Abstract
Progesterone decreases cocaine self-administration in women and in female rats. In a previous study using rats selectively bred for high (HiS) and low (LoS) saccharin intake, HiS rats escalated their cocaine intake compared to LoS rats. Our goal was to examine the effects of progesterone on the escalation of cocaine self-administration in HiS and LoS rats. Four groups of female rats were compared: HiS P (progesterone-treated), LoS P, HiS VEH (vehicle-treated), and LoS VEH. Rats were trained to self-administer 0.8 mg/kg cocaine i.v. under a fixed-ratio 1 (FR 1) schedule during daily short-access (ShA) 2-h sessions. Rats then self-administered 3 randomly-presented doses of cocaine (0.2, 0.4, 1.6 mg/kg) and then had daily 6-h long-access (LgA) sessions with 0.4 mg/kg cocaine for 21 days. Cocaine intake was then reassessed with the 4 doses under the ShA condition. Throughout the experiment, rats were treated with daily s.c. injections of P (0.5 mg/kg) or an equal volume of VEH 30 min prior to each session. During the initial ShA condition, HiS rats earned more cocaine infusions than LoS rats at all doses, and during the subsequent LgA condition, HiS rats escalated cocaine intake, while the LoS rats maintained a steady rate. Progesterone treatment potentiated escalation of cocaine intake in the HiS rats but had an opposite effect on LoS rats, attenuating their cocaine self-administration. Results from the post-LgA dose-response ShA condition, indicated that both LoS and HiS VEH and P-treated rats earned more infusions than pre-LgA, but mainly at low doses. These results suggest that genetic differences in drug abuse vulnerability contribute differentially to treatment outcomes during escalation, a critical phase of the drug abuse process.
Keywords: Selective breeding, Saccharin intake, Progesterone, Escalation, Cocaine self- administration, rat
Introduction
Rats that have been selectively bred for high (HiS) and low (LoS) saccharin intake exhibit differential vulnerability in animal models of drug abuse (Carroll et al., 2008). For example, HiS rats (vs LoS rats) acquire ethanol (Dess et al., 1998), heroin, and cocaine self-administration at increased rates and show greater cocaine-induced locomotor activity (Carroll et al., 2007a). As sweet preference is positively related to drug abuse in humans (Janowsky et al., 2003; Wronski et al., 2007), this animal model of differential vulnerability allows us to investigate genetic factors during key phases of the drug abuse process, such as escalation.
In animal experiments, steadily escalating drug intake during extended periods of long access (LgA) serves as a model of human bingeing. This behavior corresponds to changes in neurobiological reward systems that underlie the transition from intermittent drug use to abuse (Ahmed and Koob, 1998). Previous work indicates that HiS rats escalate i.v. cocaine self-administration at a faster rate compared to LoS rats (Perry et al., 2006). Female rats (Roth and Carroll, 2004) and monkeys (Carroll et al., 2005) also escalate drug intake more than males, and escalation is a defining factor in the progression toward drug abuse in humans (Fattore et al., 2009). Preclinical and clinical work indicates that this sex difference is largely attributable to the gonadal hormones estrogen and progesterone (Anker and Carroll, 2010a; Carroll and Anker, 2010), as estrogen enhanced while progesterone inhibited the escalation of cocaine self-administration in female rats (Larson et al., 2007). In clinical studies with women, estrogen was associated with increased cocaine-induced positive subjective effects (Evans et al., 2002; Sofuoglu et al., 1999), while pretreatment with progesterone attenuated these responses (Evans and Foltin, 2006; Sufouglu et al., 2001, 2002, 2004), but see Reed et al. (2010) for opposite effects with amphetamine).
Given the possible therapeutic value of progesterone during bingeing phases of drug abuse, the extent to which it may reduce escalation in an animal model of cocaine intake warrants investigation. The purpose of the present study was to compare the effects of systemic progesterone on the escalation of i.v. cocaine self-administration in rats that differ widely in drug abuse vulnerability and motivation to seek drugs of abuse, HiS and LoS rats (Carroll et al., 2008). Additionally, results from previous studies indicated that female rats and monkeys showed greater responsiveness than males to both pharmacological (Anker and Carroll, 2010b; Anker et al., 2009; Campbell et al., 2002; Carroll et al., 2001; Cosgrove and Carroll, 2004) and behavioral (i.e., alternative nondrug reinforcement such as preferred foods or wheel running/exercise) (Carroll et al., 2000; Cosgrove and Carroll, 2003; Cosgrove et al., 2002) methods of drug abuse treatment. Thus, based on the sex difference data, it was hypothesized that the more vulnerable HiS rats would show a greater reduction in cocaine intake than LoS rats. Dose-response functions during short-access (ShA) periods were also assessed before and after the LgA condition, as previous work had shown long-term increases in ShA drug intake after LgA (Larson et al., 2007; Roth and Carroll, 2004).
Methods
Subjects
Thirty-four experimentally-naïve adult female rats selectively bred at the University of Minnesota (Carroll et al., 2002) from Occidental HiS and LoS lines (Occidental College, Los Angeles, CA) served as subjects in this study. The HiS and LoS lines were cultivated through breeding pairs based on extreme saccharin phenotype scores as previously described (Carroll et al., 2008). Phenotype scores were derived from a 24-h two-bottle test (Badia-Elder et al., 1996) in which 24-h consumption of a 0.1% saccharin solution was compared to 24-h water intake [saccharin score = (saccharin ml – water baseline ml), divided by body weight, X 100]. To ensure the ecological validity of our results we only tested in progesterone in female rats that were gonadally intact.
Prior to the experiment, rats were pair-housed in plastic cages with ad libitum access to pellet chow and water with stable humidity, light-dark cycle (12 h-12 h; lights on at 06:00 h), and temperature. Chronic, indwelling catheters were implanted in the rats’ jugular vein using a procedure described in Carroll and Boe (1982). Following recovery, rats were allowed continuous access to water and were given 16 g of ground food at the end of each experimental session. All experimental procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee under Protocol 1008A87754, and they complied with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
Apparatus
Rats were individually housed in 24 custom-made (physics Department, University of Minnesota, Minneapolis, MN) octagonal operant conditioning chambers (Carroll et al., 2001) that contained a drinking spout, recessed food receptacle, 2 stimulus lights, an active and inactive lever, and a house light. Infusion equipment remained attached to the rats during and following self-administration sessions. During sessions, the house light was illuminated, and a response on the active lever resulted in the delivery of an i.v. infusion of cocaine and illumination of the lights above the lever for the length of the infusion. Responses on the inactive lever illuminated the stimulus lights above the lever for the duration of an infusion but did not result in an infusion.
Procedure
Rats recovered for 3 days following surgery and were then trained to self-administer cocaine (0.8 mg/kg) under a FR 1 schedule of reinforcement during daily 2-h sessions. Throughout training and the rest of the experiment, HiS and LoS rats received subcutaneous injections of either progesterone (P, 0.5 mg/kg; HiS P, n=9; LoS P, n =7) or vehicle (VEH, peanut oil; HiS VEH, n =9; LoS VEH, n =9) 30 min prior to each session. Rats were studied for a total approximately 50 days. Estrous cycle was not controlled for in the present experiment, as previous work has shown that cocaine, when self-administered under the long-access conditions of the present study, disrupts cycle phase (Larson et al. 2007).
Once rats acquired stable cocaine self-administration at 0.8 mg/kg cocaine, they were allowed to self-administer each of 3 doses of cocaine that were given in mixed order (0.2, 0.4, and 1.6 mg/kg) for 3 sessions of stable responding. The session length was then extended to 6 h (long access, LgA; 09:00 -15:00 h) for 21 days, and subsequently, cocaine intake was reassessed under the ShA dose-response condition (0.2, 0.4, 0.8, and 1.6 mg/kg).
Data analysis
Responses and infusions served as the primary dependent measures. Responses and infusions during LgA were averaged into seven blocks of 3 days each and analyzed using 3-factor repeated-measures ANOVAs with phenotype (LoS vs HiS) and treatment (P vs VEH) as the between-group factors and day (LgA) as the repeated measure. The number of responses and infusions during the pre- and post-LgA dose-response conditions were analyzed with separate 3-factor repeated measures ANOVAs with phenotype and treatment as the between subjects factors and dose as the repeated measures. Additional 3-factor repeated measures ANOVAs with phenotype as the between-subjects factor and dose and access condition (pre- vs post-LgA) as repeated measures were conducted. After a significant main effect, post-hoc tests were conducted using Fisher's LSD protected t tests. Statistical analyses were conducted using GB Stat (Dynamic Microsystems, Inc., Silver Spring, MD).
Results
Pre-LgA dose-response condition
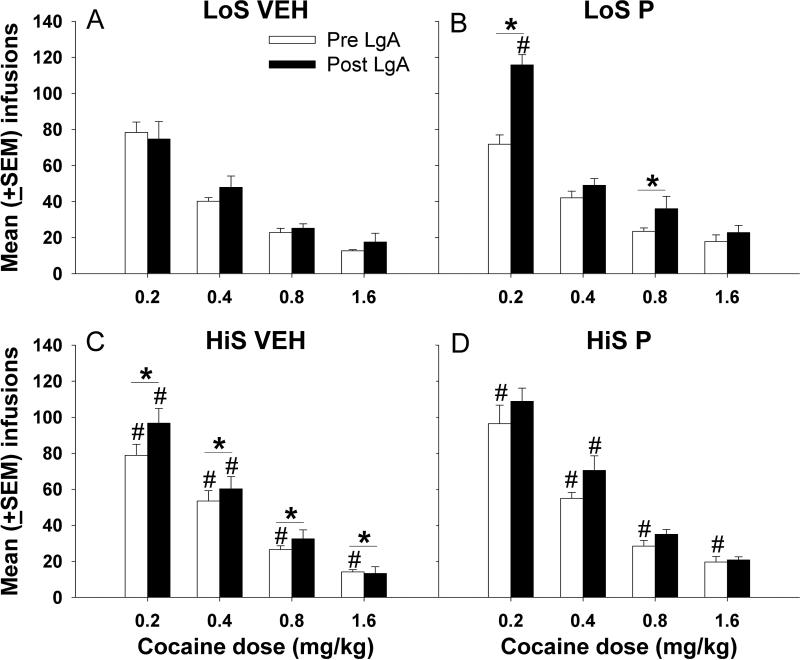
There were no significant effects of treatment (P vs VEH) on cocaine-maintained responses or cocaine infusions during the pre-LgA dose-response condition; however, HiS rats had more responses (F1, 135 = 8.43, p<0.01) (not shown) and infusions (F1, 135 = 8.67, p<0.01) (Fig 1C and 1D vs 1A and 1B) than LoS rats.
Figure 1.
Mean (±SEM) cocaine infusions obtained under the pre- and post-LgA dose-response conditions for the four groups. # denotes a significant phenotype difference for the respective dose and LgA (pre or post) condition (p<0.05). * indicates a significant within-subject difference between pre- vs. post-LgA conditions (p<0.05).
LgA
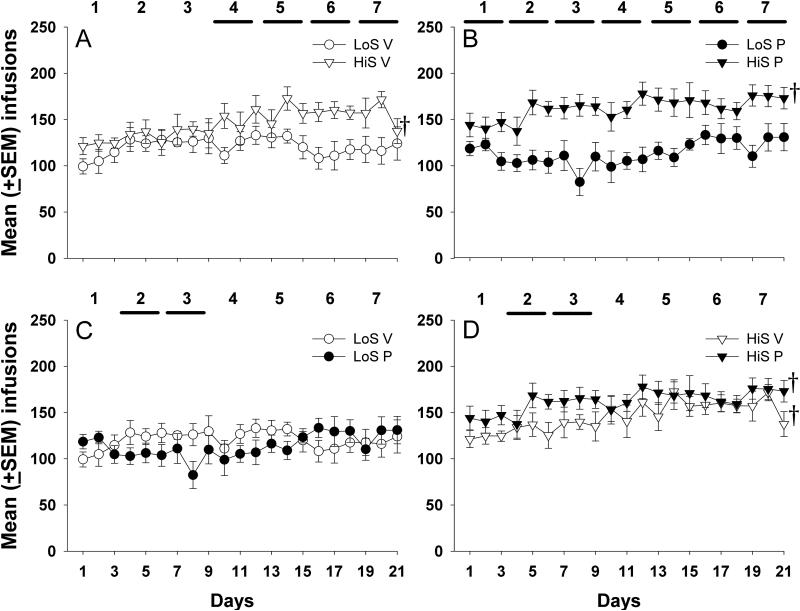
Similar to the pre-LgA condition, there was no significant effect of P treatment on cocaine-reinforced lever responses during LgA, but Fig 2A and 2B show that HiS rats had more responses than LoS rats (F1, 237 = 7.04, p<0.05) (not shown). For infusions, there was a significant main effect of phenotype (F1, 237 = 24.13, p<0.01) and a significant treatment X phenotype X day interaction (F6, 237 = 2.25, p<0.05). A subsequent post-hoc analysis indicated that both HiS groups (HiS VEH and HiS P) earned significantly more infusions during their last 3-day block compared to their first 3-day block (p<0.05) (Fig 2A, 2B, and 2D); indicating escalation of cocaine intake. In contrast, LoS rats (LoS VEH and LoS P) did not escalate their drug intake (Fig 2A, 2B, and 2C). HiS VEH rats earned more infusions than LoS VEH rats during blocks 4-7 (p<0.05) (Fig 2A), while HiS P rats earned more cocaine infusions than LoS P across all LgA blocks (p<0.05) (Fig 2B). The HiS P group earned more infusions during blocks 2 and 3 than the HiS VEH group (p<0.05) (Fig 2D). In contrast, LoS P rats earned significantly fewer cocaine infusions than LoS rats treated with VEH during blocks 2 and 3 (p<0.05) (Fig 2C).
Figure 2.
Mean (± SEM) cocaine infusions (0.4 mg/kg) are presented for each day of the LgA phase (6 hr). Horizontal lines indicate the 3-day intervals during which there were significant group differences in responses or drug deliveries (p<0.05). The † symbol indicates a significant within group difference in infusions during interval 7 (days 19-21) compared to interval 1 (days 1- 3).
Post-LgA dose-response condition
Following LgA, rats were retested under the FR 1 condition. Analyses of active lever responses (not shown) indicated a significant main effect of phenotype (F1, 115 = 15.75, p<0.01) and dose (F3,115 = 151.53, p<0.01). For infusions, there were significant main effects of treatment (F1, 115 = 14.51, p<0.01), phenotype (F1, 115 = 4.49, p<0.01), and dose (F3, 115 = 299.25, p<0.01), and a significant treatment X phenotype X dose interaction (F3, 115 = 3.27, p<0.01). Post-hoc comparisons indicated that at the 2 lowest dose of cocaine (0.2 and 0.4 mg/kg) HiS VEH rats earned more cocaine infusions than LoS VEH rats (p<0.05) (Fig 1A vs 1C), and the LoS P group earned more 0.2 mg/kg infusions than the LoS VEH group (p<0.05) (Fig 1B). LoS rats treated with progesterone earned fewer 0.4 mg/kg cocaine infusions than HiS P rats (p<0.05) (Fig 1B vs 1D). Comparison of cocaine intake under the dose-response conditions before and after LgA for each group indicated that HiS VEH rats earned more infusions post-LgA compared to pre-LgA (F1, 63 = 6.14, p<0.05) (Fig 1C), and LoS P rats earned more infusions of 0.2 and 0.8 mg/kg cocaine following LgA compared to before (p<0.05) (Fig 1B).
Saccharin preference test
Results from saccharin testing confirmed saccharin phenotype status and indicated that HiS rats consumed more saccharin than LoS rats. While HiS groups’ scores were higher than LoS groups’ scores (F1, 19 = 13.40, p<0.01), there were no significant differences in saccharin scores (±SEM) as a result of previous progesterone treatment in either LoS or HiS groups (LoS VEH = 5.8 ± 5.7; LoS P = 10.0 ± 1.3; HiS VEH = 22.3 ± 3.2; HiS P = 20.7 ± 3.0).
Discussion
In the present study, we used an animal model to examine genetic influences on receptivity to a pharmacological treatment for cocaine bingeing. The primary finding was that the pharmacological treatment (i.e., progesterone) enhanced the drug-prone and drug-resistant behavioral profiles inherent in the HiS and LoS rats under the LgA condition (as is illustrated when comparing Figure 1A to Figure 1B). Specifically, on days 3-9, progesterone (vs VEH) potentiated cocaine self-administration in the HiS rats, while it suppressed intake in the LoS rats. The emergence of effects of progesterone on cocaine self-administration that were restricted to days 3-9 suggests that animals may have become tolerant to the effects of progesterone or cocaine during LgA. Altering the dosing regimen such that progesterone was administered every other day may have prolonged its effect on LgA cocaine self-administration. The lack of a treatment effect in HiS rats on days 10-21 may be due to a ceiling effect. For example, HiS rats have a greater capacity to continue to escalate cocaine intake at the end of a 21-day LgA condition (Perry et al. 2006). Additionally, the lack of an effect on days 1-3 may have been the result of the change in session length from 2 to 6 hr. Results from several studies indicate that treatment effects and individual differences in LgA cocaine intake often emerge following several days of cocaine self-administration (Anker et al. 2011; Gipson et al. 2011; Larson et al. 2007; Mantsch et al. 2008). In contrast to the results in the LgA condition, progesterone enhanced cocaine intake at the lowest cocaine concentration in the LoS rats following the LgA period, thus illustrating a complex interaction between phenotype, treatment, phase, and dose.
Two important factors that need to be considered when comparing results from the present and previous studies are strain and selective breeding. Previous studies demonstrating an attenuating effect of progesterone on cocaine self-administration have almost exclusively used female Wistar rats, while in the present study Sprague-Dawley rats were used (for review see Anker and Carroll, 2010a). Furthermore, findings indicate that receptivity to drug abuse treatment is strongly influenced by strain. For example, Haile and Kosten (2001) demonstrated that, the effects of several D2 and D1 agonists and antagonists differed between Lewis and Fischer 344 rats, and similar to the findings with progesterone, administration of one treatment (SKF 38393) increased cocaine self-administration in Fischer 344 rats (drug abuse resistant) and decreased it in Lewis rats (drug abuse prone). An additional consideration when comparing the present results to previous findings is that rats in the present study were selectively bred for differential saccharin intake over successive generations. With each generation, the behavioral phenotype (and the underlying genes) between HiS and LoS rats has increasingly diverged. Thus, the fact that progesterone treatment facilitated cocaine self-administration in HiS rats while it had an opposite effect in LoS rats and Wistar rats could be explained by the contribution of genetic influences associated with strain (in the case of outbred Wistar rats) and selective breeding (in the case of LoS rats).
Regardless of treatment status (P or VEH), HiS rats escalated cocaine self-administration during the LgA condition, while LoS rats maintained a lower steady rate of cocaine intake. These findings agree with previous reports indicating that HiS (vs LoS) rats are more likely to escalate their cocaine intake under similar LgA conditions (Carroll et al., 2007b; Perry et al., 2006). This confirms that the HiS and LoS rat lines are useful genetic models of drug abuse vulnerability and resistance, respectively (for review see Carroll et al., 2008). Results from the ShA sessions before and after LgA provide further evidence of greater intake in HiS than LoS rats, particularly at the lower doses of cocaine as seen previously (Lynch and Carroll, 1999; Carroll et al., 2002; Roth and Carroll, 2004).
The attenuating effects of progesterone on iv cocaine self-administration have been reported in our laboratory under similar LgA conditions (Larson et al., 2007) and during other phases of drug abuse that are modeled in animals in outbred Wistar female rats (Anker et al., 2007; Anker and Carroll, 2010a). Results from clinical work provide cross-species support to these findings by indicating that progesterone treatment decreased positive subjective ratings of smoked and iv cocaine in women (Evans and Foltin, 2006; Sufouglu et al., 2001, 2002, 2004). The results of the present study suggest that while progesterone may be efficacious in decreasing responses to cocaine, this treatment effect may depend on the individual's drug abuse vulnerability.
It has been reported that females are more vulnerable to several aspects of drug abuse than males (Carroll and Anker, 2010), and they respond differentially to treatments for drug abuse (Campbell et al., 2002; Carroll et al., 2001; Cosgrove and Carroll, 2004). The present results suggest that other individual differences (sweet preference) that predict higher and lower levels of drug abuse, result in different receptivity to treatments (even in opposite ways). These results also suggest that other vulnerability markers expressed in animal models (e.g., impulsivity) should be tested for their reactivity to treatment strategies. Further evidence for genetic contributions in sensitivity to the treatment of the escalation of cocaine self-administration has been demonstrated in a recent study by Holtz and Carroll (2011). Similar to the results of the present study baclofen treatment increased the escalation of cocaine self-administration in HiS female rats and decreased it in LoS rats.
The present results highlight the importance of individual differences in susceptibility to treatment for drug abuse. Just as female-specific treatments have been suggested based on sex difference studies (Carroll and Anker, 2010; Fattore et al., 2009), development of vulnerability-specific treatments may be in order as well. The present model that links genetically-mediated markers of drug abuse vulnerability such as sweet intake to differential treatment outcomes may also prove useful for the emergent clinical strategy of pharmacogenetics (Haile and Kosten, 2009), in which genomic variations between individuals inform treatment choices.
Acknowledgements
The authors would like to thank Luke Gliddon, Emily Kidd, Jennifer Pawlik, Matthew Starr, Rachael Turner, Troy Velie, and Jeremy Williams, and Natalie Zlebnik for their technical assistance. This work was supported by NIDA grants R01 DA003240 and K05 DA015267 (MEC), P05 DA024196 (Kelvin O Lim and MEC, Directors), and F31 DA023301 (JJA).
Footnotes
Conflicts of interest: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci Biobehav Rev. 2010a;35:315–33. doi: 10.1016/j.neubiorev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010b;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology (Berl) 2009;203:63–72. doi: 10.1007/s00213-008-1371-9. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Carroll ME. Differential effects of allopregnanolone on the escalation of cocaine self-administration and sucrose intake in female rats. Psychopharmacol (Berl) 2011;212:419–429. doi: 10.1007/s00213-010-1968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia-Elder N, Kiefer SW, Dess NK. Taste reactivity in rats selectively bred for high vs low saccharin consumption. Physiol Behav. 1996;59:749–55. doi: 10.1016/0031-9384(95)02131-0. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Morgan AD, Carroll ME. Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug Alcohol Depend. 2002;66:61–69. doi: 10.1016/s0376-8716(01)00185-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Boe IN. Increased intravenous drug self-administration during deprivation of other reinforcers. Pharmacol Biochem Behav. 1982;17:563–7. doi: 10.1016/0091-3057(82)90319-7. [DOI] [PubMed] [Google Scholar]
- Carroll MN, Roth ME, Voeller RK, Nguyen PD. Acquisition of oral phencyclidine self-administration in rhesus monkeys: Effect of sex. Psychopharmacolgy. 2000;149:401–408. doi: 10.1007/s002130000389. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Campbell UC, Heideman P. Ketoconazole suppresses food restriction-induced increases in heroin self-administration in rats: sex differences. Exp Clin Psychopharmacol. 2001;9:307–16. doi: 10.1037//1064-1297.9.3.307. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161:304–13. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Batulis DK, Landry KL, Morgan AD. Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology (Berl) 2005;180:414–26. doi: 10.1007/s00213-005-2182-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anderson MM, Morgan AD. Higher locomotor response to cocaine in female (vs male) rats selectively bred for high (HiS) and low (LoS) saccharin intake. Pharmacol Biochem Behav. 2007a;88:94–104. doi: 10.1016/j.pbb.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anderson MM, Morgan AD. Regulation of cocaine self-administration in rats selectively bred for high (HiS) and low (LoS) saccharin intake. Psychopharmacology (Berl) 2007b;190:331–341. doi: 10.1007/s00213-006-0600-3. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–60. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME. Differential effects of a nondrug reinforcer, saccharin, on oral self-administration of phencyclidine (PCP) in male and female rhesus monkeys. Psychopharmacology. 2003;170:9–16. doi: 10.1007/s00213-003-1487-x. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME. Effects of bremazocine on oral phencyclidine (PCP) self-administration in male and female rhesus monkeys. Exp Clin Psychopharmacol. 2004;12:111–117. doi: 10.1037/1064-1297.12.2.111. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter R, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharm Biochem Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–8. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W. Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinology. 2009;34(Suppl 1):S227–36. doi: 10.1016/j.psyneuen.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacol (Berl) 2011;214:557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Kosten TR. Differential effects of D1- and D2-like compounds on cocaine self-administration in Lewis and Fischer 344 inbred rats. J Pharacol Exp Ther. 2001;299:509–518. [PubMed] [Google Scholar]
- Haile CN, Kosten TR. The potential of pharmacogenomics to treat drug addiction. Pharmacogenomics. 2009;10:1883–6. doi: 10.2217/pgs.09.146. [DOI] [PubMed] [Google Scholar]
- Holtz NA, Carroll ME. The effects of baclofen on the escalation of i.v. cocaine self-administration and reistatement of cocaine-seeking behavior in rats selectively bred for high (HiS) and low (LoS) saccharin intake. Pharmacol Biochem Behav. 2011 doi: 10.1016/j.pbb.2011.08.028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, Pucilowski O, Buyinza M. Preference for higher sucrose concentrations in cocaine abusing-dependent patients. J Psychiatr Res. 2003;37:35–41. doi: 10.1016/s0022-3956(02)00063-8. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461–71. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychophamacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Serge JP, Hoks MA, Francis DM, Katz ES. Surgical adrenalectomy with diurnal corticosterone replacement slows escalation and prevents the augmentation of cocaine-induced reinstatement in rats self-administering cocaine under long-access conditions. Neuropsychopharmacol. 2008;33:814–426. doi: 10.1038/sj.npp.1301464. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guidelines for the care and use of mammals in neuroscience and behavioral research. The National Academies Press; Washington D.C.: 2003. p. 209. [PubMed] [Google Scholar]
- Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacology (Berl) 2006;186:235–45. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- Reed SC, Levin FR, Evans SM. The effects of progesterone pretreatment on the responses to oral D-amphetamine in women. Hormones and Behavior. 2011;58:533–543. doi: 10.1016/j.yhbeh.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav. 2001;69:299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–5. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Wronski M, Skrok-Wolska D, Samochowiec J, Ziolkowski M, Swiecicki L, Bienkowski P, Korkosz A, Zatorski P, Kukwa W, Scinska A. Perceived intensity and pleasantness of sucrose taste in male alcoholics. Alcohol. 2007;42:75–9. doi: 10.1093/alcalc/agl097. [DOI] [PubMed] [Google Scholar]