Abstract
Introduction
Video-assisted thoracoscopic surgery (VATS) has been developed for surgical treatment of thoracic spinal tuberculosis to overcome the problems associated with a formal thoracotomy. VATS, however, is technically demanding with a difficult learning curve.
Materials and methods
We conducted a retrospective long-term follow-up study of anterior debridement and reconstruction via a thoracoscopy-assisted mini-open approach for the surgical treatment of thoracic spinal tuberculosis. There were 50 patients collected with mean age 38.3 years with thoracic spinal tuberculosis.
Results
The average operative time was 210 min (range 170–300 min), the average blood loss during operation was 550 ml (range 300–1,000 ml), and the mean chest drainage duration was 3.5 days (3–5 days). Complications occurred in 17 patients (34%). The mean follow-up was 6.5 years. There was statistically difference in VAS 3 months after surgery compared to preoperatively (P < 0.001), as well as final follow-up compared to 3 months post-op (P < 0.001). In patients with minor pulmonary impairment as measured by pulmonary function testing, 15 improved to normal and 5 had no change. In patients with moderate pulmonary impairment, 6 improved to normal and 2 improved to minor impairment at final follow-up. Neurological improvement of one to three grades had occurred in 26 patients by final follow-up. There was statistically difference in kyphotic angle 3 months after surgery compared to preoperatively (P < 0.05), as well as final follow-up compared to 3 months post-op (P < 0.001). The average correction rate of kyphotic angle was 38.7% and the loss of correction rate was 1.3% at final follow-up. No recurrent tuberculosis was found.
Conclusion
Thoracoscopy-assisted mini-open approach can provide a simple, safe, and practical treatment option with minimal invasiveness in cases of thoracic spinal tuberculosis. Successful clinical and radiographic outcomes can be achieved via anterior debridement and reconstruction at long-term follow-up.
Keywords: Spinal thoracic vertebrae, Thoracoscopy, Tuberculosis
Introduction
Anterior radical debridement and spinal fusion via thoracotomy combined with anti-tuberculosis chemotherapy is advocated by some researchers as an effective treatment for thoracic spinal tuberculosis [6, 22]. However, this procedure has been associated with substantial morbidity and long recovery time [10, 16]. Recently, video-assisted thoracoscopic surgery (VATS) has been developed for surgical treatment of thoracic tuberculosis to overcome the problems associated with a formal thoracotomy. Compared to traditional thoracotomy techniques, video-assisted thoracoscopic surgery employs a smaller incision with potentially less soft tissue disruption and better subsequent shoulder function [16]. However, the learning curve associated with VATS is a major consideration for the initial adoption of such a technique. Therefore, in order to facilitate ease of use, modified extended manipulating channels can be created to allow use of a combination of thoracoscopy and conventional spinal instruments designed to treat thoracic spine tuberculosis. Its efficacy and safety have been described in previous papers [7, 11].
Reconstruction of the anterior spinal column is another important consideration by way of VATS due to the stability of the spine can be significantly compromised after creation of a large intervertebral gap. In the early days of surgical treatment of spinal TB, strut bone grafting alone without fixation was an accepted method of treatment due to concerns of instrumentation affecting the ability to irradicate infection. However, bone graft dislodgement and kyphotic correction loss were frequent without the use of instrumentation. Huang et al. [7] treated 10 patients with tuberculous spondylitis using modified VATS anterior debridement and bone grafting without fixation. At 2-year follow-up, one patient had rib strut graft resorption and another patient experienced graft subsidence with increased kyphosis.
Recently, studies have shown that supplemental anterior fixation can avoid failure of bone grafting and improve clinical outcomes in surgical treatment of spinal tuberculosis via conventional approaches [3, 12, 21]. The safety of anterior fixation was also evaluated by Ha et al. [8]. There is, however, a paucity of research on thoracic spine tuberculosis treated by VATS with anterior instrumentation. In this study, we report on 50 consecutive patients with thoracic spinal tuberculosis who underwent anterior debridement and reconstruction via thoracoscopy-assisted mini-open approach. The purpose of this study was to assess the clinical effectiveness of this technique in achieving fusion of the spine and decompression of the spinal cord at minimum of 5-year follow-up.
Materials and methods
Patients population and evaluating variables
We conducted a retrospective cohort study at a single tertiary-care referral hospital. Approval was obtained from our Institutional Review Board before initiation of the study. Inclusion criteria include single or two-level thoracic spinal tuberculosis (T4–T12), severe back pain resistant to conservative treatment, instability and progressive deformity due to significant bony destruction, neurologic deficits with sequestered bone and disc, and large abscesses. We excluded cases with thoracic kyphosis over 45° (mean value measured from upper border of T4 to the lower border of T12) in this study due to concerns regarding adequate decompression and kyphosis correction with traditional compression implants using thoracoscopy. Other exclusion criteria included extensive pleural adhesions due to previous chest trauma or thoracic surgery, and inability to tolerate single lung ventilation. Severe respiratory compromise may be considered as an absolute contraindication [13].
There were 50 patients (30 men and 20 women) were treated and evaluated from March 1998 to December 2003 and reviewed retrospectively. The average age was 38.3 years (range 21–60 years). All patients received regular preoperative chemotherapy of isonicotinyl hydrazine, rifampin, ethambutol and streptomycin for at least 2 weeks. All patients experienced back pain with a mean duration of 11 months. 24 patients had symptoms of fever, night sweats, or weight loss in addition to back pain. The mean preoperative white blood cell count was 6.9 × 109/l (normal range 4–10 × 109/l). Average erythrocyte sediment rate (ESR) was 43.8 mm/h (normal range 0–15 mm/h), and the average concentration of C-reaction protein was 39.5 mg/l (normal range < 8 mg/l) before surgery. Neurological function was evaluated by Frankel Grading. The preoperative neurological status was Grade B in 2 cases, Grade C in 6, Grade D in 18 and Grade E in 24. Magnetic resonance image (MRI) showed a large paraspinal abscess in 42 cases, pleuritis in 15, and dural compression in 28. Of the 50 cases, 17 involved a single level, and 33 were two-level.
Perioperative measurements included operative time, blood loss, chest tube drainage, length of hospital stay, and complications. Pulmonary function test results were evaluated preoperatively and at final follow-up and expressed as percent predicted values of forced expiratory volumes in one second (FEV1). Pulmonary impairment was classified as mild (60–80 FEV1/% Predicted), moderate (40–59 FEV1/% Predicted), and severe (<40 FEV1/% Predicted) based on British Thoracic Society (BTS) criteria [2]. The clinical outcomes data were assessed preoperatively, at 3 months postoperatively and final follow-up by use of the Visual Analogue Scale (VAS) and Subjective Clinical Results. Subjective clinical results were categorized as excellent, good, fair, and poor. Excellent was defined as satisfaction with the surgery with no residual symptoms and a return to previous activities. Good was defined as improved symptoms with occasional need for analgesics, fair was defined as dissatisfaction with the surgery with residual symptoms, and poor was defined as dissatisfaction with the surgery with worsened symptoms [7].
Statistical calculations including means and standard deviations were performed using the SPSS 17.0 (SPSS Inc., Chicago, IL, USA) for Windows. A normal distribution of the data with VAS and kyphotic angle was analyzed. Student’s t test and Fisher’s Z test were used for the comparison of statistical data. Statistical significance was established at a P value less than 0.05.
Surgical technique
General anesthesia was administered with double-lumen intubation and maintained with single lung ventilation. The patient was placed in the lateral decubitus position. The side chosen to be up was located on the more severely affected side. Orientation using fluoroscopy was performed prior to skin incision. An initial 10 mm port for the thoracoscope was made in the sixth or seventh intercostal space near the anterior axillary line. After separating any adhesive pleura, a 25° thoracoscope was inserted to inspect the lung and thorax. Under thoracoscopic visualization, the level(s) of diseased vertebrae were identified by inserting needles as markers for lateral fluoroscopy. A 3–4 cm skin incision was made above the target vertebrae to provide a working channel under direct visualization of the thoracoscope. A thorough debridement was performed to remove necrotic disc, sequestra, infected granulation tissue and caseous material. Any segmental vessels visible at the operative site were ligated with double titanium clips. Thoracoscopic-assisted decompression was considered complete only when the dura was exposed in the involved segment and appeared to be devoid of compression. The removed material was sent for histopathologic examination. Interbody fusion was then performed by implanting a tricortical iliac crest autograft or titanium mesh cage filled with allograft while the assistant manually pushed the kyphotic thoracic spine forward to correct the kyphosis. Generally, titanium mesh cages were not used in elderly patients with osteoporosis or patients with disrupted endplates. Anterior single-rod fixation with the VentroFix System (Synthes, Switzerland) was placed via extended manipulating channels. Technique of compression with use of VentroFix instruments, which was similar to open procedure, was then applied to stabilize the bone strut or cage. The bone grafting and screw-rod position were confirmed with fluoroscopy. A chest tube was placed prior to wound closure.
Postoperative care
The chest tube was removed when less than 50 ml had been collected. All patients received antitubercular drugs for 12 months (Streptomycin, Isoniazid, Rifampin, and Pyrazinamide for 3 months followed by Isoniazid, Rifampin, and Pyrazinamide for 9 months). Plain radiographs of the spine were obtained at 1 week postoperatively, three months postoperatively, and at final follow-up. CT scans were obtained when the fusion status was indeterminate by plain radiographs. Bony fusion were defined as absence of motion in standing and dynamic flexion-extension positions, presence of trabecular bone bridging between the grafts and the vertebrae, and no fixation failure or focal pain at the level of the fusion [3]. Liver function and ESR were monitored carefully at regular intervals.
Results
Perioperative parameters
Surgeries were completed as planned in all patients. A left-sided approach was used in 29 patients and a right-sided approach in 21. The most cephalad fixation level was T3 while the most caudal was L1. For reconstruction of the anterior and middle columns, iliac autograft was used in 28 patients and titanium mesh bone grafting in 22. The mean operative time was 210 min (range 170–300 min). The median intraoperative blood loss was 550 ml (range 300–1,000 ml). The chest tube was removed on average 3.5 days (range 3–5 days) after surgery. The mean hospital stay was 8.8 days.
Clinical outcomes
The mean follow-up was 6.5 years (range 5.0–7.3 years). VAS back pain: for comparison of preoperatively and 3 months postoperatively (normal distribution: F = 0.010, P = 0.922), there was a statistically significant difference (P < 0.001) with Student’s t test; For comparison of 3 months postoperatively and final follow-up (not normal distribution: F = 5.039, P = 0.027), there was a statistically significant difference (P < 0.001) with Fisher’s Z test (Table 1). For subjective patient reported outcomes, excellent results were obtained in 37 patients, good in 9, fair in 3 and poor in 1, with 92% of patients having a good or excellent result. Neurological improvement of one to three grades had occurred in 26 patients by final follow-up. Based on the Frankel grading, 44% of patients with an incomplete neurological deficit (22 of 50) improved by one level, 6% patients (3 of 50) improved by two levels, and one patient improved by three levels (Table 2). 13 patients with spastic gait preoperatively achieved normal gait by clinical examination at three months after surgery. There were no cases of deterioration in neurological function, and there were no cases of scapular dysfunction and recurrence of tuberculosis noted postoperatively.
Table 1.
VAS and kyphotic angle in the 50 patients
| Pre-operative | 3 months after surgery | Final follow-up | |
|---|---|---|---|
| VAS | 8.3 ± 1.2 | 1.5 ± 0.6 | 1.2 ± 0.3 |
| Kyphotic angle (°) | 30.2 ± 4.5 | 18.5 ± 1.0 | 18.9 ± 0.8 |
Table 2.
Frankel grading for the 50 patients
| Grade | Pre-operative | 3 months after surgery | Final follow-up |
|---|---|---|---|
| A | 0 | 0 | 0 |
| B | 2 | 0 | 0 |
| C | 6 | 1 | 1 |
| D | 18 | 5 | 3 |
| E | 24 | 44 | 46 |
Radiological outcomes
The kyphotic angle was corrected from 30.2 ± 4.5° before surgery to 18.5 ± 1.0° at 3 months postoperatively, for a correction rate of 38.7%. Final X-ray film showed an average loss of kyphotic correction of 1.3% (Table 1). For comparison of the kyphotic angle between preoperatively and 3 months postoperatively (not normal distribution: F = 5.157, P = 0.025), there was a statistically significant difference (P < 0.05) with use of Fisher’s Z test; For comparison of the kyphotic angle between 3 months postoperatively and final follow-up (normal distribution: F = 0.088, P = 0.767), there was a statistically significant difference (P < 0.001) with Student’s t test. All cases achieved bony fusion at a mean time of 18.5 weeks (range 12–23 weeks). At final follow-up, there was no evidence of implant loosening or migration detected. Sagittal alignment of thoracic spine was improved, and no significant coronal malalignment was noted (Fig. 1).
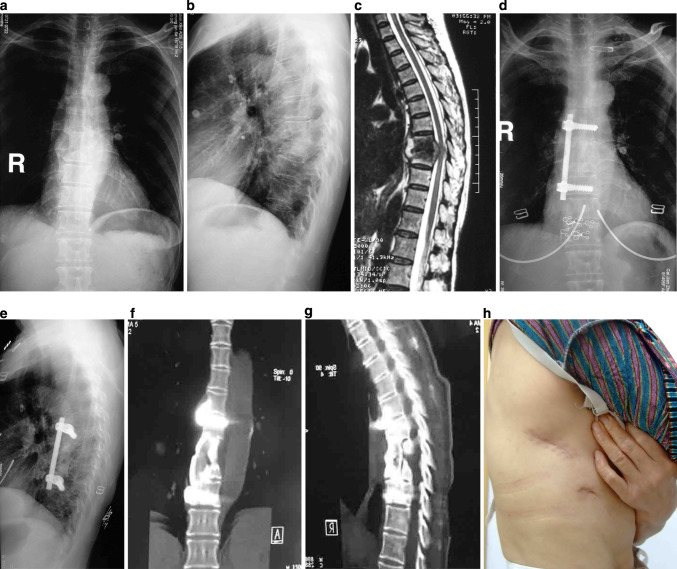
Fig.1.
53-year-old female with T8-9 tuberculosis underwent thoracoscopic-assisted anterior debridement, iliac bone autograft and fixation with single-rod Ventrofix system (Synthes Spine, Switzerland). a, b AP X-ray shows paraspinal shadow and lateral plain radiograph demonstrates a narrowed disc space at T8-9 and a kyphotic angle of 39.2°. c MRI demonstrates vertebral destruction, paravertebral and epidural abscess with compression of the spinal cord. d, e AP X-ray and lateral plain radiograph of final follow-up show that there was no fixation failure, and the postoperative kyphotic angle measured 22.8° at final follow-up. f, g Three-dimensional computer tomography scan in coronal and sagittal planes demonstrates a solid fusion. h Postoperative clinical photograph demonstrates the size of the skin incision (black arrow)
Pulmonary function test
There were 22 patients with preoperative mean percent predictive FEV1 of 82 ± 2% (range 80–90%) preoperatively and 81 ± 2% (range 80–87%) at final follow-up. 20 cases were categorized as minor preoperative pulmonary impairment with preoperative mean percent predictive FEV1 of 65 ± 3% (range 60–70%); 15 improved to normal (81 ± 1%) and 5 had no change. There were 8 cases with moderate preoperative pulmonary impairment with preoperative mean percent predictive FEV1 of 50 ± 7% (range 40–59%); 6 improved to normal (81 ± 0.5%) and 2 improved to have minor impairment (65 and 69%) at final follow-up.
Complications
There were 17 complications in this study for a rate of 34%. There were four cases of temporary intercostal neuralgia, which was attributed to the use of a hard retractor tool during the procedure. The pain treated by administration of analgesic (celecoxib, 400 mg PO Qday, for an average of 7.5 days). There were three cases of superficial wound infection which all healed by second intention. There were 10 cases of pulmonary complications with rate of 20%, three patients sustained lacerations of the lung parenchyma during dissection of adhesions between the lung and chest wall, two cases with pulmonary atelectasis, one pleural effusion, two pneumonias, one hemopneumothorax and one empyema. All of these cases with complications recovered after treatment with antibiotics, suction, or closed thoracic drainage. Of the 10 cases with pulmonary complications, 3 patients had minor preoperative pulmonary function impairment and 5 with moderate impairment. At final follow-up, 5 patients improved to normal pulmonary function and 3 continued with minor impairment. The other 2 patients had normal pulmonary function before surgery and remained normal at final follow-up.
Discussion
Thorough debridement and anterior reconstruction accompanied by anti-tuberculosis chemotherapy is an effective treatment for thoracic spinal tuberculosis, which typically results in good neurological recovery and deformity prevention [3, 4, 17]. Open anterior approaches to the thoracic spine may cause significant restriction to rehabilitation due to postoperative pain and complications [5]. Thoracoscopic techniques were introduced in the 1990s to potentially reduce the morbidity associated with open approaches [18, 21]. The range of application of anterior thoracoscopic surgery has been expanded from anterior discectomy or debridement to anterior fixation and reconstruction. Less soft tissue disruption, safety, and efficacy of thoracoscopic surgery has been shown in previous studies [4, 13, 19]. These thoracoscopic techniques, however, may be less familiar to many spine surgeons and requires much time and effort to negotiate a difficult learning curve. The two-dimensional view provided by endoscopy may cause disorientation with respect to anatomy due to magnification and a lack of depth perception, which may in turn lead to serious endoscopic complications [20]. Apart from the surgical skills required, the extra investment in thoracoscopic equipment and instruments may be problematic when cost-effectiveness is paramount.
Therefore, the initial enthusiasm for endoscopic anterior spinal procedures has shifted towards the use of a less invasive but mini open approach or thoracoscopy-assisted mini-open technique with a small incision of 3–4 cm [15, 16]. This approach provides direct visualization of the thoracic spine including vessels, nerves, and visceral structures. Under direct view, the management of vascular complications can be easier and quicker than with all-endoscopic procedures. Furthermore, a direct three-dimensional view considerably facilitates the performance of a corpectomy with direct spinal canal decompression as well as bone graft or cage insertion, placement of anterior instrumentation, and can reduce operative time [15].
It has been reported that blood loss using the thoracoscopy-assisted mini-open technique is similar to an all endoscopic technique, but the operative time is much shorter [16]. Huang et al. [7] reported 10 cases of anterior thoracoscopic surgery with two working channels of 2.5–3.5 cm or one channel of 5–6 cm. The average blood loss was 485 ml (150–850 ml), and mean surgical duration was 174 min (120–240 min). The excellent and good outcomes in Huang’s study amounted to 90% with a mean kyphotic correction rate of 37.3%. Jayaswal et al. [11] reported 23 cases of thoracic tuberculosis treated by mini-open anterior thoracoscopic surgery. The median blood loss was 780 ml (330–1,180 ml) with an average operative time of 228 min (102–324 min). Neurological improvement was shown in 94.4% of cases (17/18). In our study, VAS back pain significantly improved. 92% of patients had a good or excellent result, consistent with the subjective clinical results described by Huang et al. [7]. The average kyphotic correction was 38.7%. Neurological function in all patients with preoperative deficits improved at least one grade at final follow-up.
Anterior reconstruction and fixation is an important technique facilitated by way of VATS to minimize bone graft subsidence, maintain kyphotic correction, and improve bony fusion. The safety and efficacy of anterior fixation in surgical treatment of spinal tuberculosis via conventional approach has been described in previous studies [3, 8, 12, 21]. There is, however, a paucity of study of thoracic spine tuberculosis treated by VATS with anterior instrumentation. Huang et al. [7] performed anterior debridement and reconstruction by modified VATS without anterior instrumentation. In their study, kyphotic angles were corrected from 26.8° before surgery to 16.8° after surgery, but there was significant loss of correction at final follow-up. Jayaswal et al. [11] reported on 23 cases with thoracic spinal tuberculosis. Six of whom were treated by video-assisted thoracoscopic anterior debridement, decompression, and anterior screw-rod fixation. Average preoperative kyphosis was 39° and was corrected to 28° with minimal loss of correction at final follow-up. 22 of the 23 patients achieved a solid fusion with an average time for fusion of 16.5 weeks. There was no recurrence of disease in any patients. Overall, good results had been achieved at final follow-up. In our study, there was minimal loss of kyphotic correction of 1.3%, and solid bony fusion was obtained in all cases at an average of 18.5 weeks. There were also no cases of recurrence of tuberculosis or fixation failure. Our study is consistent with others in demonstrating the effectiveness of using anterior metal implants in the face of active infection.
It should be noted that complications of anterior thoracic surgery are still possible with the mini-open procedure. A complication rate of 24.4–31.3% was reported in some studies [9, 11, 13, 23]. Of these complications, the risk of injury to the pulmonary parenchyma, such as lung laceration, atelectasis, pneumonia or empyema is significant concerns. Hodgson et al. [6] treated 412 cases with Pott’s disease of spine via open transthoracic procedure. In their study, major complications included ileus, pneumonia, hemothorax, pneumothorax, cystitis, and wound infection, and there were 4 deaths in the first 100 cases with secondary to cardiac failure, hepatic failure, and pneumonia. In this study, the pulmonary complication rate was 20%, which is consistent with rates in open procedures. All lung complications including atelectasis, hemopneumothorax, pneumonia and empyema improved with perioperative pulmonary therapy, which has been previously described [1, 7]. These pulmonary complications are usually due to lung parenchyma laceration during mobilization of adhesed lungs, and mild or moderate lung lacerations can be repaired through mini-open access. Conversion to an open procedure may be necessary for more extensive lacerations [7].
Changes in pulmonary function secondary to lung complications who underwent thoracoscopic surgery was not analyzed in the afore-mentioned studies. With regard to pulmonary function test, percent predicted values of forced expiratory volumes in one second (FEV1) is often expressed as one of main parameters due to can provide an adequate assessment of the volume and flow function. Kim [14] reported 64 AIS patients who underwent an anterior spinal instrumentation and fusion through an open thoracotomy, pulmonary function impairment often occurred at 2 years postoperative. In our study, the thoracoscopic mini-open procedure did not negatively affect preoperative pulmonary function, meanwhile, prevent worsening of lung function for those with preoperative pulmonary impairment. 23 of 28 cases with preoperative pulmonary impairment recovered their lung function to some degree at long-term follow-up. We suspect that a thoracoscopic-assisted anterior mini-open approach may help decrease respiratory complications compared to open thoracotomy.
There were, however, some limitations to this retrospective study. First, we did not have a control group for comparison, such as patients undergoing an all-endoscopic technique or traditional thoracotomy. In addition, there should be some analysis of the relationship of complications with pulmonary impairment and patient-reported outcomes.
In conclusion, anterior mini-open approach assisted by thoracoscopy can be a safe and effective technique for anterior debridement and reconstruction of thoracic tuberculosis. This technique is a feasible option for the treatment of thoracic tuberculosis while both minimizing the risk of complications and allowing easier intervention for potential intraoperative complications.
Conflict of interest
None.
References
- 1.Akgül AG, Örki A, Örki T, Yüksel M, Arman B (2011) Approach to empyema necessitatis. World J Surg 35:981–984. doi:10.1007/s00268-011-1035-5 [DOI] [PubMed]
- 2.Pearson MG, Alderslade R, Allen SC, et al. BTS guidelines for the management of chronic obstructive pulmonary disease. The COPD Guidelines Group of the Standards of Care Committee of the BTS. Thorax. 1997;52:S1–S28. doi: 10.1136/thx.52.2008.S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai LY, Jiang LS, Wang W, Cui YM. Single-stage anterior autogenous bone grafting and instrumentation in the surgical management of spinal tuberculosis. Spine (Phila Pa 1976) 2005;30:2342–2349. doi: 10.1097/01.brs.0000182109.36973.93. [DOI] [PubMed] [Google Scholar]
- 4.Dickman CA, Rosenthal D, Karahalios DG, Paramore CG, Mican CA, Apostolides PJ, Lorenz R, Sonntag VK. Thoracic vertebrectomy and reconstruction using a microsurgical thoracoscopic approach. Neurosurgery. 1996;38:279–293. doi: 10.1097/00006123-199602000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Faciszewski T, Winter RB, Lonstein JE, Denis F, Johnson L. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults. A review of 1223 procedures. Spine (Phila Pa 1976) 1995;20:1592–1599. doi: 10.1097/00007632-199507150-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson AR, Stock FE, Fang HS, Ong GB. Anterior spinal fusion. The operative approach and pathological findings in 412 patients with Pott’s disease of the spine. Br J Surg. 1960;48:172–178. doi: 10.1002/bjs.18004820819. [DOI] [PubMed] [Google Scholar]
- 7.Huang TJ, Hsu RW, Chen SH, Liu HP. Video-assisted thoracoscopic surgery in managing tuberculous spondylitis. Clin Orthop Relat Res. 2000;379:143–153. doi: 10.1097/00003086-200010000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976) 2005;30:38–43. doi: 10.1097/01.brs.0000154674.16708.af. [DOI] [PubMed] [Google Scholar]
- 9.Huang TJ, Hsu RW, Sum CW, Liu HP. Complications in thoracoscopic spinal surgery: a study of 90 consecutive patients. Surg Endosc. 1999;13:346–350. doi: 10.1007/s004649900987. [DOI] [PubMed] [Google Scholar]
- 10.Ikard RW. Methods and complications of anterior exposure of the thoracic and lumbar spine. Arch Surg. 2006;141:1025–1034. doi: 10.1001/archsurg.141.10.1025. [DOI] [PubMed] [Google Scholar]
- 11.Jayaswal A, Upendra B, Ahmed A, Chowdhury B, Kumar A. Video-assisted thoracoscopic anterior surgery for tuberculous spondylitis. Clin Orthop Relat Res. 2007;460:100–107. doi: 10.1097/BLO.0b013e318065b6e4. [DOI] [PubMed] [Google Scholar]
- 12.Jin D, Qu D, Chen J, Zhang H. One-stage anterior interbody autografting and instrumentation in primary surgical management of thoracolumbar spinal tuberculosis. Eur Spine J. 2004;13:114–121. doi: 10.1007/s00586-003-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapoor SK, Agarwal PN, Jain BK, Jr, Kumar R. Video-assisted thoracoscopic decompression of tubercular spondylitis: clinical evaluation. Spine (Phila Pa 1976) 2005;30:E605–E610. doi: 10.1097/01.brs.0000182328.03082.e2. [DOI] [PubMed] [Google Scholar]
- 14.Kim YJ, Lenke LG, Bridwell KH, Cheh G, Sides B, Whorton J. Prospective pulmonary function comparison of anterior spinal fusion in adolescent idiopathic scoliosis: thoracotomy versus thoracoabdominal approach. Spine (Phila Pa 1976) 2008;33:1055–1060. doi: 10.1097/BRS.0b013e31816fc3a5. [DOI] [PubMed] [Google Scholar]
- 15.Kossmann T, Jacobi D, Trentz O. The use of a retractor system (SynFrame) for open, minimal invasive reconstruction of the anterior column of the thoracic and lumbar spine. Eur Spine J. 2001;10:396–402. doi: 10.1007/s005860100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin R, Matusz D, Hasharoni A, Scharf C, Lonner B, Errico T. Mini-open thoracoscopically assisted thoracotomy versus video-assisted thoracoscopic surgery for anterior release in thoracic scoliosis and kyphosis: a comparison of operative and radiographic results. Spine J. 2005;5:632–638. doi: 10.1016/j.spinee.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Moon MS. Tuberculosis of the spine. Controversies and a new challenge. Spine (Phila Pa 1976) 1997;22:1791–1797. doi: 10.1097/00007632-199708010-00022. [DOI] [PubMed] [Google Scholar]
- 18.McAfee PC, Regan JR, Zdeblick T, Zuckerman J, Picetti GD, 3rd, Heim S, Geis WP, Fedder IL. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery. A prospective multicenter study comprising the first 100 consecutive cases. Spine (Phila Pa 1976) 1995;20:1624–1632. doi: 10.1097/00007632-199507150-00012. [DOI] [PubMed] [Google Scholar]
- 19.Mückley T, Schütz T, Schmidt MH, Potulski M, Bühren V, Beisse R. The role of thoracoscopic spinal surgery in the management of pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976) 2004;29:E227–E233. doi: 10.1097/00007632-200406010-00023. [DOI] [PubMed] [Google Scholar]
- 20.Ozdemir HM, Us AK. The role of anterior spinal instrumentation and allograft fibula for the treatment of Pott disease. Spine (Phila Pa 1976) 2003;28:474–479. doi: 10.1097/01.BRS.0000048666.17934.17. [DOI] [PubMed] [Google Scholar]
- 21.Regan JJ, Guyer RD. Endoscopic techniques in spinal surgery. Clin Orthop Relat Res. 1997;335:122–139. [PubMed] [Google Scholar]
- 22.Upadhyay SS, Sell P, Saji MJ, Sell B, Hsu LC. Surgical management of spinal tuberculosis in adults. Hong Kong operation compared with debridement surgery for short and long term outcome of deformity. Clin Orthop Relat Res. 1994;302:173–182. [PubMed] [Google Scholar]
- 23.Watanabe K, Yabuki S, Konno S, Kikuchi S. Complications of endoscopic spinal surgery: a retrospective study of thoracoscopy and retroperitoneoscopy. J Orthop Sci. 2007;12:42–48. doi: 10.1007/s00776-006-1086-x. [DOI] [PubMed] [Google Scholar]