For centuries, opioid drugs have been the mainstay of chronic pain treatment. However, over time analgesic tolerance develops, leaving few treatment options. Here we show that PDGFR-β-mediated signaling is sufficient to cause morphine tolerance and necessary for its behavioral expression. PDGFR-β inhibition selectively eliminates morphine tolerance in rats. PDGFR-β inhibitors are widely used and well-tolerated, suggesting that clinical translation of our findings could reduce the tremendous suffering endured by chronic pain patients.
The platelet-derived growth factor receptor (PDGFR) is a receptor tyrosine kinase (RTK) that exerts profound effects on n-methyl-D-aspartate receptor (NMDAR) function1. NMDARs play a mechanistic role in opioid tolerance2, but clinically, NMDAR antagonists have been ineffective or neurotoxic3. The mu opioid receptor (MOR) has been shown to transactivate the PDGFR-β4 and other RTKs5, but the clinical significance of this effect remains unknown. Clinical PDGFR inhibitors do not cross the blood-brain barrier (BBB)6. We reformulated imatinib (Gleevec®) to improve brain penetration, then determined whether PDGFR-mediated signaling modulated opioid tolerance.
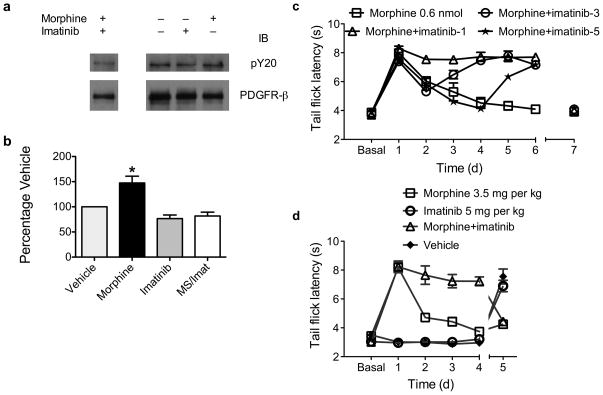
We treated MOR-transfected C6 glioma cells with 1 and 10μM morphine for varying times and performed immunoprecipitation/immunoblotting (IP/IB) to quantify PDGFR phosphorylation. Morphine did not activate PDGFR-α (data not shown) but did activate PDGFR-β 40 minutes after treatment (Supplementary fig. 1). Morphine significantly increased phosphorylation at 10 nM - 1 μM concentrations (Supplementary fig. 2). Activation did not follow a standard dose-response curve, but appeared threshold-based. The MOR agonist fentanyl induced a similar magnitude and pattern of PDGFR-β activation (Supplementary fig. 3). Morphine did not activate PDGFR-β in non-transfected cells (Supplementary fig. 4). We then treated rats with either 0.6 nmol morphine, 10 μg imatinib, or both drugs intrathecally (i.t.), and harvested spinal cords 40 min. later. The substantia gelatinosa was microdissected and IP/IB performed. Morphine caused a 47% increase in PDGFR-β phosphorylation, which was blocked by imatinib (Fig. 1a,b) and naloxone (Supplementary fig. 5).
Figure 1. Morphine activates the PDGFR-β, and PDGFR-β inhibition blocks tolerance.
a: Animals were treated with 0.6 nmol morphine, 10 μg imatinib, morphine + imatinib (MS/Imat), or vehicle for 40 min. Lumbar spinal cords were then harvested, and the substantia gelatinosa microdissected. Individual lysates were prepared for each animal, and immunoprecipitation (IP) was performed with anti-PDGFR-β. Samples were then run on SDS-PAGE gels and immunoblotted (IB) with anti-phospotyrosine (pY20). Blots were then stripped and reprobed with anti-PDGFR-β to control for IP efficiency. A representative IP/IB experiment is shown with irrelevant lanes removed. b: Graphic summary of the data. Morphine caused a 47% increase in PDGFR-β phosphorylation. Data presented as mean +/− s.d. F(3,19) = 13.8; P < 0.0001 (one-way ANOVA); * P < 0.05 vs. all other treatment groups by Bonferroni multiple comparison post-tests. n = 5 – 6 independent animals per treatment group. c: Animals were treated daily with intrathecal (i.t.) injection of either 1) 0.6 nmol morphine; 2) morphine + 10 μg imatinib begun on Day 1 (Morphine+imatinib-1); 3) morphine + imatinib begun on Day 3; or 4) morphine + imatinib begun on Day 5. On Day 7, all animals received morphine alone (indicated by discontinuous lines between days 6 and 7). Analgesic responses were monitored using tail-flick latency. All data presented as seconds +/− s.e.m. Treatment F(3,32) = 18.5, Day F(6,224) = 160, Interaction F(21,224) = 22.0; all P < 0.0001 (2-way ANOVA). n = 9 animals per treatment group. d: Animals were treated for 4 days with subcutaneous (s.c.) injection of either 1) 3.5 mg/kg morphine; 2) 5 mg/kg imatinib; 3) morphine and imatinib; or 4) vehicle. On day 5, all animals received morphine alone. Treatment F(3,32) = 90.2, Day F(5,160) = 44.5, Interaction F(15,160) = 41.2; all P < 0.0001 (2-way ANOVA). n = 9 animals per treatment group.
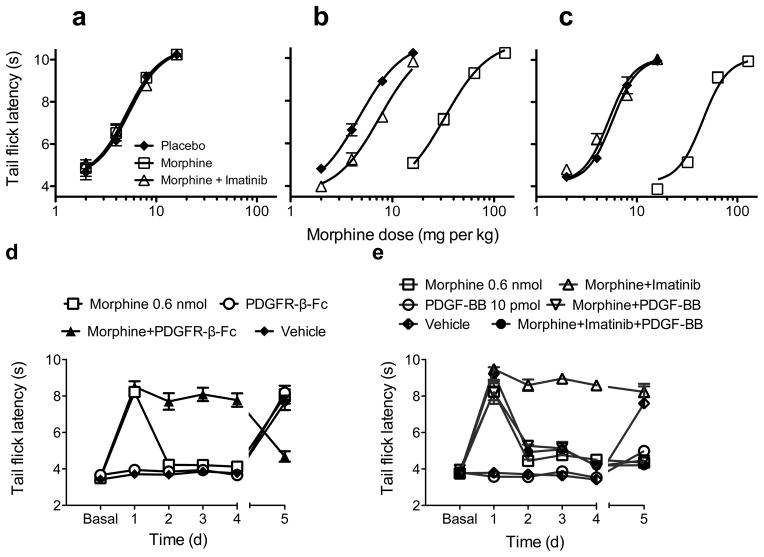
We then determined whether imatinib could prevent tolerance. We administered 0.6 nmol morphine i.t. for seven days, and began co-administration of 10 μg imatinib on days one, three, or five. On day 1, comparison of imatinib-treated to morphine-treated rats demonstrated that imatinib did not alter the analgesic potency of morphine (Fig. 1c). Imatinib administered from day 1 completely eliminated morphine tolerance. Initiation of treatment on days 3 or 5 reversed tolerance within two days (Fig. 1c), demonstrating reversal of established tolerance by imatinib. On day 7, all animals received morphine alone. Surprisingly, all animals were tolerant to morphine, indicating that imatinib only temporarily reversed the processes that cause tolerance. Systemic imatinib also reversed opioid tolerance. Neither imatinib nor vehicle was analgesic. Also, prolonged administration of imatinib or vehicle did not interfere with morphine analgesia (Fig. 1d). We then investigated whether imatinib reversed tolerance after continuous, high-dose morphine. We determined morphine dose-response curves by subcutaneous (s.c.) injection of escalating morphine doses in naive rats (Fig. 2a), then implanted 150 mg of continuous-release morphine (or placebo) pellets7. Five days later, dose-response curves were determined again. 30 min before testing, half of the morphine-pelleted rats received 5 mg/kg imatinib s.c, while the other half and placebo-pelleted animals received vehicle. Remarkably, imatinib reversed profound morphine tolerance (95% CI of ED50 ratio morphine/placebo pelleted animals, 6.1–7.8; 95% CI of morphine pelleted and imatinib treated/placebo pelleted ED50, 1.4–1.8; Fig. 2b). This procedure was repeated the following day. Analogous to i.t. administration (Fig. 1c), imatinib completely reversed profound morphine tolerance (95% CI morphine/placebo pelleted ED50, 6.9–8.9; 95% CI morphine pelleted and imatinib treated/placebo pelleted ED50, 0.8–1.0; Fig. 2c).
Figure 2. Imatinib reverses profound morphine tolerance, and its effects are mediated by the PDGFR-β.
3 groups of 8 opioid naive rats received subcutaneous (s.c.) injection of 2 mg/kg morphine. Analgesia was assessed using tail flick latency (TFL) 30 min later. 15 min after TFL testing, the s.c. morphine dose was doubled and testing repeated until TFL values exceeded the cutoff value of 10 seconds. a: baseline dose response curves. After baseline testing, 2 groups of 8 rats had 2–75 mg continuous release morphine pellets implanted under isoflurane anesthesia, while the third group received 2 placebo pellets. b: On day 5 after pellet implantation, the animals underwent dose response testing. 30 min prior to the initial morphine injection, one group of morphine pelleted rats received 5 mg/kg imatinib s.c., while the other morphine pelleted group and the placebo pelleted animals were injected with an equivalent volume of vehicle. Imatinib significantly reduced an approximately 7-fold ED50 shift in morphine tolerant animals to approximately 1.5-fold. c: Day 6 dose-response results. The procedure described above was repeated the following day. Imatinib completely reversed an approximately 8-fold ED50 shift in morphine tolerant animals. All data presented as seconds +/− s.e.m. n = 8 animals per treatment group. d: Animals were treated daily for 4 days with intrathecal (i.t.) injection of either 1) 0.6 nmol morphine; 2) 10 ng PDGFR-β-Fc fragment (PDGFR-β-Fc); 3) morphine+PDGFR-β-Fc; or 4) vehicle. On day 5, PDGFR-β-Fc, vehicle, and Morphine+PDGFR-β-Fc groups received morphine alone, while the morphine group received morphine and PDGFR-β-Fc (indicated by discontinuous lines between days 4 and 5). All data presented as seconds +/− s.e.m. Treatment F(3,192) = 84.8, Day F(5,192) = 64.4, Interaction F(15,160) = 37.8; all P<0.0001 (2-way ANOVA). n = 6 animals for PDGFR-β-Fc and vehicle groups, n = 12 for morphine and morphine+PDGFR-β-Fc groups. e: Animals received daily i.t. injections of either 1) 0.6 nmol morphine; 2) 10 pmol PDGF-BB; 3) morphine and 10 μg imatinib; 4) morphine and 10 pmol PDGF-BB; 5) morphine, imatinib, and PDGF-BB; or 6) vehicle for 4 days. On day 5, the PDGF-BB and vehicle groups received morphine alone, and all other groups continued their previous treatments (indicated by discontinuous lines between days 4 and 5). All data presented as seconds +/− s.e.m. Treatment F(5,31) = 236, Day F(5,155) = 73.8, Interaction F(25,155) = 20.4; all P < 0.0001 (2-way ANOVA). n = 5–8 animals per group.
Another possible explanation for this effect is that opioid tolerance unmasked a latent analgesic effect of imatinib. We treated animals with of 10 mg/kg morphine s.c. twice daily for 2, 5, 8, or 10 days. In the first three groups, after morphine was discontinued rats received 5 mg/kg imatinib alone to complete a 10 daycourse. Imatinib was not analgesic (Supplementary fig. 6). Opioids act through the mu opioid receptor (MOR), a Gi/o-activating G-protein coupled receptor (GPCR)8. α-2 adrenoreceptor agonists activate Gi/o-coupled GPCRs and can cause analgesia9. Therefore, we hypothesized that imatinib would inhibit tolerance to clonidine. We administered 5 μg clonidine or clonidine and 10 μg imatinib i.t. for 10 days. Imatinib did not inhibit clonidine analgesic tolerance (Supplementary fig. 7), suggesting that tolerance inhibition is opioid-specific.
While selective, imatinib is not PDGFR-specific10. Also, the time to peak PDGFR activation was longer than previous examples of transactivation11, suggesting another mechanism could be involved. We administered 0.6 nmol morphine alone or with 10 ng PDGFR-β Fc fusion protein (PDGFR-β-Fc), which scavenges released PDGF-B, i.t. for 4 days. Morphine with PDGFR-β-Fc completely reversed tolerance without augmenting analgesia (Fig. 2d). PDGFR-β-Fc or vehicle alone were not analgesic. On day 5, morphine-treated animals received morphine and PDGFR-β-Fc, while other groups received morphine. Animals that received morphine after 4 days of morphine and PDGFR-β-Fc were profoundly tolerant. PDGFR-β-Fc completely restored analgesia in tolerant animals, and PDGFR-β-Fc or vehicle did not alter morphine analgesia. PDGFR-β-Fc also blocked morphine-induced PDGFR-β phosphorylation in stably transfected C6 cells (Supplementary fig. 8), further supporting the concepts that tolerance inhibition is PDGFR-β-selective and is due to opioid-induced release of PDGF-B.
It is possible that PDGF release causes “apparent” tolerance either by decreasing morphine analgesia or decreasing basal response latencies (i.e, inducing thermal hyperalgesia). We administered 0.6 nmol morphine, 10 pmol PDGF-BB, vehicle, morphine + 10 μg imatinib, morphine + PDGF-BB, or morphine + imatinib + PDGF-BB i.t. for 4 days. On day 5, vehicle or PDGF-BB treated rats received morphine while all others continued previous treatments. PDGF-BB did not alter baseline tail-flick responses (Fig. 2e). Analgesic responses of animals receiving morphine or morphine and PDGF-BB were similar, indicating that PDGF-BB did not interfere with morphine analgesia or become anti-analgesic over time. However, PDGF-BB completely abolished tolerance inhibition by imatinib. Conversely, animals given PDGF-BB for 4 days were tolerant when challenged with morphine even though they had never received opioids, indicating that PDGFR-β activation could directly cause morphine tolerance. We replicated this finding by giving vehicle or 10 pmol PDGF-BB i.t. for 4 days, then measuring paw withdrawal latency. Baselines remained stable. On day 5, animals received 0.6 nmol morphine. Vehicle-treated animals showed robust analgesia, while PDGF-BB-treated animals were completely tolerant (Supplementary fig. 9). To determine whether this effect was opioid-specific, rats were given 10 pmol PDGF-BB or vehicle i.t. for 4 days then challenged with 5 μg clonidine on day 5. Both groups had robust analgesic responses (Supplementary fig. 10), suggesting that tolerance induction by PDGF-BB is opioid-specific.
Our findings conclusively demonstrate that PDGFR-β antagonism completely eliminates morphine tolerance. When PDGFR-β activation was blocked, tolerance was reversed, while PDGF-BB administration alone caused tolerance, indicating that phosphorylation of the PDGFR-β is sufficient to cause morphine tolerance and necessary for its behavioral expression. The scavenging experiments in Fig. 2d and Supplementary fig. 8 demonstrated that morphine-induced PDGF release, not direct transactivation, stimulated the PDGFR-β. Our finding that opioid-induced PDGFR-β activation in vitro appears to be uniform above a threshold concentration is consistent with the hypothesis that tolerance is mediated by opioid-induced PDGF release.
PDGFR-β activation inhibits NMDARs1. Therefore, if common signaling pathways mediated both effects, PDGFR-β agonists, rather than inhibitors, might block tolerance. However, the behavioral effects of these signals are quite different. PDGF-BB does not alter morphine analgesia or baseline responses, and does not alter the rate of morphine tolerance development (see Fig. 2e). NMDA reduces morphine analgesia, induces thermal hyperalgesia and accelerates the development of morphine tolerance 12,13. Unlike PDGFR-β inhibition, NMDAR antagonists can cause analgesia14 and sustained reversal of tolerance2. Taken together, these findings suggest that the NMDAR and PDGFR-β modulate tolerance independently. If the NMDAR is not involved, then what are possible explanations for this effect? Based on our findings, we postulate that PDGFR-β inhibition blocks tolerance utilizing two mechanisms: A rapid effect causing most of the reversal; and a slower process that completely restores analgesia (see Figs. 1c and 2a,b, and c). The initial reversal may be due to rapid post-translational modification of analgesic effector(s) after PDGFR-β antagonist administration, while changes in transcriptional or translational regulation of effector molecules could account for delayed effects. This hypothesis is outlined in Supplemental fig. 11. Given the widespread use of imatinib and morphine, it appears surprising that tolerance inhibition has not been previously observed. We hypothesize that imatinib levels needed to inhibit tolerance are not currently achieved in the CNS.
Opioids and PDGFR-β have opposing effects on several putative analgesic mediators. For example, opioids increase while PDGFR-β decreases current amplitudes of voltage-sensitive calcium channels and voltage-activated potassium channels15–17. Conversely, opioids decrease, while PDGFR-β increases the non-selective cation current17,18. Logically, PDGFR-β antagonism could reverse tolerance by actions upon some (or all) of these effectors. Opioids and PDGFR-β also activate some common intracellular signaling molecules, such as PI3K, PLCγ/PKC, and MAP kinase cascades17,19. If one or more of these substrates causes tolerance, PDGFR-β inhibitors could lead to rapid changes in the post-translational modification of relevant targets. Opioids and PDGFR-β activate many transcription factors, such as CREB, AP-1, STAT, and NF-kB, and also modulate translational machinery20. We propose that transcriptional or translational modulation could underlie the delayed phase of tolerance reversal.
In conclusion, we have demonstrated that inhibiting PDGFR-β signaling selectively eliminates morphine analgesic tolerance without altering acute analgesic effects of morphine in rats. We also found that morphine-induced PDGFR-β signaling is necessary and sufficient for the behavioral expression of morphine tolerance. These findings could have profound clinical implications for the untold millions suffering from chronic intractable pain.
Supplementary Material
Acknowledgments
We thank J. Dulin, T. Sylvester, and C Schultz for technical support. This work was funded by grants from the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism (USA) to H.B.G. We dedicate this work to the memory of our colleague and dear friend Bing Mo, who tragically passed away before these studies were completed.
All animal study protocols were approved by the MD Anderson Cancer Center Institutional Animal Care and Use Committee.
References
- 1.Valenzuela CF, et al. Platelet-derived growth factor induces a long-term inhibition of N-methyl-D-aspartate receptor function. J Biol Chem. 1996;271:16151–16159. doi: 10.1074/jbc.271.27.16151. [DOI] [PubMed] [Google Scholar]
- 2.Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- 3.Rice ASC, Hill RG. New treatments for neuropathic pain. Annu Rev Med. 2006;57:535–551. doi: 10.1146/annurev.med.57.121304.131324. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Farooqui M, Gupta K. Morphine Stimulates Vascular Endothelial Growth Factor-Like Signaling in Mouse Retinal Endothelial Cells. Current Neurovascular Research. 2006;3:171–180. doi: 10.2174/156720206778018767. [DOI] [PubMed] [Google Scholar]
- 5.Belcheva MM, Szucs M, Wang D, Sadee W, Coscia CJ. mu-Opioid receptor-mediated ERK activation involves calmodulin-dependent epidermal growth factor receptor transactivation. J Biol Chem. 2001;276:33847–33853. doi: 10.1074/jbc.M101535200. [DOI] [PubMed] [Google Scholar]
- 6.Dai H, Marbach P, Lemaire M, Hayes M, Elmquist WF. Distribution of STI-571 to the brain is limited by P-glycoprotein-mediated efflux. J Pharmacol Exp Ther. 2003;304:1085–1092. doi: 10.1124/jpet.102.045260. [DOI] [PubMed] [Google Scholar]
- 7.Gold LH, Stinus L, Inturrisi CE, Koob GF. Prolonged tolerance, dependence and abstinence following subcutaneous morphine pellet implantation in the rat. Eur J Pharmacol. 1994;253:45–51. doi: 10.1016/0014-2999(94)90755-2. [DOI] [PubMed] [Google Scholar]
- 8.Gutstein H, Akil H. Opioid Analgesics. In: Brunton L, Lazo J, Parker K, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw-HIll; New York: 2006. pp. 547–590. [Google Scholar]
- 9.Aghajanian GK, Wang YY. Common alpha 2- and opiate effector mechanisms in the locus coeruleus: intracellular studies in brain slices. Neuropharmacology. 1987;26:793–799. doi: 10.1016/0028-3908(87)90054-2. [DOI] [PubMed] [Google Scholar]
- 10.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 11.Herrlich A, et al. Ligand-independent activation of platelet-derived growth factor receptor is a necessary intermediate in lysophosphatidic, acid-stimulated mitogenic activity in L cells. Proc Natl Acad Sci U S A. 1998;95:8985–8990. doi: 10.1073/pnas.95.15.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolesnikov Y, Jain S, Wilson R, Pasternak GW. Lack of morphine and enkephalin tolerance in 129/SvEv mice: evidence for a NMDA receptor defect. J Pharmacol Exp Ther. 1998;284:455–459. [PubMed] [Google Scholar]
- 13.Malmberg AB, Yaksh TL. Spinal nitric oxide synthesis inhibition blocks NMDA-induced thermal hyperalgesia and produces antinociception in the formalin test in rats. Pain. 1993;54:291–300. doi: 10.1016/0304-3959(93)90028-N. [DOI] [PubMed] [Google Scholar]
- 14.Coderre TJ, Van Empel I. The utility of excitatory amino acid (EAA) antagonists as analgesic agents. I Comparison of the antinociceptive activity of various classes of EAA antagonists in mechanical, thermal and chemical nociceptive tests. Pain. 1994;59:345–352. doi: 10.1016/0304-3959(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 15.Black MJ, Woo Y, Rane SG. Calcium channel upregulation in response to activation of neurotrophin and surrogate neurotrophin receptor tyrosine kinases. J Neurosci Res. 2003;74:23–36. doi: 10.1002/jnr.10748. [DOI] [PubMed] [Google Scholar]
- 16.Timpe LC, Fantl WJ. Modulation of a voltage-activated potassium channel by peptide growth-factor receptors. J Neurosci. 1994;14:1195–1201. doi: 10.1523/JNEUROSCI.14-03-01195.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams J, Christie M, Manzoni O. Cellular and synaptic adaptions mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 18.Frace AM, Gargus JJ. Activation of single-channel currents in mouse fibroblasts by platelet-derived growth factor. Proceedings of the National Academy of Sciences. 1989;86:2511–2515. doi: 10.1073/pnas.86.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: Effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.