Abstract
Purpose
Restoration of the anterior spinal profile and regular load-bearing is the main goal treating anterior spinal defects in case of fracture. Over the past years, development and clinical usage of cages for vertebral body replacement have increased rapidly. For an enhanced stabilization of rotationally unstable fractures, additional antero-lateral implants are common. The purpose of this study was the evaluation of the biomechanical behaviour of a recently modified, in situ distractible vertebral body replacement (VBR) combined with a newly developed antero-lateral polyaxial plate and/or pedicle screws and rods using a full corpectomy model as fracture simulation.
Methods
Twelve human spinal specimens (Th12–L4) were tested in a six-degree-of-freedom spine tester applying pure moments of 7.5 Nm to evaluate the stiffness of three different test instrumentations using a total corpectomy L2 model: (1) VBR + antero-lateral plate; (2) VBR, antero-lateral plate + pedicle screws and rods and (3) VBR + pedicle screws and rods.
Results
In the presented total corpectomy defect model, only the combined antero-posterior instrumentation (VBR, antero-lateral plate + pedicle screws and rods) could achieve higher stiffness in all three-movement planes than the intact specimen. In axial rotation, neither isolated anterior instrumentation (VBR + antero-lateral plate) nor isolated posterior instrumentation (VBR + pedicle screws and rods) could stabilize the total corpectomy compared to the intact state.
Conclusions
For rotationally unstable vertebral body fractures, only combined antero-posterior instrumentation could significantly decrease the range of motion (ROM) in all motion planes compared to the intact state.
Keywords: Vertebral body replacement, Biomechanics, In situ distractible cage, Antero-lateral plate, Fracture, Spine
Introduction
Different causes like tumours, infections and fractures can produce instabilities of the anterior vertebral column [8, 16, 32]. Posterior instrumentation with pedicle screws and rods is a widely accepted and proved standard procedure for instabilities of the thoracic and lumbar spine [3, 33]. However, isolated posterior instrumentations are often accompanied by complications like a loss of correction or implant failure. Therefore, especially in thoracolumbar burst fractures, an anterior approach for reconstruction and stabilization of the anterior vertebral column is often necessary to restore regular spinal column loading [3, 10, 15, 17, 25, 26, 32, 33]. Additional anterior approaches to the spine and the 360° fusion are also reasonable from the biomechanical point of view: different biomechanical studies, dealing with a total corpectomy defect model to simulate a rotationally instable fracture, reported the highest stiffness in all motion planes for this kind of fractures treated with 360° fusion [15, 17, 25].
Up to now, a tricortical iliac crest bone graft has been the gold standard for vertebral body replacement. However, several authors observed increased morbidity and complications harvesting strut grafts from the iliac crest [2, 13, 17, 18, 20, 28, 36]. Complications, such as pseudarthrosis, graft collapse with kyphotic deformity and graft extrusion were reported [12, 14, 19]. As an alternative, cages for vertebral body replacement in the thoracolumbar spine are used more frequently [1, 4, 5, 15, 17, 25]. One of the first widely used and most popular non-distractible VBR is the meshed titanium cage (DePuy AcroMed, Sulzbach, Germany), according to Lowery and Harms [21]. Recently, also in situ distractible VBRs have been developed. These implants offer some surgical advantages like in situ distractibility and adjustment. However, influenced by the type of vertebral body replacement system, various authors reported differences in the biomechanical behaviour of non-expandable and in situ expandable VBRs especially concerning stiffness [15, 17, 25].
Thoracolumbar fractures type A3.1 according to Magerl et al. [22] can be treated by single anterior procedures. In rotationally unstable fractures type B or type C, the spinal profile should be restored by combined anterior–posterior procedures. Therefore, cages or iliac crest grafts along with an anterior or antero-lateral plate or a rod screw system can be used [9, 11]. Construct stiffness seems to be influenced by anterior plate/rod devices [6, 9]. The different implants are varying in the number of fixation points, modes of screw anchorage and number or type of screw connection (polyaxial vs. angular stable). Positive experiences with height adjustable implants lead to the development of in situ expandable and reducible plates to correct kyphotic spinal angulations or deformities after spinal tumours or fractures [31]. Encouraging results with locking plates in fracture treatment of extremities lead to the application of “angular stable systems” for the thoraco-lumbar spine [31].
The present study evaluated the stiffness of different instrumentations in a total corpectomy defect model using a modified in situ distractible VBR combined with a newly developed antero-lateral polyaxial plate and/or an established pedicle screws and rods. To the author’s best knowledge, the implants used in the actual study have not been tested together in a biomechanical setting until now. Therefore, the purpose of this study was to test, whether the modified vertebral body replacement system and the new additional polyaxial antero-lateral plate have similar biomechanical results in the stabilization of a total vertebral body resection model, than other in situ distractible or non-distractible cages and other additional antero-lateral polyaxial plates.
Materials and methods
Specimens, preparation and defect model
Twelve fresh frozen human cadaveric spines (Th12–L4) of six male and six female donors were used for biomechanical stability testing following total corpectomy and subsequent instrumentation. The average age of the specimens at death was 73 ± 11 years (range 56–89 years; median: 73 years). The medical history of all donors excluded diseases compromising the mechanical properties of the thoracolumbar spine. Bone mineral density (BMD) was evaluated using a pre-interventional quantitative computed tomography (CT) scan (GE Lightspeed16, GE Medical Systems, Waukesha, WI, USA) including European Forearm Phantom calibration (EFP; QMR GmbH, Möhrendorf, Germany). The BMD was measured as a standard laboratory procedure to allow for comparison of bone quality with previous and upcoming experiments and to rule out severely osteoporotic specimens. The measured average BMD was 75.15 ± 19.60 mg/cm3.
Specimens were kept frozen at −20°C and vacuum sealed in two plastic bags until the definite date of biomechanical testing. 12 h before testing, the specimens were thawed over night at 6°C according to Panjabi et al. [24]. Preparation was performed at room temperature right before testing. All muscular tissue was removed and ligaments, discs, capsules as well as supporting structures were preserved. After preparation of Th12–L4, the upper part of the cranial (Th12) and the lower part of caudal (L4) vertebrae were embedded in polymethyl-methacrylate cement (PMMA, Technovit 3040, Heraeus Kulzer, Wehrheim, Germany) ensuring the middle vertebra (L2) was aligned horizontally. Flanges were centrally mounted to the PMMA embeddings. The specimens were rigidly fixed to the frame of the spine tester. Screws for the fixation of the three-dimensional motion analysis system (Winbiomechanics, Zebris Isny, Germany) were fixed to the anterior side of the vertebrae Th12, L1, L3 and L4. Before testing, anterior-posterior and lateral x-rays (BV 25, Phillips, The Netherlands) of all instrumented specimens were performed to check the correct position of pedicle screws, antero-lateral plates and VBRs.
After biomechanical testing of the intact specimens as described below, a total corpectomy L2 was performed simulating a rotationally unstable vertebral fracture. For surgical preparation, specimens were fixed to a customized X-ray jig allowing a 360° rotation. The preparation steps and the corpectomy were performed using regular surgical instruments. The adjacent intervertebral discs L1/2 and L2/3 were removed and the anterior and posterior longitudinal ligaments were cut and resected. The pedicles of each specimen were dissected close to the vertebral body with an oscillating saw and the vertebral body L2 was removed en-bloc.
Implants
All implants were used according to the recommendations of the manufacturer and were implanted in a standardized way as described below.
Pedicle screws and rods
For posterior instrumentation, pedicle screws and rods of the “krypton” system (Ulrich medical, Ulm, Germany) were used. Pedicle screws, connectors, locking screws, rods and cross-connectors are manufactured of titanium alloy. The pedicle entrance points were identified according to the anatomical landmarks and marked with k-wires. The position of the k-wires was controlled with biplanar fluoroscopy. Following identification of the entrance points, they were decortized with the awl. Finally, pedicles were prepared with the cutter before pedicle screws were drilled in. Screw length and diameter of the pedicle screws were determined before implantation by means of CT scan measurements. Screw length was chosen, that screws span 2/3 of the vertebral body depth. Screw diameter was chosen, that screws filled the pedicle. To improve the rotational stability, the internal fixator was combined with a cross-connector fixed to the two internal fixator rods (Fig. 1a).
Fig. 1.
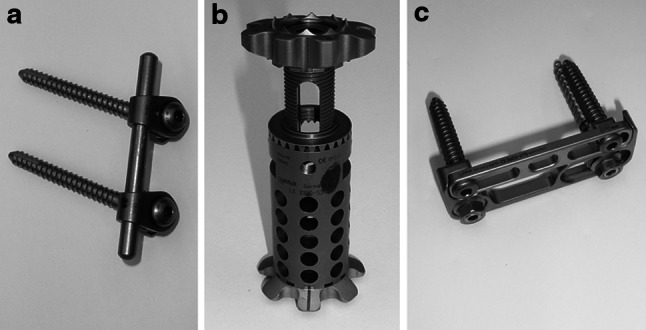
a Vertebral body replacement system (obeliscPro, ulrich medical, Ulm, Germany). b Antero-lateral polyaxial plate (golden gate, ulrich medical, Ulm, Germany). c Angular stable internal fixator (Krypton, ulrich medical, Ulm, Germany)
Expandable VBR
After corpectomy the anterior column was reconstructed using the recently modified VBR “obeliscPro” (Ulrich medical, Ulm, Germany). The implant is manufactured of titanium alloy and consists of a center piece with one or two round and different angulated modular endplates. To prevent dislocation, the endplates are armed with small spikes (Fig. 1b). The necessary height of the implant can be adjusted with a bevel gear drive unit. The adjusted height arrests and is additionally fixed in position by means of a locking screw. To prevent segmental overdistraction insertion and distraction of the cage was performed under fluoroscopic control until the small spikes of the endplates had completely penetrated into the cortical part of the vertebral bodies.
Antero-lateral polyaxial plate system
The antero-lateral plate “golden gate” (Ulrich medical, Ulm, Germany) and its components are also manufactured of titanium alloy. The system consists of a low profile footplate (C-plate) and a fixing plate (gate), both of them with a variable length (Fig. 1c). For fixing the implant to the vertebrae, two k-wires were drilled under fluoroscopic control in the sagittal plane of the vertebras L1 and L3. After bone decortication, two cannulated polyaxial screws were placed mono-cortically into the vertebras. For assembling “golden gate” to the vertebras, the C-plate must be mounted to the heads of the polyaxial screws via connectors. The gate was inserted and locked onto the C-plate by fixing screws. For compression of the specimen during implantation, an axial preload of 200 N was applied via the X-ray jig by dead weights.
Biomechanical testing
For biomechanical testing a 6 degrees of freedom spine tester described by Knop et al. [17] was used (Fig. 2). Testing was performed according to the recommendations for implant testing of Panjabi et al. [23] and Wilke et al. [34]. To avoid tissue dehydration, all specimens were kept moist with physiological saline solution throughout testing, according to international standards [24, 35]. All tests were performed at room temperature.
Fig. 2.
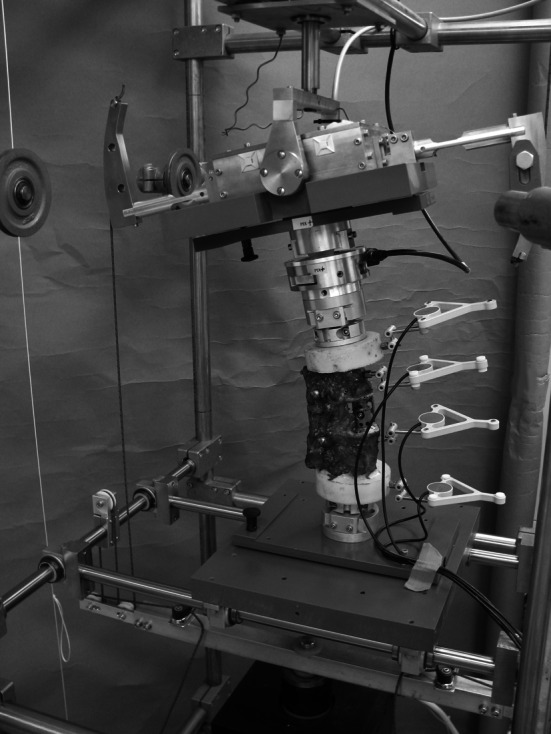
Test setup for the flexibility test in the spine tester with a three-dimensional motion analysis system
Flexibility tests were performed in the three motion planes flexion–extension, lateral bending right/left and axial rotation right/left using pure moments of ±7.5 Nm [34]. Moments and forces induced at the cranial end of the specimen were continuously recorded by a six-component load cell (Schunk FT Delta SI 660-60, Lauffen/Neckar, Germany). Segmental motions from Th12–L4 were measured using an ultrasound based motion analysis system (Winbiomechanics, Zebris©, Isny, Germany). The range of motion (ROM) and the neutral zone (NZ) of the bridged segment L1–L3 were determined from hysteresis curves [34]. ROM was calculated of the minimum and maximum angular displacement during minimum and maximum bending moment of each motion plane. The opening of the hysteresis curve at 0 Nm bending moment was taken as NZ. To allow preconditioning of the specimens and to minimize the visoelastic effect only the third load cycle was evaluated and used for further analysis and comparisons. To compensate for initial differences in ROM of the used specimens, the calculated ROM for all instrumented states of one specimen were normalized to the intact state of corresponding specimen.
Study protocol
The following test sequences were performed. To reduce potential effects caused by implant removal during the test procedure step (b) and (d) were alternated for all 12 specimens:
intact specimen (“int”)
isolated anterior instrumentation with VBR + antero-lateral plate (“ant”)
combined antero-posterior instrumentation with VBR, antero-lateral plate + pedicle screws and rods (“360”)
isolated posterior instrumentation with VBR + pedicle screws and rods (“post”)
Statistical analysis
Statistical analysis was carried out using the SPSS software package (SPSS® V18.0, SPSS Inc, Chicago, USA). Data comparison was performed using one-way repeated measures ANOVA with Bonferroni corrections for multiple comparisons. Significances were defined for P values below 0.05 for all statistical test methods. A trend was specified for P values between 0.05 and 0.1.
Results
Flexion/extension
Compared to the intact specimen in flexion/extension only the combined antero-posterior instrumentation “360” (VBR, antero-lateral plate + pedicle screws and rods) and the isolated posterior instrumentation “post” (VBR pedicle screws and rods) showed a higher stiffness than the intact state. The isolated anterior instrumentation “ant” (VBR + antero-lateral plate) was not able to restore intact stiffness (Table 1). Significant differences could be evaluated between the intact specimens “int” and the combined antero-posterior instrumentation “360” (P = 0.004), between the isolated anterior instrumentation “ant” and the combined antero-posterior instrumentation “360” (P < 0.0001) and between the isolated anterior instrumentation “ant” and the isolated posterior instrumentation “post” (P = 0.016).
Table 1.
Normalized mean ranges of motion (ROM) (%) ± SD in flexion/extension
| State of specimens and test instrumentations | Normalized mean ROM (%) ± SD |
|---|---|
| “int” (intact) | 100 |
| “ant” (VBR and antero-lateral plate) | 114 ± 30 |
| “360” (VBR, antero-lateral plate and internal fixateur fixator) | 53 ± 29 |
| “post” (VBR and internal fixator) | 79 ± 34 |
Axial rotation
Compared to the intact specimen in axial rotation only combined antero-posterior instrumentation “360” (VBR, antero-lateral plate + pedicle screws and rods) showed a higher stiffness than the intact state. Neither isolated anterior instrumentation “ant” (VBR + antero-lateral plate) nor isolated posterior instrumentation “post” (VBR + pedicle screws and rods) could restore the stiffness of the intact specimens (Table 2). Significant differences could be evaluated between the isolated anterior instrumentation “ant” and the combined antero-posterior instrumentation “360” (P < 0.0001) and between the combined antero-posterior instrumentation “360” and the isolated posterior instrumentation “post” (P < 0.001).
Table 2.
Normalized mean ranges of motion (ROM) (%) ± SD in axial rotation
| State of specimens and test instrumentations | Normalized mean ROM (%) ± SD |
|---|---|
| “int” (intact) | 100 |
| “ant” (VBR and antero-lateral plate) | 161 ± 48 |
| “360” (VBR, antero-lateral plate and internal fixateur fixator) | 73 ± 37 |
| “post” (VBR and internal fixator) | 143 ± 47 |
Lateral bending
In lateral bending all instrumentations showed a higher stiffness in comparison to the intact specimens. The combined antero-posterior instrumentation “360” (VBR, antero-lateral plate + pedicle screws and rods) showed the highest reduction of range of motion (Table 3). The combined antero-posterior instrumentation “360” showed a significant reduction in ROM compared to the intact specimens (“int”) (P = 0.002) and the isolated anterior (“ant”) and posterior (“post”) instrumentations (P = 0.001).
Table 3.
Normalized mean ranges of motion (ROM) (%) ± SD in lateral bending
| State of specimens and test instrumentations | Normalized mean ROM (%) ± SD |
|---|---|
| “int” (intact) | 100 |
| “ant” (VBR and antero-lateral plate) | 89 ± 43 |
| “360” (VBR, antero-lateral plate and internal fixateur fixator) | 45 ± 32 |
| “post” (VBR and internal fixator) | 82 ± 34 |
Discussion
The purpose of this study was to evaluate the biomechanical behaviour of an in situ distractible VBR and an antero-lateral plate and/or combined with pedicle screws and rods using a human cadaveric corpectomy defect model. Until now, the VBR and the antero-lateral plate used in this study have not been tested together using a human spine specimen.
Similar to our test scenario Rohlmann et al. [27] evaluated the biomechanical behaviour of an in situ distractible VBR combined with pedicle screws and rods in a total corpectomy L3 model using a three-dimensional, non-linear finite element model of the lumbar spine. After posterior instrumentation and VBR implantation, no significant differences regarding the three-dimensional stiffness could be observed.
Knop et al. [17] compared a non-expandable VBR and an in situ expandable VBR, each of them combined with an anterior instrumentation and/or pedicle screws and rods in 12 human cadaveric spines using a total L1 corpectomy defect model. The authors evaluated an isolated anterior instrumentation and combined antero-posterior instrumentation. Combined antero-posterior instrumentations showed significantly higher stability in flexion/extension and lateral bending compared to isolated anterior or posterior instrumentations, independent of the used VBR (expandable or non-expandable). In axial rotation no differences could be observed and no implant combination was able to restore the rotational stability of the intact specimens. Comparing two vertebral body replacement implants, significantly higher stability was noted for the expandable VBR combined with isolated pedicle screws and rods for extension, lateral bending and axial rotation. The authors could not observe any differences between the expandable and non-expandable VBR combined with isolated anterior instrumentation. The same biomechanical results could be observed in our own present study: The VBR combined with posterior pedicle screws and rods showed significant higher stability in the stabilization of a total corpectomy defect model for extension, lateral bending and axial rotation than isolated anterior or posterior instrumentations.
Similar to our own and the results of Knop et al. [17] were reported by Khodadadyan-Klostermann et al. [15] and Pflugmacher et al. [25]. The authors compared the stability of three in situ expandable VBRs and one non expandable VBR combined with pedicle screws and rods and/or an anterior instrumentation with a locked angular stable plate (“LCDCP”) using a total corpectomy model L1. Overall, the authors could not find any differences in the biomechanical performance between the non-expandable and in situ expandable VBRs. The isolated anterior instrumentations (VBR + antero-lateral plate) showed a significantly lower stiffness and a higher range of motion than the intact specimens. An additional posterior instrumentation significantly increased the stiffness and reduced the range of motion in all motion planes. The highest stiffness and the lowest range of motion were reported for combined antero-posterior instrumentation (VBR, anterior locking plate + pedicle screws and rods). The results of Khodadadyan-Klostermann et al. [15] and Pflugmacher et al. [25] were confirmed also by our own results. An additional plate stabilisation of a VBR and pedicle-screw/rod construct improves the stability of an instrumented total corpectomy defect model and shows the highest stiffness and most reduced range of motion in all motion planes.
Results of an isolated anterior vertebral body replacement were reported by Claes et al. [7] and Schulte et al. [29]. Claes et al. [7] evaluated the stability of a vertebral body replacement prototype for the metastatic spine. The implant consisted of bone-integrating biocompatible materials, a bioglass-polyurethan spacer (PU-C) with integrated plate and screws of carbon-fibre reinforced polyetheretherketone (CF-PEEK), which can be used to fix the spacer antero-lateral at the cranial and caudal adjacent vertebral endplates. The biomechanical testing was performed using a total corpectomy L1 defect model at six human lumbar spine specimens. The PU-C spacer combined with CF-PEEK plate was compared with other anterior instrumentations: a modular segmental spinal system spacer + a lateral placed compression plate system, a modular segmental spinal system spacer + a lateral placed 90-mm long stabilization system and the PU-C spacer + a new 100-mm long antero-lateral placed device system. The authors showed the highest stiffness in all three anatomical directions for the PU-C spacer + the CF-PEEK plate. After corpectomy and instrumentation of the defect by the various implant systems, ROM in flexion/extension was smaller than in the intact spine for all instrumentations. In lateral bending the instrumentation with PU-C spacer + CF-PEEK plate and the PU-C spacer + the new 100-mm long antero-lateral placed device system reduced the RoM, while in axial rotation only the instrumentation with the PU-C spacer + the CF-PEEK plate showed a reduced ROM compared to the intact spine. Therefore, they concluded, that the prototype showed biomechanically comparable results to other studies investigating anterior plating systems or anterior compression plates. These biomechanical in vitro results [7] could be confirmed by Schulte et al. [29] who investigated the same PU-C spacer + the CF-PEEK plate in a prospective in vivo study in five patients with metastatic lesions at the lumbar spine (L1–L4) which were treated by total corpectomy. The authors described a good primary stability in all cases. Follow-up using CT and MRI revealed a progressive osseous integration of the PU-C spacer in the four patients surviving more than 6 months. Results obtained from imaging methods were verified by biomechanical investigation of an explanted autopsy specimen. The biomechanical and in vivo results of Claes et al. [7] and Schulte et al. [29] could not be compared directly to our own evaluations, because the implants used by the authors were manufactured with varying design and material. In vivo usage of conventional VBRs combined with pedicle screws/rods and anterior plating provides also a good primary stability.
Biomechanical analysis using isolated anterior instrumentations of a total corpectomy defect model were also performed by Disch et al. [9]. The authors combined an in situ expandable VBR with an anterior angle stable plating system and a polyaxial plating-system. The angular stable system combined with a VBR showed better results in lateral bending and axial rotation than the combination of VBR and polyaxial plating system. In flexion/extension no differences between the different anterior instrumentations could be observed. Similar to the own results, the isolated anterior instrumentations, only reduced the intact range of motion in lateral bending (ROM). In flexion/extension and axial rotation, the ROM could also not be reduced to the magnitude of the intact specimens. In accordance to our own results, Disch et al. [9] showed, that isolated anterior instrumentations with VBRs and additional anterior plating (polyaxial and angular stable systems) are not suitable in the stabilisation of total corpectomy defect models.
Biomechanical test set-ups of the spine have well known limitations. Due to the in vitro model used in the current experiments any influences of spinal muscles cannot be assessed. Secondary influences of in vivo factors, such as tissue healing and bony consolidation cannot be analysed. Therefore, transferring results of biomechanical investigations to the clinical situation remains difficult. Regarding these limitations, our investigations showed comparable results to the literature [9, 15, 17, 25].
A stabilization of a total corpectomy model in all three motion planes could only be achieved by combined antero-posterior instrumentation “360” (VBR, antero-lateral plate + pedicle screws and rods). Neither isolated anterior instrumentation “ant” (VBR and antero-lateral plate) nor isolated posterior instrumentation “post” (VBR + pedicle screws and rods) could stabilize the total corpectomy defect model in axial rotation to values in the range of the intact specimen. Regarding our own biomechanical results and the reported literature, we recommend combined antero-posterior instrumentation in clinical usage to achieve stability when treating rotationally unstable vertebral fractures.
Conclusion
For a rotationally unstable vertebral body fracture—in this study simulated by a total corpectomy model—only combined antero-posterior instrumentation showed a higher stiffness than the intact specimens, in all motion planes. Therefore, the combined antero-posterior instrumentation should be used when treating rotationally unstable fractures, to obtain primary fracture stability comparable to pre-fractured values.
Acknowledgments
All implants used in the present study were provided for free by Ulrich medical, Ulm, Germany. The study was supported by institutional funds of Ulrich medical, Ulm, Germany.
Conflit of interest
None.
References
- 1.Alici E, Alku OZ, Dost S. Prosthesis designed for vertebral body replacement. J Biomech. 1990;23(8):799–809. doi: 10.1016/0021-9290(90)90027-Z. [DOI] [PubMed] [Google Scholar]
- 2.Banwart JC, Knop C, Lange U, Blauth M. Effect of a crosslink or cerclage on the mechanical stability of an internal fixator. Orthopäde. 1999;28:714–722. [PubMed] [Google Scholar]
- 3.Been HD. Anterior decompression and stabilization of thoracolumbar burst fractures by the use of the Slot-Zielke device. Spine. 1991;16:70–77. doi: 10.1097/00007632-199101000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Blauth M, Knop C, Bastian L, Lobenhoffer P. New developments in surgery of the injured spine. Orthopäde. 1997;26:437–449. doi: 10.1007/s001320050110. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard JA, Koka A, Bensusan JS, Stevenson S, Emery SE. Effects of irradiation on posterior spinal fusions. A rabbit model. Spine. 1994;19(16):1836–1841. doi: 10.1097/00007632-199408150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Brodke DS, Gollogly S, Bachus KN, Mohr RA, Nguyen BK. Anterior thoracolumbar instrumentation: stiffness and load sharing characteristics of plate and rod systems. Spine. 2003;28:1794–1801. doi: 10.1097/01.BRS.0000083201.55495.0E. [DOI] [PubMed] [Google Scholar]
- 7.Claes L, Schultheiss M, Wolf S, Wilke HJ, Arand M, Kinzl L. New radiolucent system for vertebral body replacement its stability in comparison to other systems. J Biomed Mater Res. 1999;48:82–89. doi: 10.1002/(SICI)1097-4636(1999)48:1<82::AID-JBM14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 8.Cybulski GR, Douglas RA, Meyer PR, Rovin AR. Complications in three-column cervical spine injuries requiring anterior–posterior stabilisation. Spine. 1992;17:253–256. doi: 10.1097/00007632-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Disch AC, Knop C, Schaser KD, Blauth M, Schmoelz W. Angular stable anterior plating following thoracolumbar corpectomy reveals superior segmental stability compared to conventional polyaxial plate fixation. Spine. 2008;33(13):1429–1437. doi: 10.1097/BRS.0b013e318175c342. [DOI] [PubMed] [Google Scholar]
- 10.Eysel P, Hopf C, Füderer S. Kyphotic deformities in fractures of the thoracolumbar spine. Orthopäde. 2001;30:955–964. doi: 10.1007/s001320170009. [DOI] [PubMed] [Google Scholar]
- 11.Gebhard F, Schultheiss M (2008) Surgical treatment of fractures of the lumbar spine. In: Käfer W, Cakir B, Mattes T, Reichel H (eds) Orthopaedic spine surgery. An instructional course book. Heidelberg, Steinkopff, pp 129–136
- 12.Gertzbein SD, Court-Brown CM, Jacobs RR, Marks P, Martin C, Stoll J, Fazl M, Schwartz M, Rowed D. Decompression and circumferential stabilization of unstable spinal fractures. Spine. 1988;13(8):892–895. doi: 10.1097/00007632-198808000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Goulet JA, Senunas LE, De Silva GL, Greefield ML. Autogenous iliac crest bone graft. Clin Orthop. 1997;339:76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Kaneda K, Taneichi H, Abumi K, Hashimoto T, Satoh S, Fujiya M. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Joint Surg Am. 1997;79(1):69–83. doi: 10.2106/00004623-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Khodadadyan-Klostermann C, Schaefer J, Schleicher P, Pflugmacher R, Eindorf T, Haas NP, Kandziora F. Expandable cages: biomechanical comparison of different cages for ventral spondylodesis in the thoracolumbar spine. Chirurg. 2004;75:694–701. doi: 10.1007/s00104-003-0786-4. [DOI] [PubMed] [Google Scholar]
- 16.Knop C, Blauth M, Bühren V, Hax PM, Kinzl L, Mutschler W, Pommer A, Ulrich C, Wagner S, Weckbach A, Wentzensen A, Wörsdörfer O. Surgical treatment of injuries of the thoracolumbar transition. 1: Epidemiology. Unfallchirurg. 1999;102(12):924–935. doi: 10.1007/s001130050507. [DOI] [PubMed] [Google Scholar]
- 17.Knop C, Lange U, Bastian L, Blauth M. Three-dimensional motion analysis with Synex. Comparative biomechanical test series with a new vertebral body replacement for the thoracolumbar spine. Eur Spine J. 2000;9:472–485. doi: 10.1007/s005860000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kossmann T, Ertel W, Platz A, Trentz O. Combined surgery for fractures of the thoraco-lumbar junction using the inlay-span method. Orthopäde. 1999;28(5):432–440. doi: 10.1007/s001320050368. [DOI] [PubMed] [Google Scholar]
- 19.Kostuik JP. Anterior fixation for burst fractures of the thoracic and lumbar spine with or without neurological involvement. Spine. 1988;13(3):286–293. doi: 10.1097/00007632-198803000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14(12):1324–1331. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Lowery GL, Harms J (1996) Titanium surgical mesh for vertebral defect replacement and intervertebral spacers. In: Thalgott JS, Aebi M (eds) Manual of internal fixation of the spine. Lippincott-Raven, Philadelphia, pp 127–146
- 22.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 23.Panjabi MM, Krag M, Summers D, Videman T. Biomechanical time-tolerance of fresh cadaveric human spine specimens. J Orthop Res. 1985;3(3):292–300. doi: 10.1002/jor.1100030305. [DOI] [PubMed] [Google Scholar]
- 24.Panjabi MM. Biomechanical evaluation of spinal fixation devices: I. A conceptual framework. Spine. 1988;13(10):1129–1134. doi: 10.1097/00007632-198810000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Pflugmacher R, Schleicher P, Schaefer J, Scholz M, Ludwig K, Khodadadyan-Klostermann C, Haas NP, Kandziora F. Biomechanical comparison of expandable cages for the vertebral body replacement in the thoracolumbar spine. Spine. 2004;29(13):1413–1419. doi: 10.1097/01.BRS.0000129895.90939.1E. [DOI] [PubMed] [Google Scholar]
- 26.Reinhold M, Schmoelz W, Canto F, Krappinger D, Blauth M, Knop C. A new distractible implant for vertebral body replacement: biomechanical testing of four implants for the thoracolumbar spine. Arch Orthop Trauma Surg. 2009;29(10):1375–1382. doi: 10.1007/s00402-009-0823-y. [DOI] [PubMed] [Google Scholar]
- 27.Rohlmann A, Zander T, Fehrmann M, Klöckner C, Bergmann G. Influence of implants for vertebral body replacement on the mechanical behaviour of the lumbar spine. Orthopäde. 2000;3:503–507. doi: 10.1007/s00132-001-0293-6. [DOI] [PubMed] [Google Scholar]
- 28.Sawin PD, Traynelis VC, Menezes AH. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88(2):255–265. doi: 10.3171/jns.1998.88.2.0255. [DOI] [PubMed] [Google Scholar]
- 29.Schulte M, Schultheiss M, Hartwig E, Wilke HJ, Wolf S, Sokiranski R, Fleitner T, Kinzl L, Claes L. Vertebral body replacement with bioglas-polyurethane composite in spine metastases–clinical, radiological and biomechanical results. Eur Spine J. 2000;9(5):437–444. doi: 10.1007/s005860000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultheiss M, Hartwig E, Kinzl L, Claes L, Wilke HJ. Thoracolumbar fracture stabilization: comparative biomechanical evaluation of a new video-assisted implantable system. Eur Spine J. 2004;13:93–100. doi: 10.1007/s00586-003-0640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thalgott JS, Kabins MB, Timlin M, Fritts K, Giuffre JM. Four year experience with the AO anterior thoracolumbar locking plate. Spinal Cord. 1997;35(5):286–291. doi: 10.1038/sj.sc.3100399. [DOI] [PubMed] [Google Scholar]
- 32.Ulmar B, Cakir B, Huch K, Puhl W, Richter M. Expandable titanium cages in vertebral body replacement. Z Orthop. 2004;142(6):449–455. doi: 10.1055/s-2004-820345. [DOI] [PubMed] [Google Scholar]
- 33.Wilke HJ, Wenger K, Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998;7(2):148–154. doi: 10.1007/s005860050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilke HJ, Jungkunz B, Wenger K, Claes LE. Spinal segment range of motion as a function of in vitro test conditions: effects of exposure period, accumulated cycles, angular deformation rate, and moisture condition. Anat Rec. 1998;251(1):15–19. doi: 10.1002/(SICI)1097-0185(199805)251:1<15::AID-AR4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 35.Wippermann BW, Schratt HE, Steeg S, Tscherne H. Complications of spongiosa harvesting of the ilial crest. A retrospective analysis of 1191 cases. Chirurg. 1997;68:1286–1291. doi: 10.1007/s001040050361. [DOI] [PubMed] [Google Scholar]
- 36.Vahldiek MJ, Panjabi MM. Stability potential of spinal instrumentations in tumor vertebral body replacement surgery. Spine. 1998;23:543–550. doi: 10.1097/00007632-199803010-00006. [DOI] [PubMed] [Google Scholar]


