Abstract
Introduction
The purpose of this experimental study was to analyse cervical spine kinematics after 1-level and 2-level total disc replacement (TDR) and compare them with those after anterior cervical arthrodesis (ACA) and hybrid construct. Kinematics and intradiscal pressures were also investigated at adjacent levels.
Methods
Twelve human cadaveric spines were evaluated in different testing conditions: intact, 1 and 2-level TDR (Discocerv™, Scient’x/Alphatec), 1 and 2-level ACA, and hybrid construct. All tests were performed under load control protocol by applying pure moments loading of 2 N m in flexion/extension (FE), axial rotation (AR) and lateral bending (LB).
Results
Reduction of ROM after 1-level TDR was only significant in LB. Implantation of additional TDR resulted in significant decrease of ROM in AR at index level. A second TDR did not affect kinematics of the previously implanted TDR in FE, AR and LB. One and 2-level arthrodesis caused significant decrease of ROM in FE, AR and LB at the index levels. No significant changes in ROM were observed at adjacent levels except for 1-level arthrodesis in FE and hybrid construct in AR. When analysis was done under the displacement-control concept, we found that 1 and 2-constructs increased adjacent levels contribution to global ROMC3–C7 during FE and that IDP at superior adjacent level increased by a factor of 6.7 and 2.3 for 2-level arthrodesis and hybrid constructs, respectively.
Conclusion
Although 1- and 2-level TDR restored only partially native kinematics of the cervical spine, these constructs generated better biomechanical conditions than arthrodesis at adjacent levels limiting contribution of these segments to global ROM and reducing the amount of their internal stresses.
Keywords: Biomechanics, Biomechanical testing, Cervical spine, Artificial disc, Disc replacement, Kinematics, Intradiscal pressure, Multilevel
Introduction
Total disc replacement (TDR) in the cervical spine has been progressively introduced to address the adverse effects of traditional anterior cervical discectomy and fusion (ACDF): stiffness, pseudarthrosis, donor site morbidity, mechanical failure and adjacent segment degeneration [1]. By preserving some amount of motion and reproducing physiologic kinematics of cervical spine more closely, TDR may reduce stresses on adjacent discs and thus potentially reduce the incidence of adjacent segment disease which has been estimated to an annual incidence of 3% per year [2–4].
Recent prospective and comparative studies reported that short-term results after TDR were at least as good as those reported for ACDF [5–12]. In fact, TDR was recently approved in the US by the Food and Drug Administration (FDA) for treatment of one-level cervical spondylosis.
Because TDR is an emerging technology with the need for evaluation, most laboratory and clinical studies first investigated safety and efficacy of cervical TDR at one-level [5–19]. However, multi-segmental cervical spondylosis is not rare in clinical practice. Benefits in terms of stresses reduction on adjacent segments may be more important for multilevel procedure than for mono-segmental surgery considering that loss of mobility is greater when multiple segments are fused. Recently, some authors reported clinical experience of TDR inserted at two levels or above previous fusion [20–22] and their results suggested that clinical outcomes after multilevel TDR were at least as good as those observed for single-level TDR. Although multilevel TDR could be an attractive option to treat multilevel cervical disc disease, there is still no consensus about the different treatment options: multilevel ACDF, multilevel TDR or hybrid constructs (arthrodesis combined with TDR).
In vitro investigations are helpful to understand the biomechanical behaviour of motion preserving technologies and quantify changes in instrumented- and adjacent-levels. Although these laboratory evaluations represent an important preclinical step, there is a lack of objective data about the biomechanical behaviour of multilevel TDR in the cervical spine. Indeed, only few laboratory studies involving multilevel TDR have been reported [23, 24] and we found only one In vitro study comparing 2-level TDR versus the standard surgical procedure, i.e. 2-level arthrodesis [24]. In addition, to our knowledge, there is no biomechanical study investigating multilevel TDR with both kinematics and intradiscal pressures measurements.
Through an in vitro human cadaveric investigation, the objective of the present study was then to compare the biomechanical behaviour of 1- and 2-level cervical disc prosthesis versus standard anterior cervical arthrodesis and hybrid construct by measuring changes in cervical kinematics at instrumented levels. A secondary objective was to analyse kinematics and intradiscal pressures (IDP) at adjacent levels.
Materials and methods
Spinal specimen preparation
Twelve fresh adult cervical spines from C2 to T2 were harvested en bloc from human cadavers coming from the department of anatomy of the University. There were six male and six female cadaver specimens, with a mean age at death of 62 ± 6.4 years [55–77].
Prior to biomechanical tests, plain radiographs were performed to exclude specimens with tumoral, degenerative or traumatic pathology. Once harvested, each spine was immediately conserved in plastic bags at −20°C. The day before biomechanical testings, all spines were thawed at +4°C for 12 h and at room temperature on the day of testing. In preparation for biomechanical testing, all soft tissues including para-vertebral muscles were removed while preserving spinal ligaments, facet joint capsules, discs and bony elements.
The proximal vertebra (C2) was fixed in a container using a low fusion point alloy (MCP 70, MCP Metalspecialities Inc, Fairfield, CT) whereas the distal vertebra (T2) was firmly mounted in a specific device designed with metallic rods and screws and fixed to the testing platform.
Biomechanical tests protocol
In order to avoid tissue dehydration, the specimens were kept moistened with 0.9% NaCl physiologic serum spray during the tests.
Biomechanical tests were performed under load control by applying loads to the upper vertebra (C2) which was allowed to move unconstrained in 6-degrees-of-freedom. Using a system of weights and pulleys pure moments loading were successively applied along three axes in flexion/extension (FE), lateral bending (LB) and axial rotation (AR) to a 2 N m maximum moment loading with 0.2 N m steps. Three loading cycles were applied for preconditioning the specimen. In flexion–extension, spine specimens were tested with and without applying a 50 N compressive preload using the follower load method described by Patwardhan et al. [25].
Measurement of angular and linear displacements was obtained using a three-dimensional optoelectronic measurement system (POLARIS™ VICRA system, Northern Digital Inc, Waterloo, ON) connected to an acquisition and data processing system. Typical load–displacement curves were obtained for each different testing condition allowing for the determination of the ROM.
Variation of motion between intact and instrumented spines was calculated as following:
100 × [ROM of instrumented spine − ROM of intact spine]/ROM of intact spine].
Testing conditions
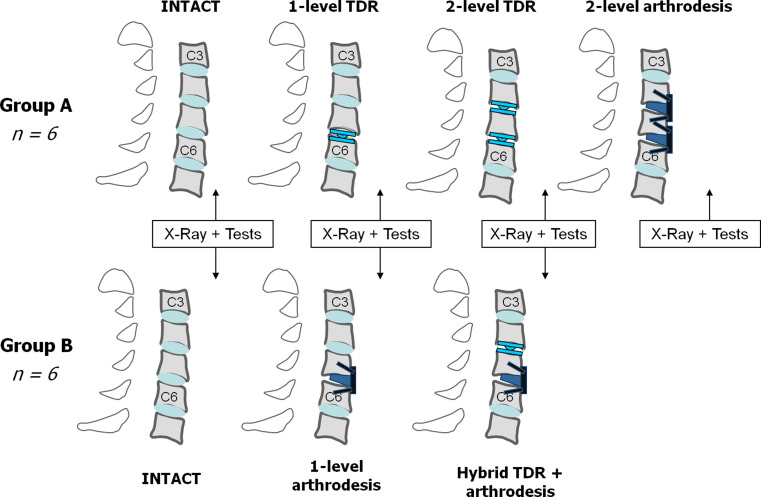
To avoid excessive number of testing conditions per spinal specimen, spines were divided into two groups (A and B) of six specimens each with different testing conditions evaluated in each group (Fig. 1).
Fig. 1.
Testing conditions. Twelve human cadaveric spines from C2 to T2 were divided in two groups. In group A, specimens were evaluated intact, after TDR at C5–C6, after implantation of a second TDR at C4–C5, and finally after arthrodesis at C4–C5 and C5–C6. In group B, specimens were evaluated intact, after arthrodesis at C5–C6 and finally after additional TDR at C4–C5 (hybrid construct)
In group A, spines were tested in the following four different conditions (Fig. 2):
Intact
After 1-level total disc replacement (TDR)
After 2-level TDR
After 2-level arthrodesis
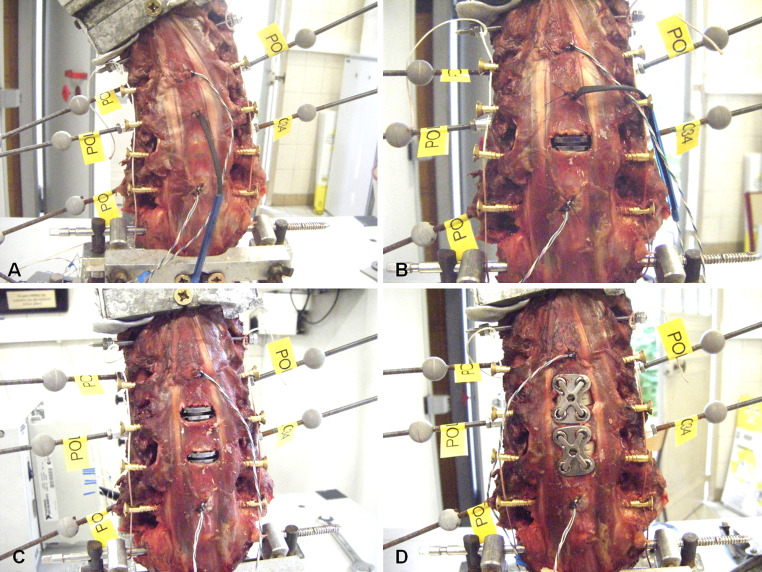
Fig. 2.
Group A: intact (a), 1-level TDR (b), 2-level TDR (c) and 2-level arthrodesis (d). Cervical specimens were mounted on the set up with reflective markers fixed on each vertebra from C3 to C7 to allow for measurement of 3D displacements using an optoelectronic measurement system. Pressure sensors were inserted in adjacent discs to measure IDP during flexibility tests
In group B, spines were tested in the following three different conditions (Fig. 3):
Intact
After 1-level arthrodesis
After hybrid construct
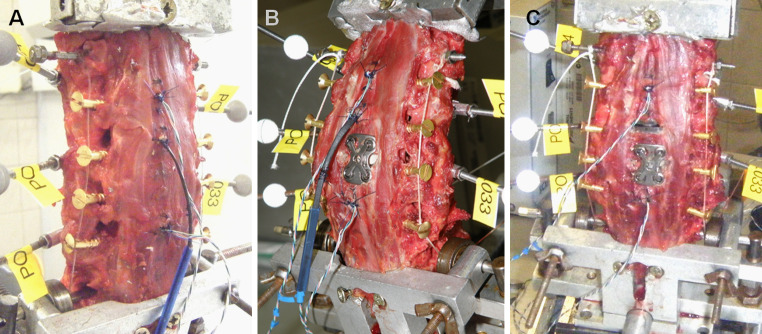
Fig. 3.
Group B: intact (a), 1-level arthrodesis (b) and hybrid construct (c). Guiding screws to apply follower compressive preload were placed between the anterior and posterior ridges of the transverse processes from C3 to C7 (b)
The TDR consisted of a ball-and-socket device with cranial geometric center and ceramic-on-ceramic bearing surfaces (Discocerv™, Scient’x/Alphatec Spine Inc., Carlsbad, CA, USA); and the arthrodesis was performed by means of an interbody PEEK cage (Samarys™, Scient’x/Alphatec Spine Inc., Carlsbad, CA, USA) combined with an anterior cervical plating (ACP) system (Secuplaque™, Scientx/Alphatec Spine Inc., Carlsbad, CA, USA).
After incision of the anterior longitudinal ligament, complete discectomy was performed at the involved level while preserving the vertebral endplates and uncovertebral joints. We kept the longitudinal posterior ligament intact but posterior annulus was totally removed. The implant was then placed with the objective of an optimal position in both frontal and antero-posterior planes. Using high-speed burr (Medtronic Inc., Minneapolis, MN, USA) moderate drilling of vertebral endplates was realized to optimize implant positioning. The size of the implant was determined according to the distraction effect during implant positioning.
Biplanar stereoradiographic X-rays
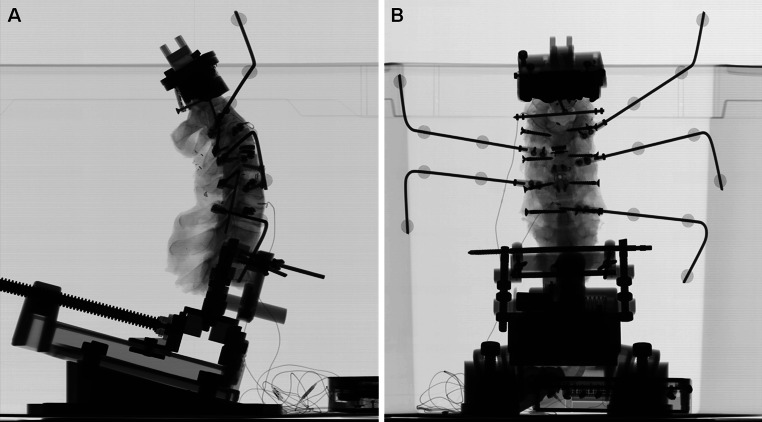
The anatomical frame of each vertebra and the local frame of its associated reflective markers were determined using 3D reconstructions that were obtained from EOS™ biplanar X-ray system prior each biomechanical test [26, 27] (Fig. 4). Accuracy in linear and angular measurements was previously calculated to 0.5 mm and 0.5°, respectively. Biplanar X-rays also served to verify implant positioning in both frontal and sagittal planes.
Fig. 4.
Biplanar X-rays. Prior to biomechanical tests, precise location of reflective markers and intradiscal load sensors was checked using lateral (a) and AP (b) radiographic views. Positioning of implants was also verified prior each flexibility test
Intradiscal pressure measurement
Special pressure sensors (EPL-B02-100P; Entran, Fairfield, NJ) were placed into the C3–C4 intervertebral disc allowing for measurement of IDP at this level during experimental tests (Fig. 3). They were inserted so that the pressure sensitive area was located at the anterior third of the intervertebral space in the AP plane, at the mid-height of the disc and aligned with the midline in the frontal plane.
Adjacent levels
As recommended by Panjabi et al. [28–30], to analyse and understand changes in kinematics and IDP at adjacent levels, we used the concept of displacement-control protocol to compare intact and instrumented spines (i.e. equivalent to hybrid testing protocol). ROMs and IDPs at adjacent levels were thus compared under equivalent ROMC3–C7 corresponding to the maximal ROMC3–C7 of the stiffest condition in flexion (i.e. 2-level arthrodesis for group A and hybrid construct for group B).
Contribution of adjacent segments was then expressed in % of ROMC3–C7 and calculated as following:
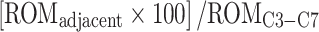 |
Statistical analysis
The statistical analysis was performed using specific software (XLSTAT Software, Addinsoft SARL, Paris, France). Comparison between group A and B in terms of age and flexibility was performed using independent-samples Mann–Whitney test. For each group, statistical comparison of ROM and IDP between intact and instrumented spines was carried out using paired-samples Wilcoxon test. All p values were considered statistically significant for a p value less than 0.05.
Results
The mean age was 62.3 ± 5 years [55–69] for group A and 61.8 ± 8.1 years [55–77] for group B, p = 0.575. Sex ratio was 1 for each group. No statistical difference was found in terms of global ROMC3–C7 between the two groups (Table 1).
Table 1.
Maximal ROMC3–C7 at 2 N m in flexion–extension, axial rotation and lateral bending
| FE | AR | LB | |
|---|---|---|---|
| Group A | |||
| Intact | 52 ± 10 [37–63] | 44 ± 9 [27–51] | 43.5 ± 6.5 [32–51] |
| 1-level TDR | 51.5 ± 10 [39–67] | 41.5 ± 11 [23–53] | 41.5 ± 6 [30–49] |
| 2-level TDR | 48 ± 10 [34–61] | 39.5 ± 10 [20–50] | 39 ± 7 [26–45] |
| 2-level arthrodesis | 32** ± 7 [25–42] | 26.5** ± 9 [13–39] | 27.5** ± 5 [20–33] |
| Group B | |||
| Intact | 57.5 ± 8.5 [47–71] | 46.5 ± 6.5 [39–57] | 43.5 ± 6 [35–51] |
| 1-level arthrodesis | 49** ± 7 [41–59] | 39.5** ± 6.5 [32–47] | 38** ± 4.5 [33–45] |
| Hybrid construct | 45.5** ± 7 [37–53] | 36.5** ± 5.5 [29–43] | 35.5** ± 4.5 [27–41] |
Mean are expressed in degrees, ± standard deviation [min–max]
** p < 0.05 (instrumented vs. intact spines)
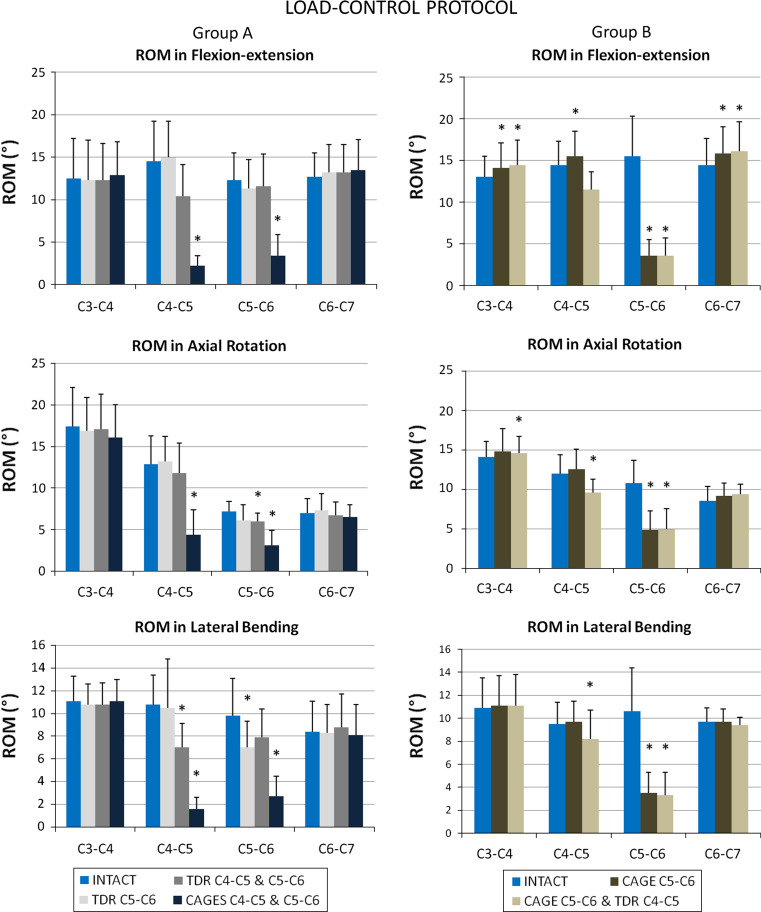
Results of segmental ROM from C3–C4 to C6–C7 are presented in Fig. 5 for all testing conditions. In flexion–extension all spinal specimens were tested with and without follower preload (results are presented in Table 2), however changes in ROM between intact and preloaded spines were never more than 1°.
Fig. 5.
Segmental ROM in FE (upper), AR (middle) and LB (lower) for intact and instrumented spines. Results for group A are presented on the left and for group B on the right. Error bars indicate 1 standard deviation and statistical significance is indicated as asterisk when p was <0.05
Table 2.
ROM in flexion–extension for groups A and B (with/without follower preload)
| Group A | Preload | Intact | TDR C5–C6 | TDR C4–C5 and C5–C6 | Arthrd. C4–C5 and C5–C6 |
|---|---|---|---|---|---|
| C3–C4 | – | 12.5 ± 4.5 |
12.5 ± 4.5 NS |
12.5 ± 4.5 NS |
13 ± 4 NS |
| Follower | 13 ± 4.5 |
12.5 ± 4.5 NS |
12.5 ± 4 NS |
13 ± 4 NS |
|
| C4–C5 | – | 14.5 ± 4.5 |
15 ± 4 NS |
10.5 ± 3.5 NS |
2 ± 1 p = 0.028 |
| Follower | 15 ± 4.5 |
15.5 ± 4 p = 0.028 |
10.5 ± 3.5 NS |
2.5 ± 1.5 p = 0.028 |
|
| C5–C6 | – | 12.5 ± 3 |
11.5 ± 3.5 NS |
11.5 ± 4 NS |
3.5 ± 2.5 p = 0.028 |
| Follower | 12.5 ± 3 |
12 ± 3.5 NS |
11 ± 4 NS |
3.5 ± 3 p = 0.028 |
|
| C6–C7 | – | 12.5 ± 3 |
13 ± 3.5 NS |
13.5 ± 3.5 NS |
13.5 ± 3.5 NS |
| Follower | 13 ± 3 |
13.5 ± 3 p = 0.046 |
13.5 ± 3.5 p = 0.046 |
14 ± 3.5 NS |
| Group B | Preload | Intact | Arthrd. C5–C6 | TDR C4–C5 and Arthrd. C5–C6 | |
|---|---|---|---|---|---|
| C3–C4 | – | 13 ± 2.5 |
14 ± 3 p = 0.028 |
14.5 ± 3 p = 0.028 |
|
| Follower | 13 ± 2.5 |
14 ± 3 NS |
14.5 ± 3 p = 0.046 |
||
| C4–C5 | – | 14.5 ± 3 |
15.5 ± 3 p = 0.028 |
11.5 ± 2 NS |
|
| Follower | 14.5 ± 2.5 |
15.5 ± 2.5 NS |
10.5 ± 2 p = 0.046 |
||
| C5–C6 | – | 15.5 ± 5 |
3.5 ± 2 p = 0.028 |
3.5 ± 2 p = 0.028 |
|
| Follower | 16.5 ± 4.5 |
3.5 ± 2 p = 0.028 |
4 ± 2 p = 0.028 |
||
| C6–C7 | – | 14.5 ± 3 |
16 ± 3 p = 0.028 |
16 ± 3.5 p = 0.028 |
|
| Follower | 15 ± 3 |
16 ± 3 p = 0.028 |
16.5 ± 3 p = 0.028 |
Mean are expressed in degrees, ± standard deviation
NS Not Significant; statistical significance for instrumented versus intact spines
Instrumented levels
Compared to intact spines, implantation of 1-level TDR resulted in significant decrease of ROM only in LB from 10 ± 3 to 7 ± 2.5° (p = 0.046; mean variation of −28%). Two-level TDR was associated with significant reduction of ROM at C5–C6 from 7 ± 1.5 to 6 ± 1° in AR (p = 0.046; mean variation of −16%) and at C4–C5 from 11 ± 2.5 to 7 ± 2° in LB (p = 0.028; mean variation of −35%).
As expected, 1- and 2-level arthrodesis resulted in significant reduction of ROM at the two instrumented levels in FE, LB and AR.
Hybrid construct (arthrodesis at C5–C6 and TDR at C4–C5) caused significant reduction of ROM in the three loading conditions at the arthrodesis level and also in AR and LB at the arthroplasty level.
Adjacent levels
Changes in kinematics and IDP at adjacent levels were analysed using the concept of displacement-control protocol, i.e. comparison of segmental ROMs and IDPs between intact and instrumented spines was performed under equivalent global ROMC3–C7 corresponding to the maximal ROMC3–C7 of the stiffest condition in flexion (i.e. 2-level arthrodesis for group A and hybrid construct for group B).
Ranges of motion
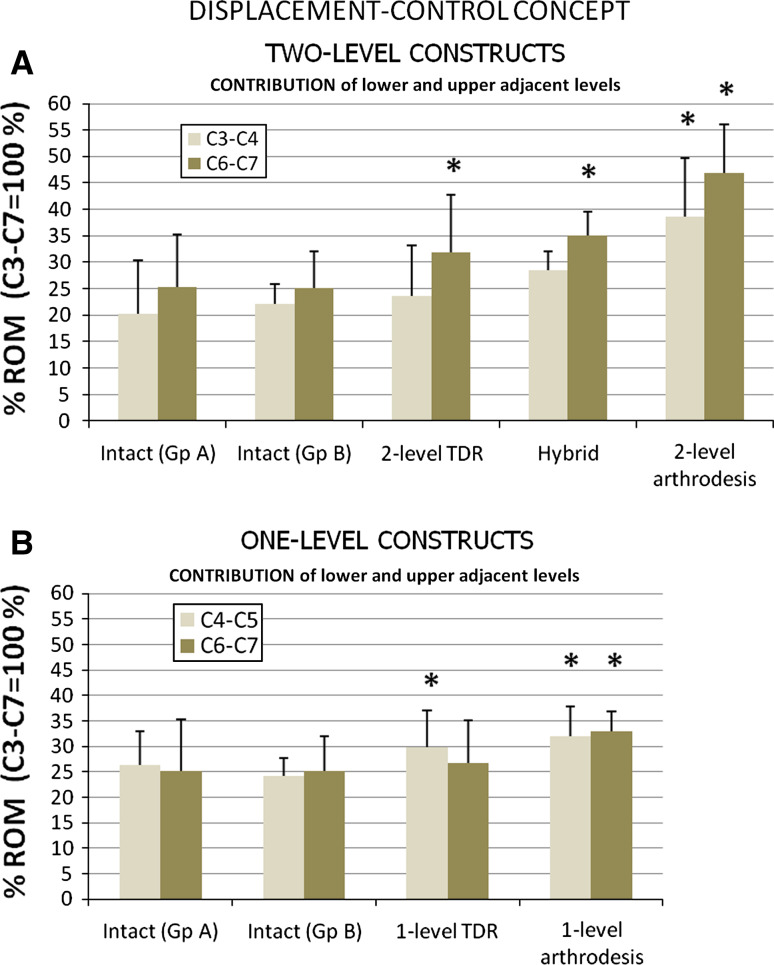
Contributions of adjacent levels (i.e. C4–C5 and C6–C7 for 1-level constructs; C3–C4 and C6–C7 for 2-level constructs) to ROMC3–C7 in flexion–extension are presented in Fig. 6.
Fig. 6.
Contribution of lower and upper adjacent levels to global ROMC3–C7 for two-level constructs (a) and for one-level constructs (b). Error bars indicate 1 standard deviation and statistical significance is indicated as asterisk when p was <0.05
As suspected, 2-level arthrodesis resulted in increase of contribution of both upper and lower adjacent levels (from 20 to 38.5%, p = 0.028 for C3–C4; and from 25 to 47%, p = 0.028 for C6–C7). Significant changes in contribution were only noted at lower level for hybrid and 2-level TDR constructs (from 25 to 35%, p = 0.028; and from 25 to 32%, p = 0.035, respectively).
Concerning 1-level constructs, arthrodesis caused increase of contribution at both upper and lower adjacent levels (from 24 to 32% for C4–C5, p = 0.028; and from 25 to 33% for C6–C7, p = 0.028) whereas significant changes in contribution was only noted at upper level for 1-level TDR (from 26 to 30%, p = 0.046).
Intradiscal pressures
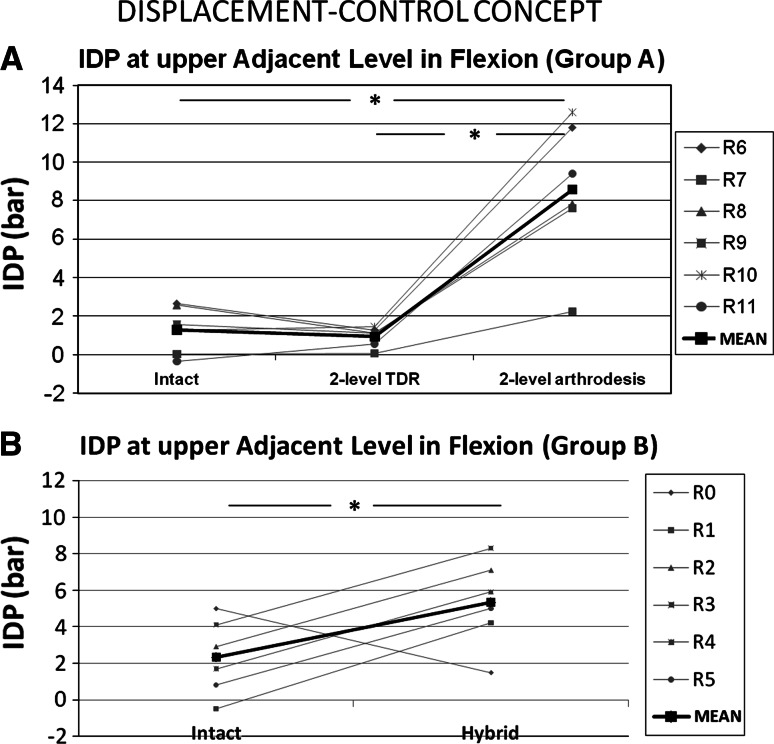
IDPs at upper adjacent level (i.e. C3–C4) are presented in flexion for 2-level constructs in Fig. 7.
Fig. 7.
Comparison of intradiscal pressure in flexion at upper adjacent level (C3–C4) between intact and two-level instrumented spines: group A (a) and group B (b). Statistical significance is indicated as asterisk (*) when p was <0.05
Group A Compared to intact spines, and under equivalent ROMC3–C7 (mean = 19.4 ± 6°), 2-level TDR slightly decreased IDP from 1.29 ± 1.25 to 0.93 ± 0.52 bar but this was not significant; whereas 2-level arthrodesis strongly increased IDP from 1.29 ± 1.25 to 8.58 ± 3.71 bar (p = 0.028; increase by a factor of 6.7).
Group B Compared to intact spines, and under equivalent ROMC3–C7 (mean = 27.9 ± 3°), hybrid construct increased IDP from 2.3 ± 2.07 to 5.3 ± 2.38 bar (p = 0.046; increase by a factor of 2.3).
Discussion
This study permitted to quantify 3D kinematics and changes in IDP after implantation of cervical TDR in different conditions: 1-level, hybrid and 2-level constructs; and then compare with the standard surgical treatment namely ACA.
Instrumented levels
Contrary to a number of biomechanical studies previously published [16–18], we did not find that implantation of TDR at 1 or 2 levels could restore completely native kinematics of the cervical spine. We noted that ROM after TDR was systematically reduced for the three loading conditions, especially in AR and LB, even if the difference was not always significant. This was slightly more marked for the additional TDR placed at C4–C5 above a previously implanted TDR implanted at C5–C6. As an example, compared to intact spines, variation could be −35% at C4–C5 in LB for 2-level TDR construct and −28% at C5–C6 in LB for 1-level TDR. Restoration of 3D motion after TDR during experimental tests is variable through the literature. Snyder et al. [31] reported also that ROM after TDR was significantly reduced in lateral bending suggesting that uncinates could potentially limit motion in LB. They proposed resection of uncinates to restore the extent of 3D motions more completely.
In fact, although some authors reported no difference in ROM between intact and instrumented spines [16–18], we noted that ROM in some of these studies was most often reduced but the difference was not statistically significant. As an example, Puttlitz et al. [17] tested six human cervical spines intact and instrumented with TDR (ProDisc-C, Synthes) by applying 3D pure moments of 1 N m. ROM of instrumented spines was measured to 73 and 72% of intact spines in AR and LB, respectively; however, the authors concluded that there was no difference between the two tested conditions because difference was no statistically significant.
Most In vitro studies, as in our report, investigated ball-and-socket design. These types of cervical disc prosthesis consist of 3-degrees-of-freedom joint (3D rotations but no translation) with a fixed COR during motion (considering bearing surfaces remain congruent) and are thus considered as constrained TDR. Considering that native intervertebral disc permits 6-degrees-of-freedom mobility, complete restoration of natural kinematics is unlikely with such implants and this is in accordance with our results. Kinematic conflicts in the facet joints and/or uncovertebral areas may logically result in limitations of ROM in some directions of motion. Results from In Vivo studies also suggest that extent of motion is only partly restored after TDR implantation. As an example, ROM in flexion–extension ranges from approximately 6 to 10° in most clinical and radiographic studies [6, 7, 9, 11, 32] whereas physiologic ROM is rather around 15/20° in normal and asymptomatic population [33–37]. With less constrained TDR design results from further In vitro studies may probably be different but at the moment objective data are lacking from the literature.
Otherwise, contrary to some studies reported in the lumbar spine [38], we did not observe destabilizing effect for 2-level TDR. There was no hypermobility of TDR placed above a previously implanted TDR. In addition, a second TDR did not affect the biomechanical behaviour of the previously implanted TDR. We just noted that the upper TDR was slightly less mobile than the lower one but this difference could be related to the cervical spine level (C4–C5 vs. C5–C6) rather than the upper or lower location of the TDR. Otherwise, hybrid construct permitted to analyse the biomechanical behaviour of TDR placed above cervical anterior arthrodesis. We noted near similar kinematics in comparison with TDR placed alone or above a previously implanted TDR. Our results are in complete accordance with those reported by Phillips et al. [23].
As expected, one- and -two-level arthrodesis induced strong reduction of ROM for the three loading conditions. However, we noted that limitation of motion was more marked in FE and LB (mean reduction by ~80%) than in axial rotation (mean reduction by ~60%) as previously reported [15, 18, 24]. One must care that when fusion will be acquired, reduction of ROM will be certainly greater than that observed during In vitro testing corresponding only to primary stability.
Adjacent levels
There are still controversies using load-controlled versus displacement-controlled protocol during experimental testing [28, 39–41]. Using pure moments loading, as in our experimental protocol, for all the testing conditions, one ensured that the load magnitude does not vary along the spinal segment and between the testing conditions. Loads applied to a segment are then independent of the flexibility of the adjacent segments limiting the interest of such protocol to analyse effects of implants on adjacent segments behaviour. As an example, we did not find any significant difference at 2 N m between 2-level TDR and 2-level arthrodesis for the three loading conditions. On the other hand, applying the same angular displacement to the different testing conditions (based on intact ROM), as proposed by Panjabi et al. [28, 29] (hybrid protocol) exposes to the risk of excessive loads and disco-ligamentar injuries for the stiffest constructs, especially for multilevel arthrodesis construct. Hence, in our study, we used load-controlled protocol for biomechanical tests whereas we applied the concept of displacement-control protocol to compare the biomechanical behaviour of adjacent levels between the different testing conditions. In our opinion, this method permitted to highlight the changes in kinematics and IDP at adjacent segments.
Although 1- and 2-level TDR restored only partly cervical kinematics at instrumented levels, their implantation induced only minimal changes in ROM at adjacent levels.
On the contrary, and as expected, one- and two-level arthrodesis increased the contribution of upper and lower adjacent levels to global ROMC3–C7 during FE.
In the literature, we found only two studies investigated In vitro multilevel TDR [23, 24]. The first one was reported in 2009 by Phillips et al. [23] who tested six human cervical spines in three different conditions: intact, after TDR at C5–C6 and after additional TDR at C6–C7. In this study, there was no comparison between TDR and arthrodesis. Biomechanical tests were done under load-controlled protocol by applying 1.5 N m pure moments in FE, AR and LB. The authors found that 2-level TDR did not affect ROM at upper adjacent level (C4–C5) in FE and AR but observed a small increase in LB. In 2010, Cunningham et al. [24] conducted an In vitro human cadaveric study comparing the ROM of eight cervical spines tested in six different conditions: intact, 1-level TDR, 1-level ACA, hybrid, 2-level TDR and 2-level ACA. They used hybrid testing protocol described by Panjabi et al. [28, 29]. No IDP was registered during biomechanical tests. They found that instrumented-level ROM was preserved with 1- and 2-level TDR under all loading modes, and that ROM at the levels adjacent to the TDR remained essentially unchanged whereas ROM at lower adjacent level increased after 2-level ACA by a factor of 2, 1.6 and 1.2 in FE, AR and LB, respectively.
Concerning IDP, no significant differences were observed between intact spines and those instrumented with 2-level TDR during flexion whereas IDP increased by a factor of 6.7 for 2-level arthrodesis constructs. To our knowledge, this is the first study which compared adjacent level IDPs between multilevel TDR versus multilevel arthrodesis. Measurement of IDP is essential to evaluate change in internal stresses at adjacent level considering that minimal change in ROM can lead to a great change in IDP. Increase of IDP at proximal and distal levels to a 1-level simulated fusion was first reported in 2002 by Eck et al. [42]. In 2005 Dmitriev et al. [18] have also reported increase of IDP at adjacent level after 1-level cervical arthrodesis. These authors compared TDR versus ACA at C5–C6 and analysed IDP at both proximal and distal levels using hybrid protocol. They found that IDP in flexion–extension increased by a factor of 2.3 at C6–C7 and 1.4 at C4–C5 after ACA. Chang et al. [43] confirmed these findings through a human cadaveric study reporting that in arthroplasty-treated specimens, the IDP showed little difference from that of the intact spine at both proximal and distal levels, whereas in fusion-treated specimens, the IDP increased at the posterior disc on extension and at the anterior disc on flexion at the proximal level.
In our study, compared to intact spines and multilevel TDR, multilevel arthrodesis resulted in exaggerated contribution of adjacent segments to global ROM. In addition, multilevel arthrodesis increased the amount of internal stresses at these segments. These findings may potentially result in accelerated adjacent segment disease. In addition, as mentioned above, when fusion is acquired reduction of ROM will be certainly greater than that observed during In vitro testing, making the fused segment more rigid, this may logically exaggerate increase stresses at adjacent levels.
Limitations of the study
Restoration of native kinematics of the cervical spine implies to restore not only the physiologic 3D ROM but also the nature of the motion. This study, as most In vitro studies [44], focused mainly on the extent of motion with limitations considerations for the quality of motion. Complete biomechanical evaluation of spinal kinematics after TDR necessitates using other investigational methods such as finite element methods or In Vivo imaging techniques.
As mentioned above, the results may be different with a different TDR design especially a less constrained prosthesis. Consequently, our results are only valid for similar TDR design and under the same testing conditions.
Finally, to simulate physiologic compressive load provided by paraspinal muscles and thus more closely simulate In Vivo conditions, we used follower preload in FE during experimental tests. However, laboratory protocols cannot simulate the complexity of human musculature action and thereby caution has to be exercised for direct comparison between our results and In Vivo conditions.
Conclusion
In conclusion, although implantation of TDR at 1 or 2 levels restored only partially native kinematics of the cervical spine, 1- and 2-level TDR generated better biomechanical conditions than ACA at adjacent levels limiting contribution of these segments to global ROM and also reducing the amount of internal stresses at these segments. In addition, implantation of a second cervical TDR did not generate hypermobility of the instrumented spine and did not affect the biomechanical behaviour of the previously implanted TDR. These biomechanical findings support the concept of multilevel arthroplasty in the cervical spine and suggest that multilevel TDR should be considered as alternative options to treat cervical multilevel degenerative disc disease, even if future clinical studies are necessary to ensure long-term safety of multilevel TDR.
Acknowledgments
Conflict of interest
Cédric Barrey and Gilles Perrin are consultant for Scient’X-Alphatec Spine company. Scient’X-Alphatec Spine Co. provided research support for this work to Dr. Skalli.
Abbreviations
- ROM
Range of motion
- FE
Flexion–extension
- AR
Axial rotation
- LB
Lateral bending
- TDR
Total disc replacement
- ACP
Anterior cervical plate
- ACA
Anterior cervical arthrodesis
References
- 1.Huang RC, Wright TM, Panjabi MM, Lipman JD. Biomechanics of nonfusion implants. Orthop Clin North Am. 2005;36:271–280. doi: 10.1016/j.ocl.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of previous anterior cervical arthrodesis. J Bone Joint Surg (Am) 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Durbhakula MM, Ghiselli G. Cervical total disc replacement, part I: rationale, biomechanics, and implant types. Orthop Clin North Am. 2005;36:349–354. doi: 10.1016/j.ocl.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Galbusera F, Bellini CM, Brayda-Bruno M, Fornari M. Biomechanical studies on cervical total disc arthroplasty: a literature review. Clin Biomech. 2008;23:1095–1104. doi: 10.1016/j.clinbiomech.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Goffin J, Van Calenbergh F, Van Loon J, Casey A, Kehr P, Liebig K, et al. Intermediate follow-up after treatment of degenerative disc disease with the Bryan® cervical disc prosthesis: single-level and bi-level. Spine. 2003;28:2673–2678. doi: 10.1097/01.BRS.0000099392.90849.AA. [DOI] [PubMed] [Google Scholar]
- 6.Porchet F, Metcalf N. Clinical outcomes with the prestige II cervical disc: preliminary results from a prospective randomised clinical trial. Neurosurg Focus. 2004;17:E6. doi: 10.3171/foc.2004.17.3.6. [DOI] [PubMed] [Google Scholar]
- 7.Bertagnoli R, Duggal N, Pickett GE, Wigfield CC, Gill SS, Karg A, et al. Cervical total disc replacement, part two: clinical results. Orthop Clin North Am. 2005;36:255–262. doi: 10.1016/j.ocl.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Pickett GE, Rouleau JP, Duggal N. Kinematic analysis of the cervical spine following implantation of an artificial cervical disc. Spine. 2005;30:1949–1954. doi: 10.1097/01.brs.0000176320.82079.ce. [DOI] [PubMed] [Google Scholar]
- 9.Sasso RC, Smucker JD, Hacker RJ, Heller JG. Clinical outcomes of BRYAN cervical disc arthroplasty: a prospective randomized controlled multicenter trial with 24 month follow-up. J Spinal Disord Tech. 2007;20:481–491. doi: 10.1097/BSD.0b013e3180310534. [DOI] [PubMed] [Google Scholar]
- 10.Bhadra AK, Raman AS, Casey AT, Crawford RJ. Single-level cervical radiculopathy: clinical outcome and cost-effectiveness of four techniques of anterior cervical discectomy and fusion and disc arthroplasty. Eur Spine J. 2009;18:232–237. doi: 10.1007/s00586-008-0866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heller JG, Sasso RC, Papadopoulos SM, Anderson PA, Fessler RG, Hacker RJ, et al. Comparison of Bryan cervical disc arthroplasty with anterior cervical decompression and fusion. Clinical and radiographic results of a randomized, controlled, clinical trial. Spine. 2009;34:101–107. doi: 10.1097/BRS.0b013e31818ee263. [DOI] [PubMed] [Google Scholar]
- 12.Goffin J, Van Loon J, Van Calenbergh F, Lipscomb B. A clinical analysis of 4- and 6-year follow-up results after cervical disc replacement surgery using the Bryan cervical disc prosthesis. J Neurosurg Spine. 2010;12:261–269. doi: 10.3171/2009.9.SPINE09129. [DOI] [PubMed] [Google Scholar]
- 13.Murrey D, Janssen M, Delamarter R, Goldstein J, Zigler J, Tay B, et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 2009;9:275–286. doi: 10.1016/j.spinee.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Wigfield C, Gill S, Nelson R, Langdon I, Metcalf N, Robertson J. Influence of an artificial cervical joint compared with fusion on adjacent-level motion in the treatment of degenerative cervical disc disease. J Neurosurg. 2002;96:S17–S21. doi: 10.3171/spi.2002.96.1.0017. [DOI] [PubMed] [Google Scholar]
- 15.MacAfee PC, Cunningham B, Dmitriev A, Hu N, Woo Kim S, Cappuccino A, Pimenta L. Cervical disc replacement–porous coated motion prosthesis: a comparative biomechanical analysis showing the key role of the posterior longitudinal ligament. Spine. 2003;28:S176–S185. doi: 10.1097/01.BRS.0000092219.28382.0C. [DOI] [PubMed] [Google Scholar]
- 16.DiAngelo DJ, Roberston JT, Metcalf NH, McVay BJ, Davis RC. Biomechanical testing of an artificial cervical joint and an anterior cervical plate. J Spinal Disord Tech. 2003;16:314–323. doi: 10.1097/00024720-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Puttlitz CM, Rousseau MA, Xu Z, Hu S, Tay BK, Lotz JC. Intervertebral disc replacement maintains cervical spine kinematics. Spine. 2004;29:2809–2814. doi: 10.1097/01.brs.0000147739.42354.a9. [DOI] [PubMed] [Google Scholar]
- 18.Dmitriev AE, Cunningham BW, Hu N, Sell G, Vigna F, McAfee PC. Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: an in vitro human cadaveric model. Spine. 2005;30:1165–1172. doi: 10.1097/01.brs.0000162441.23824.95. [DOI] [PubMed] [Google Scholar]
- 19.Barrey C, Mosnier T, Jund J, Perrin G, Skalli W. In vitro evaluation of a ball-and-socket cervical disc prosthesis with cranial geometric center. J Neurosurg Spine. 2009;11:538–546. doi: 10.3171/2009.6.SPINE0949. [DOI] [PubMed] [Google Scholar]
- 20.Pimenta L, McAfee PC, Cappuccino A, Cunningham BW, Diaz R, Coutinho E. Superiority of multilevel cervical arthroplasty outcomes versus single-level outcomes: 229 consecutive PCM prostheses. Spine. 2007;32:1337–1344. doi: 10.1097/BRS.0b013e318059af12. [DOI] [PubMed] [Google Scholar]
- 21.Cheng L, Nie L, Zhang L, Hou Y. Fusion versus Bryan cervical disc in two-level cervical disc disease: a prospective, randomized study. Int Orthop. 2009;33:1347–1351. doi: 10.1007/s00264-008-0655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips FM, Allen TR, Regan JJ, Albert TJ, Cappuccino A, Devine JG, et al. Cervical disc replacement in patients with and without previous adjacent level fusion surgery: a prospective study. Spine. 2009;34:556–565. doi: 10.1097/BRS.0b013e31819b061c. [DOI] [PubMed] [Google Scholar]
- 23.Phillips FM, Tzermiadianos MN, Voronov LI, Havey RM, Carandang G, Dooris A, et al. Effect of two-level total disc replacement on cervical spine kinematics. Spine. 2009;34:E794–E799. doi: 10.1097/BRS.0b013e3181afe4bb. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham BW, Hu N, Zorn CM, McAfee PC. Biomechanical comparison of single and two-level cervical arthroplasty versus arthrodesis: effect on adjacent-level spinal kinematics. Spine J. 2010;10:341–349. doi: 10.1016/j.spinee.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Patwardhan AG, Havey RM, Ghanayem AJ, Diener H, Meade KP, Dunlap B, Hodges SD. Load-carrying capacity of the human cervical spine in compression is increased under a follower load. Spine. 2000;25:1548–1554. doi: 10.1097/00007632-200006150-00015. [DOI] [PubMed] [Google Scholar]
- 26.Dubousset J, Charpak G, Dorion I, Skalli W, Lavaste F, Deguise J, et al. A new 2D and 3D imaging approach to musculoskeletal physiology and pathology with low-dose radiation and the standing position: the EOS system. Bull Acad Natl Méd. 2005;189:287–297. [PubMed] [Google Scholar]
- 27.Rousseau MA, Laporte S, Chavary-Bernier E, Lazennec JY, Skalli W. Reproducibility of measuring the shape and three-dimensional position of cervical vertebrae in upright position using the EOS stereoradiography system. Spine. 2007;32:2569–2572. doi: 10.1097/BRS.0b013e318158cba2. [DOI] [PubMed] [Google Scholar]
- 28.Panjabi MM, Cholewicki J, Nibu K, et al. Criticial load of the human cervical spine: an In Vitro experimental study. Clin Biomech. 1998;13:11–17. doi: 10.1016/S0268-0033(97)00057-0. [DOI] [PubMed] [Google Scholar]
- 29.Panjabi MM, Crisco JJ, Vasavada A, Oda T, Cholewicki J, Nibu K et al (2001) Mechanical properties of the human cervical spine as shown by three-dimensional load-displacement curves. Spine 26:2692–2700 [DOI] [PubMed]
- 30.Kim SH, Chang UK, Chang JC, Chun KS, Lim TJ, Kim DH. The changes in range of motion after a lumbar spinal arthroplasty with Charité™ in the human cadaveric spine under physiologic compressive follower preload: a comparative study between load control protocol and hybrid protocol. J Korean Neurosurg. 2009;46:144–151. doi: 10.3340/jkns.2009.46.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder JT, Tzermiadianos MN, Ghanayem AJ, Voronov LI, Rinella A, Dooris A, et al. Effect of uncovertebral joint excision on the motion response of the cervical spine after total disc replacement. Spine. 2007;32:2965–2969. doi: 10.1097/BRS.0b013e31815cd482. [DOI] [PubMed] [Google Scholar]
- 32.Mummaneni PV, Burkus JK, Haid RW, Traynelis VC, Zdeblick TA (2007) Clinical and radiographic analysis of cervical disc arthroplasty compard with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine 6:198–209 [DOI] [PubMed]
- 33.White AA, Panjabi MM. Clinical Biomechanics of the Spine. 2. Philadelphia: Lippincott; 1990. [Google Scholar]
- 34.Amevo B, Worth D, Bogduk N. Instantaneous axes of rotation of the typical cervical motion segments: a study in normal volunteers. Clin Biomech. 1991;6:111–117. doi: 10.1016/0268-0033(91)90008-E. [DOI] [PubMed] [Google Scholar]
- 35.Dvorak J, Panjabi M, Novotny J, Antinnes J. In vivo flexion/extension of the normal cervical spine. J Orthop Res. 1991;9:828–834. doi: 10.1002/jor.1100090608. [DOI] [PubMed] [Google Scholar]
- 36.Watier B (1997) Etude expérimentale du rachis cervical: comportement mécanique in vitro et cinématique in vivo [thesis]. Paris: Ecole Nationale Supérieure d’Arts et Métiers, Arts et Metiers Paris-Tech
- 37.Bogduk N, Mercer S. Biomechanics of the cervical spine. I: normal kinematics. Clin Biomech. 2000;15:633–648. doi: 10.1016/S0268-0033(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham BW HUN, Beatson HJ, Serhan H, Sefter JC, McAfee PC. Revision strategies for single- and two-level total disc arthroplasty procedures: a biomechanical perspective. Spine J. 2009;9:735–743. doi: 10.1016/j.spinee.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Goel VK, Wilder DJ, Pope MH, Edwards WT. Controversy: biomechanical testing of the spine. Load-controlled versus displacement-controlled analysis. Spine. 1995;20:2354–2357. doi: 10.1097/00007632-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Wilke H-J, Wenger K, Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998;7:148–154. doi: 10.1007/s005860050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goel VK, Panjabi MM, Patwardhan AG, Dooris AP, Serhan H. Test protocols for evaluation of spinal implants. J Bone Joint Surg. 2006;88-A:103–109. doi: 10.2106/JBJS.E.01363. [DOI] [PubMed] [Google Scholar]
- 42.Eck JC, Humphreys SC, Lim TH, Jeong ST, Kim JG, Hodges SD, et al. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine. 2002;27:2431–2434. doi: 10.1097/00007632-200211150-00003. [DOI] [PubMed] [Google Scholar]
- 43.Chang UK, Kim DH, Lee MC, Willenberg R, Kim SH, Lim J. Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J Neurosurg Spine. 2007;7:33–39. doi: 10.3171/SPI-07/07/033. [DOI] [PubMed] [Google Scholar]
- 44.Wen N, Lavaste F, Santin JJ, Lassau JP. Three-dimensional biomechanical properties of the human cervical spine in vitro. I. Analysis of normal motion. Eur Spine J. 1993;2:2–11. doi: 10.1007/BF00301048. [DOI] [PubMed] [Google Scholar]