Abstract
Chronic intake of high fat diet is known to alter brain neurotransmitter systems that participate in the central regulation of food intake. Dopamine (DA) system changes in response to high fat diet have been observed in the hypothalamus, important in the homeostatic control of food intake, as well as within the central reward circuitry (ventral tegmental area (VTA), nucleus accumbens (NAc) and prefrontal cortex (PFC)), critical for coding the rewarding properties of palatable food and important in hedonically-driven feeding behavior. Using a mouse model of diet-induced obesity (DIO), significant alterations in expression in dopamine-related genes were documented in adult animals, and the general pattern of gene expression changes was opposite within the hypothalamus versus the reward circuitry (increased versus decreased, respectively). Differential DNA methylation was identified within the promoter regions of tyrosine hydroxylase (TH) and dopamine transporter (DAT) and the pattern of this response was consistent with the pattern of gene expression. Behaviors consistent with increased hypothalamic DA and decreased reward circuitry DA were observed. These data identify differential DNA methylation as an epigenetic mechanism linking chronic intake of high fat diet with altered dopamine-related gene expression, and this response varies by brain region and DNA sequence.
Keywords: reward, hypothalamus, DNA methylation, dopamine transporter, tyrosine hydroxylase, food intake
Introduction
Increased intake of palatable, energy dense foods is a critical variable that drives weight gain and increases the risk for obesity. Central nervous system (CNS) circuitry that governs food intake includes both hypothalamic structures, as well as the central reward circuitry (ventral tegmental area (VTA), nucleus accumbens (NAc) and prefrontal cortex (PFC), and these different brain regions participate in homeostatic and hedonically driven feeding, respectively(Berthoud & Morrison 2008, Lutter & Nestler 2009, Vucetic & Reyes 2010). Chronic consumption of high fat or high sugar diets and the resultant obesity are known to change gene expression and function within both hypothalamic (Briggs et al. 2010) and reward-related circuitry (Johnson & Kenny 2010). Food intake patterns can become dysregulated in obesity, leading to an overall increase in food intake. Additionally, there is support in the literature for the idea that obese individuals (Stice et al. 2008) and obese animals (Stice et al. 2008, Cottone et al. 2009, Johnson & Kenny 2010, Davis et al. 2008) demonstrate decreased responding within the central reward system, which may further contribute to increased consumption of palatable foods, as animals/people seek to mitigate the negative state of diminished reward system function (Johnson & Kenny 2010, Koob & le Moal 2008) by consuming foods that are rewarding.
Dopamine plays a critical role in the regulation of food intake. DA action in the hypothalamus plays a role in promoting food intake (Meguid et al. 2000), and DA activity in the reward circuitry is associated with the rewarding aspects of food (Volkow et al. 2010) (e.g., fat (Rada et al. 2010) or sucrose (Rada et al. 2005) ingestion result in dopamine release in NAc). Importantly, dopamine dysfunction has been identified in both obese individuals and animals. In the hypothalamus, chronic intake of high fat diet leads to altered dopamine expression and function within the hypothalamus (Lee et al. 2010a, Li et al. 2009, Huang et al. 2005). Further, it has been shown that this altered DA function is a direct response to the increased fat consumption per se, as opposed to the resultant obesity (Li et al. 2009). Additionally, dopamine dysfunction has been noted within the mesocorticolimbic circuitry. Decreased dopamine D2 receptor expression and function in obese rats (Wang et al. 2001, Johnson & Kenny 2010, Stice et al. 2008), and obese humans (Wang et al. 2001, Johnson & Kenny 2010, Stice et al. 2008), decreased extracellular dopamine in the striatum in DIO rats (Geiger et al. 2009) and decreased D1 receptor expression in the NAc of obesity prone rats on a high fat diet (Alsiö et al. 2010) all support the idea of decreased dopamine activity in the reward circuitry in obesity.
Little to nothing is known about the mechanisms that drive gene expression changes in the CNS in response to diet and/or obesity. Epigenetic gene regulation, including DNA methylation, and histone modifications, represent a pathway through which organisms can rapidly adapt to environmental challenges. The dopaminergic system has been shown to be vulnerable to prenatal and early postnatal alterations in maternal diet (Teegarden et al. 2009, Marichich et al. 1979, Palmer et al. 2008, Chen et al. 1997) and we have demonstrated the DNA methylation plays an important role in driving some of these changes (Vucetic et al. 2010a, Vucetic et al. 2010b). The prenatal period is known to be a critical period in brain development, but whether epigenetic modifications during the postnatal period may play a role in driving changes in dopaminergic gene expression has not been addressed and is one focus of the present studies.
MATERIALS AND METHODS
Animals and experimental model
C57BL/6J females were bred to DBA/2J males (The Jackson Laboratory, Bar Harbor, ME). At weaning, half the pups were placed on a high fat diet (Test Diet, Richmond, IN #58G9; 18.5% protein, 60% fat and 20.5% carbohydrate), and half continued on the control diet (#5755; 18.5% protein, 12% fat and 69.5% carbohydrate). Body weights were recorded weekly, and male mice (n=5-9/group) were used in all experiments. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania.
Genomic DNA and Total RNA isolation from brain
Animals were euthanized with an overdose of carbon dioxide, followed by cervical dislocation, a method consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. After the animals were killed, brains were rapidly removed and placed in RNAlater (Ambion, Austin, TX) for four hours before dissections. Brain dissections were performed as previously described (Vucetic et al. 2010a, Reyes et al. 2003, Cleck et al. 2008). Genomic DNA (gDNA) and total RNA were isolated simultaneously using AllPrep DNA/RNA Mini Kit (Qiagen).
Gene expression analysis by quantitative Real-Time PCR
For each individual sample, 500 ng of total RNA was used in reverse transcription using High Capacity Reverse Transcription Kit (ABI, Foster City, CA). Expression of target genes was determined by quantitative RT-PCR using gene-specific TaqMan probes with TaqMan Gene Expression Master Mix (ABI) on the ABI7900HT Real-Time PCR Cycler. Probes used for RT-PCR are listed in supplemental material. The relative amount of each transcript was determined using delta Ct values as previously described (Pfaffl 2001). Changes in gene expression were calculated against endogenous, unchanged GAPDH standard.
Methylated DNA Immunoprecipitation (MeDIP) Assay
MeDIP assay was performed as described (Weber et al. 2005). Methylated DNA was immunoprecipitated using 10 μg of mouse monoclonal 5-methylcytidine antibody (Eurogentec) or mouse pre-immune serum. Enrichment in the MeDIP fraction was determined by quantitative RT-PCR using ChIP-qPCR Assay Master Mix (SuperArray) on the ABI7900HT Real-Time PCR Cycler. For all genes examined, primers were obtained from Superarray (ChIP-qPCR Assays (−01) kb tile, SuperArray) for the amplification of genomic regions spanning the CpG sites located approximately 300-500 bp upstream of the transcription start sites (see Supplemental Material for primer sequences). MeDIP results were expressed as fold enrichment of immunoprecipitated DNA for each specific site. To calculate differential occupancy fold change (% enrichment), the MeDIP DNA fractions’ Ct values were normalized to the Input DNA fraction Ct value (see Supplemental Material). Finally, the normalized level of DNA methylation at a particular site was expressed as relative to control group set to 1.
Metabolic measurements
Animals (n=4/group) were tested in the Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments, Columbus, OH), which monitors food and water intake, indirect calorimetry, and x-axis activity. Animals had unrestricted access to powdered chow through a feeder located in the middle of the cage floor. At least one week prior to testing, animals were housed in the CLAMS overnight to acclimate to powdered food and a novel cage. While in the CLAMS cages, animals had ad libitum access to powdered diet and water. Food intake was normalized to body weight. For weeks 8-20, animals were placed in the cages one hour prior to lights out and were removed 20 hrs later. Data from the 12 hr dark period and 6 hr light period was analyzed and reported. Food intake response to HF diet. A separate cohort of animals (n=6-8/group) were tested at 14 months of age. For this experiment, animals were run in the CLAMS for 4 nights and data from the four 12 hr dark periods were averaged and analyzed. All mice in this experiment were tested twice in the cages, once with access to the control diet and once with access to the high fat diet. The order of presentation was counterbalanced.
Statistical analyses
Data, presented as means ± s.e.m., were analyzed using Prism 4 (GraphPad) and Excel tools for statistical analysis. Two-way ANOVA (group × time) was used to analyze the CLAMS data, while Student’s t-test was used to analyze differences in gene expression between DIO and control animals. A Bonferroni correction was applied for comparison across multiple brain regions (TH and DAT: α=.05/2 brain regions= .025; all other genes: α =.05/4 brain regions=.0125). Otherwise, a p-value of 0.05 or lower was considered significant.
Results
Mice had continuous access to control diet (control) or 60% high fat diet (diet-induced obese, DIO) from weaning (at 3 weeks of age) until the time of sacrifice at 20 weeks. Two-way ANOVA for body weight revealed a significant interaction (F(2,12)=19.4, p<.002) between diet and time and a main effect for diet, (F(1,12)=14.6, p<.009), with HF fed animals gaining significantly more weight over time, as would be predicted (Supplemental Figure 1).
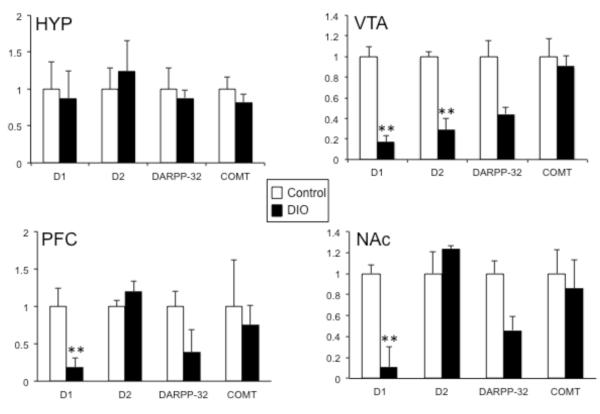
At 20 weeks of age, dopamine-related gene expression was examined within the hypothalamus and reward circuitry. We focused our initial analysis on two genes important to dopamine function that are expressed within both the hypothalamus and the VTA; tyrosine hydroxylase (TH, the rate limiting enzyme in DA synthesis) and dopamine transporter, (DAT, critical for clearing DA from the synapse). In the hypothalamus, both TH and DAT mRNA were significantly increased approximately 3 fold (t(12)=2.2, p=.024, t(12)=3.1, p=.005, respectively), while in the VTA, expression of TH and DAT mRNA was significantly repressed (t(15)=2.5, p=.011, t(13)=2.6, p=.011, respectively) (Fig 1). Dopamine dysfunction, particularly hypofunction, has been reported in both obese humans and in animal models of obesity, therefore, mRNA expression for additional dopamine-related genes was examined within hypothalamus and the reward circuitry (VTA, NAc and PFC). Levels of D1, D2, DARPP-32 and COMT did not differ in the hypothalamus, however within the reward circuitry, a general decrease in dopaminergic gene expression was observed (Fig 2). The D1 receptor was decreased in areas that receive significant DA innervation, NAc and PFC (t(10)=8.6, p<.0001, t(12)=4.1, p=.0007, respectively), while D2 levels remained unchanged in these regions. Both receptor subtypes (D1 and D2) were significantly downregulated in the VTA (t(5)=6.9, p=.0005, t(4)=5.9, p=.002, respectively). Further, DARPP-32, which signals downstream of both receptor subtypes, was decreased in all three areas of the reward circuitry, however the difference was only statistically reliable in the PFC (t(11)=3.7, p=.002, VTA and NAc demonstrated a nonsignificant trend (p=.04 and p=.02, respectively, n.s. after Bonferroni correction). COMT, which degrades DA, was not altered in any brain region examined.
Figure 1. Long-term exposure to high-fat (HF) diet alters TH and DAT mRNA expression.
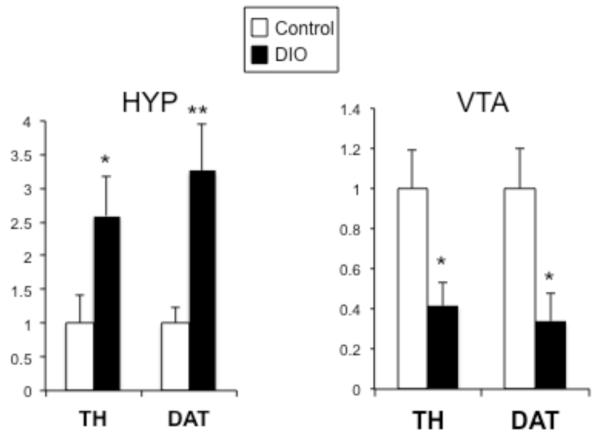
Gene expression was measured in the hypothalamus (HYP; left) and ventral tegmental area (VTA; right) of control (white bars) and diet-induced obese (DIO) mice (black bars) using quantitative real time PCR. TH and DAT mRNA were significantly increased in the HYP, while TH and DAT mRNA were significantly decreased in the VTA. *p<.025, **p<.005 (n=7-9/group)
Figure 2. Long-term exposure to high-fat (HF) diet affects dopamine-related gene expression in the reward circuitry.
Dopamine-related gene expression (D1, D2, DARPP-32, COMT) was measured in the hypothalamus (HYP) and regions of the central reward circuitry (VTA, NAc and PFC) of control and diet-induced obese (DIO) mice using quantitative real time PCR. These genes were unchanged in the HYP, but D1, D2 and DARPP-32 levels were reduced in the reward-related regions in DIO animals and the effect varied by region. COMT levels were not altered in any region studied. *p<.025, **p<.005 (n=7/group in HYP, PFC, NAc; n=4/group in VTA)
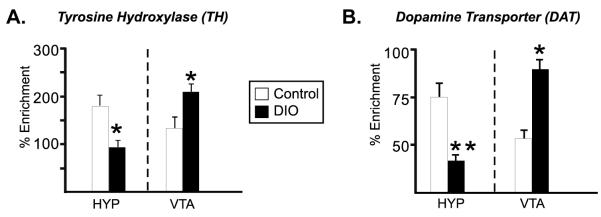
In an effort to determine whether chronic HF diet could alter DNA methylation patterns, DNA methylation was interrogated within the promoter region of specific target genes using the MeDIP assay. For both TH (Fig 3A, left) and DAT (Fig 3B, left) promoter methylation was significantly decreased in the hypothalamic samples (p<.05). Interestingly, the opposite pattern was observed in the VTA, such that there was a significant increase in DNA methylation within the promoter regions of both TH and DAT (Fig 3A, B, right, p<.05 and p<.01, respectively). Levels of methylation within the GAPDH promoter did not differ (data not shown). The direction of the changes in gene expression are consistent with what would be predicted based on the observed patterns of change in DNA methylation (e.g., increased methylation with transcriptional repression).
Figure 3. DNA methylation status of TH and DAT promoters in DIO mice.
Genomic DNA was isolated from dissected HYP and VTA of control (white bars) and obese (DIO) mice (black bars), sheared by sonication and immunoprecipitated with 5-methylcytosine antibody. The enrichment of DNA methylation relative to input genomic DNA in the promoter region of TH or DAT was quantified by qPCR. DIO mice displayed decreased methylation of TH and DAT within the HYP. The opposite effect was observed in the VTA, with both promoters showing significantly more methylation. GAPDH methylation was not altered in DIO mice (data not shown). Values are mean ± s.e.m. *p <0.05, **p< 0.01, n=6/group, Two-tailed t-test.
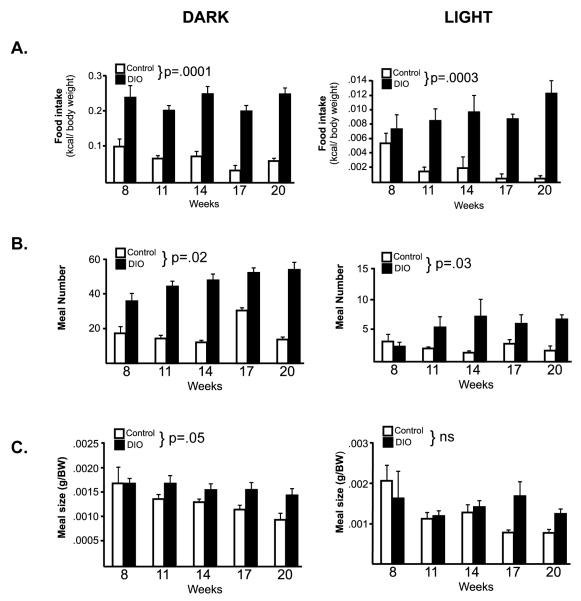
To evaluate, feeding and related metabolic and reward behaviors, the pattern of food intake was evaluated in control and DIO mice in metabolic chambers. Food intake, as well as locomotor activity and metabolic rate (oxygen consumption) were evaluated every 3 weeks from 8-20 weeks of age, while on their respective diets (controls on control diet, DIO animals on high fat diet, n=4/group). During the 12hr dark period, DIO animals consumed significantly more calories than control animals (main effect group; F(1,24)=85.8, p=.0001, Fig 4A). This pattern persisted into the light period as well, with DIO animals eating significantly more calories than controls (main effect group; F(1,24)=55.9, p=.0003). When food intake was measured solely by grams consumed rather than kcal consumed, the identical pattern was observed (data not shown). An analysis of meal patterns revealed that DIO animals ate significantly more meals in both the dark and the light (DARK: F(1, 24)= 10.1, p<.02; LIGHT: F(1,24)=7.5, p=.03, Figure 4B), and meal size was significantly increased during the dark period (F(1, 24)= 5.78, p<.05, Figure 4C). DIO animals tended to be less active during the dark, however, this difference was not statistically reliable (p=.08, Supplemental Figure 1), and activity patterns did not differ during the light. With regard to metabolic rate, there was a significant group × time interaction (F(4,24)= 3.8, p=.0156), such that the significant effect of change over time (F(4,24)=10.5, p<.0001) differed between the two groups, while the decrease in metabolic rate over time (F(4,24)=17.8, p<.0001) during the light period was not different between the groups (Supplemental Figure 1).
Figure 4. Food intake analysis of DIO mice.
(a) Food intake, (b) meal number, and (c) meal size were evaluated in control (white bars) and DIO mice (black bars) (n=4/group). DIO mice were hyperphagic, with a significant increase in food intake, meal number and meal size during the dark period (LEFT). Food intake and meal number were significantly elevated during the light period as well (RIGHT).
We have previously reported that these DIO animals have a reduced preference for sucrose, (Vucetic et al. 2011). To test whether alterations in response to rewarding stimuli would be observed in other contexts, a separate cohort of mice was tested at late middle age (14 months). In this experiment, 12 hr overnight food intake was evaluated in control and DIO animals two times, once fed the control diet and once fed the HF diet. Normal weight animals ate significantly more food when given access to the HF diet, increasing their intake 47% when given HF diet. In contrast, DIO animals did not increase their intake when fed the HF diet (t(12)=2.3, p<.05).
Discussion
In the present manuscript, we have demonstrated that chronic consumption of a high fat diet in mice is associated with significant changes in dopaminergic gene expression and that the pattern of this change differs depending on the brain region (hypothalamic versus reward-related circuitry). Importantly, differential DNA methylation within the promoter regions of TH and DAT differ in a pattern consistent with the observed gene expression changes. It was particularly striking that genes expressed in different brain regions (but with presumably identical promoter sequences) were differentially methylated. These data indicate that differential DNA methylation (hypo- or hyper-promoter methylation) in response to chronic HF diet is not a response that occurs broadly, affecting genes similarly across different brain regions. Rather, changes in DNA methylation within the promoter regions of specific genes must be secondary to some other aspect unique to the circuitry, for example, the pattern of neuronal activation in response to the HF diet or a hormonal/endocrine stimulus secondary to the HF diet consumption.
Importantly, we observed behavioral responses that are consistent with the gene expression changes. Increased dopamine within the hypothalamus in known to promote food intake. In the DIO mice, we observed increased food intake (both as kcal/bw and as grams/bw) and increased meal number, in both the dark and light periods, as well as increased meal size in the dark. It has been shown that increased food intake, particularly within the light period of nocturnal animals like mice, is a primary contributor to increased weight gain (Arble et al. 2009). We also observed a decreased preference for sucrose (reported previously (Vucetic et al. 2011)) and a lack of increase in food consumption when presented with the HF diet, a response that was observed in the normal weight controls. These behaviors are consistent with the gene expression findings that demonstrate an overall decrease in dopaminergic gene expression within the central reward circuitry. Collectively, these behaviors have the potential to promote obesity in two distinct ways; (1) through an increase in food intake and (2) by increasing the drive for palatable food, as the animal with a blunted response to palatable foods may seek and/or consume these food relatively more than a normal animal in order to reach the same rewarding response.
Dopamine dysregulation in obesity has been documented in other animal models. In a model similar to the present experiments, mice were fed a 35% HF diet from age 3-15 weeks, and gene expression in the hypothalamus was investigated (Lee et al. 2010a). Paralleling the present findings, these authors reported upregulation of TH and DAT, with no change in D1 or D2 receptor expression. Another group focused specifically on the ventromedial hypothalamus (as opposed to total hypothalamus) and found decreased TH expression rather than increased (Li et al. 2009), however in that study the HF diet administration started much later (at 12 weeks of age) and was maintained for a shorter length of time (8 weeks as opposed to 17+ weeks), highlighting two key variables that affect how high fat diet can affect dopamine function, the developmental timeperiod and length of administration of the high fat diet. Highlighting this point is the finding that 4 weeks of HF diet administration (relatively short exposure) to 12 week old animals (relatively older animals) was ineffective in altering gene expression levels of TH, DAT, D1 or D2 within hypothalamus (de Leeuw van Weenen et al. 2009).
Dopamine dysregulation within reward circuitry has been observed as well, and most findings report a decreased level of gene/protein expression and/or function of the dopamine system within the reward circuitry. D2 downregulation has been documented in response to chronic high fat diet and obesity (Johnson & Kenny 2010), coupled with a decrease in brain reward threshold that persisted for 14 days after the removal of the HF diet. In a recent notable publication, rats were fed a high fat/high sucrose diet for 5 weeks beginning at 8 weeks of age, and were classified as obesity prone or resistant depending on weight gain (Alsiö et al. 2010). Obesity prone rats showed a decrease in D1 receptor in the NAc (similar to our findings). This decrease persisted after 18 days of withdrawal from the palatable diet. The decrease in D1 expression was linked to the consumption of the palatable diet, rather than weight gain per se, because a group that was pair fed restricted amounts of the palatable diet (consumed the diet but did not gain weight) also showed a decrease in D1 expression. A convergent finding comes from rats fed a restricted amount of HF diet that showed reduced DA turnover in the NAc in response to HF diet consumption in the absence of significant weight gain (Davis et al. 2008). In mice, DAT binding in the NAc was decreased after 20 days on HF diet, and persisted for at least 7 days after removal of the high fat diet (South & Huang 2008). Examination of obesity prone and obesity resistant rats consuming a control diet and therefore not different in weight found that obesity prone rats showed decreased levels of dopamine in the NAc, suggesting that the reduction in dopamine did not require the animal to be obese (Rada et al. 2010). However, these rats had been fed a HF diet for 5 days to classify them as obesity prone or resistant, so the dopamine dysfunction may have been linked to a differential response to the HF diet. Additionally, basal and amphetamine-induced dopamine release in striatum are reduced in obese rats fed a chronic high fat diet (Geiger et al. 2009). Importantly, similar phenomenon have also been observed in humans, as obese women when compared to normal weight women show reduced activation in the caudate in response to consumption of a palatable solution (Stice et al. 2008).
An important consideration in all of these reports is the relative importance of the consumption of the HF diet versus the resultant obesity. In our study (and others (Johnson & Kenny 2010, Geiger et al. 2009)), it is not possible to distinguish which of these stimuli may drive the changes in dopamine related gene expression. However, in other studies described above ((Alsiö et al. 2010, Davis et al. 2008, Rada et al. 2010)) dopaminergic changes are observed in animals fed a HF diet in the absence of obesity, suggesting that consumption of the HF diet, rather than the obesity and associated physiological changes, is sufficient to alter dopamine system function.
Given that there is support that these changes in dopamine-related gene expression persist after the removal of the diet (detailed in previous paragraph (Alsiö et al. 2010, South & Huang 2008, Johnson & Kenny 2010)), we examined whether epigenetic modifications may contribute to changes in gene expression. DNA methylation in particular may represent an epigenetic modification that is relatively stable over time. We focused our detailed analyses on TH and DAT, given the opposite pattern of responses that was observed in the reward circuitry and the hypothalamus. As predicted, we found that DNA methylation levels in the promoter regions of TH and DAT, paralleled the gene expression changes (increased methylation related to decreased expression). These data identify differential DNA methylation as a mechanistic link between consumption of high fat diet and/or obesity and the resultant changes in dopamine-related gene expression. Differential DNA methylation in the brain in response to diverse early life challenges has been reported (Coupé et al. 2010, Murgatroyd et al. 2009, Vucetic et al. 2010a, Vucetic et al. 2010b, Weaver et al. 2004, Plagemann et al. 2009, Niculescu & Lupu 2009, Niculescu et al. 2006), however, similar findings in the postnatal brain are more limited. BDNF and Fkbp5 are differentially methylated during memory consolidation (Lubin et al. 2008) and in response to chronic corticosterone exposure (Lee et al. 2010b), respectively. In DIO mice, hypomethylation of melanocortin 4 receptor has been reported in whole brain (Widiker et al. 2010), while we have recently reported differential DNA metylation within the μ-opioid receptor promoter in reward circuitry regions. Given reports of the persistence of dopaminergic gene alteration in the absence of the high fat diet (Alsiö et al. 2010, South & Huang 2008, Johnson & Kenny 2010), the question of whether differential DNA methylation will persist with removal of the diet or normalization of body weight and whether DNA methylation is responsible for persistent changes in gene expression represents important next steps in this line of investigation.
The obesity epidemic affects the majority of Americans, and it is clear that a better understanding of how chronic intake of high fat diet affects the development and function of the CNS is critical. The present data have extended the literature supporting dopaminergic dysfunction in obesity by assessing a number of dopaminergic related genes across numerous brain regions and importantly identifying epigenetic mechanisms linking chronic high fat diet intake to these alterations. Behavioral modifications, including controlling overall food intake and choosing healthier food options, are a key to initiating and maintaining weight loss. Yet for the majority of obese patients, these behavioral modifications are at a minimum extremely difficult or worse yet, impossible to initiate and maintain. A better understanding of the CNS adaptations that occur during the development of obesity will prove critical in designing both better behavioral and pharmacological therapeutics for obesity.
Supplementary Material
Figure 5. DIO mice show decreased intake of a rewarding food.
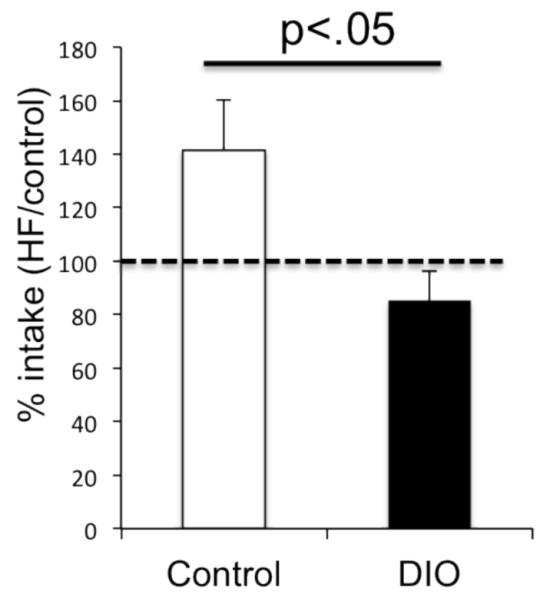
Food intake was measured two separate times during the dark period in control and DIO mice, once when fed the control diet and once when fed the HF diet (counterbalanced order). Control mice increased their food intake when presented with the palatable HF diet, while DIO mice failed to show this response.
Acknowledgements
The current work was supported by NIH DK064086 (Reyes) and MH087978 (Reyes).
Footnotes
Disclosure statement: The authors have nothing to disclose.
Disclosure/Conflicts of Interest
Z Vucetic, K. Totoki, J. Carlin and TM Reyes declare no conflicts of interest, financial or otherwise. Z. Vucetic, K. Totoki and J. Carlin contributed scientific experimentation, TM Reyes designed and supervised the experiments, performed the analyses, and wrote the manuscript.
References
- Alsiö J, Olszewski PK, Norbäck AH, Gunnarsson ZE, Levine AS, Pickering C, Schiöth HB. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience. 2010;171:779–787. doi: 10.1016/j.neuroscience.2010.09.046. [DOI] [PubMed] [Google Scholar]
- Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151:10. doi: 10.1210/en.2010-0556. [DOI] [PubMed] [Google Scholar]
- Chen JC, Turiak G, Galler J, Volicer L. Postnatal changes of brain monoamine levels in prenatally malnourished and control rats. Int J Dev Neurosci. 1997;15:527–263. doi: 10.1016/s0736-5748(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Cleck JN, Ecke LE, Blendy JA. Endocrine and gene expression changes following forced swim stress exposure during cocaine abstinence in mice. Psychopharmacology (Berl) 2008;201:15–28. doi: 10.1007/s00213-008-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, et al. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupé B, Amarger V, Grit I, Benani A, Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology. 2010;151:702–713. doi: 10.1210/en.2009-0893. [DOI] [PubMed] [Google Scholar]
- Davis JF, Tracy AL, Schurdak JD, yp MH, Lipton JW, Clegg DJ, Benoit SC. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci. 2008;122:1257–1263. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw van Weenen JE, Hu L, Zelm KJ-V, Vries M. G. d., Tamsma JT, Romijn JA, Pijl H. Four weeks high fat feeding induces insulin resistance without affecting dopamine release or gene expression patterns in the hypothalamus of C57Bl6 mice. Brain Res. 2009;1250:141–148. doi: 10.1016/j.brainres.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XF, Yu Y, Zavitsanou K, Han M, Storlien L. Differential expression of dopamine D2 and D4 receptor and tyrosine hydroxylase mRNA in mice prone, or resistant, to chronic high-fat diet-induced obesity. Brain Res Mol Brain Res. 2005;135:150–161. doi: 10.1016/j.molbrainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience. 2010:13. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, yMoal M. Addiction and the brain antireward system. Ann Rev Psychol. 2008:59. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Lee AK, Mojtahed-Jaberi M, Kyriakou T, Astarloa EA, Arno M, Marshall NJ, Brain SD, O’Dell SD. Effect of high-fat feeding on expression of genes controlling availability of dopamine in mouse hypothalamus. Nutrition. 2010a;26:411–422. doi: 10.1016/j.nut.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Tamashiro KL, Yang X, et al. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010b;151:4332–4343. doi: 10.1210/en.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, South T, Han M, Chen J, Wang R, Huang XF. High-fat diet decreases tyrosine hydroxylase mRNA expression irrespective of obesity susceptibility in mice. Brain Res. 2009;1268:181–189. doi: 10.1016/j.brainres.2009.02.075. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 2009;139:629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marichich ES, Molina VA, Orsingher OA. Persistent changes in central catecholaminergic system after recovery of perinatally undernourished rats. J Nutr. 1979:109. doi: 10.1093/jn/109.6.1045. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Lupu DS. High fat diet-induced maternal obesity alters fetal hippocampal development. Int J Dev Neurosci. 2009;27:627–633. doi: 10.1016/j.ijdevneu.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Brown AS, Keegan D, Siska LD, Susser E, Rotrosen J, Butler PD. Prenatal protein deprivation alters dopamine-mediated behaviors and dopaminergic and glutamatergic receptor binding. Brain Res. 2008;1237:62–74. doi: 10.1016/j.brainres.2008.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;20:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Brunn M, et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol. 2009;587:4963–4976. doi: 10.1113/jphysiol.2009.176156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Rada P, Bocarsly ME, Barson JR, Hoebel BG, Leibowitz SF. Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol Behav. 2010;101:394–400. doi: 10.1016/j.physbeh.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes T, Walker JR, DeCino C, Hogenesch JB, PE S. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci. 2003;23:5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South T, Huang XF. High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochem Res. 2008;33:598–605. doi: 10.1007/s11064-007-9483-x. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teegarden SL, Scott AN, Bale TL. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience. 2009;162:924–932. doi: 10.1016/j.neuroscience.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2010 doi: 10.1016/j.tics.2010.11.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Reyes TM. Chronic high fat diet drives postnatal epigenetic regulation of μ-opioid receptor in the brain. Neuropsychopharmacology. 2011;36:1199–1206. doi: 10.1038/npp.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal High-Fat Diet Alters Methylation and Gene Expression of Dopamine and Opioid-Related Genes. Endocrinology. 2010a;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med. 2010;2:577–593. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I, Reyes TM. Early life protein restriction alters dopamine circuitry. Neuroscience. 2010b;168:359–370. doi: 10.1016/j.neuroscience.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:1883. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Weaver I, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, MJ M. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weber M, Davies JD, Wittig D, Oakeley EJ, Haase M, Wan L, Lam L, Schübeler D. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet. 2005;37:853–862. doi: 10.1038/ng1598. [DOI] [PubMed] [Google Scholar]
- Widiker S, Karst S, Wagener A, Brockmann GA. High-fat diet leads to a decreased methylation of the Mc4r gene in the obese BFMI and the lean B6 mouse lines. J Appl Genet. 2010;51:193–197. doi: 10.1007/BF03195727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.