Abstract
Objective
Based on the neuroprotective effects of granulocyte colony-stimulating factor (G-CSF) on experimental spinal cord injury, we initiated a clinical trial that evaluated the safety and efficacy of neuroprotective therapy using G-CSF for patients with worsening symptoms of compression myelopathy.
Methods
We obtained informed consent from 15 patients, in whom the Japanese Orthopaedic Association (JOA) score for cervical myelopathy decreased two points or more during a recent 1-month period. G-CSF (5 or 10 μg/kg/day) was intravenously administered for five consecutive days. We evaluated motor and sensory functions of the patients and the presence of adverse events related to G-CSF therapy.
Results
G-CSF administration suppressed the progression of myelopathy in all 15 patients. Neurological improvements in motor and sensory functions were obtained in all patients after the administration, although the degree of improvement differed among the patients. Nine patients in the 10-μg group (n = 10) underwent surgical treatment at 1 month or later after G-CSF administration. In the 10-μg group, the mean JOA recovery rates 1 and 6 months after administration were 49.9 ± 15.1 and 59.1 ± 16.3%, respectively. On the day following the start of G-CSF therapy, the white blood cell count increased to more than 22,700 cells/mm3. It varied from 12,000 to 50,000 and returned to preadministration levels 3 days after completing G-CSF treatment. No serious adverse events occurred during or after treatment.
Conclusion
The results indicate that G-CSF administration at 10 μg/kg/day is safe for patients with worsening symptoms of compression myelopathy and may be effective for their neurological improvement.
Keywords: Neuroprotective therapy, Granulocyte colony-stimulating factor, Compression myelopathy, Clinical trial
Introduction
Chronic compression of the spinal cord by osteophytes and ossification of the posterior longitudinal ligament (OPLL) causes compression myelopathy [1, 6]. Such myelopathy usually progresses with a slow, stepwise decline in function. In some patients, however, motor paresis and paresthesia rapidly progress with mild or no trauma. According to a previous study, the severity of compression myelopathy rapidly worsened in almost 5% of patients [19]. Rapidly worsening compressive myelopathy results in severe neurological deficits with poor functional recovery because of limited axonal regeneration [1, 3, 6, 24]. To date, early surgical treatment has been the only effective therapy [17, 25].
Granulocyte colony-stimulating factor (G-CSF) is a 19.6 kDa glycoprotein. This cytokine promotes survival, proliferation, and differentiation of cells in the neutrophil lineage [13, 16]. Furthermore, G-CSF can mobilize both immature and mature bone marrow cells into the peripheral blood. As a result, it is used clinically for patients with leukocytopenia and for donors of peripheral blood-derived hematopoietic stem cells for transplantation. Several recent reports have indicated that G-CSF also has nonhematopoietic functions and can potentially be used for the treatment of neuronal injury, including stroke and neurodegenerative diseases [4, 7, 9, 18, 20]. We previously demonstrated that G-CSF promoted the restoration of damaged spinal cord tissue and the recovery of neural function in experimental spinal cord injury in both mice and rats [8, 14]. In addition, we showed that G-CSF promoted the migration of bone marrow-derived cells into the damaged spinal cord, suppressed apoptosis of neuronal cells and oligodendrocytes, protected myelin, decreased inflammation, and promoted angiogenesis [8, 14]. Based on these results, we have suggested that G-CSF is a candidate for neuroprotective therapy for worsening symptoms of compression myelopathy.
Recently, we began a phase I and IIa clinical trial for the purpose of evaluating the safety and efficacy of neuroprotective therapy using G-CSF for patients with worsening symptoms of compression myelopathy. In the present study, we evaluated the results of this trial.
Methods
This clinical trial was performed with the approval of the Institutional Review Board of our university. We recruited patients 20–75 years of age, in whom the Japanese Orthopaedic Association (JOA) score for cervical myelopathy decreased two points or more during a recent 1-month period. We excluded patients in the following categories: (1) those with intracranial pathologies (e.g., tumors, infection, or ischemia); (2) those having a history of major bleeding requiring blood transfusion or a history of leukopenia, thrombocytopenia, or hepatic or renal dysfunction, severe heart failure, or splenomegaly; (3) those with evidence of malignant disease within the past 5 years. We also excluded patients who were pregnant or nursing. Eligible patients gave informed consent for participation in the trial.
In the first stage of this trial, G-CSF (5 μg/kg/day) was intravenously administered for five consecutive days (the 5-μg group). We conducted an open-label study, and a control group was not used. We evaluated common criteria for adverse event reporting, version 3.0. We also evaluated the patients’ severity of myelopathy, using JOA scores (cervical myelopathy scores range from 0 to 17, thoracic myelopathy scores range from 0 to 11) [10]. We then evaluated their motor and sensory functions by calculating scores of muscle power, touch sensation, and pain sensation according to the American Spinal Injury Association (ASIA) score (motor scores range from 0 to 100, light touch and pin prick scores range from 0 to 112) [11]. In the present study, two orthopedic spine surgeons specializing in cervical and thoracic spine surgery evaluated patients’ neurological status independently every month until 6 months after G-CSF administration, and calculated the mean data. In addition, we analyzed hematological data from the treated patients. During the first stage (the 5-μg group), we did not restrict the time of surgery of patients and performed surgical treatment according to the patients’ directives.
At the second stage, G-CSF (10 μg/kg/day) was similarly administered for five consecutive days (the 10-μg group). We evaluated adverse events, JOA score, scores of muscle power, touch sensation and pain sensation, and hematological data, as done with the 5-μg group. A major difference of the study design between the 5-μg group and the 10-μg group was a restriction of the time of surgery after G-CSF administration. In the 10-μg group, to evaluate neurological improvement resulting from neuroprotective therapy with G-CSF, we planned to follow patients without surgical treatment for 1 month after G-CSF administration. When patients were given informed consent documents, we explained our plans regarding the time of surgery, and we administered G-CSF only to those patients who agreed with the protocol. One month after G-CSF administration, we performed surgical treatment according to the patients’ wishes. But when myelopathy progressed and patients wanted to initiate surgery, we abandoned the original schedule and performed surgery according to the patients’ requests regardless of the timing relative to G-CSF administration.
Statistical analysis was performed using a Mann–Whitney U test. A p value <0.05 was considered statistically significant. Results are presented as mean values ± standard deviation of the mean.
Results
The 5-μg group
Between June 2008 and May 2009, a total of five patients were enrolled in the first stage of this trial, and all the patients had cervical and/or thoracic myelopathy due to ossification of the spinal ligament, such as OPLL and ossification of the ligamentum flavum (OLF) (Table 1). In all five of the patients, the JOA score decreased two points or more over a recent 1-month period (Table 2). Neurological improvements in both motor and sensory functions were observed in all five patients by the seventh day following the start of G-CSF administration, though the degree of the improvement differed depending on the patient (Table 4). The five patients underwent surgical treatment after G-CSF administration; one patient underwent posterior decompression and four patients posterior decompression with instrumented fusion. The time between the first day of G-CSF administration and surgery ranged from 9 to 115 days.
Table 1.
Patients who underwent G-CSF therapy
| Case no. | Dose of G-CSF (μg/kg/day) | Age (years)/gender | Diagnosis | Most stenotic level | Surgical procedure | Time of surgery after G-CSF administration (days) | Follow-up period after G-CSF administration (months) |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 61/M | T-OLF | T10–11 | PD (T2–3, T9–11) | 49 | 6 |
| 2 | 5 | 68/M | T-OPLL | T4–5 | PDF (T1–T7) | 10 | 6 |
| 3 | 5 | 51/M | T-OPLL | T1–2 | PDF (C7–T5) | 10 | 6 |
| 4 | 5 | 37/M | T-OPLL | T3–4 | PDF (T1–T10) | 9 | 6 |
| 5 | 5 | 35/M | C- and T-OPLL | C6–7 | PDF (C2–T4) | 115 | 6 |
| 6 | 10 | 46/M | T-OPLL | T7–8 | PDF (T4–T11) | 59 | 6 |
| 7 | 10 | 67/M | C-OPLL | C5–6 | NS | NS | 6 |
| 8 | 10 | 75/M | C-OPLL | C3–4 | PDF (C2–T1) | 49 | 6 |
| 9 | 10 | 64/M | C-OPLL | C3–4 | PDF (C2–T1) | 41 | 6 |
| 10 | 10 | 32/M | T-OPLL | T7–8 | PDF (T4–T12) | 29 | 6 |
| 11 | 10 | 67/M | T-OLF | T11–12 | PD (T10–12) | 33 | 6 |
| 12 | 10 | 46/M | CSM | C5–6 | PD (C3–7) | 94 | 6 |
| 13 | 10 | 66/M | CSM | C4–5 | PD (C3–7) | 73 | 6 |
| 14 | 10 | 67/M | CSM | C4–5 | PDF (C2–T1) | 67 | 6 |
| 15 | 10 | 74/M | CSM | C7–T1 | PD (C7–T1) | 30 | 6 |
M male, T thoracic, OLF ossification of ligamentum flavum, PD posterior decompression, OPLL ossification of the posterior longitudinal ligament, PDF posterior decompression with instrumented fusion, C cervical, NS no surgery
Table 2.
JOA score before and after G-CSF administration (5 μg group)
| Case no. | JOA score | Recovery rate | ||
|---|---|---|---|---|
| 1 month before administration | Immediately before administration | 6 months after administration | 6 months after administration | |
| 1 | 6/11 | 1/11 | 4/11 | 30.0 |
| 2 | 5.5/11 | 3/11 | 8/11 | 62.5 |
| 3 | 7/11 | 3.5/11 | 11/11 | 100.0 |
| 4 | 6/11 | 2/11 | 6.5/11 | 50.0 |
| 5 | 4.5/17 | 2.5/17 | 6.5/17 | 27.6 |
| Mean ± SD | 54.0 ± 26.4 | |||
Recovery rate = (postoperative score − preoperative score/full score − preoperative score) × 100 (%)
JOA score Japanese Orthopaedic Association score (cervical myelopathy: 0–17 points, thoracic myelopathy: 0–11 points)
Table 4.
Scores of muscle power, touch sensation, and pain sensation before and after G-CSF administration
| Group | Before | Time after initiating G-CSF administration | ||
|---|---|---|---|---|
| 7 d | 1 m | 6 m | ||
| Muscle power | ||||
| 5 μg | 81.3 ± 12.1 | 89.3 ± 9.9 | 95.5 ± 5.7 | |
| 10 μg | 91.5 ± 6.7 | 98.2** ± 3.0 | 99.5** ± 0.9 | |
| Touch sensation | ||||
| 5 μg | 78.5 ± 7.4 | 77.0 ± 8.4 | 99.5 ± 16.0 | |
| 10 μg | 92.5 ± 14.3 | 98.3 ± 15.4 | 106.6* ± 5.9 | |
| Pain sensation | ||||
| 5 μg | 78.5 ± 7.4 | 79.5 ± 12.4 | 98.0 ± 15.4 | |
| 10 μg | 89.0 ± 14.5 | 100.5* ± 11.3 | 106.0* ± 6.1 | |
Scores of muscle power, touch sensation and pain sensation was defined according to the American Spinal Injury Association score (motor: 0–100, light touch and pin prick: 0–112). Before: immediately before G-CSF administration
7 d 7 days after G-CSF administration, 1 m 1 month after G-CSF administration, 6 m 6 months after G-CSF administration
* p < 0.05 compared with that before G-CSF administration
** p < 0.01 compared with that before G-CSF administration
One day after the start of G-CSF therapy, the white blood cell (WBC) count increased to more than 15,200 cells/mm3 (Table 5). It remained elevated (from 15,200 to 43,200) during the administration, and returned to preadministration levels within 3 days of the final G-CSF treatment. G-CSF selectively mobilized cells of the neutrophil lineage, while neither monocytes nor lymphocytes were affected (Table 5). There was no change in inflammation during G-CSF administration, as indicated by C-reactive protein levels, except for an instance of surgical site infection (Table 5). One patient (case 4) developed a surgical site infection 14 days after G-CSF administration (5 days after surgery). The infection was relieved by debridement of the infection site and administration of antibiotics. No relation was found between the infection and the G-CSF administration. No other adverse event occurred during or after the administration.
Table 5.
Blood data before and after G-CSF administration
| Group | Baseline | After G-CSF administration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 day | 2 days | 3 days | 4 days | 5 days | 6 days | 7 days | 14 days | 1 month | 6 months | ||
| 5 μg | |||||||||||
| WBC (×103/mm3) | 7.2 ± 1.6 | 26.7* ± 10.7 | 25.0* ± 5.5 | 24.9* ± 6.6 | 23.3* ± 9.3 | 20.8* ± 9.6 | 10.4 ± 3.2 | 8.2 ± 2.4 | 8.2 ± 2.4 | 7.3 ± 2.8 | 7.2 ± 0.4 |
| Neutrophils (×103/mm3) | 4.5 ± 1.5 | 22.1* ± 9.2 | 20.9* ± 5.8 | 20.6* ± 6.1 | 19.0* ± 7.7 | 151.9* ± 7.7 | 6.8 ± 2.8 | 5.1 ± 2.0 | 5.9 ± 2.4 | 4.7 ± 2.3 | 4.1 ± 0.1 |
| CRP (mg/dl) | 0.7 ± 1.2 | 0.8 ± 1.3 | 0.8 ± 1.3 | 0.8 ± 1.1 | 0.8 ± 1.0 | 0.7 ± 1.0 | 0.7 ± 1.0 | 0.8 ± 0.9 | 4.6*,a ± 6.9 | 2.9*,a ± 6.1 | 0.2 ± 0.2 |
| 10 μg | |||||||||||
| WBC (×103/mm3) | 6.1 ± 1.6 | 29.3* ± 4.8 | 31.5* ± 5.6 | 35.2* ± 7.2 | 27.8* ± 9.3 | 25.1* ± 8.0 | 10.5 ± 2.8 | 6.7 ± 1.6 | 4.8 ± 1.9 | 6.0 ± 1.9 | 6.8 ± 2.1 |
| Neutrophils (×103/mm3) | 3.5 ± 1.1 | 25.4* ± 4.2 | 25.1* ± 8.8 | 29.8* ± 6.2 | 22.4* ± 7.7 | 20.0* ± 6.5 | 6.6 ± 2.2 | 3.9 ± 1.2 | 2.8 ± 1.4 | 3.4 ± 1.2 | 4.0 ± 1.6 |
| CRP (mg/dl) | 0.3 ± 0.8 | 0.6 ± 1.3 | 1.1 ± 2.6 | 1.6 ± 3.4 | 1.4 ± 2.4 | 1.8 ± 2.9 | 2.0 ± 4.3 | 1.7 ± 3.3 | 0.7 ± 1.2 | 0.4 ± 0.5 | 0.1 ± 0.1 |
* p < 0.05 compared with the baseline level
aIncrease due to the surgical site infection of case 4
The 10-μg group
Between July 2009 and February 2010, a total of ten patients were enrolled in the second stage of this trial: six patients had cervical and thoracic myelopathy because of ossification of the spinal ligament, such as OPLL and OLF, and four patients had cervical spondylotic myelopathy (CSM) (Table 1). In all ten of the patients, the JOA score had decreased two points or more over a recent 1-month period (Table 3). One month after administration, the mean JOA recovery rate was 49.9 ± 15.1% (Table 3), and the muscle power score was significantly improved compared with that before G-CSF administration (Table 4). Nine patients underwent surgical treatment at 1 month or later after G-CSF administration. Six months after administration, the mean JOA recovery rate was 59.1 ± 16.3% (Table 2), and scores of muscle power, touch sensation, and pain sensation were significantly improved compared with those before G-CSF administration (Table 4). One day after the start of G-CSF therapy, the WBC count increased to more than 22,700 (Table 5). It remained elevated (up 12,500 to 50,000) during the administration, and returned to preadministration levels within 3 days of the final G-CSF treatment. G-CSF successfully mobilized cells of the neutrophil lineage, but neither monocytes nor lymphocytes were affected (Table 5). There was no significant change in inflammation during G-CSF administration, as indicated by C-reactive protein levels (Table 5). No adverse event occurred during or after the administration.
Table 3.
JOA score before and after G-CSF administration (10 μg group)
| Case no. | JOA score | Recovery rate | ||||
|---|---|---|---|---|---|---|
| 1 month before administration | Immediately before administration | 1 month after administration | 6 months after administration | 1 month after administration | 6 months after administration | |
| 6 | 7.5/11 | 5.5/11 | 9/11 | 9/11 | 63.6 | 63.6 |
| 7 | 16.5/17 | 11.5/17 | 14/17 | 14/17 | 45.5 | 45.5 |
| 8 | 16/17 | 8.5/17 | 14.5/17 | 14.5/17 | 70.6 | 70.6 |
| 9 | 14/17 | 9.5/17 | 14.5/17 | 15/17 | 66.7 | 73.3 |
| 10 | 6/11 | 4/11 | 6/11 | 6/11 | 28.6 | 28.6 |
| 11 | 6/11 | 4/11 | 6.5/11 | 6.5/11 | 35.7 | 35.7 |
| 12 | 14/17 | 11.5/17 | 14/17 | 16/17 | 45.5 | 81.8 |
| 13 | 12/17 | 7.5/17 | 13/17 | 14/17 | 57.9 | 68.4 |
| 14 | 6/17 | 0/17 | 4.5/17 | 11/17 | 26.5 | 64.7 |
| 15 | 7.5/11 | 5/11 | 8.5/11 | 8.5/11 | 58.3 | 58.3 |
| Mean ± SD | 49.9 ± 15.1 | 59.1 ± 16.3 | ||||
Recovery rate = (postoperative score − preoperative score/full score − preoperative score) × 100 (%)
JOA score Japan Orthopaedic Association score (cervical myelopathy: 0–17 points, thoracic myelopathy: 0–11 points)
Case presentation
Case 7
A 67-year-old man was admitted to our hospital with a complaint of progression of myelopathy. Over the preceding 2 weeks, a loss of muscle power in his upper and lower extremities had rapidly progressed, and gait disturbance developed. Previously, he had undergone surgical treatment for cervical myelopathy because of OPLL: C3–C7 laminoplasty at 64 years of age. After that operation, he could run and slight numbness was present at his finger; his JOA scale score was 16.5 points at 1 month before administration.
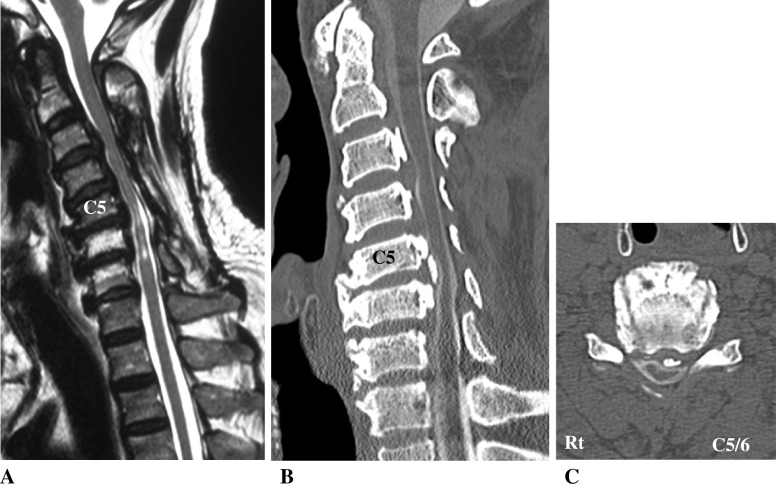
On admission, he showed severe loss of sensation below the C6–T1 dermatome level, and muscle strength of his upper extremities decreased to 2–4/5 and lower extremities decreased to 4/5 in manual muscle testing. He could not walk without a cane for assistance. Deep tendon reflexes were hyperactive in bilateral triceps tendons and lower extremities, and Babinski’s sign was positive bilaterally. His bladder function was normal, and his JOA score was 11.5 points. Examination with computed tomography (CT) and magnetic resonance (MR) imaging showed anterior compression of the spinal cord by segmental type OPLL at C3–C7 (Fig. 1). Especially at C5–C6, an ossified mass caused severe anterior compression to the spinal cord.
Fig. 1.
Case 7. T2-weighted midsagittal MR image (a) and a CT midsagittal reconstruction plane (b) and CT axial plane at C5–C6 (c) showing anterior compression of the spinal cord by ossification of posterior longitudinal ligament (OPLL) at C5–C6
He underwent G-CSF administration (10 μg/kg/day) for 5 days. On the fourth day of G-CSF administration, he felt improved muscle strength in both arms and legs. The G-CSF-induced improvement of motor and sensory functions reached a peak level 2 weeks after G-CSF administration; he could walk without a cane, and no deterioration occurred during the following 6 months. He felt no difficulties in daily life, and he returned to his work 3 months after G-CSF administration.
Discussion
In June 2008, we started a phase I and IIa clinical trial that evaluated the safety and efficacy of neuroprotective therapy using G-CSF for patients with worsening symptoms of compression myelopathy. During the first stage of this trial, G-CSF (5 μg/kg/day) was intravenously administered for five consecutive days. The results indicated that neurological improvements in both motor and sensory functions were obtained in all patients, although the degree of improvement differed depending on the patient. No serious adverse events occurred during or after the administration. Previous studies of G-CSF therapy for acute myocardial infarction, acute cerebral infarction, and amyotrophic lateral sclerosis [2, 5, 12, 15, 21–23, 26, 27] have used a dose of 10 μg/kg/day G-CSF for five consecutive days (Table 6). Therefore, we administered 10 μg G-CSF/kg/day intravenously for five consecutive days for the second stage of this trial. No adverse events occurred, and all patients have shown neurological improvements. This suggests that G-CSF therapy at a dose of 10 μg/kg/day for 5 days is safe for patients with worsening symptoms of compression myelopathy.
Table 6.
Clinical trials using G-CSF injection
| Author [ref.] | Sample size | Clinical scenario | G-CSF dose (μg/kg/day) | Route of administration | Duration of G-CSF therapy (days) | Peak WBC count (×103/μl) |
|---|---|---|---|---|---|---|
| Engelmann et al. [2] | 23 | AMI | 10 | s.c. | 5 | 42.9 ± 25.7 |
| Ince et al. [5] | 15 | AMI | 10 | s.c. | 6 | 55 ± 8 |
| Nefussy et al. [12] | 19 | ALS | 5 | s.c. | 4 | 30.0 ± 7.2 |
| Ripa et al. [15] | 39 | AMI | 10 | s.c. | 6 | 51 ± 8 |
| Shyu et al. [21] | 7 | CI | 15 | s.c. | 5 | 42.9 ± 9.6 |
| Takano et al. [22] | 18 | AMI | 2.5 | s.c. | 5 | 29.4 ± 9 |
| Valgimigli et al. [23] | 10 | AMI | 5 | s.c. | 4 | 35 ± 11 |
| Zohlnhofer et al. [27] | 56 | AMI | 10 | s.c. | 5 | 48 ± 15 |
| Our cases | 5 | Myelopathy | 5 | i.v. | 5 | 26.7 ± 10.7 |
| 10 | Myelopathy | 10 | i.v. | 5 | 35.2 ± 7.2 |
CI cerebral infarction, AMI acute myocardial infarction, ALS amyotrophic lateral sclerosis, s.c. subcutaneous injection, i.v. intravenous injection
In the present study, the increase of WBC counts after G-CSF administration was lower than that in other clinical studies using G-CSF [2, 5, 12, 15, 21–23, 26, 27]. One of the reasons for this seems to be that we performed G-CSF therapy for a chronic disease, whereas other studies performed G-CSF therapy for the acute phase of disease. In addition, we suggest that the route of G-CSF administration could contribute to the lower WBC increases in the present study. G-CSF was intravenously administered in our study, while it was subcutaneously administered in other studies [2, 5, 12, 15, 21–23, 26, 27].
In the ten patients enrolled in the second stage of this trial, G-CSF suppressed the progression of myelopathy. In addition, neurological improvements in both motor and sensory functions were obtained in all patients. The study design was open-label, and no control group was instituted. In spite of such limitations, the present results indicate that G-CSF had a neuroprotective effect on worsening symptoms of compression myelopathy.
In this trial, one patient (case 7) did not choose surgery because neurological recovery after the G-CSF administration was evident. In other cases, neurological improvement was also obtained though the degree of improvement differed among individual cases. This result indicated that other cases in addition to case 7 might have been able to avoid surgery. We suggest that by introducing G-CSF neuroprotective therapy, extremely conservative treatment may be possible for patients with worsening symptoms of compression myelopathy, and surgical treatment can be avoided in some patients.
We had planned a third stage for this clinical trial with G-CSF administration of 15 μg/kg/day for 5 days. Based on the present results, however, we cancelled the third stage because G-CSF therapy at a dose of 10 μg/kg/day caused sufficient neurological improvement. In addition, G-CSF therapy at a dose of 10 μg/kg/day increased WBC counts to 50,000 cells/mm3 in one patient. Thus, it is possible that G-CSF therapy at a dose of 15 μg/kg/day could cause side effects.
We intend to advance to a phase IIb clinical trial for accurate assessment of the efficacy of G-CSF therapy. Based on the present results, we will use G-CSF at a dose of 10 μg/kg/day for 5 days. The study design will be a multi-center prospective controlled clinical trial, and a control group without G-CSF administration will be incorporated. By undertaking this phase IIb clinical trial, we wish to establish the efficacy of the G-CSF neuroprotective therapy for patients with worsening symptoms of compression myelopathy. To date, there have been no reports of a drug that improves neurological status in patients with worsening symptoms of compression myelopathy. If the efficacy and safety of G-CSF treatment for worsening symptoms of compression myelopathy are established and clinical use of G-CSF neuroprotective therapy is approved, a novel and effective approach for the treatment of this disorder will be available.
Acknowledgments
This work was supported by a Health Labour Science Research Grant of Japan.
Conflict of interest
None.
References
- 1.Baba H, Maezawa Y, Imura S, et al. Quantitative analysis of the spinal cord motoneuron under chronic compression: an experimental observation. J Neurol. 1996;243:109–116. doi: 10.1007/BF02443999. [DOI] [PubMed] [Google Scholar]
- 2.Engelmann MG, Theiss HD, Hennig-Theiss C, et al. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) trial. J Am Coll Cardiol. 2006;48:1712–1721. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 3.Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine. 1998;23:2730–2737. doi: 10.1097/00007632-199812150-00012. [DOI] [PubMed] [Google Scholar]
- 4.Gibson CL, Jones NC, Prior MJ, et al. G-CSF suppresses edema formation and reduces interleukin-1β expression after cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:431–439. doi: 10.1038/sj.jcbfm.9600033. [DOI] [PubMed] [Google Scholar]
- 5.Ince H, Petzsch M, Kleine HD, et al. Prevention of left ventricular remodeling with granulocyte colony-stimulating factor after acute myocardial infarction: final 1-year results of the Front-integrated Revascularization and Stem Cell Liberation in Evolving Acute Myocardial Infarction by Granulocyte Colony-Stimulating Factor (FIRSTLINE-AMI) Trial. Circulation. 2005;112:173–180. doi: 10.1161/CIRCULATIONAHA.105.541433. [DOI] [PubMed] [Google Scholar]
- 6.Kameyama T, Hashizume Y, Ando T, et al. Spinal cord morphology and pathology in ossification of the posterior longitudinal ligament. Brain. 1995;118:263–278. doi: 10.1093/brain/118.1.263. [DOI] [PubMed] [Google Scholar]
- 7.Kawada H, Takizawa S, Takanashi T, et al. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113:701–710. doi: 10.1161/CIRCULATIONAHA.105.563668. [DOI] [PubMed] [Google Scholar]
- 8.Koda M, Nishio Y, Kamada T, et al. Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow-derived cells into injured spinal cord and promotes functional recovery after compression-induced spinal cord injury in mice. Brain Res. 2007;1149:223–231. doi: 10.1016/j.brainres.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 9.Komine-Kobayashi M, Zhang N, Liu M, et al. Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice. J Cereb Blood Flow Metab. 2006;26:402–413. doi: 10.1038/sj.jcbfm.9600195. [DOI] [PubMed] [Google Scholar]
- 10.Masaki Y, Yamazaki M, Okawa A, et al. An analysis of factors causing poor surgical outcome in patients with cervical myelopathy due to ossification of the posterior longitudinal ligament: anterior decompression with spinal fusion versus laminoplasty. J Spinal Disord Tech. 2007;20:7–13. doi: 10.1097/01.bsd.0000211260.28497.35. [DOI] [PubMed] [Google Scholar]
- 11.Maynard FM, Jr, Bracken MB, Creasey G, et al. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord. 1997;35:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- 12.Nefussy B, Artamonov I, Deutsch V, et al. Recombinant human granulocyte-colony stimulating factor administration for treating amyotrophic lateral sclerosis: a pilot study. Amyotroph Lateral Scler. 2010;11:187–193. doi: 10.3109/17482960902933809. [DOI] [PubMed] [Google Scholar]
- 13.Nicola NA, Metcalf D, Matsumoto M, et al. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983;258:9017–9023. [PubMed] [Google Scholar]
- 14.Nishio Y, Koda M, Kamada T, et al. Granulocyte colony-stimulating factor attenuates neuronal death and promotes functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol. 2007;66:724–731. doi: 10.1097/nen.0b013e3181257176. [DOI] [PubMed] [Google Scholar]
- 15.Ripa RS, Jorgensen E, Wang Y, et al. Stem cell mobilization induced by subcutaneous granulocyte colony-stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation. 2006;113:1983–1992. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- 16.Roberts AW. G-CSF: a key regulator of neutrophil production, but that’s no all! Growth Factors. 2005;23:33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- 17.Sampath P, Bendebba M, Davis JD, et al. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine. 2000;25:670–676. doi: 10.1097/00007632-200003150-00004. [DOI] [PubMed] [Google Scholar]
- 18.Schäbitz WR, Kollmar R, Schwaninger M, et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34:745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt MH, Quinones-Hinojosa A, Rosenberg WS. Cervical myelopathy associated with degenerative spine disease and ossification of the posterior longitudinal ligament. Semin Neuronal. 2002;22:143–148. doi: 10.1055/s-2002-36537. [DOI] [PubMed] [Google Scholar]
- 20.Schneider A, Kuhn HG, Schäbitz WR. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle. 2005;4:1753–1757. doi: 10.4161/cc.4.12.2213. [DOI] [PubMed] [Google Scholar]
- 21.Shyu WC, Lin SZ, Lee CC, et al. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 2006;174:927–933. doi: 10.1503/cmaj.051322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takano H, Hasegawa H, Kuwabara Y, et al. Feasibility and safety of granulocyte colony-stimulating factor treatment in patients with acute myocardial infarction. Int J Cardiol. 2007;122:41–47. doi: 10.1016/j.ijcard.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Valgimigli M, Rigolin GM, Cittanti C, et al. Use of granulocyte-colony stimulating factor during acute myocardial infarction to enhance bone marrow stem cell mobilization in humans: clinical and angiographic safety profile. Eur Heart J. 2005;26:1838–1845. doi: 10.1093/eurheartj/ehi289. [DOI] [PubMed] [Google Scholar]
- 24.Wada S, Yone K, Ishidou Y, et al. Apoptosis following spinal cord injury in rats and preventative effect of N-methyl-d-aspartate receptor antagonist. J Neurosurg. 1999;91:98–104. doi: 10.3171/spi.1999.91.1.0098. [DOI] [PubMed] [Google Scholar]
- 25.Wang YL, Tsau JC, Huang MH. The prognosis of patients with cervical spondylotic myelopathy. Kaohsiung J Med Sci. 1997;13:425–431. [PubMed] [Google Scholar]
- 26.Zhang Y, Wang L, Fu Y, et al. Preliminary investigation of effect of granulocyte colony stimulating factor on amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:430–431. doi: 10.3109/17482960802588059. [DOI] [PubMed] [Google Scholar]
- 27.Zohlnhofer D, Ott I, Mehilli J, et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA. 2006;295:1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]