Abstract
Mephedrone (4-methylmethcathinone) is a β-ketoamphetamine with close structural analogy to substituted amphetamines and cathinone derivatives. Abuse of mephedrone has increased dramatically in recent years and has become a significant public health problem in the US and Europe. Unfortunately, very little information is available on the pharmacological and neurochemical actions of mephedrone. In light of the proven abuse potential of mephedrone and considering its similarity to methamphetamine and methcathinone, it is particularly important to know if mephedrone shares with these agents an ability to cause damage to dopamine nerve endings of the striatum. Accordingly, we treated mice with a binge-like regimen of mephedrone (4X 20 or 40 mg/kg) and examined the striatum for evidence of neurotoxicity 2 or 7 days after treatment. While mephedrone caused hyperthermia and locomotor stimulation, it did not lower striatal levels of dopamine, tyrosine hydroxylase or the dopamine transporter under any of the treatment conditions used presently. Furthermore, mephedrone did not cause microglial activation in striatum nor did it increase glial fibrillary acidic protein levels. Taken together, these surprising results suggest that mephedrone, despite its numerous mechanistic overlaps with methamphetamine and the cathinone derivatives, does not cause neurotoxicity to dopamine nerve endings of the striatum.
Keywords: mephedrone, dopamine, neurotoxicity, β-ketoamphetamines, microglial activation, methamphetamine
The β-ketoamphetamines are psychostimulants and include such substances as cathinone, methcathinone, mephedrone (4-methylmethcathinone; MEPH) and 3,4-methylenedioxypyrovalerone. Some of these agents are psychoactive ingredients found in khat (Catha edulis Forsk), an evergreen shrub that has been cultivated and chewed as a recreational and socializing drug in Africa and the Arabian peninsula for centuries (Feyissa and Kelly 2008). β-ketoamphetamines have recently moved into western societies in the form of so-called “bath salts”, synthetic powders that are sold legally in commercial establishments and head-shops under such names as Ivory Wave, Red Dove or Scarface. These compounds have been called “natural” amphetamines and their effects on humans are quite profound and can range from increased alertness to psychoses and depression (Kelly 2011, Schifano et al. 2011, Winstock et al. 2011, Brunt et al. 2010). The β-ketoamphetamines are inexpensive and readily synthesized in clandestine labs. They are also being abused at an increasing rate across the US and Europe. Emergency room admissions for treatment after intoxication with these agents have more than doubled from 2010 to 2011 according to the CDC. Emerging evidence of the high addictive potential and craving associated with the β-ketoamphetamines has very recently set off alarms of concern at numerous US governmental agencies that monitor drug abuse trends to include NIDA, the White House Office of National Drug Control Policy, and the DEA. Most of the β-ketoamphetamines are classified as DEA Schedule I compounds and mephedrone and related drugs are now banned by all member states of the European Monitoring Centre for Drugs and Drug Addiction.
Almost as alarming as the rise in abuse of the β-ketoamphetamines is the paucity of data on their mechanisms of action and particularly their ability to damage the central nervous system, especially in light of the structural analogy of cathinone, methcathinone and methylone to amphetamine, methamphetamine (METH) and 3,4-methylenedioxymethamphetamine (MDMA), respectively (Kelly 2011). The only difference between these drug classes is the presence of the β-keto moiety on the cathinones (Gibbons and Zloh 2010). Wagner and colleagues (Wagner et al. 1982) first suggested the possibility that cathinone could be neurotoxic when they showed long-lasting reductions in dopamine (DA) and DA uptake sites in rat striatum after repeated drug administration. The β-ketoamphetamines share with the substituted amphetamines a high potency in blocking transporters for DA and serotonin (5-HT; DAT and SERT, respectively) (Cozzi and Foley 2003, Cozzi et al. 1999, Rothman et al. 2003, Metzger et al. 1998, Fleckenstein et al. 2000, Nagai et al. 2007, Meltzer et al. 2006) and causing monoamine release in vitro (Kalix and Glennon 1986, Kalix 1984, Gygi et al. 1997, Rothman et al. 2003) and in vivo (Pehek et al. 1990, Banjaw and Schmidt 2006, Gygi et al. 1997, Kehr et al. 2011). Like METH, at least cathinone is a powerful inhibitor of monoamine oxidase B (Nencini et al. 1984). Oral administration of Catha edulis extract to rats leads to a long-term reduction in striatal DA levels (Banjaw and Schmidt 2005). Methcathinone has been shown to cause persistent reductions in function of both DA and 5-HT nerve endings manifested as inhibition of tryptophan hydroxylase and tyrosine hydroxylase (TH), depletion of DA and 5-HT neurotransmitters and inhibition of DA and 5-HT uptake into synaptosomes (Gygi et al. 1997, Gygi et al. 1996, Sparago et al. 1996). Methcathinone intoxication also leads to significant hyperthermia (Rockhold et al. 1997). PET imaging studies in abstinent methcathinone users have revealed reduced striatal DAT density, an effect that is highly suggestive of a loss of DA terminals (McCann et al. 1998). The coincident stimulation of DA release and inhibition of its uptake and breakdown, when combined with hyperthermia, mirror the critical elements underlying the neurotoxicity associated with METH (Kuhn et al. 2008, Yamamoto et al. 1998, Yamamoto and Bankson 2005, Krasnova and Cadet 2009, Cadet et al. 2007, Fleckenstein et al. 2007).
MEPH is now one of the most commonly abused drugs behind cannabis, MDMA and cocaine (Morris 2010, Winstock et al. 2011). MEPH is consumed in a binge-like fashion (i.e., “stacking”) and is often taken with other drugs such as cannabis and MDMA (Schifano et al. 2011). MEPH is found increasingly in tablets sold as ecstasy and its use will likely eclipse that of MDMA as the purity of this latter drug continues to fall (Brunt et al. 2010). What is more, MEPH induces stronger feelings of craving in humans by comparison to MDMA (Brunt et al. 2010) and users who snort MEPH rate it as more addictive than cocaine (Winstock et al. 2011). By comparison to substituted amphetamines and cathinone derivatives, the neurochemical actions of MEPH have scarcely been studied. Emerging data has shown that MEPH causes locomotor activation (Motbey et al. 2011). It also increases synaptic levels of DA by virtue of its interaction with the DAT, resulting in increased release and inhibition of reuptake (Kehr et al. 2011, Hadlock et al. 2011, Martinez-Clemente et al. 2011). Surprisingly little is known about the neurotoxic potential of MEPH beyond the recent observation that repeated treatment of rats causes persistent serotonergic deficits (Hadlock et al. 2011). We report presently that binge-like administration of high doses of MEPH causes significant hyperthermia and hyperactivity but does not decrease striatal levels of DA, TH or DAT. MEPH does not cause microglial activation and glial fibrillary acidic protein (GFAP) levels are unaltered, suggesting that it does not cause damage to DA nerve endings of the striatum.
Materials and methods
Materials
MEPH hydrochloride, (+) METH hydrochloride, pentobarbital, horseradish peroxidase (HRP)-conjugated Isolectin B4 (ILB4; from Griffonia simplicifolia), 3, 3’-diaminobenzidine, paraformaldehyde, Triton X-100, Tween 20, DA, methanol, EDTA, all buffers, and HPLC reagents were purchased from Sigma-Aldrich (St. Louis, MO). CitriSolv and Permount were products of Fisher Scientific (Pittsburgh, PA). Bicinchoninic acid protein assay kits were obtained from Pierce (Rockford, IL). Polyclonal antibodies against rat TH were produced as previously described (Kuhn and Billingsley 1987). Monoclonal antibodies against rat DAT were generously provided by Dr. Roxanne A. Vaughan (University of North Dakota, Grand Forks, ND). Rabbit polyclonal antibodies against GFAP were purchased from Thermo Scientific (Rockford, IL). HRP-conjugated secondary antibodies were obtained from Amersham (Piscataway, NJ) and Jackson ImmunoResearch Laboratories (West Grove, PA).
Animals
Female C57BL/6 mice (Harlan, Indianapolis, IN) weighing 20–25 g at the time of experimentation were housed 5 per cage in large shoe-box cages in a light (12 h light/dark) and temperature controlled room. Female mice were used because they are known to be very sensitive to neuronal damage by the neurotoxic amphetamines and to maintain consistency with our previous studies of METH neurotoxicity (Thomas et al. 2010, Thomas et al. 2004a, Thomas et al. 2008a, Thomas et al. 2008b, Thomas et al. 2009, Thomas and Kuhn 2005). Mice had free access to food and water. The Institutional Care and Use Committee of Wayne State University approved the animal care and experimental procedures. All procedures were also in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Pharmacological, physiological and behavioral procedures
Mice were treated with a binge-like regimen of MEPH comprised of 4 injections of 20 or 40 mg/kg with a 2 h interval between each injection. This treatment regimen is known to cause extensive DA nerve ending damage when used for the substituted amphetamines and cathinone derivatives. The doses of MEPH used presently were determined empirically in pilot experiments. Lower doses of MEPH (e.g., 4X 5–10 mg/kg) were not neurotoxic to the DA neuronal system and did not cause changes in body temperature (data not shown). MEPH doses higher than 4X 40 mg/kg were not tested to avoid complications associated with cardiotoxicity (Meng et al. 2012). Mice were treated with a neurotoxic regimen of METH (4X 5 mg/kg with 2 h between injections) in select experiments (specified below). Controls received injections of physiological saline on the same schedule used for MEPH. All injections were given via the i.p. route. Mice were sacrificed 2 or 7 days after the last MEPH treatment. Body temperature was monitored by telemetry using IPTT-300 implantable temperature transponders from Bio Medic Data Systems, Inc. (Seaford, DE). Temperatures were recorded non-invasively every 20 min starting 60 min before the first METH injection and continuing for 9 h thereafter using the DAS-5001 console system from Bio Medic. Locomotor activity was measured in a locomotor activity apparatus comprised of four transparent plastic cages (AccuScan Instruments, Columbus, OH; 21 cm × 21 cm × 30 cm) each covered by a removable perforated plastic lid. Mice were placed in the center of the cage immediately after each injection of MEPH for 60 min and total activity, distance traveled, movement time and stereotyped episodes were recorded automatically and analyzed by Fusion software (AccuScan Instruments). Stereotyped episodes are operationally defined as repeated breaks of the same infrared light beam before and after a rest period (i.e., no stereotypy) ≥ 1 s. Mice were returned to home cages for 60 min prior to the next MEPH injection.
Determination of striatal DA content
Striatal tissue was dissected bilaterally from brain after treatment and stored at −80°C. Frozen tissues were weighed and sonicated in 10 volumes of 0.16 N perchloric acid at 4°C. Insoluble protein was removed by centrifugation and DA was determined by HPLC with electrochemical detection as previously described for METH (Thomas et al. 2010, Thomas et al. 2009).
Determination of TH and DAT protein levels by immunoblotting
The effects of MEPH on striatal TH and DAT levels were determined by immunoblotting as an index of toxicity to striatal DA nerve endings. Mice were sacrificed by decapitation after treatment and striatum was dissected bilaterally. Tissue was stored at −80°C. Frozen tissue was disrupted by sonication in 1% SDS at 95°C and insoluble material was sedimented by centrifugation. Protein was determined by the bicinchoninic acid method and equal amounts of protein (70 µg/lane) were resolved by SDS-polyacrylamide gel electrophoresis and then electroblotted to nitrocellulose. Blots were blocked in Tris buffered saline containing Tween 20 (0.1% v/v) and 5% non-fat dry milk for 1 h at room temperature. Primary antibodies were added to blots and allowed to incubate for 16 h at 4°C. Blots were washed 3X in Tris-buffered saline to remove unreacted antibodies and then incubated with HRP-conjugated anti-IgG secondary antibody (1:4000) for 1 h at room temperature. Immunoreactive bands were visualized by enhanced chemiluminescence and the relative densities of TH- and DAT-reactive bands were determined by imaging with a Kodak Image Station (Carestream Molecular Systems, Rochester, NY) and analyzing with ImageJ software (NIH).
Assessment of glial status in striatum
Microglial activation was assessed by staining fixed brain sections with HRP-conjugated ILB4 as developed by Streit (Streit 1990) and as previously described in our studies with METH (Thomas et al. 2004b, Thomas et al. 2008b). At the time of sacrifice, mice were deeply anesthetized with pentobarbital (120 mg/kg) and perfused transcardially with ice-cold 4% paraformaldehyde in phosphate buffered saline (PBS). Brains were removed and stored overnight in fixative at 4°C. Sections of 50 µm thickness were cut through the striatum. Sections were floated into PBS containing 0.3% H2O2 for 30 min, washed once in PBS + 0.1% Triton X-100, then incubated in fresh PBS + 0.1% Triton X-100 for an additional 30 min. Microglia were labeled with HRP-conjugated ILB4 (10 µg/ml in PBS + 0.1% Triton X-100) overnight at 4°C. Excess ILB4 was removed by 3 washes with PBS + 0.1% Triton X-100 (5 min each) followed by a single wash in PBS before exposure to 3,3’-diaminobenzidine substrate (0.1 mg/ml) in PBS for 25 min. After 3 washes with PBS, all sections were transferred to glass slides, air dried and dehydrated through a series of graded ethanol washes. Sections were incubated in Citrisolv for 5 min then cover-slipped under Permount. Astrocytic activation was assessed by immunohistochemistry using antibodies against GFAP (1:500 dilution) according to the same procedures described above for ILB4 histochemical staining except that GFAP was visualized using HRP-linked goat anti-rabbit secondary antibodies. Brain sections from drug-treated mice were processed simultaneously with controls to normalize staining among treatment groups. Glial reactivity was viewed under the light microscope and the number of stained cells observed after various treatments was quantified using MicroSuite Five Software (Olympus, Center Valley, CA). Cell counts were made by persons blinded to the treatment conditions. Counts were taken from two non-adjacent fields (40 µm2) of four independent sections from all like-treated mice, bilaterally, generating an average count for each treated subject.
Data analysis
The effects of drug treatments on striatal DA, TH and DAT content (Figs. 1 and 2) were tested for significance by one-way ANOVA followed by Tukey’s multiple comparison test in GraphPad Prism 5. Tests for glial activation (Figs. 3 and 4) used a 1-way ANOVA followed by Tukey’s for data obtained at the 2d time point (four groups) and a t-test was used to compare data obtained at the 7d time point (two groups). Results of MEPH on core body temperature (Fig. 5) over time were compared to controls using a two-way repeated measures ANOVA followed by Bonferroni’s test to determine significant differences in body temperature at individual times. MEPH effects on locomotor activity, distance traveled, movement time and stereotyped episodes (Fig. 6) were tested for significance by two-way ANOVA followed by Bonferroni multiple comparison test. Differences were considered significant if p < 0.05.
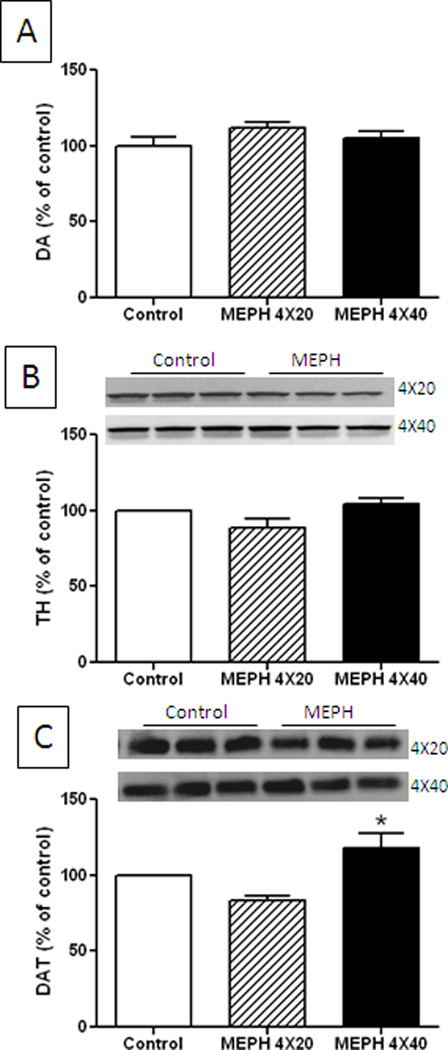
Fig. 1.
Effect of MEPH on DA nerve endings of the striatum 2d after treatment. Mice (n = 6 per group) were treated with MEPH in doses of 4X 20 mg/kg or 4X 40 mg/kg and the levels of DA (A), TH (B) and DAT (C) were determined at 2 days after the last injection of MEPH. Immunoblots show only 3 representative samples for each treatment group. All immunoblots were scanned and presented as means ± SEM relative to controls. The effects of either dose of MEPH on DA, TH and DAT were not significantly different from control or from each other with the exception that DAT levels after the higher dose of MEPH were significantly increased over those of the lower dose (p < 0.05, one-way ANOVA followed by Tukey’s test).
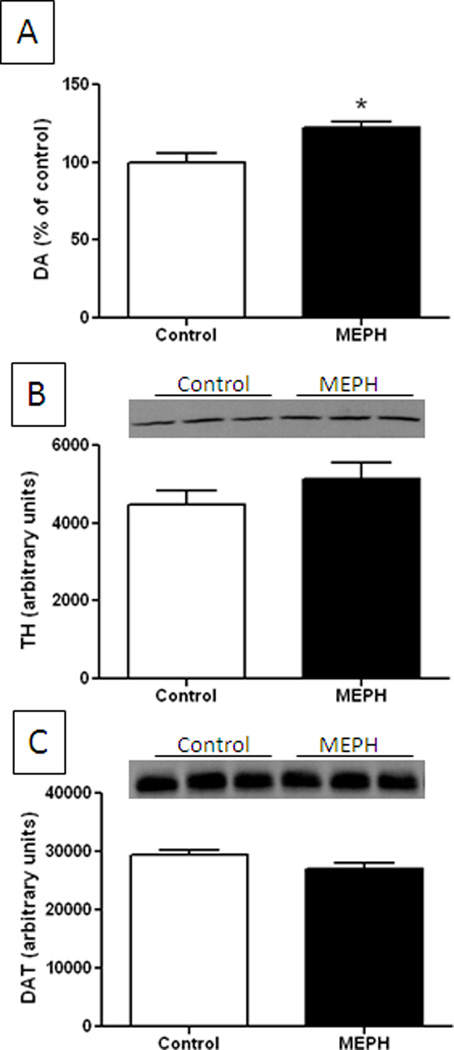
Fig. 2.
Effect of MEPH on DA nerve endings of the striatum 7d after treatment. Mice (n = 6 per group) were treated with MEPH (4X 40 mg/kg) and the levels of DA (A), TH (B) and DAT (C) were determined at 7 days after the last injection of MEPH. Immunoblots show only 3 representative samples for each treatment group. All immunoblots were scanned and presented as means ± SEM relative to controls. The effects of MEPH on TH or DAT were not significantly different from control but DA levels were increased significantly (p < 0.05. one-way ANOVA followed by Tukey’s test).
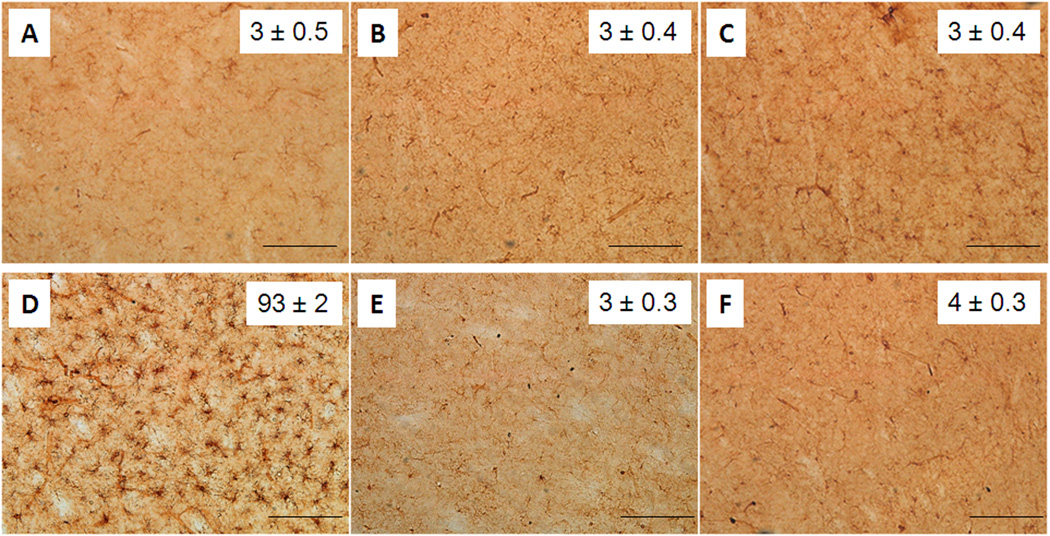
Fig. 3.
Effect of MEPH on striatal microglia. Mice (n = 4 per group) were treated with 4X 20 mg/kg or 4X 40 mg/kg MEPH and striatum was analyzed for microglial activation by histochemical staining of sections with ILB4. Microglia counts were obtained as described in Materials and Methods and are presented as means ± SEM within each panel. Treatment conditions and time of sacrifice after treatments are (A) control 2d, (B) 4X 20 mg/kg MEPH 2d, (C) 4X 40 mg/kg MEPH 2d, (D) 4X 5 mg/kg METH 2d, (E) control 7d, and (F) 4X 40 MEPH 7d. None of the MEPH treatment conditions were significantly different from control but the effect of METH on microglial activation was (p < 0.0001, one-way ANOVA followed by Tukey’s test). The scale bars represent 50 µm.
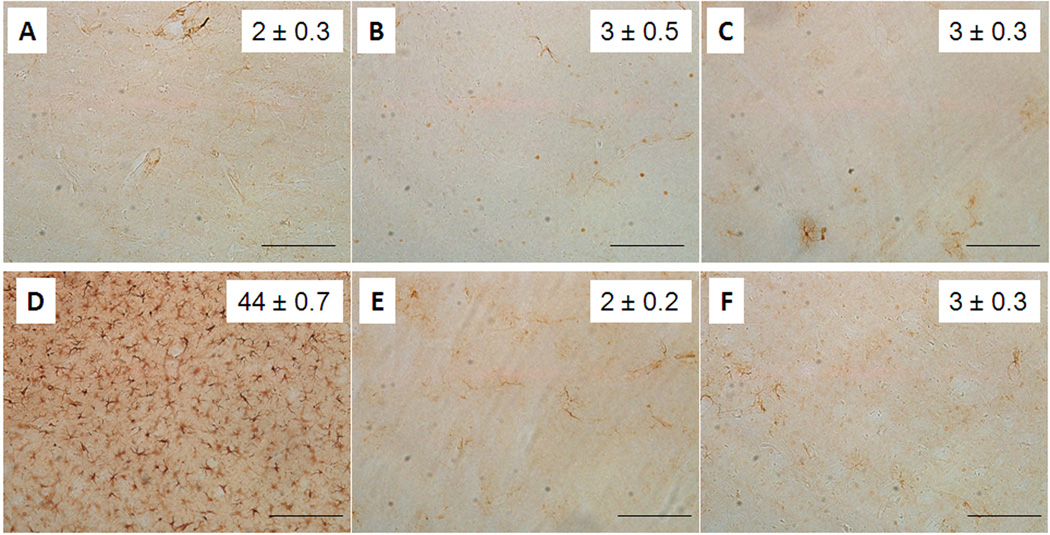
Fig. 4.
Effect of MEPH on striatal astrocyte expression of GFAP. Mice (n = 4 per group) were treated with 4X 20 mg/kg or 4X 40 mg/kg MEPH and striatum was analyzed for GFAP expression by immunohistochemistry. Counts of GFAP-positive astrocytes were obtained as described in the Materials and Methods and are presented as means ± SEM within each panel. Treatment conditions and time of sacrifice after treatments are (A) control 2d, (B) 4X 20 mg/kg MEPH 2d, (C) 4X 40 mg/kg MEPH 2d, (D) 4X 5 mg/kg METH 2d, (E) control 7d, and (F) 4X 40 MEPH 7d. None of the MEPH treatment conditions were significantly different from control. but the effect of METH was (p < 0.0001, one-way ANOVA followed by Tukey’s test). The scale bars represent 50 µm.
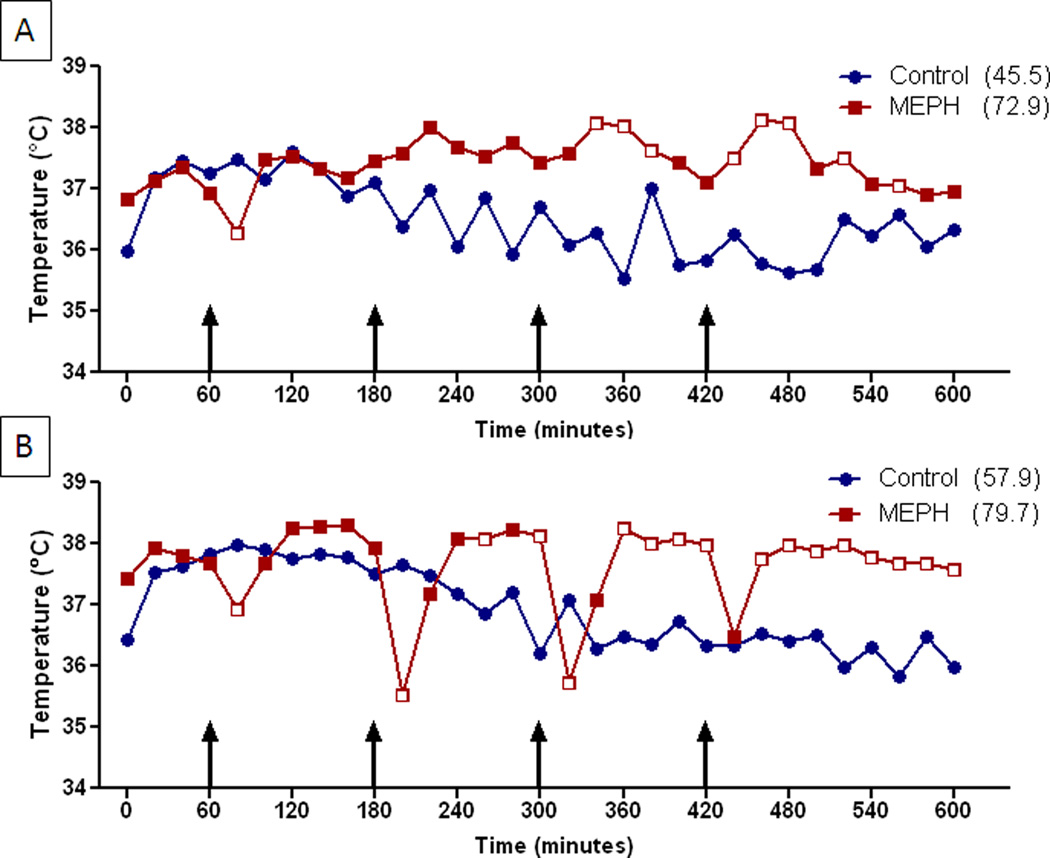
Fig. 5.
Effects of MEPH on core body temperature. Mice (n = 6 per group) were treated with 4X 20 mg/kg (A) or 4X 40 mg/kg (B) and core body temperatures were recorded by telemetry at 20 min intervals for 9 h after the first injection. Results are presented as group means. SEMs are omitted for the sake of clarity and were always < 10% of the respective mean values. Injections of MEPH are indicated by arrows. Areas under the curve (AUC) for MEPH treatment groups were calculated using GraphPad Prism 5 with respect to an arbitrary baseline set to 35°C for each group and are presented in parenthesis after each treatment condition. The main effects of 4X 20 mg/kg MEPH (A) and 4X 40 mg/kg MEPH (B) and their interaction were significantly different (p < 0.0001 for each; two-way repeated measures ANOVA). Significant differences between drug and controls at individual time points (p < 0.05, Bonferroni’s test) are indicated by open red symbols ( ) whereas those that do not differ are closed red symbols (
) whereas those that do not differ are closed red symbols ( ).
).
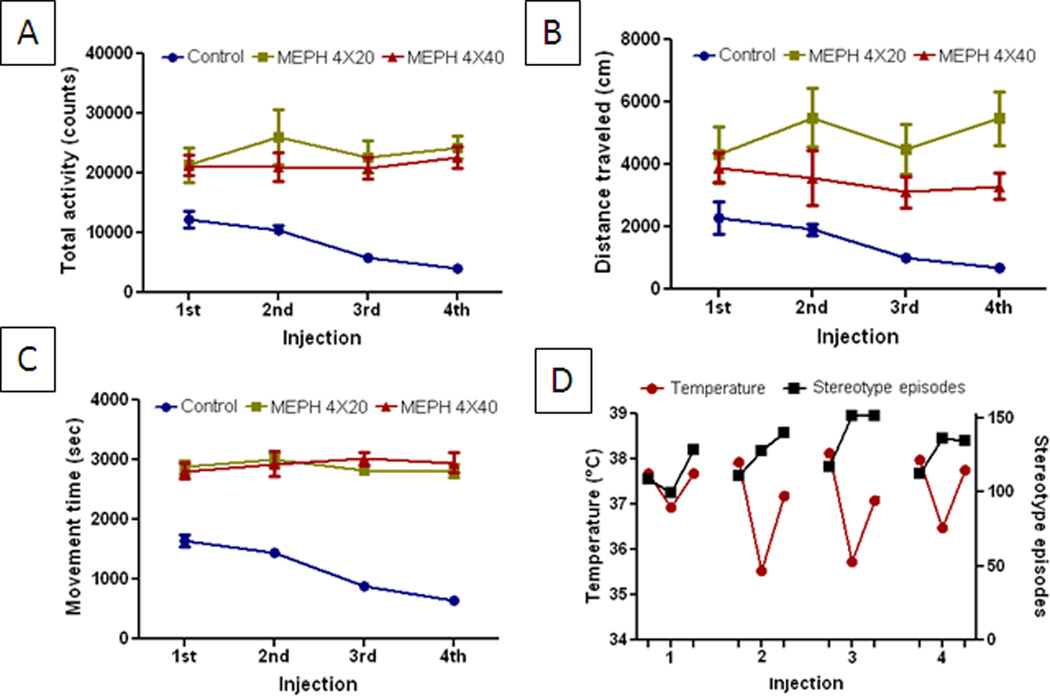
Fig. 6.
Effect of MEPH on locomotor activity. Mice (n= 6 per group) were treated with 4X 20 mg/kg or 4X 40 mg/mg MEPH and placed in locomotor activity monitors. Total activity counts (A), distance traveled (B), movement time (C) and stereotyped episodes (D) were recorded automatically for 60 min after each of the 4 MEPH injections. Data are presented as means ± SEM. The main effect of drug treatment was significantly different from controls for total activity, distance traveled and movement time (p < 0.0001 for all, two-way ANOVA). The main effect of drug dose was not significant for total activity, movement time and stereotyped episodes but was for distance traveled (p < 0.0001, two-way ANOVA followed by Bonferroni’s test).
Results
Effects of MEPH on striatal DA nerve endings
Mice were treated with a binge-like regimen of MEPH and the effects on DA, TH and DAT were determined to assess damage to striatal DA nerve endings in the same manner used for the study of METH neurotoxicity. Fig. 1A shows that MEPH at doses (4X for each) of 20 or 40 mg/kg did not change striatal DA content 2d after treatment. It can also be seen in Fig. 1B that neither dose of MEPH changed the levels of TH. However, the high dose of MEPH caused a slight but significant increase in DAT by comparison to the low dose while the low dose of MEPH was not different from control DAT levels (Fig. 1C). Because MEPH can cause damage to 5-HT nerve endings in rats when tested 7d after treatment (Hadlock et al. 2011), we extended the analysis of DA nerve ending markers to this time point in mice at the higher MEPH dose of 40 mg/kg. The results in Fig. 2A show that MEPH actually caused a slight but significant increase in striatal DA content 7d after treatment and it did not change the levels of TH (Fig. 2B) or DAT (Fig. 2C) in agreement with results in Fig. 1.
Effects of MEPH on glial activation
The effects of MEPH on microglia are presented in Fig. 3. It can be seen that MEPH at doses of 20 (Fig. 3B) or 40 mg/kg (Fig. 3C) did not result in microglial activation 2d after treatment. Very few ILB4-positive microglia were observed in striatum from either control (Fig. 3A and 3E) or drug-treated mice. By comparison, METH (4X 5 mg/kg) caused a robust and significant microglial activation in striatum as shown in Figure 3D. Microglial activation was also not observed if the time of sacrifice of MEPH-treated mice was extended from 2d to 7d (Fig. 3F). Striatum was also analyzed for astrocytic activation via measures of GFAP and the results are shown in Fig. 4. It is evident that GFAP stained astrocytes were not changed from control (Fig. 4A and 4E) after either dose of MEPH (Fig. 4B and 4C) 2d after treatment but a large and significant increase was seen in METH-treated mice as expected (Fig. 4D). Changes in GFAP were likewise not seen if the time of analysis was extended to 7d after the last MEPH injection (Fig. 4F).
Effects of MEPH on core body temperature
Core temperatures of MEPH-treated mice were monitored by telemetry throughout the period of drug treatment and the results are presented in Fig. 5. It can be seen that 20 mg/kg MEPH caused an increase in body temperature that rose above control about 2.5 h after the first injection and remained elevated for the duration of the treatment period (Fig. 5A). The higher dose of 40 mg/kg MEPH caused a somewhat different body temperature response. It can be seen in Fig. 5B that each injection of MEPH caused an immediate drop in body temperature and the magnitude of this drop increased with successive injections. These transient reductions in body temperature reached 2–2.5°C after the second and third injections and diminished to a drop of 1.5°C after the fourth injection. These drops in body temperature returned to and then exceeded controls within 40 min and remained elevated until the next injection. By normalizing changes in body temperature to controls, it became clear that MEPH-treated mice were hyperthermic for ~70% of time after treatment (420 min), hypothermic for 20% of the time (120 min) and normothermic for 10% of time (60 min). The effect of MEPH on core body temperature was significant by comparison to controls for both doses.
Effects of MEPH on locomotor activity
MEPH caused locomotor activation as shown in Fig. 6. Doses of 20 or 40 mg/kg caused significant increases in total locomotor counts (Fig. 6A), distance traveled (Fig. 6B), movement time (Fig. 6C) and stereotyped episodes (Fig. 6D), effects that persisted throughout the entire treatment period. By comparison, controls showed habituation in all measures of locomotor activity over time. The main effect of drug treatment was significantly different from controls for all measures of activity. The main effect of drug dose was not significant for total activity, movement time or stereotyped episodes but was for distance traveled. Whereas mice treated with METH show more constant activity, MEPH treated mice show cyclic bouts of explosive and stereotyped activity followed by short periods of inactivity (defined operationally as stereotyped episode) as shown in Fig. 6D.
Discussion
MEPH abuse is increasing at an alarming rate (Winstock et al. 2011, Brunt et al. 2010) justifying the acute need for additional research into its mechanisms of action. MEPH has numerous elements in common with substituted amphetamines and cathinone derivatives to include the following: 1) MEPH is a β-ketoamphetamine with a structure very similar to methcathinone and, by extension, to METH (Kelly 2011, Gibbons and Zloh 2010, Schifano et al. 2011, Maurer 2010); 2) MEPH can increase the synaptic levels of DA by simultaneously stimulating release (Kehr et al. 2011) and blocking reuptake (Kehr et al. 2011, Hadlock et al. 2011, Martinez-Clemente et al. 2011) probably by direct binding to the DAT (Martinez-Clemente et al. 2011); and 3) it is a psychostimulant that increases motility (Motbey et al. 2011) and it sustains self-administration by rats to an extent that exceeds METH (Hadlock et al. 2011). In light of these similarities and considering in addition that the substituted amphetamines and cathinone derivatives cause damage to DA nerve endings of the striatum, we predicted that MEPH would share this property as well.
Persistent reductions in DA nerve ending-specific markers such as DA itself, TH and DAT are well accepted indicators of damage caused by drugs such as METH and MPTP. Testing these same markers presently to assess the neurotoxic potential of MEPH revealed a surprising lack of effect of this drug on the DA nerve ending. Administration of high doses of MEPH consistent with those ingested by human abusers of this drug (Schifano et al. 2011), in a binge-like regimen used to study both METH and MPTP neurotoxicity, failed to uncover evidence of even mild toxicity. These markers were assessed 2d after the last injection of MEPH, a time at which the neurotoxic effects of METH have reached their maximum in mice (Thomas et al. 2004b, Thomas et al. 2008b, Thomas et al. 2009). To ensure that a toxic response in the striatum to MEPH was not delayed, we tested mice 7d after the highest dose of MEPH used presently (i.e., 40 mg/kg) and again did not observe any evidence of striatal DA nerve ending damage.
Regional markers of damage to the striatum, such as activation of microglia and astrocytes, were also assessed after treatment of mice with MEPH. In agreement with results obtained for DA, TH and DAT, results for MEPH indicated that microglia and astrocytes were not activated 2d or 7d after drug treatment. Glial activation is pronounced 2d after treatment of animals with METH (Thomas et al. 2004b, LaVoie et al. 2004) but the present results were clear in that striatal microglia were not activated and GFAP expression was not changed by MEPH, even after treatment with very high doses. Increased GFAP expression is a robust and reliable indicator of METH-induced damage to the striatum (O'Callaghan and Miller 1993, O'Callaghan and Miller 1994). These observations point to the conclusion that MEPH, despite its numerous similarities to the neurotoxic amphetamines and cathinone derivatives, does not cause damage to DA nerve endings of the striatum.
The failure to see neurotoxicity after MEPH is all the more surprising in light of the fact that it caused a significant hyperthermia in mice, as is seen after treatment of animals with METH (Bowyer et al. 1994, Albers and Sonsalla 1995, Cadet et al. 2007, Johnson-Davis et al. 2003, Miller and O'Callaghan 1994, Miller and O'Callaghan 1995). Each injection of MEPH at the lower dose of 20 mg/kg led to a gradual increase in body temperature which reached maximum about 2.5 h after the initial injection and remained elevated through the rest of the recording session. The response to the higher dose of MEPH was interesting in that after the second injection, body temperatures fell dramatically (2–2.5°C) and then quickly reversed to levels that were hyperthermic. This cycle of hypothermia-hyperthermia was repeated after the third and fourth injections of MEPH. In total, body temperatures were elevated above control for much longer than they were reduced (420 min vs 120 min, respectfully) after the binge treatment regimen of MEPH. Finally, we observed that MEPH caused a significant locomotor hyperactivity at both 20 and 40 mg/kg. This increase in activity was seen in all facets of hypermotility to include number of beam breaks in the activity meter, distance traveled and time spent moving. While we did not compare MEPH to METH presently, and despite the fact that both drugs are psychostimulants, the effect of MEPH was qualitatively different from METH. MEPH-treated mice showed bursts of running around the cage perimeter with little traversing of the cage interior space. The running bouts were interspersed with periods of relative inactivity. We also noted when giving the 4 injections of either dose of the binge-like regimen, mice became harder to handle with each injection and the handling associated with the injections seemed to provoke the running bursts. These results agree well with the observations of Motbey and colleagues (Motbey et al. 2011) showing that MEPH causes locomotor activation in rats.
While our manuscript was in preparation, it was reported that MEPH causes long-term deficiencies in 5-HT neurochemical function of rats (Hadlock et al. 2011) suggesting the possibility that MEPH is like MDMA in specifically targeting the 5-HT neuronal system for damage. This is an interesting possibility that requires more investigation because MDMA can also cause damage to DA nerve endings of the striatum (Yamamoto and Bankson 2005, Steele et al. 1994, Green et al. 2003, Cadet et al. 2007), it causes hyperthermia (Vorhees et al. 2010, Sprague et al. 2003, O'Shea et al. 2002) and it also increases glial activation (Thomas et al. 2004a, Torres et al. 2010, Orio et al. 2009, O'Callaghan and Miller 1994, Pu et al. 1996). It is known that rats are more sensitive than mice to 5-HT nerve ending damage after treatment with METH or MDMA but it is clear that mice, like humans, readily show DA neuronal deficits after intoxication with the substituted amphetamines or cathinone derivatives (Fleckenstein et al. 2007, Yamamoto et al. 2010, Cadet et al. 2007, Fleckenstein et al. 2000). Thus, MEPH may be even more selective than MDMA in that its neurotoxicity is directed solely at the 5-HT neuronal system.
In summary, the results of the present experiments were contrary to our expectation that MEPH would cause neurotoxicity to DA nerve endings of the striatum. This expectation was prompted by the fact that MEPH exerts the same effects that are thought to be essential for METH-induced damage to the DA neuronal system to include increased release of DA, inhibition of reuptake and increases in locomotor activity and core body temperature. Several factors could explain why MEPH is not neurotoxic to the DA neuronal system. First, it may be possible that it’s DAT blocking properties make its neurochemical actions more like those of nomifensine or cocaine. These drugs increase the synaptic levels of DA but they do not cause neurotoxicity. Second, MEPH may not cause reactive oxygen stress (Yamamoto and Bankson 2005, Yamamoto et al. 1998, Krasnova and Cadet 2009, Cadet and Krasnova 2009, Gibb et al. 1990, Fleckenstein et al. 2007) and mitochondrial dysfunction (Yamamoto and Bankson 2005, Brown et al. 2005, Burrows et al. 2000), two factors that are important components of the METH neurotoxic cascade. Third, MEPH may not induce excitotoxicity in striatum via increased efflux of glutamate as is seen after METH treatment (Yamamoto et al. 2010, Tata and Yamamoto 2008, Mark et al. 2007, Stephans and Yamamoto 1995, Yamamoto et al. 1998). Fourth, the hypothermic periods seen after MEPH may act to diminish the contribution of hyperthermia to neurotoxicity. If indeed MEPH does not cause oxidative stress, microglial activation, mitochondrial dysfunction or excitotoxicity, it may well be the case that it would actually protect against METH toxicity as do other DAT blockers (Pu et al. 1994, Schmidt and Gibb 1985, Marek et al. 1990). These possibilities are currently under examination and will not only yield important information on the actions of MEPH but they may also offer clues into the neurotoxic mechanisms associated with METH.
Acknowledgements
This work was supported by grants from NIH/NIDA and the Department of Veterans Affairs. We thank Dr. Roxanne Vaughan for the generous gift of DAT monoclonal antibody.
Abbreviations used
- 5-HT
serotonin
- AUC
area under the curve
- DA
dopamine
- DAT
dopamine transporter
- GFAP
glial fibrillary acidic protein
- HRP
horseradish peroxidase
- ILB4
Isolectin B4
- MDMA
3,4-methylenedioxymethamphetamine
- MEPH
mephedrone
- METH
methamphetamine
- PBS
phosphate-buffered saline
- SERT
serotonin transporter
- TH
tyrosine hydroxylase
Footnotes
The authors declare that they have no conflict of interest related to the publication of this article.
References
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J. Pharmacol. Exp. Ther. 1995;275:1104–1114. [PubMed] [Google Scholar]
- Banjaw MY, Schmidt WJ. Behavioural sensitisation following repeated intermittent oral administration of Catha edulis in rats. Behav. Brain Res. 2005;156:181–189. doi: 10.1016/j.bbr.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Banjaw MY, Schmidt WJ. Catha edulis extract and its active principle cathinone induce ipsilateral rotation in unilaterally lesioned rats. Behav. Pharmacol. 2006;17:615–620. doi: 10.1097/01.fbp.0000236273.10418.2b. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J. Pharmacol. Exp. Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Brown JM, Quinton MS, Yamamoto BK. Methamphetamine-induced inhibition of mitochondrial complex II: roles of glutamate and peroxynitrite. J. Neurochem. 2005;95:429–436. doi: 10.1111/j.1471-4159.2005.03379.x. [DOI] [PubMed] [Google Scholar]
- Brunt TM, Poortman A, Niesink RJ, van den Brink W. Instability of the ecstasy market and a new kid on the block: mephedrone. J. Psychopharmacol. (Oxf) 2010 doi: 10.1177/0269881110378370. epub ahead of print Sep 10, 2010. [DOI] [PubMed] [Google Scholar]
- Burrows KB, Gudelsky G, Yamamoto BK. Rapid and transient inhibition of mitochondrial function following methamphetamine or 3,4-methylenedioxymethamphetamine administration. Eur. J. Pharmacol. 2000;398:11–18. doi: 10.1016/s0014-2999(00)00264-8. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. Int. Rev. Neurobiol. 2009;88:101–119. doi: 10.1016/S0074-7742(09)88005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Foley KF. Methcathinone is a substrate for the serotonin uptake transporter. Pharmacol. Toxicol. 2003;93:219–225. doi: 10.1046/j.1600-0773.2003.pto930504.x. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Sievert MK, Shulgin AT, Jacob P, 3rd, Ruoho AE. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur. J. Pharmacol. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Kelly JP. A review of the neuropharmacological properties of khat. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1147–1166. doi: 10.1016/j.pnpbp.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Gibb JW, Hanson GR. Differential effects of stimulants on monoaminergic transporters: pharmacological consequences and implications for neurotoxicity. Eur. J. Pharmacol. 2000;406:1–13. doi: 10.1016/s0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Gibb JW, Johnson M, Hanson GR. Neurochemical basis of neurotoxicity. Neurotoxicology. 1990;11:317–321. [PubMed] [Google Scholar]
- Gibbons S, Zloh M. An analysis of the 'legal high' mephedrone. Bioorg. Med. Chem. Lett. 2010;20:4135–4139. doi: 10.1016/j.bmcl.2010.05.065. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy") Pharmacol. Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Gygi MP, Fleckenstein AE, Gibb JW, Hanson GR. Role of endogenous dopamine in the neurochemical deficits induced by methcathinone. J. Pharmacol. Exp. Ther. 1997;283:1350–1355. [PubMed] [Google Scholar]
- Gygi MP, Gibb JW, Hanson GR. Methcathinone: an initial study of its effects on monoaminergic systems. J. Pharmacol. Exp. Ther. 1996;276:1066–1072. [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, et al. 4-Methylmethcathinone(mephedrone): neuropharmacological effects of a designer stimulant of abuse. J. Pharmacol. Exp. Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Davis KL, Fleckenstein AE, Wilkins DG. The role of hyperthermia and metabolism as mechanisms of tolerance to methamphetamine neurotoxicity. Eur. J. Pharmacol. 2003;482:151–154. doi: 10.1016/j.ejphar.2003.09.063. [DOI] [PubMed] [Google Scholar]
- Kalix P. Effect of the alkaloid (-)-cathinone on the release of radioactivity from rat striatal tissue prelabelled with 3H-serotonin. Neuropsychobiology. 1984;12:127–129. doi: 10.1159/000118124. [DOI] [PubMed] [Google Scholar]
- Kalix P, Glennon RA. Further evidence for an amphetamine-like mechanism of action of the alkaloid cathinone. Biochem. Pharmacol. 1986;35:3015–3019. doi: 10.1016/0006-2952(86)90380-1. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared to MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and serotonin levels in nucleus accumbens of awake rats. Br. J. Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JP. Cathinone derivatives: A review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3:439–453. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Billingsley ML. Tyrosine hydroxylase: Purification from PC-12 cells, characterization, and production of antibodies. Neurochem. Int. 1987;11:463–475. doi: 10.1016/0197-0186(87)90036-2. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine disposition in the presynaptic process regulates the severity of methamphetamine-induced neurotoxicity. Ann. N.Y. Acad. Sci. 2008;1139:118–126. doi: 10.1196/annals.1432.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp. Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Vosmer G, Seiden LS. Dopamine uptake inhibitors block long-term neurotoxic effects of methamphetamine upon dopaminergic neurons. Brain Res. 1990;513:274–279. doi: 10.1016/0006-8993(90)90467-p. [DOI] [PubMed] [Google Scholar]
- Mark KA, Quinton MS, Russek SJ, Yamamoto BK. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J. Neurosci. 2007;27:6823–6831. doi: 10.1523/JNEUROSCI.0013-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur. Neuropsychopharmacol. 2011 doi: 10.1016/j.euroneuro.2011.07.009. epub ahead of print Aug 6, 2011. [DOI] [PubMed] [Google Scholar]
- Maurer HH. Chemistry, pharmacology, and metabolism of emerging drugs of abuse. Ther. Drug Monit. 2010;32:544–549. doi: 10.1097/FTD.0b013e3181eea318. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J. Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J. Med. Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Cao J, Kang J, Ying X, Ji J, Reynolds W, Rampe D. Mephedrone, a new designer drug of abuse, produces acute hemodynamic effects in the rat. Toxicol. Lett. 2012;208:62–68. doi: 10.1016/j.toxlet.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Metzger RR, Hanson GR, Gibb JW, Fleckenstein AE. 3-4-Methylenedioxymethamphetamine-induced acute changes in dopamine transporter function. Eur. J. Pharmacol. 1998;349:205–210. doi: 10.1016/s0014-2999(98)00196-4. [DOI] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J. Pharmacol. Exp. Ther. 1994;270:752–760. [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol. Neurobiol. 1995;11:177–192. doi: 10.1007/BF02740694. [DOI] [PubMed] [Google Scholar]
- Morris K. UK places generic ban on mephedrone drug family. Lancet. 2010;375:1333–1334. doi: 10.1016/s0140-6736(10)60559-4. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, 'meow'): acute behavioural effects and distribution of Fos expression in adolescent rats. Addiction Biol. 2011 doi: 10.1111/j.1369-1600.2011.00384.x. epub ahead of print Oct 13, 2011. [DOI] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur. J. Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Nencini P, Amiconi G, Befani O, Abdullahi MA, Anania MC. Possible involvement of amine oxidase inhibition in the sympathetic activation induced by khat (Catha edulis) chewing in humans. J. Ethnopharmacol. 1984;11:79–86. doi: 10.1016/0378-8741(84)90097-7. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. Quantification of reactive gliosis as an approach to neurotoxicity assessment. NIDA Res. Monogr. 1993;136:188–212. [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J. Pharmacol. Exp. Ther. 1994;270:741–751. [PubMed] [Google Scholar]
- O'Shea E, Easton N, Fry JR, Green AR, Marsden CA. Protection against 3,4-methylenedioxymethamphetamine-induced neurodegeneration produced by glutathione depletion in rats is mediated by attenuation of hyperthermia. J. Neurochem. 2002;81:686–695. doi: 10.1046/j.1471-4159.2002.00844.x. [DOI] [PubMed] [Google Scholar]
- Orio L, Llopis N, Torres E, Izco M, O'Shea E, Colado MI. A Study on the Mechanisms by Which Minocycline Protects Against MDMA ('Ecstasy')-Induced Neurotoxicity of 5-HT Cortical Neurons. Neurotox Res. 2009;18:187–199. doi: 10.1007/s12640-009-9120-3. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Schechter MD, Yamamoto BK. Effects of cathinone and amphetamine on the neurochemistry of dopamine in vivo. Neuropharmacology. 1990;29:1171–1176. doi: 10.1016/0028-3908(90)90041-o. [DOI] [PubMed] [Google Scholar]
- Pu C, Broening HW, Vorhees CV. Effect of methamphetamine on glutamate-positive neurons in the adult and developing rat somatosensory cortex. Synapse. 1996;23:328–334. doi: 10.1002/(SICI)1098-2396(199608)23:4<328::AID-SYN11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Pu C, Fisher JE, Cappon GD, Vorhees CV. The effects of amfonelic acid, a dopamine uptake inhibitor, on methamphetamine-induced dopaminergic terminal degeneration and astrocytic response in rat striatum. Brain Res. 1994;649:217–224. doi: 10.1016/0006-8993(94)91067-7. [DOI] [PubMed] [Google Scholar]
- Rockhold RW, Carlton FB, Jr, Corkern R, Derouen L, Bennett JG, Hume AS. Methcathinone intoxication in the rat: abrogation by dextrorphan. Ann. Emerg. Med. 1997;29:383–391. doi: 10.1016/s0196-0644(97)70351-2. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J. Pharmacol. Exp. Ther. 2003;307:138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- Schifano F, Albanese A, Fergus S, et al. Mephedrone (4-methylmethcathinone; 'meow meow'): chemical, pharmacological and clinical issues. Psychopharmacology (Berl) 2011;214:593–602. doi: 10.1007/s00213-010-2070-x. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Gibb JW. Role of the dopamine uptake carrier in the neurochemical response to methamphetamine: effects of amfonelic acid. Eur. J. Pharmacol. 1985;109:73–80. doi: 10.1016/0014-2999(85)90541-2. [DOI] [PubMed] [Google Scholar]
- Sparago M, Wlos J, Yuan J, Hatzidimitriou G, Tolliver J, Dal Cason TA, Katz J, Ricaurte G. Neurotoxic and pharmacologic studies on enantiomers of the N-methylated analog of cathinone (methcathinone): a new drug of abuse. J. Pharmacol. Exp. Ther. 1996;279:1043–1052. [PubMed] [Google Scholar]
- Sprague JE, Banks ML, Cook VJ, Mills EM. Hypothalamic-pituitary-thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy) J. Pharmacol. Exp. Ther. 2003;305:159–166. doi: 10.1124/jpet.102.044982. [DOI] [PubMed] [Google Scholar]
- Steele TD, McCann UD, Ricaurte GA. 3,4-Methylenedioxymethamphetamine (MDMA, "Ecstasy"): pharmacology and toxicology in animals and humans. Addiction. 1994;89:539–551. doi: 10.1111/j.1360-0443.1994.tb03330.x. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BY. Effect of repeated methamphetamine administrations on dopamine and glutamate efflux in rat prefrontal cortex. Brain Res. 1995;700:99–106. doi: 10.1016/0006-8993(95)00938-m. [DOI] [PubMed] [Google Scholar]
- Streit WJ. An improved staining method for rat microglial cells using the lectin from Griffonia simplicifolia (GSA I-B4) J. Histochem. Cytochem. 1990;38:1683–1686. doi: 10.1177/38.11.2212623. [DOI] [PubMed] [Google Scholar]
- Tata DA, Yamamoto BK. Chronic stress enhances methamphetamine-induced extracellular glutamate and excitotoxicity in the rat striatum. Synapse. 2008;62:325–336. doi: 10.1002/syn.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Angoa Perez M, Francescutti-Verbeem DM, Shah MM, Kuhn DM. The role of endogenous serotonin in methamphetamine-induced neurotoxicity to dopamine nerve endings of the striatum. J. Neurochem. 2010;115:595–605. doi: 10.1111/j.1471-4159.2010.06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci. Lett. 2004a;367:349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Methamphetamine-induced neurotoxicity and microglial activation are not mediated by fractalkine receptor signaling. J. Neurochem. 2008a;106:696–705. doi: 10.1111/j.1471-4159.2008.05421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J. Neurochem. 2008b;105:605–616. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Increases in cytoplasmic dopamine compromise the normal resistance of the nucleus accumbens to methamphetamine neurotoxicity. J. Neurochem. 2009;109:1745–1755. doi: 10.1111/j.1471-4159.2009.06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. Cyclooxygenase-2 is an obligatory factor in methamphetamine-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2005;313:870–876. doi: 10.1124/jpet.104.080242. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J. Pharmacol. Exp. Ther. 2004b;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Torres E, Gutierrez-Lopez MD, Borcel E, Peraile I, Mayado A, O'Shea E, Colado MI. Evidence that MDMA ('ecstasy') increases cannabinoid CB2 receptor expression in microglial cells: role in the neuroinflammatory response in brain. J. Neurochem. 2010;113:67–78. doi: 10.1111/j.1471-4159.2010.06578.x. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, He E, Skelton MR, Graham DL, Schaefer TL, Grace CE, Braun AA, Amos-Kroohs R, Williams MT. Comparison of (+)-methamphetamine, +/--methylenedioxymethamphetamine (MDMA), (+)-amphetamine and +/--fenfluramine in rats on egocentric learning in the Cincinnati water maze. Synapse. 2010;65:368–378. doi: 10.1002/syn.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Preston K, Ricaurte GA, Schuster CR, Seiden LS. Neurochemical similarities between d,l-cathinone and d-amphetamine. Drug Alcohol Depend. 1982;9:279–284. doi: 10.1016/0376-8716(82)90067-9. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Crit. Rev. Neurobiol. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Gudelsky GA, Stephans SE. Amphetamine neurotoxicity: Roles for dopamine, glutatamate, and oxidative stress. In: Qureshi GA, editor. Progress in HPLC-HPCE. Vol. 7. Bombay: VSP; 1998. pp. 223–244. [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann. N.Y. Acad. Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]