med Chem. 2003;46:5567–70. doi: 10.1021/jm034164c. [DOI] [PubMed] [Google Scholar]
- Licata SC, Platt DM, Cook JM, Sarma PV, Griebel G, Rowlett JK. Contribution of GABAA receptor subtypes to the anxiolytic-like, motor, and discriminative stimulus effects of benzodiazepines: studies with the functionally selective ligand SL651498 [6-fluoro-9-methyl-2-phenyl-4-(pyrrolidin-1-ylcarbonyl)-2,9-dihydro-1H-pyridol[3,4-b]indol-1-one] J Pharmacol Exp Ther. 2005;313:1118–25. doi: 10.1124/jpet.104.081612. [DOI] [PubMed] [Google Scholar]
- Licata SC, Platt DM, Cook JM, Van Linn ML, Rowlett JK. Contribution of alpha1 subunit-containing gamma-aminobutyric acidA (GABAA) receptors to motor-impairing effects of benzodiazepines in squirrel monkeys. Psychopharmacology (Berl) 2009;203:539–46. doi: 10.1007/s00213-008-1401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–4. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Maurissen JP, Marable BR, Andrus AK, Stebbins KE. Factors affecting grip strength testing. Neurotoxicol Teratol. 2003;25:543–53. doi: 10.1016/s0892-0362(03)00073-4. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–43. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;6:587–92. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Larsen JS, Mathiasen C, Jacobsen TA, Munro G, Erichsen HK, et al. NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile], a unique subtype-selective GABAA receptor positive allosteric modulator: in vitro actions, pharmacokinetic properties and in vivo anxiolytic efficacy. J Pharmacol Exp Ther. 2008;327:954–68. doi: 10.1124/jpet.108.138859. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD, Cook J, Ma C. Selective antagonism of the ataxic effects of zolpidem and triazolam by the GABAA/alpha1-preferring antagonist beta-CCt in squirrel monkeys. Psychopharmacology (Berl) 2002;164:151–9. doi: 10.1007/s00213-002-1189-9. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Tornatzky W, Cook JM, Ma C, Miczek KA. Zolpidem, triazolam, and diazepam decrease distress vocalizations in mouse pups: differential antagonism by flumazenil and beta-Carboline-3-carboxylate-t-butyl ester (beta-CCt) J Pharmacol Exp Ther. 2001;297:247–53. [PubMed] [Google Scholar]
- Rowlett JK, Cook JM, Duke AN, Platt DM. Selective antagonism of GABAA receptor subtypes: an in vivo approach to exploring the therapeutic and side effects of benzodiazepine-type drugs. CNS Spectr. 2005;10:40–8. doi: 10.1017/s1092852900009895. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–98. doi: 10.1146/annurev.pharmtox.44.101802.121429. Review. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, et al. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol. 2002;451:103–10. doi: 10.1016/s0014-2999(02)02191-x. [DOI] [PubMed] [Google Scholar]
- Savić MM, Obradović DI, Ugresić ND, Cook JM, Yin W, Bokonjić DR. Bidirectional effects of benzodiazepine binding site ligands in the elevated plus-maze: differential antagonism by flumazenil and beta-CCt. Pharmacol Biochem Behav. 2004;79:279–90. doi: 10.1016/j.pbb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Savić MM, Obradović DI, Ugresić ND, Cook JM, Sarma PV, Bokonjić DR. Bidirectional effects of benzodiazepine binding site ligands on active avoidance acquisition and retention: differential antagonism by flumazenil and beta-CCt. Psychopharmacology (Berl) 2005;180:455–65. doi: 10.1007/s00213-005-2170-1. [DOI] [PubMed] [Google Scholar]
- Savić MM, Huang S, Furtmüller R, Clayton T, Huck S, Obradović DI, et al. Are GABAA receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists? Neuropsychopharmacology. 2008;33:332–9. doi: 10.1038/sj.npp.1301403. [DOI] [PubMed] [Google Scholar]
- Savić MM, Milinković MM, Rallapalli S, Clayton T, Sr, Joksimović S, Van Linn M, Cook JM. The differential role of alpha1- and alpha5-containing GABA(A) receptors in mediating diazepam effects on spontaneous locomotor activity and water-maze learning and memory in rats. Int J Neuropsychopharmacol. 2009;12:1179–93. doi: 10.1017/S1461145709000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon HE, Guzman F, Cook JM. beta-Carboline-3-carboxylate-t-butyl ester: a selective BZ1 benzodiazepine receptor antagonist. Life Sci. 1984;35:2227–36. doi: 10.1016/0024-3205(84)90464-8. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv Pharmacol. 2006;54:231–63. doi: 10.1016/s1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- van Rijnsoever C, Täuber M, Choulli MK, Keist R, Rudolph U, Mohler H, et al. Requirement of alpha5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J Neurosci. 2004;24:6785–90. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster JC, Volkerts ER, Verbaten MN. Effects of alprazolam on driving ability, memory functioning and psychomotor performance: a randomized, placebo-controlled study. Neuropsychopharmacology. 2002;27:260–9. doi: 10.1016/S0893-133X(02)00310-X. [DOI] [PubMed] [Google Scholar]