Abstract
Introduction
The treatment of rigid and severe scoliosis and kyphoscoliosis is a surgical challenge. Presurgical halo-gravity traction (HGT) achieves an increase in curve flexibility, a reduction in neurologic risks through gradual traction on a chronically tethered cord and an improvement in preoperative pulmonary function. However, little is known with respect to the ideal indications for HGT, its appropriate duration, or its efficacy in the treatment of rigid deformities.
Materials and methods
To investigate the use of HGT in severe deformities, we performed a retrospective review of 45 patients who had severe and rigid scoliosis or kyphoscoliosis. The analysis focused on the impact of HGT on curve flexibility, pulmonary function tests (PFTs), complications and surgical outcomes in a single spine centre.
Results
PFTs were used to assess the predicted forced vital capacity (FVC%). The mean age of the sample was 24 ± 14 years. 39 patients had rigid kyphoscoliosis, and 6 had scoliosis. The mean apical rotation was 3.6° ± 1.4°, according to the Nash and Moe grading system. The curve apices were mainly in the thoracic spine. HGT was used preoperatively in all the patients. The mean preoperative scoliosis was 106.1° ± 34.5°, and the mean kyphosis was 90.7° ± 29.7°. The instrumentation used included hybrids and pedicle screw-based constructs. In 18 patients (40%), a posterior concave thoracoplasty was performed. Preoperative PFT data were obtained for all the patients, and 24 patients had ≥3 assessments during the HGT. The difference between the first and the final PFTs during the HGT averaged 7.0 ± 8.2% (p < .001). Concerning the evolution of pulmonary function, 30 patients had complete data sets, with the final PFT performed, on average, 24 months after the index surgery. The mean preoperative FVC% in these patients was 47.2 ± 18%, and the FVC% at follow-up was 44.5 ± 17% (a difference that did not reach statistical significance). The preoperative FVC% was highly predictive of the follow-up FVC% and the response during HGT. The mean flexibility of the scoliosis curve during HGT was only 14.8 ± 11.4%, which was not significantly different from the flexibility measures achieved on bending radiographs or Cotrel traction radiographs. In rigid curves, the Cobb angle difference between the first and final radiographs during HGT was only 8° ± 9° for scoliosis and 7° ± 12° for kyphosis. Concerning surgical outcomes, 13 patients (28.9%) experienced minor and 15 (33.3%) experienced major complications. No permanent neurologic deficits or deaths occurred. Additional surgery was indicated in 12 patients (26.7%), including 7 rib-hump resections. At the final evaluation, 69% of the patients had improved coronal balance, and at a mean follow-up of 33 ± 23.3 months, 39 patients (86.7%) were either satisfied or very satisfied with the overall outcome.
Conclusion
The improvement of pulmonary function and the restoration of sagittal and coronal balance are the main goals in the treatment of severe and rigid scoliosis and kyphoscoliosis. A review of the literature showed that HGT is a useful tool for selected patients. Preoperative HGT is indicated in severe curves with moderate to severe pulmonary compromise. HGT should not be expected to significantly improve severe curves without a prior anterior and/or posterior release. The data presented in this study can be used in future studies to compare the surgical and pulmonary outcomes of severe and rigid deformities.
Keywords: Halo-gravity traction, Severe scoliosis, Kyphoscoliosis, Pulmonary function, Surgical treatment
Introduction
The correction of severe and rigid scoliosis and kyphoscoliosis is a surgical challenge. Significant pulmonary compromise is frequently present adding challenge to already surgically daunting procedures. The aim of surgery in these patients is to halt the progress of a disabling deformity, to diminish the size of the curve and to restore trunk balance while improving the patient’s quality of life, providing cardiopulmonary stability and avoiding long-term sequelae from restrictive lung disease, painful degeneration and curve decompensation [1–6]. Especially in kyphoscoliosis, surgery is designed to reduce the tension placed on the spinal cord in regions with sharp kyphosis or scoliosis.
Current surgical strategies include aggressive anterior and posterior column release and osteotomies, either with a front–back or a posterior-only approach. With the extent of the surgical release and segmental instrumentation, the potential for curve correction is increased; however, so is the potential for neurologic compromise [5, 7–9]. Therefore, in the past, preoperative halo-gravity traction (HGT) was recommended. It was thought to reduce the severity of scoliosis and improve pulmonary function before surgery [10–13]. However, these recommendations and current practice lack evidence, and the role of HGT within different correction methods remain to be defined. In addition, there is a paucity of data concerning the risks of reconstructive surgery, particularly in patients with severe scoliosis and pulmonary impairment, who need surgical treatment the most.
Because of the growing number of reports using modern pedicle screw systems in combination with aggressive releases for the correction of severe curves [8, 9, 11, 14–21], the authors scrutinised the value of HGT within treatment modalities applied in the past decade by analysing a sample of patients with severe, rigid (kypho-)scoliosis. We sought to quantify the impacts of preoperative HGT on pulmonary function and curve correction to more precisely define the indications for HGT.
Being aware of the normal decline in pulmonary function with ageing [22] and the known risk factors for cardiopulmonary decompensation over the long term, such as an FVC% below 45% and scoliotic curves exceeding 90°–100° [3, 23], the analysis of pulmonary function test (PFT) data is decisive in the treatment of severe scoliosis. There are data on moderate curves and their pulmonary evolution, but the available reports lack data on severe scoliosis [2–5, 20]. Therefore, by a retrospective analysis of surgical and pulmonary outcomes, we aimed to clarify the connections between the following issues: spinal deformity, pulmonary function and surgical results.
Methods and materials
Study sample
Our database was analysed to identify all the patients with scoliosis or kyphoscoliosis who underwent HGT before instrumented correction (preop-HGT) and who were able to undergo PFTs. The minimum follow-up was 12 months. A total of 45 cases fulfilled the criteria, and their medical records and radiographs were retrospectively reviewed.
One patient with Pott’s disease underwent a non-instrumented kyphoscoliosis correction using a greffe antérieur procedure and thus was excluded from the analysis of the main sample when evaluating radiographs and PFT. She was, however, included in a subgroup analysis of patients with ‘severe curves and progressive neurologic deficits’ because of the scarcity of such data.
Clinical assessment
The subjects’ medical charts were analysed for preoperative body height and weight at the final clinical visit. Neurological deficits were categorised according to the Ranawat classification. The patients were stratified according to whether they had suffered progressive neurological deficits during the 6 months prior to surgery, chronic neurologic deficits or no deficit. The patients were also categorised as ambulatory or non-ambulatory. Complications during the treatment course were noted and classified as minor or major according to Glassmann [24]. The number and details of medical and surgical complications as well as subsequent and revision surgeries were recorded. Revision surgeries were defined as those related to a complication (e.g., a rod fracture). Complications were defined as early if they occurred within 3 months of the surgery.
Radiographic analysis
The major scoliosis and kyphosis (Cobb angle) was extracted from biplanar, full-standing, standard radiographs preoperatively, postoperatively and at follow-up as well as from bending radiographs and radiographs performed with the patient in HGT or in Cotrel traction (traction radiographs).
On the full-standing radiographs, the coronal balance was measured by dropping a C7 plumb line and measuring its offset from the mid-sagittal sacral line (in cm). When the coronal C7 plumb line was ≤2 cm to the left or right of the mid-sagittal line, the spine was defined as balanced. Sagittal balance was measured by dropping a C7 plumb line. If it fell posterior to the hip axis, the patient was defined as being in global sagittal balance. If it fell anterior, the patient was in sagittal imbalance.
We defined the preoperative radiographs as the neutral radiographs. During HGT, the final radiograph taken directly before the first surgical procedure was defined as the final-HGT radiograph, and the first HGT radiograph taken was defined as the 1st-HGT-radiograph. The radiograph with the best Cobb angle correction during HGT was defined as the preop-HGT radiograph and was used for calculating the HGT flexibility for scoliosis and kyphosis. The preoperative analysis of radiographs included the grading of apical vertebral rotation according to the classification of Nash and Moe (4 types), with a fifth degree added for off-the-plane rotation. The calculation of curve flexibility and correction rates was performed on radiographs during HGT, during Cotrel traction, and on standard bending films as previously described [25, 26]. To put the postoperative curve correction into perspective considering the related preoperative flexibility, we calculated the Cincinnati correction index (CCI) [26]. Preoperative MRI and post-myelogram CT scans were reassessed for intraspinal anomalies. Changes in construct alignment, failure of instrumentation and evidence of non-union were noted. A diagnosis of non-union was made when there was radiographic evidence of non-union or instrumentation failure or motion during surgical exploration. Because of the severe deformities and reduced quality of some radiographs, some measurements could not be obtained.
Pulmonary function tests
The patients were assessed using a standard ultrasound spirometer in the sitting position (Spiroson, v1.2D, ndd-Medizintechnik, Germany). The pulmonary function value reported was forced vital capacity (FVC), which is expressed as a percentage of the predicted value (FVC%) [27]. PFTs were performed before HGT was initiated (defined as the preop-FVC%) and during HGT at several time points (defined as the 1st-, 2nd-, and 3rd-HGT-FVC%). Of the serial FVC% assessments during HGT, the best FVC% (defined as best-HGT FVC%) and the final FVC% (final-HGT FVC%) were recorded. During the study period, the patients were subjected to postoperative PFTs (postop-FVC%) and follow-up PFTs (follow-up-FVC%).
According to the American Thoracic Society’s guidelines for the severity of pulmonary impairment [28], ‘no’ pulmonary impairment was considered when the FVC% was >80% of the predicted value, ‘mild’ when the FVC% was ≤80% but >65%, ‘moderate’ when the FVC% was ≤65% but >50%, and ‘severe’ when the FVC% was ≤50%. For all the time points, a negative value of FVC%-change during the treatment period indicated a decline in FVC%.
Based on the preop-FVC% and the number of times that PFTs were performed, we calculated the time-dependent relationships between the HGT-FVC% changes (the difference in FVC% at distinct time points during HGT compared with the FVC% before the initiation of HGT). The absolute effect of HGT on PFTs was analysed by calculating the difference between the preop-FVC% before the initiation of HGT and the final HGT-FVC%.
Patients were defined as ‘pulmonary responders’ to HGT if their pulmonary function (in terms of the FVC%) improved by at least 5% and as ‘non-responders’ if there was an improvement or decrease of 0–5%; a decline was noted if the FVC% dropped more than 5% during HGT. Likewise, the impact of surgical treatment on PFT results was analysed by comparing the preop-FVC% to the follow-up FVC% in patients with both preoperative and follow-up PFTs performed ≥6 months after the operation. Patients were classified as having improved (>5% FVC% increase), stable (±5% FVC% change) or worsened (>5% FVC% decrease).
Postoperative pulmonary complications were recorded as suggested by Liang [68]. Because of the scarcity of PFT data on the treatment of patients with severe scoliosis, patients without complete PFT data sets were included. The numbers of patients with PFT data at given time points are reported as statistically valid numbers in the results section and the subgroup analysis.
Halo-gravity traction and surgical strategy
The indications for HGT were combinations of the following criteria: extensive curve size and rigidity; a progressive neurological deficit in a decompensating large curve with the spinal cord being draped over the apex; the presence of a sick cord and the goal of preoperative adaptation of the cord to corrective stress; the presence of intraspinal anomalies that might cause acute tension during the surgical correction; and poor PFT results. The target weight during HGT was ~30–50% of body weight. The patients cooperated with daily neurologic checks. The pins were tightened every 2 days. The patients were encouraged to be in their wheelchairs or walkers, all had pulmonary monitoring and training, and daily exercises were performed to reduce the effects of deconditioning. HGT-related complications were recorded. HGT was halted if PFTs had improved or plateaued, if curve improvement was judged to be maximised or if a neurologic deficit had improved.
During the study period, coronal curves >80°–90° with a flexibility <30% and sagittal curves >70° were defined as severe and rigid. Patients underwent open anterior release with subsequent HGT if it was determined to be suitable by the anaesthesiologist, based on the final-HGT-FVC%. Likewise, patients had concave thoracoplasty (CTP = multiple concave rib osteotomies) [25, 29] for posterior spinal release and cosmesis if it was judged acceptable after considering pulmonary concerns. The instrumentation involved single (7%), dual (67%) and triple screw-rod constructs (27%), with/without cross-links, using hybrid or pedicle screw-only constructs, mainly in terms of an instrumented end-level distraction spondylodesis. In the period studied, only scoliosis distraction and translation manoeuvres were applied, and there were no vertebral column resections (VCRs). Iliac crest grafts were used.
Statistical analysis
To include continuous variables and binary outcome parameters, we performed correlation analyses between radiographic and pulmonary parameters and risk factor identification for adverse events, such as revision surgery, major complications, an FVC% <40% [30, 31] and non-union. Descriptive statistics included an analysis of means ± 1 standard deviation and ranges. The results are reported as rounded numbers. Paired Student’s t tests and Pearson’s correlation coefficients were used to test for significant differences and relationships. Fisher’s exact test or Pearson’s test was used to analyse cross-tabulation tables. A p value <5% indicated a statistically significant correlation or difference. The analyses were performed using Statistica 6.1 (StatSoft, Tulsa, USA).
Results
Sample characteristics
The sample included 45 patients, 31 female (69%) and 14 male (31%), with a mean age of 24 ± 14 (9–56) years and follow-up of 33 ± 24 (12–108) months. 39 patients (87%) had kyphoscoliosis, and 6 (13%) had scoliosis. The aetiology was congenital in 23 patients (51%), neuromuscular in 7 (16%), and idiopathic in 5 (11%). A total of five patients (11%) suffered from scoliosis associated with neurofibromatosis. Another five patients (11%) had syndromatic or post-thoracotomy scoliosis.
Fifteen patients (33%) underwent an anterior release, and 18 (40%) a CTP. A greffe antérieur procedure was performed in four patients (9%). The posterior fusion extended to the lumbosacral junction in four patients and to the cervicothoracic junction in three. All the patients wore a brace for 6 months postoperatively.
Preoperatively, all except two patients were ambulatory. A total of ten patients had neurologic deficits. Five had a chronic non-progressive deficit, and five presented with a progressive neurologic deficit that had initiated ≤6 months before admission. At follow-up, all patients except one were ambulatory. The preoperative imaging studies of nine patients revealed signs of a sick cord with or without neurological deficits, and nine showed intraspinal pathologies, including four cases of syrinx and four cases of split cord malformation (SCM). 6 patients (13%) had undergone prior spinal surgery.
The body height at the index treatment averaged 149 ± 13 (118–176) cm, and weight was 47 ± 14 (23–88) kg. In 34 patients with a complete set of data, the preoperative height was 147 ± 13 (118–171) cm and the height at follow-up was 154 ± 12 (124–182) cm. The increase averaged 7 ± 5 cm (−5 to 24 cm; p < .0001). The mean body weight was 43 ± 12 (23–68) kg preoperatively and 48 ± 11 (28–73) kg at follow-up. The average increase was 5 ± 6 kg (−4 to 27 kg; p < .0001).
Halo-gravity traction
All the patients underwent HGT prior to surgery. In 15 patients (33%), HGT preceded an anterior release, with continued HGT until posterior spinal fusion. Only the HGT radiographs and the FVC% before the anterior release were evaluated. The duration of HGT was 30 ± 14 (11–81) days. The maximum load applied was 33 ± 9 (13–52) % of each patient’s body weight.
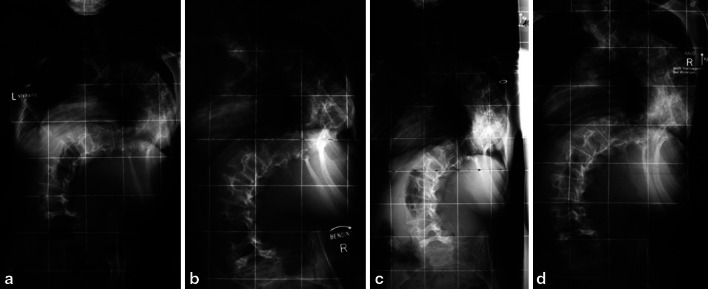
The mean time between the application of the halo-ring and the first HGT radiographs was 7 days, until the 2nd-HGT radiographs it was 22 days (n = 28), and until the 3rd-radiographs it was 27 days (n = 15). The number of serial radiographs was 2 ± 1 (1–5). There were no serious complications from HGT. A set of composite radiographs is presented in Fig. 1.
Fig. 1.
Composite radiograph series of preoperative halo-gravity traction (HGT). Example of a 14-year-old patient with kyphoscoliosis, height 139 cm, and weight 29 kg. He had Cobb angles of 147° on preoperative full-spine standing radiographs and 129° on convex bending. At the beginning of HGT, he had a Cobb angle of 123°; on day 3 and on the final-HGT-radiograph directly before surgery, the Cobb angle was 117°. During HGT, the preop-FVC% of 39% increased to 48% until final pulmonary function testing before surgery, indicating a modest improvement in the Cobb angle but a valuable improvement in pulmonary function related to the straightening of the compensatory curves
Pulmonary function tests
The preop-FVC% in 42 patients was 49.5 ± 22% (14–120%; n = 42). The preop-FVC% correlated with the extent of the scoliosis on the neutral radiographs (p = .02, r = −.4, n = 42), whereas the location of the apex or the amount of kyphosis had no impact on the FVC%. The 1st-HGT-FVC% was 47 ± 21% (21–102%; n = 24), the 2nd-HGT-FVC% was 43 ± 18% (17–82%; n = 15) and the 3rd-HGT-FVC% was 50 ± 30% (20–100%; n = 6). At the time of the index surgery, 6 patients (14%) had no or mild pulmonary impairment, 7 patients (17%) had moderate pulmonary impairment and 26 (62%) had severe pulmonary impairment.
To summarise the course of HGT, the final HGT-FVC% was 49.5 ± 20% (19–100%; n = 25). The number of days in HGT until the final HGT-FVC% was assessed was 19 ± 10. We analysed the effect of HGT on pulmonary function in terms of FVC% changes. Complete data sets were available for 24 patients. At the end of HGT, 13 (54%) were identified as responders, 10 (42%) as non-responders and 1 patient showed a decline. The preop-FVC% in this group of patients was 42 ± 20 (14–98) %, and the final HGT-FVC% was 49 ± 20 (19–100) %. The difference averaged 7 ± 8% (−5 to 27%, n = 24) and was significant (p < .0004). Notably, the difference between the preop-FVC% and the 1st-HGT-FVC% was 5 ± 7% (−7 to 23%; n = 24), which was significant (p < .005), whereas the difference between the 1st-HGT-FVC% and the final HGT-FVC% did not reach significance (p = .05). In a subset of 18 patients with a preop-FVC% <40%, the increase until the final-HGT-FVC% was 9 ± 8.5% (−5 to 27%, p. < 00002).
The statistical analysis of the relationship between HGT and pulmonary function showed that the HGT-FVC% largely depended on the preop-FVC% (p < .0001, r = .9, n = 24), but not on the other variables. The duration of HGT did not have a significant impact on the evolution of the FVC%, nor did the amount of Cobb angle change during HGT. However, even with a moderate surgical correction in rigid curves, there was a strong trend towards improvement from the preop-FVC% to the follow-up-FVC% with a reduction in the magnitude of scoliosis (p = .06, r = −.34, n = 31). That is, pulmonary function improved with better curve correction.
Other than in scoliosis, the difference between the kyphosis on the 1st-HGT radiographs and that on the final HGT radiographs significantly correlated with the FVC% difference between the 1st-HGT-FVC% and the 2nd-HGT-FVC% in the subset of patients with relevant data available (p = .02, r = −.8, n = 9), indicating better FVC% with improved kyphosis correction during HGT.
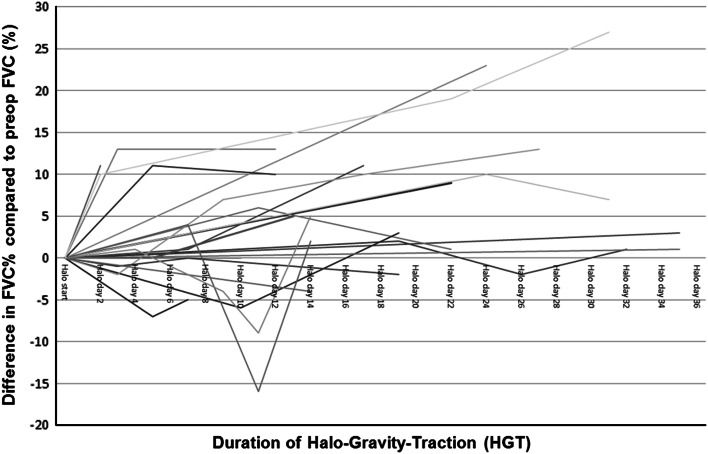
Based on the serial measurements, we calculated the time-dependent relationships between FVC% changes in 24 patients. The results are graphically displayed in Fig. 2. Statistics revealed that the aetiology of scoliosis had no impact on FVC%-changes.
Fig. 2.
The graph illustrates the time-dependent relationships between FVC% and the duration of HGT compared to the preop-FVC% in a subset of 24 patients. Changes in FVC% were calculated as differences from the FVC% before the initiation of HGT. Notably, a few cases temporarily worsened with final recovery, and a few improved beyond an FVC of 10%
The postop-FVC% averaged 29 ± 19% (11–81%; n = 12). The difference between the preop-FVC% and the postop-FVC% was −7.5 ± 9 (−20 to 11) %, which indicated a decline. The follow-up-FVC% was 45 ± 17% (24–100%; n = 31). Concerning the evolution of pulmonary function, 30 patients had complete datasets, with the final PFT performed 24 (12–74) months after surgery. The preop-FVC% in these patients was 47 ± 18 (18–98) %, and the follow-up-FVC% was 44 ± 17 (24–100) %. The mean degree of preoperative kyphosis in this group was 91°, and the mean degree of scoliosis was 115°. The difference between the postop-FVC% and the follow-up-FVC% averaged 2 ± 19% (−37 to 28%; n = 10), whereas the difference between the preop-FVC% and the follow-up-FVC% was 3 ± 12% (−37 to 18%, n = 30), which was not significant (p = .2). The FVC% improved >5% in nine patients (30%) and >10% in two. 12 patients (40%) remained stable, with an FVC% of ±5%, and 9 (30%) declined >5%. The main findings are illustrated in Fig. 3.
Fig. 3.
Illustration of the course of pulmonary function from the preoperative state to follow-up. Patients with complete data for preop-FVC%, HGT-FVC% and follow-up-FVC% are marked in dark grey. Patients with complete data for preop-FVC% and follow-up-FVC% are marked in light grey
A statistical analysis showed that the follow-up-FVC% was strongly dependent on the preop-FVC% (p = .0001, r = .8, n = 30) and that the length of the follow-up (in months) had no impact on the difference in FVC% evolution (p = .5). In contrast, we noticed a significant correlation between anterior release and the FVC%-change from preoperative to follow-up (p = .006). There was a 10% decline in the FVC% if an anterior release was performed but an increase of 2% if it was not performed. With an anterior release, the correction rates for scoliosis (35 vs. 30%) and kyphosis (26 vs. 21%) were increased, but the differences were not significant. We did not identify any other single risk factor with an impact on the follow-up-FVC% or a preoperative threshold predicting a pulmonary complication.
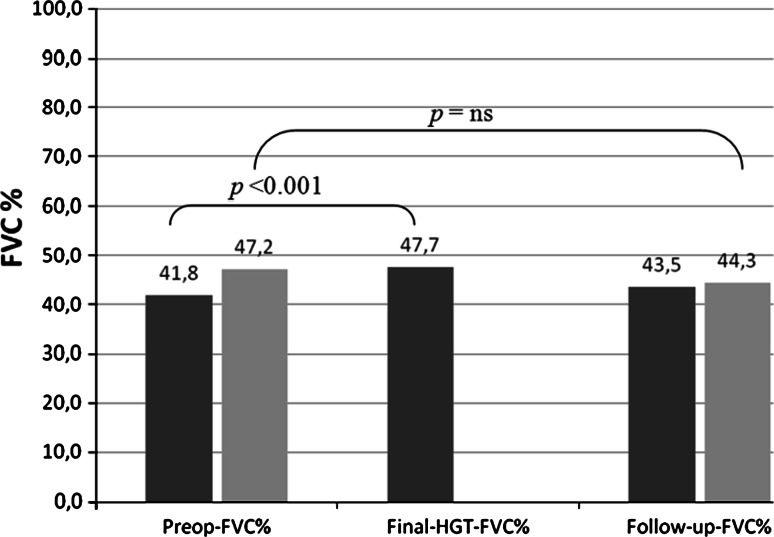
Considering the course of pulmonary function before surgery, during the HGT and at follow-up, 18 patients had complete data sets, with the final PFT performed 16 months after surgery. In these patients, the preop-FVC% was 42 ± 19 (18–98) %, the final-HGT-FVC% was 48 ± 19 (19–100) % and the follow-up FVC% was 43.5 ± 18 (24–100) %. The preoperative degrees of kyphosis and scoliosis were 96° and 118°, respectively. The results illustrated in Fig. 4 show that pulmonary function could be increased by HGT and maintained at the preoperative level until follow-up.
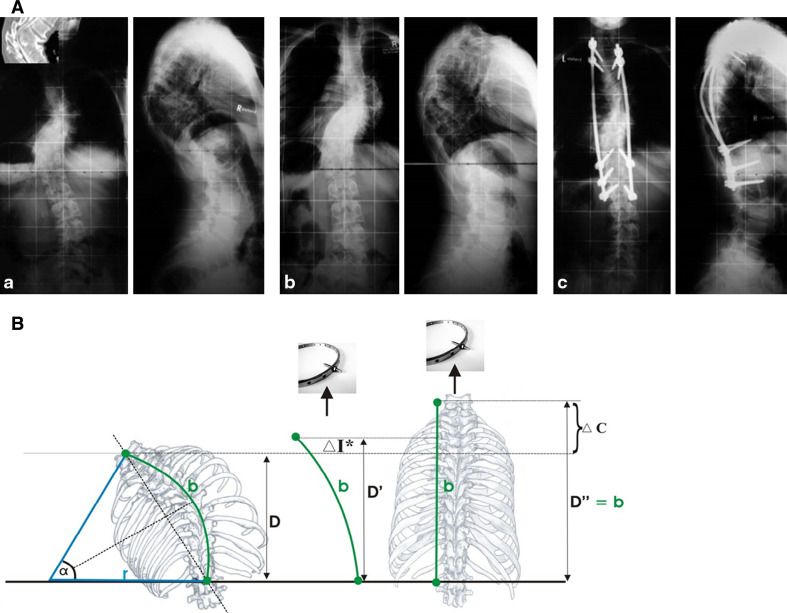
Fig. 4.
A A clinical example of improvement in neurological function during HGT. a A 13-year-old patient with congenital kyphoscoliosis and progressive neurologic deficit (Nurick III, Ranawat IIIa, incontinency, leg weakness), a scoliosis of 63° and a kyphosis of 75°. b After 11 days in HGT, the neurology had improved, as had the kyphosis (slightly). c Treatment with an anterior release T6–8 and T11–L1 and 2-staged PSF T6–L2. At 48 months’ follow-up, the patient was defined as Ranawat II. The patient was satisfied. B Clinical rationale for the improvement of neurologic symptoms during HGT. Rigid kyphoscoliosis can decompensate, and tensioning of the spinal cord being draped over the kyphotic or scoliotic apex might create a myelopathic spinal cord with progressive neurologic deficits. Improvements in progressive neurological deficits in selective cases with decompensated curves can occur during HGT. The effect is induced by a slight curve correction. This, in turn, causes a release of the apical tether on the spinal cord. With partial correction (image in the middle), the tension on the spinal cord is reduced, which allows the cord to recover preoperatively. The clinical observations can be put into an algebraic equation, as illustrated the length of the spinal cord sector can be estimated using the formula: 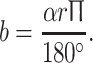 During HGT, the trunk height increases (D vs. D′), and the kyphotic and scoliotic curves are reduced slightly, but rigid curves do not fully correct, which should be avoided. With slight curve correction, the apical tension on the cord is reduced. If HGT is applied until complete straightening of the spine occurs (see D″), tension-induced myelopathy may result
During HGT, the trunk height increases (D vs. D′), and the kyphotic and scoliotic curves are reduced slightly, but rigid curves do not fully correct, which should be avoided. With slight curve correction, the apical tension on the cord is reduced. If HGT is applied until complete straightening of the spine occurs (see D″), tension-induced myelopathy may result
Radiographic results
Kyphosis
The major kyphosis was located in the high thoracic region (C7–T4) in 7 patients (16%), the thoracic region (T5–T10) in 28 (62%) and the thoracolumbar region (T11–L2) in 10 (22%). The preoperative kyphosis was 91° ± 30° (4°–138°) on the neutral radiographs and 76° ± 27° (19°–122°) on the final-HGT radiographs, a significant difference (p < .0001). The kyphosis on the traction radiographs was 73° ± 28° (21°–135°, n = 17), also significantly different from the neutral radiographs (p = .004).
The HGT-flexibility of kyphosis was 17 ± 19% (−29° to 72°), and the traction-flexibility was 18 ± 18% (−9 to 51%, n = 17). The difference between the traction and HGT radiographs was 0.5° ± 13° (−26° to 20°; n = 17), and the difference in flexibility was 4 ± 13% (−22 to 22%; n = 17). Neither difference was significant.
On the 1st-HGT radiographs, kyphosis measured 80° ± 25° (15°–130°); on the final HGT-radiographs, it was 74° ± 25° (19°–122°). The improvement was 7° ± 12° (−8° to 41°, n = 27, p < .03).
The degree of postoperative kyphosis was 70° ± 26° (16°–122°), and the correction was 21° ± 18° (−31° to 58°) or 23 ± 24% (−65 to 70%) (p < .0001). The difference between the HGT kyphosis and the postoperative kyphosis was 5° ± 15° (−37° to 41°; p = .05). The amount of correction was significantly correlated with the HGT-flexibility (p < .0001, r = .7, n = 42) and the traction-flexibility (p = .002, r = .7, n = 17).
At follow-up, the kyphosis measured 76° ± 25° (31°–127°), representing a significant loss of correction (−5 ± 11°, −31° to 13°; p = .005). Patients who had undergone a CTP had a significantly higher rate of kyphosis recurrence (9° vs. 2°, p = .02).
Preoperatively, 14 patients (33%) displayed a decompensated sagittal imbalance, whereas 40 (89%) had achieved sagittal balance at follow-up. In 11 patients (24%), sagittal balance had improved; 32 (71%) maintained their balance; and 2 displayed reduced sagittal balance.
Scoliosis
The major scoliosis was high thoracic (C7–T4) in 2 patients (4%), thoracic (T5–10) in 33 (73%), thoracolumbar (T11–L2) in 7 (16%) and lumbar (L2–4) in 3 (7%). Preoperatively, the apical vertebral rotation was 3.5° ± 1.5°. The preoperative major scoliosis measured 106° ± 35° (13°–151°), and the best Cobb angle on the HGT radiographs was 93° ± 27° (12°–129°). The difference was 16° ± 12° (−9° to 41°; p < .0001).
Scoliosis on the bending radiographs was corrected to 101° ± 24° (49°–150°, n = 35) with a bending-flexibility of 10 ± 12% (−15° to 42°, n = 35), and it was significantly different from the neutral radiographs (p < .0001). The degree of scoliosis on the traction radiographs was 101° ± 24° (47°–141°, n = 18) with a traction flexibility of 12 ± 12% (−7 to 37%), which was significantly different from the neutral radiographs (p < .0001), but not from the values achieved for bending-flexibility (p = .5). The HGT-flexibility was 15 ± 11 (−9.7 to 40) %. Notably, in terms of Cobb angles, the difference between the HGT-flexibility and the bending-flexibility was 4° ± 13° (−27° to 40°, n = 34). This difference was not significant (p = .1) and nor was the 3° ± 14° (28°–23°, n = 18; p = .4) difference between the HGT-flexibility and the traction-flexibility.
The scoliosis on the 1st-HGT radiographs was 98° ± 30° (16°–143°; n = 43). The improvement in scoliosis from the 1st-HGT radiograph (101° ± 30°, 20°–143°; n = 32) to the final-HGT radiograph (94° ± 26°, 14°–127°; n = 32) was significant (p = .0003), but only 8° ± 9° (−10° to 29°; n = 32). With increasing scoliosis, the difference between the Cobb angle on the traction radiographs and on the best-HGT radiographs decreased (p = .01, r = −.6, n = 18). That is, the larger the curve, the more rigid it was, and the smaller was the effect of HGT in reducing the curve in comparison with a simple Cotrel traction.
Notably, the HGT-flexibility correction was not correlated with the duration of HGT or the different weights applied. The ‘deformity correction’ and ‘trunk height increase’ frequently observed during HGT occurred in the more flexible compensatory curves.
The postoperative scoliosis was 76° ± 32° (2°–142°), and the correction averaged 31° ± 17° (0°–61°), or 33 ± 19% (0–90%; p < .0001). In terms of the CCI, the correction was 2.7 when assessed on HGT radiographs (n = 39) and 7.3 when assessed during bending (n = 35). According to the CCI, the correction rate was in the range of previously published results (Table 1). The scoliosis correction did not correlate with the duration of HGT or the size of the weights applied but was rather a function of the preoperative curve size (r = .4, p = .007).
Table 1.
Results of surgical correction of severe and rigid scoliosis and kyphoscoliosis
| Author: technique applied | Preop coronal Cobb | Postop coronal Cobb | Follow-up coronal Cobb | Coronal flexibility (%) | Correction (%) | Cincinnati index |
|---|---|---|---|---|---|---|
| Current study: PSF w/ and w/o ant rel and CTP | 106 | 70 | 75 | 10 | 34 | 2.7a |
| Lonner: ant release and Ponte and PSF-PS [50] | 97 | 51 | 53 | 11 | 47 | 4.3 |
| Lenke: pVCR and PSF-PS [14] | 85 | 42 | 68 | 12 | 51 | 4.3 |
| Yamin: ant release and HGT and PSF-Hybrids [15] | 111 | 39 | 40 | 13 | 65 | 5.0 |
| Lenke: pVCR and PSF-PS [14] | 90 | 44 | 40 | 16 | 51 | 3.2 |
| Buchowski: 50% release, temporary internal distraction and PSF [7] | 104 | 20 | 22 | 16 | 81 | 5.1 |
| Wang: multilevel pVCR and PSF-PS [57] | 80 | 32 | 34 | 18 | 60 | 3.3 |
| Suk: pVCR and PSF-PS [8] | 111 | 50 | 54 | 18 | 55 | 3.1 |
| Suh: multilevel segm periapical post 360° release and PSF-PS [19] | 118 | 49 | 50 | 20 | 58 | 2.9 |
| Sponseller: PSF-Hybrid w/ VCR in 30% [11] | 105 | 60 | 61 | 21 | 43 | 2.0 |
| Hempfing: ant-rel and cotrel traction and PSF-Hybrids [25] | 90 | 37 | 39 | 21 | 59 | 2.8 |
| Sponseller: HGT and PSF-Hybrid [2, 11, 34] | 93 | 57 | 58 | 22 | 39 | 1.8 |
| Bullman: VDS-Zielke and PSF-PS (w or w/o prior post release) [65] | 93 | 31 | 33 | 23 | 67 | 2.9 |
| Qiu: ant rel and HGT and PSF-Hybrids [16] | 96 | 57 | 59 | 23 | 41 | 1.8 |
| Li: ant rel and PSF-Hybrids [58] | 98 | 51 | 54 | 24 | 48 | 2.0 |
| Qiu: ant rel and HGT and PSF-Hybrids [16] | 92 | 40 | 43 | 24 | 57 | 2.4 |
| Lenke: pVCR for kyphoscoliosis [14] | 91 | 44 | 46 | 25 | 52 | 2.1 |
| Wang: PSF-PS and memory rods [21] | 93 | 29 | 32 | 26 | 69 | 2.7 |
| Tokunaga: multilevel ant VCR and PSF [56] | 107 | 56 | 59 | 26 | 48 | 1.8 |
| Di Silvestre: PSF-Hybrids (2× PSO) [9] | 92 | 34 | 45 | 27 | 63 | 2.3 |
| Lenke: pVCR for idiopathic scoliosis [14] | 104 | 34 | 36 | 27 | 67 | 2.5 |
| Kuklo: PSF-PS [17] | 100 | 32 | 33 | 29 | 68 | 2.3 |
| Watanabe: ant rel and PSF-PS (7×), PSF-PS (10×) w/ VCR 3× [18] | 119 | 40 | 40 | 33 | 66 | 2.0 |
| Watanabe: VDS-Zielke and PSF-Hybrids, 1× VDS-Zielke only [18] | 108 | 28 | 42 | 38 | 74 | 1.9 |
| Hamzaoglu: halo-fem-traction and PSF [62] | 122 | 60 | 64 | 39 | 51 | 1.3 |
| Mean | 95 | 44 | 47 | 19 | 54 | 2.7 |
Published results on curve rigidity (including flexibility data)
pVCR posterior vertebral column resection, PSF posterior spinal fusion, ant rel anterior release, CTP concave thoracoplasty/multiple concave sided rib osteotomies, PS pedicle screw-based constructs, HGT halo-gravity traction, Ponte Ponte-type osteotomies, hybrid constructs using pedicle screws, hooks and wires
aCincinnati index, calculated from flexibility on HGT-radiographs
The difference between the degree of scoliosis on the preop-HGT radiographs and the postoperative radiographs was 15° ± 17° (−19° to 66°, n = 43) (p < .0001). At follow-up, the mean scoliosis measured 84° ± 31° (5°–143°), which represented a loss of correction of −6° ± 13° (−35° to 17°; p = .003).
Preoperatively, the coronal C7 plumb line was −1 ± 4 (−8.5 to 5.5) cm off the sacral midline; 25 patients (56%) were out of balance. This value was −1 ± 3 (−8 to 7) cm postoperatively and −1 ± 2 (−5 to 5) cm at follow-up. Overall, 31 patients (69%) exhibited improved coronal balance. Although 16 patients remained out of balance, the ranges of the C7 plumb line (−5 to 5 cm) were modest, and acceptable trunk balance could be achieved in most of the severe deformities.
Surgical results
Complications from surgery were documented in 20 patients (44%), of whom 15 (33%) had a major complication. 3 had significant pulmonary complications related to recalcitrant atelectasis, and pulmonary decompensation indicating recurrent ventilation in two patients. The other complications included coronal decompensation with need for revision (1), pancreatitis (3), recalcitrant non-union indicating two revisions (1), temporary sensory dysfunction of the lower extremities (1), loss of kyphosis correction and decubitus from bracing (1), high thoracic junctional kyphosis (2), chylothorax (1), rod fracture and non-union (1), cardiac arrhythmia (1), deep wound infection requiring I and D (2), bladder dysfunction in a patient with recurrent endometritis (1), asymptomatic rod fracture with loss of correction (1) and adjacent curve decompensation. There was no permanent neurovascular compromise.
In 12 patients (27%), additional surgery was performed; 7 of these underwent revision surgery. 7 patients received a rib-hump resection. The latter had larger postoperative scoliosis (99° vs. 71°, p = .03). Patients who had initially undergone a CTP were less likely to need a subsequent rib-hump resection (p = .02).
At follow-up, patients were asked whether they were satisfied with the global outcome. 13 patients (29%) reported that they were ‘very satisfied’, 26 (58%) were ‘satisfied’ and 3 were ‘not satisfied/not dissatisfied’ or just ‘not satisfied’.
Evolution of patients with preoperative progressive neurological deficits
Five patients with kyphoscoliosis and progressive neurological deficits from a decompensating curve showed improvement after the initiation of HGT. The mean age of the two males and three females was 24 ± 17 years (9–48) at the time of surgery. Their kyphosis was related to congenital multi-level mixed-failure (2), neurofibromatosis (1), post-laminectomy syndrome (1) and tuberculosis (1). 2 patients had syrinxes. On admission, three patients had Ranawat IIIa, and two had IIIb. All the patients displayed spasticity, and three had progressive bowel and bladder incontinency. During HGT, 13 ± 6 kg was applied for 22 ± 11 days (11–39). The kyphosis measured 93° ± 16° (72°–108°) preoperatively and 76° ± 17° (64°–101°) on the best post-HGT radiographs. The major scoliosis Cobb angle was 34° ± 23° (15°–63°) preoperatively and 26° ± 27° (2°–68°) on the post-HGT radiographs. No patients experienced complications related to HGT or worsened neurologically. In contrast, during HGT, the neurological deficits of two patients improved, with reduced weakness and an improved gait. All patients displayed improved spasticity, hyperreflexia, subjective weakness and dysaesthesia while in HGT. At follow-up, two and three patients achieved Ranawat grades of I and II, respectively (The patient with Pott’s disease was followed up for only 6 months). In summary, HGT was shown to improve progressive neurological deficits in decompensated rigid and non-rigid curves with a sick cord. The clinical rationale is illustrated in Fig. 4.
Discussion
Pulmonary function
Severe scoliotic and kyphotic deformities affect the thoracic cage, disturbing skeletal, muscular and diaphragmatic function and reducing respiratory system compliance (Fig. 5) [28, 32]. Hence, respiratory function, rather than curve size, is the predominant problem, which has a bearing on physical function and mortality [4]. Long-term studies on the natural course of pulmonary impairment and severe scoliosis have shown that there is no spontaneous resolution but rather a slight decline of PFT over time [22], and particularly with curves >100° and/or FVC% <45%, patients suffer long-term sequelae, including reduced pulmonary endurance with an increased mortality rate compared with those who experience respiratory failure in adulthood [2, 4, 5, 23]. At scoliosis >100°, respiratory system compliance is decreased to levels comparable to adult respiratory distress syndrome [28]. Untreated severe (kypho-)scoliosis has a poor prognosis, accounting for up to 5% of chronic ventilator failures in adulthood [2, 5, 28, 33, 34–36]. Hence, early surgery is recommended and is said to confer improvements in pulmonary function and increases in life expectancy [3–5, 33, 35, 37]. However, surgery, while successful in curve correction, often cannot overcome the multifactorial genesis of pulmonary impairment [3, 35, 38]. Radiographic studies have correlated impaired pulmonary function with increased scoliosis, as was reported in our study, along with the degree of vertebral rotation, the deformity of the thoracic cage, the number of vertebrae involved in the thoracic curve, the degree of thoracic hyperkyphosis or lordosis and the curve location [3, 6, 28, 36, 39, 40]. The definitive Cobb angle at which PFT results worsen significantly has not been identified. Spinal deformity is the major determinant of respiratory impairment in severe scoliosis; however, the relationship is complex, and its severity of respiratory impairment cannot be inferred from radiographs alone. One must consider the patient’s age, respiratory muscle weakness and irreversible structural parenchymal damage and altered breathing mechanisms, which may have hindered several of our patients from improvement in PFT [3, 28, 35]. In our sample, 79% of the patients had moderate or severe pulmonary impairment preoperatively; 30% of the patients improved slightly, 40% maintained their preoperative level and 30% showed a decline at follow-up. Our results echo those of prior studies on patients with severe pulmonary impairment: Payo [31] reported on 17 neuromuscular scolioses, with FVC <40% and mean patient age of 13 years. The preoperative Cobb angle was 88° and was corrected to 37°. The FVC% was 26% preoperatively and 29% at follow-up. In a study by Gill [41] of eight paediatric patients with a mean scoliosis of 70°, the FVC% was 20% preoperatively and 18% at follow-up. Wazeka [20] reported on 21 scolioses with mean age of 11 years and a preop-FVC% of 30. Curve size averaged 82° and was corrected to 35°; 50% of the patients had 1-year PFT data, but only three showed pulmonary improvement. As in prior studies, we did not identify a significant increase in PFTs values until follow-up; rather, we observed stabilisation at the preoperative level. When nutritional status was used as a marker of pulmonary status [42], our analysis of body weight showed a significant postoperative gain, and clinical respiratory improvement was frequently confirmed.
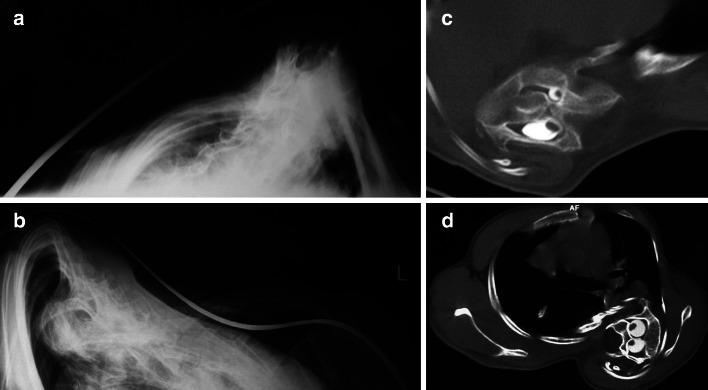
Fig. 5.
Illustration of the clinical conditions and concerns with pulmonary compromise in severe scoliosis and kyphoscoliosis. a, b Axial views of a sharp rib hump related to severe curves. c, d Stress the amount of axial rotation and intraspinal anomalies frequently seen in patients with severe scoliosis and kyphoscoliosis
Only a few reports exist on severe curves and the related severe pulmonary impairment [5, 30, 35, 36, 41]. Most spirometry data are derived from moderate idiopathic curves with no to mild pulmonary impairment and are conflicting. While some report an insignificant improvement or a decline in PFT within 2 years [39, 43], others describe significant improvements of ~2–11% [44–47]. Newton [39] identified preoperative PFT values as the best predictor of 2-year pulmonary function in these populations. In our study on severe curves, the preop-FVC% was also highly predictive of the follow-up-FVC%. There is only one study by Rizzi [5] on 35 adults with which to compare our results: 26 patients had kyphoscoliosis, and 77% had 1-year PFTs. The degrees of kyphosis and scoliosis averaged 132° and 136°, respectively. 12 patients had died at a mean age of 43 years, the cause being cardiopulmonary in all. In 19 patients with kyphoscoliosis, the preop-FVC% was 27%, improving to only 32% at follow-up. The authors identified a ‘good evolution’ group with an FVC%-increase from 31 to 39% and a ‘fair/poor evolution group’. Almost all the patients in the fair/poor pulmonary evolution group had kyphoscoliosis; their main characteristics were worse preop-FVC% and less correction, indicating a strong correlation between curve correction and pulmonary improvement [20]. In our study, the change from the preop-FVC% to the follow-up-FVC% averaged 3%, which substantiates Rizzi’s observations. It has also been suggested that the correction of vertebral rotation and rib cage deformity significantly contributes to pulmonary improvement in severe curves [40, 48]. Accordingly, in moderate scolioses treated using segmental pedicle screw constructs, Kim [43] demonstrated a significantly increased respiratory function compared with the use of less rigid constructs. Similarly, Smiljanic [35], using the anterior open approach for instrumented derotation, reported on 31 scolioses of 108° corrected to 37° and noted an improvement of the preop-FVC% from 66 to 78% at a 12-month follow-up. Concerning posterior vertebral derotation, Nepple [49] described a 14-year-old patient with a thoracic scoliosis of 149°, a flexibility of 33% and an FVC% of 31%. After an anterior release and a posterior correction with 2-level VCR and bilateral CTP, the curve was reduced to 43°. At 3 years postoperative, the thoracic curve measured 48°, and the FVC% was 52%. These results emphasise that the degree of 3D curve correction might also be decisive for pulmonary improvement in severe scoliosis.
The impact of the approach to PFTs in severe scoliosis is poorly documented. Some studies on modest curves have reported a temporary decline in PFT results after transthoracic correction [3, 35, 44, 45, 47]. In our study on severe curves, patients who did not undergo an anterior release had a small but significant FVC% increase from the postoperative period to follow-up. On the contrary, the FVC lost by the anterior approach could not be compensated by improved curve correction. In total, 40% of the patients in our series underwent a CTP. Currently, several authors use CTP as part of the posterior release and to reduce the concave rib valley [20, 37, 49–52]. Limited data exist regarding the impact of CTP on PFT results [51] and its effect on posterior release [29]. Our statistical analyses could not quantify the impact of CTP on the PFT results. In posterior-only fusions performed with a CTP, we observed a decrease in the FVC% of ~5–10% (unpublished results). Hence, there is some residual pulmonary compromise caused by CTP that usually does not have a clinical effect in patients with mild pulmonary impairment but might be an issue in severe curves. In combination with the anterior release, this factor might have prevented some of our patients from pulmonary improvement.
Halo-gravity traction
In the past, HGT was said to make surgery less complex, to improve safety and to achieve a gradual correction [11, 13, 36, 37]. However, the literature lacks evidence of the benefits of HGT on severe curves [13, 34]. Even the appropriate duration of HGT has not been identified [7, 10, 11, 42, 49, 53], with reports ranging from weeks to months [10–12, 34]. Letts [12] analysed the time-dependent correction of scoliosis during halo-femoral traction in eight patients. Corrections from 82° to 34° (40%) were obtained, and most of the improvement occurred within the first week. Letts recommended that HGT should not be continued for >3 weeks. Our results showed that preop-HGT did not induce a meaningful release effect in rigid curves. A mean 16° correction was achieved with HGT, whereas the difference between the 1st- and final-HGT-radiographs was only 8°. The flexibility on HGT radiographs is supposed to be increased compared with that of bending or traction radiographs. However, the difference between both was not significant. In a study by Seller [54] on neuromuscular scoliosis, the surgical correction rates did not differ with or without preop-HGT. In a study by Flierl [55] on 22 neuromuscular scolioses, HGT did not improve surgical outcomes. In Rizzi’s study [5] of 19 kyphoscolioses treated with HGT for 3 weeks (8 had complete data), the correction due to HGT was from 129° to 97°, and the HGT-flexibility was 23%. Our results also support those of a larger, 9-centre study on severe scoliosis [11]. 30 patients were treated with preop-HGT for 5 weeks, and 23 without. The scolioses measured 93° and 105°, the flexibility was 22 and 21%, and the correction was 62 and 59%, respectively, and the differences were not significant. The study indicated that the real effect of HGT on rigid curves might be overestimated. Finally, Park [53] reported on 20 scoliosis patients with an age of 11 years who underwent HGT for a mean of 4 weeks. In a subset of patients who underwent surgery, 46% of the total coronal curve correction was estimated to have been achieved with HGT. The curve correction was 27% in bending and 31% in final-HGT radiographs. A calculation of the correction due to HGT each week as a percentage of the total correction indicated that the scoliosis had corrected by 66% at 2 weeks, by an additional 22% at 3 weeks and by an additional 9% at 4 weeks, showing that most of the correction had occurred within the first 3 weeks. That study included more flexible curves than ours, but like the prior reports, it supports our findings: We showed that in severe, rigid curves, flexibility on HGT radiographs was not significantly different from that on bending radiographs, and most of the correction was obtained within the first 2 weeks. Finally, Tokunaga [56] reported on anterior column release with subsequent HGT and final posterior correction in 21 curves that were >80°. After the anterior release, the correction due to HGT increased from 24 to 38% after 1 week, to 42% after 2 weeks and to 45% after 3 weeks. Most of the final correction (49%) was achieved by the anterior release and the first week of HGT, emphasising that HGT gains more importance after aggressive releases.
Impact of HGT on neurological function
Deformity correction in the presence of a neurological deficit with the spinal cord being draped over the apex of the deformity or tensioned by a septum in an SCM poses significant risks. In these cases, moderate correction has generally been recommended to achieve global balance; alternatively, a detethering procedure preceding the scoliosis correction has been advocated [37, 57–59]. In general, there is no uniform advice on how best to manage these patients. Our study provides additional data regarding the use of preop-HGT in patients with severe curves and progressive neurologic deficits due to a decompensating kyphoscoliosis. We noted neurologic improvement in these patients. Rigid curves were only slightly reduced with HGT, thus allowing for the relaxation of the tension on the cords draped over the curves’ apices (Fig. 4b). In a recent analysis [60] of 147 patients with VCRs, 29% had abnormal preop-neurologic findings, and 27% experienced an intraoperative neurologic event. Whether preop-HGT is beneficial in reducing neurologic deficits in such patients with ‘sick cords’ should be analysed.
HGT and pulmonary function
Preoperative medical and pulmonary improvement in a patient with severe deformity can reduce surgical risks [42]. As a non-surgical tool, nocturnal nasal intermittent positive pressure ventilation can significantly increase the FVC% [32]. In addition, intensive preoperative pulmonary conditioning in Duchenne patients by a pulmonary trainer, as performed in our patients, can improve PFT results [61]. Preop-HGT remains another possibility for preoperative medical and pulmonary improvement. Since the 1970s, HGT has been recommended for patients whose pulmonary function was so poor that immediate surgery might have been lethal [13]. However, the current literature lacks reproducible data regarding the impact of HGT on PFT results [13, 16]. Savini [36] reported on 76 patients with mean age of 15 years and Cobb angle of 110°, some treated with preop-HGT, all undergoing Harrington fusions. They noted a correlation between pulmonary and angular improvement. Details regarding PFTs were lacking. Swank [34], reporting on 20 adults with 135° scoliosis and 127° kyphosis, noted improvements in PFT results in some undergoing HGT but an unpredictable decline or no improvement in others. In Rizzi’s study [5] on severe curves, he reported that preop-HGT proved beneficial for the improvement of FVC. 19 patients underwent HGT for 3 weeks. The preop-FVC% was 27%, and the HGT-FVC% (data from 12 patients) was 34%, representing an improvement of 7%, similar to our results. Sink [42] reported on patients with scoliosis of 83° and HGT for 13 weeks before and/or after an anterior release. Preop-HGT was recommended until a decline or plateau was observed, but the PFT data were not provided. In a study by Nepple [49], the FVC% improved from 31 to 47% after 4 weeks of HGT. In summary, preop-HGT is a useful tool to improve a patient’s pulmonary condition. Our study showed that a significant increase of 9% in the FVC% was achieved by HGT in patients with severe pulmonary impairment and a mean scoliosis curvature of 114°. HGT made a significant contribution to the patients’ postoperative pulmonary evolution, with a minority of patients showing a significant decline at follow-up, although anterior approaches were performed in 33% and CTP in 40% of the patients.
Surgical correction of severe curves
There are conflicting data as to whether maximising curve correction in severe scoliosis changes outcomes. The problems arise with the lack of uniform definitions of severe and rigid curves [1, 9, 13, 15, 19, 50, 56, 58, 62]. Generally, severe curves have a Cobb angle >80°–90°, are rigid when the flexibility is <20–30% and bend down to 70°–80° at best. Silvestre [9] stated that in severe scoliosis, the aim should be not to maximise the correction but rather to obtain acceptable balance. This strategy was also followed in our study. We noted a mean curve flexibility of 10% (15% during HGT) and a surgical correction of 33%, resulting in a CCI of 2.7 in HGT. Our results approximated those of Watanabe [18], who reported on 68 curves >100°. The mean flexibility in a hook subgroup was 23%, and the mean correction was 33%. In a study by Rinella [10] of 33 patients, scoliosis of 84° was corrected by 46%. Qiu [16] reported on 60 patients with a scoliosis of 92° and a flexibility of 38%. Using hybrids and HGT after anterior releases, the surgical correction was 39%. With a rigidity of 10–15%, our sample is unique, representing an ideal base for comparison to studies on more aggressive osteotomies and segmental instrumentations. To improve pulmonary function, it might be warranted to adapt techniques optimising curve correction [4, 14, 48]. As the smallest common denominator for increasing the correction, the extent of the release was shown to be decisive regardless of the instrumentation used [63]. A VCR achieves the most aggressive release, and the number of studies reporting correction rates of ~50–65% in rigid curves is growing [8, 14, 57, 64]. To put our results in perspective, a review of the literature on surgery for severe curves was performed (Table 1). We identified 19 studies; the patient age averaged 19 years, and the sample size 18. The preoperative scoliosis was 100°, the flexibility was 23%, and the curves were corrected to 42° postoperatively; this value declined to 48° at follow-up averaging 41 months. The scoliosis correction was 57%, but the CCI was 2.7. The patients’ kyphosis measured 55° preoperatively, 33° postoperatively and 34° at follow-up. Only a few authors reported a >50% correction in curves >90° using a posterior-only approach with segmental pedicle screw instrumentations. 6 studies reported at least one significant neurologic deficit postoperatively. Complications occurred in one-third of the patients.
In light of the potential neurological risk, particularly with VCR, alternatives should be discussed. Li [58] reported on 31 patients with a Cobb angle of 98°, a flexibility of 24% and a correction of 49% using an anterior and posterior release followed by segmental hybrid instrumentation. Suh [19] reported the results of posterior periapical segmental osteotomies and instrumentation in 13 patients. They achieved a 58% correction in a mean scoliosis of 108° with a flexibility of 20%. Olgun [52] reported on 17 kyphoscoliosis patients. Using pedicle screw instrumentation combined with multiple chevron osteotomies and CTP, rigid scolioses of 69° were corrected by 62%, and kyphoses were corrected from 76° to 49°. Bullmann [65] reported on 33 patients treated using anterior instrumentation (some had undergone prior posterior release) and posterior pedicle screw-based correction. A mean scoliosis of 93° with a flexibility of 23% was corrected by 67%. In another study [15] on 21 patients with a mean scoliosis of 111° and a flexibility of 13%, a 62% correction was achieved after the application of a halo-femoral traction Ilizarov-type device following an anterior release. In summary, there are alternatives to VCR for severe and rigid but rather harmonic curves, and a multilevel segmental release and instrumentation might be sufficient to maximise correction and improve pulmonary function. Further research should identify the ideal strategy and whether temporary HGT after an anterior/posterior release plays an important role in maximising correction and improving pulmonary function.
Complications
In surgery for severe scoliosis, the incidence of major complications [24] is reported to be 20–40% [19, 57, 58]. With VCR, temporary neurologic deficits have been seen in up to 30% of patients [5, 8, 11, 14, 15, 56, 57, 60, 65]. It must be emphasised that in our study, there was no permanent neurologic decline. Numerous case reports exist concerning potential halo-related complications [66]. One study [67] reported a rate of 53%, with 10% requiring surgery and 31% suffering from transient HGT-related neurologic compromise. However, no major HGT-related complications occurred in the series by Rinella [10] or in ours. Strict protocols, including daily pin checks, re-tightening of pins and prudent hygiene are decisive in avoiding complications.
In contrast to mild idiopathic scolioses, in which transthoracic procedures usually do not have a negative long-term impact on pulmonary function [44, 47], the data regarding severe curves are conflicting. Suh [19] observed that in severe neuromuscular scoliosis, opening the chest caused significant deterioration of lung function. In a study by Liang [68] on 48 patients with a mean curve of 90° treated by anterior release and posterior fusion, the preop-FVC% (<50%) had a significant impact on the pulmonary complication incidence. The addition of a thoracoplasty procedure was an independent risk factor. Analysing 298 scoliosis surgeries [1], Zhang noted that with a preop-FVC% of 60–80%, the pulmonary complication rate was 3%; with an FVC% of <40%, it was 32%. In another study, pulmonary complications increased as the FVC% dropped to <30% [61]. Rawlins [30] reported on 32 patients with a mean age of 13 years, kyphosis of 79°, scoliosis of 87° and preop-FVC% of <40%; 19% experienced pulmonary complications, and 9% needed tracheostomies. In Rizzi’s study [5], 40% of the patients experienced postoperative respiratory complications, and in Payo’s [31] study of 17 patients with an FVC <40%, postoperative complications occurred in 58%, and 24% experienced respiratory failure. In Wazeka’s study [20] of 21 patients with an FVC% of 32%, 19% required prolonged ventilation, and 40% experienced respiratory complications. One major risk factor identified was an anterior approach. In a study by Udink ten Cate [69] of 46 neuromuscular scolioses, those requiring prolonged ventilation had a mean preop-FVC% of 34%, compared with 67% in those without prolonged ventilation. To summarise the prior studies and ours, in patients with severe pulmonary impairment, there is an increased risk of pulmonary complications, and the benefit derived from an anterior approach in terms of better curve correction should be weighed against the risk of pulmonary impairment that this approach confers. In our study, complications occurred in 44% of the patients, with major complications observed in 33%; however, only three patients had significant pulmonary complications, which emphasises the importance of preoperative pulmonary conditioning. The other complications were of various aetiologies, including 11% that were instrumentation-related. Failures with merely end-level instrumentation highlight that in severe curves, rather rigid constructs with multiple anchors should be selected.
Conclusion
Increased information on HGT allows its use to be refined. Preoperative HGT is indicated for severe (kypho-)scoliosis with moderate to severe pulmonary compromise, an FVC% <50% or an FVC% <60% if an anterior transthoracic surgery is scheduled. If the FVC% has improved or plateaued at 2 weeks, surgery should be considered. If there is a rare, ongoing improvement, the gain in pulmonary function can be maximised until it plateaus. HGT can also be useful in progressive neurological deficits, reducing the tension placed on the cord through slight curve reduction. HGT should not be expected to soften severe and rigid curves to a meaningful extent without a prior anterior and/or posterior release, and flexibility can be assessed on bending and Cotrel traction films.
No VCRs were performed in our study, and there were no neurological deficits. Thus, our sample may serve as a control for studies using more aggressive techniques. If these techniques not only significantly correct the curve magnitude and rotation but also confer significant pulmonary improvement, these procedures might be the ideal strategy despite their inherent surgical risks. Future analyses of pulmonary function in patients with severe curves will provide conclusive evidence for the justification of high-risk deformity surgeries.
Conflict of interest
None.
References
- 1.Zhang JG, Wang W, Qiu GX, Wang YP, Weng XS, Xu HG. The role of preoperative pulmonary function tests in the surgical treatment of scoliosis. Spine. 2005;30:218–221. doi: 10.1097/01.brs.0000150486.60895.a1. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein SL. Natural history. Spine. 1999;24:2592–2600. doi: 10.1097/00007632-199912150-00006. [DOI] [PubMed] [Google Scholar]
- 3.Pehrsson K, Danielsson A, Nachemson A. Pulmonary function in adolescent idiopathic scoliosis: a 25 year follow-up after surgery or start of brace treatment. Thorax. 2001;56:388–393. doi: 10.1136/thorax.56.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitale MG, Matsumoto H, Bye MR, Gomez JA, Booker WA, Hyman JE, Roye DP. A retrospective cohort study of pulmonary function, radiographic measures, and quality of life in children with congenital scoliosis. Spine. 2008;33:1242–1249. doi: 10.1097/BRS.0b013e3181714536. [DOI] [PubMed] [Google Scholar]
- 5.Rizzi PE, Winter RB, Lonstein JE, Denis F, Perra JH. Adult spinal deformity and respiratory failure: surgical results in 35 patients. Spine. 1996;22:2517–2530. doi: 10.1097/00007632-199711010-00011. [DOI] [PubMed] [Google Scholar]
- 6.Jackson RP, Simmons EH, Striptinis D. Coronal and sagittal plane spinal deformities correlating with back pain and pulmonary function adult idiopathic scoliosis. Spine. 1989;14:1391–1397. doi: 10.1097/00007632-198912000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Buchowski JM, Skaggs DL, Sponseller PD. Temporary internal distraction as an aid to correction of severe scoliosis. J Bone Joint Surg A. 2007;89:297–309. doi: 10.2106/JBJS.G.00163. [DOI] [PubMed] [Google Scholar]
- 8.Suk Se-II, Kim J-H, Kim W-J, Lee S-M, Chung E-R, Nah K-H. Posterior vertebral column resection for severe spinal deformities. Spine. 2002;27:2374–2382. doi: 10.1097/00007632-200211010-00012. [DOI] [PubMed] [Google Scholar]
- 9.Di Silvestre M, Bakaloudis G, Lolli F, Vommaro F, Martikos K, Parisini P. Posterior fusion only for thoracic adolescent idiopathic scoliosis of more than 80°: pedicle screws versus hybrid instrumentation. Eur spine J. 2008;17:1336–1349. doi: 10.1007/s00586-008-0731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinella A, Lenke L, Whitaker C, Kim Y, Park S-S, Peelle M, Edwards C, Bridwell K. Perioperative halo-gravity traction in the treatment of severe scoliosis and kyphosis. Spine. 2005;30:475–482. doi: 10.1097/01.brs.0000153707.80497.a2. [DOI] [PubMed] [Google Scholar]
- 11.Sponseller PD, Takenaga RK, Newton P, Boachie O, Flynn J, Letko L, Betz R, Bridwell K, Gupta M, Marks M, Bastrom T. The use of traction in the treatment of severe spinal deformity. Spine. 2008;33:2305–2309. doi: 10.1097/BRS.0b013e318184ef79. [DOI] [PubMed] [Google Scholar]
- 12.Letts RM, Palakar G, Bobechko WP. Preoperative skeletal traction in scoliosis. J Bone Joint Surg A. 1975;57:616–619. [PubMed] [Google Scholar]
- 13.Winter RB, Lonstein JE. The Moe maximal correction test to determine true curve flexibility. A historical note with current application. Spine. 2010;35:1733–1735. doi: 10.1097/BRS.0b013e3181c65179. [DOI] [PubMed] [Google Scholar]
- 14.Lenke LG, Sides BA, Koester LA, Hensley M, Blanke KM. Vertebral column resection for the treatment of severe spinal deformity. Clin Orthop Relat Res. 2010;468:687–699. doi: 10.1007/s11999-009-1037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamin S, Li L, Tianjun G, Yupeng Z. Staged surgical treatment for severe and rigid scoliosis. J Orthop Surg Res. 2008;3:1–9. doi: 10.1186/1749-799X-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu Y, Liu Z, Zhu F, Wang B, Yu Y, Zhu Z, Qian B, Ma W. Comparison of effectiveness of Halo-femoral traction after anterior spinal release in severe idiopathic and congenital scoliosis: a retrospective study. J Orthop Surg Res. 2007;2:1–7. doi: 10.1186/1749-799X-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuklo TR, Lenke LG, OBrien MF, Lehman RA, Jr, Polly DW, Jr, Schroeder TM. Accuracy and efficacy of thoracic pedicle screws in curves more than 90 degrees. Spine. 2005;30:222–226. doi: 10.1097/01.brs.0000150482.26918.d8. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe K, Lenke LG, Bridwell KH, Kim YJ, Kim Y-W, Kim YB, Hensley M, Stobbs G. Comparison of radiographic outcomes for the treatment of scoliotic curves greater than 100 degrees. Wires versus hooks versus screws. Spine. 2008;33:1084–1092. doi: 10.1097/BRS.0b013e31816f5f3a. [DOI] [PubMed] [Google Scholar]
- 19.Suh SW, Modi HN, Yang J, Song H-R, Jang K-M. Posterior multilevel vertebral osteotomy for correction of severe and rigid neuromuscular scoliosis. A preliminary study. Spine. 2009;34:1315–1320. doi: 10.1097/BRS.0b013e3181a028bc. [DOI] [PubMed] [Google Scholar]
- 20.Wazeka AN, DiMaio MF, Boachie-Adjei O. Outcome of pediatric patients with severe restrictive lung disease following reconstructive spine surgery. Spine. 2004;29:528–533. doi: 10.1097/01.BRS.0000103344.25246.64. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Zheng G, Zhang X, Zhang Y, Xiao S, Wang Z. Comparative analysis between shape memory alloy-based correction and traditional correction technique in pedicle screws constructs for treating severe scoliosis. Eur Spine J. 2010;19:394–399. doi: 10.1007/s00586-009-1207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burrows B, Cline MG, Knudson RJ, Taussig LM, Lebowitz MD. A descriptive analysis of the growth and decline of the FVC and FEV1. Chest. 1983;83:717–724. doi: 10.1378/chest.83.5.717. [DOI] [PubMed] [Google Scholar]
- 23.Pehrsson K, Larsson S, Oden A, Nachemson A. Long-term follow-up of patients with untreated scoliosis. A study of mortality, causes of death, and symptoms. Spine. 1992;17:1091–1096. doi: 10.1097/00007632-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Glassmann SD, Hamill CL, Bridwell KH, Schwab FJ, Dimar JR, Lowe TG. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine. 2007;32:2764–2770. doi: 10.1097/BRS.0b013e31815a7644. [DOI] [PubMed] [Google Scholar]
- 25.Hempfing A, Ferraris L, Koller H, Rump J, Metz-Stavenhagen P. Is anterior release effective to increase flexibility in idiopathic thoracic scoliosis? Assessment by traction films. Eur Spine J. 2007;16:515–520. doi: 10.1007/s00586-006-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vora V, Crawford A, Babekhir N, Boachie-Adjei O, Lenke L, Peskin M, Charles G, Kim Y. A pedicle screw construct gives an enhanced posterior correction of adolescent idiopathic scoliosis when compared with other constructs: myth or reality. Spine. 2007;32:1869–1874. doi: 10.1097/BRS.0b013e318108b912. [DOI] [PubMed] [Google Scholar]
- 27.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J. 1993;6:5–40. [PubMed] [Google Scholar]
- 28.Murray RJ. Respiratory manifestations of extrapulmonary disorders. Philadelphia: Elsevier Saunders; 2007. [Google Scholar]
- 29.Hempfing A, Koller H, Ferraris L, Meier O (2008) The effect of concave rib osteotomies on spinal flexibility in AIS: a biomechanical intra-operative assessment of spinal rigidity using a strain gauge distraction device. In: IMAST 2008, Hong Kong, China
- 30.Rawlins BA, Winter RB, Lonstein JE, Denis FM, Kubic PT, Wheeler WB, Ozolins AL. Reconstructive spine surgery in pediatric patients with major loss in vital capacity. J Pediatr Orthop. 1996;16:284–292. doi: 10.1097/01241398-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Payo J, Perez-Grueso FS, Fernandez-Baillo N, Garcia A. Severe restrictive lung disease and vertebral surgery in a pediatric population. Eur Spine J. 2009;18:1905–1910. doi: 10.1007/s00586-009-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez C, Ferris G, Diaz J, Inmaculada F, Nunez J, Marin J. Kyphoscoliotic ventilatory insufficiency: effects of long-term intermittent positive-pressure ventilation. Chest. 2003;124:857–862. doi: 10.1378/chest.124.3.857. [DOI] [PubMed] [Google Scholar]
- 33.Koumbourlis AC. Scoliosis and the respiratory system. Pediatr Respir Rev. 2006;7:152–160. doi: 10.1016/j.prrv.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Swank SM, Winter RB, Moe JH. Scoliosis and cor pulmonale. Spine. 1982;7:343–354. doi: 10.1097/00007632-198207000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Smiljanic I, Kovac V, Cimic M. Changes in pulmonary function parameters after surgical treatment of idiopathic scoliosis. Coll Antropol. 2010;33:145–152. [PubMed] [Google Scholar]
- 36.Savini R, Parisini P, Vicenzi G. Respiratory function in severe scoliosis before and after treatment (a review of 76 cases) Ital J Orthop Traumatol. 1976;2:247–259. [PubMed] [Google Scholar]
- 37.Sucato DJ. Management of severe spinal deformity. Scoliosis and kyphosis. Spine. 2010;35:2186–2192. doi: 10.1097/BRS.0b013e3181feab19. [DOI] [PubMed] [Google Scholar]
- 38.Kearon C, Viviani GR, Kirkley A, Killian KJ. Factors determining pulmonary function in adolescent idiopathic thoracic scoliosis. Am Rev Respir Dis. 1993;148:288–294. doi: 10.1164/ajrccm/148.2.288. [DOI] [PubMed] [Google Scholar]
- 39.Newton PO, Perry A, Bastrom TP, Lenke LG, Betz RR, Clements D, D’Andrea L. Predictors of change in postoperative pulmonary function in adolescent idiopathic scoliosis. Spine. 2007;32:1875–1882. doi: 10.1097/BRS.0b013e31811eab09. [DOI] [PubMed] [Google Scholar]
- 40.Adam CJ, Cargill SC, Askin GN. Computed tomographic-based volumetric reconstruction of the pulmonary system in scoliosis. Trends in lung volume and lung volume asymmetry with spinal curve severity. J Pediatr Orthop. 2007;27:677–681. doi: 10.1097/BPO.0b013e318425ee. [DOI] [PubMed] [Google Scholar]
- 41.Gill I, Eagle M, Mehta JS, Gibson MJ, Bushby YK, Bullock R. Correction of neuromuscular scoliosis in patients with preexisting respiratory failure. Spine. 2006;31:2478–2483. doi: 10.1097/01.brs.0000239215.87174.8f. [DOI] [PubMed] [Google Scholar]
- 42.Sink EL, Karol LA, Sanders J, Birch JG, Jonston CE, Herring JA. Efficacy of perioperative halo-gravity traction in the treatment of severe scoliosis in children. J Pediatr Orthop. 2001;21:519–524. doi: 10.1097/00004694-200107000-00020. [DOI] [PubMed] [Google Scholar]
- 43.Kim YJ, Lenke LG, Bridwell KH, Cheh G, Whorton J, Sides B. Prospective pulmonary function comparison following posterior segmental spinal instrumentation and fusion of adolescent idiopathic scoliosis. Spine. 2007;32:2685–2693. doi: 10.1097/BRS.0b013e31815a7b17. [DOI] [PubMed] [Google Scholar]
- 44.Yaszay B, Jazayeri R, Lonner B. The effect of surgical approaches on pulmonary function in adolescent idiopathic scoliosis. J Spinal Disord. 2009;22:278–283. doi: 10.1097/BSD.0b013e31816d2530. [DOI] [PubMed] [Google Scholar]
- 45.Izatt MT, Harvey JR, Adam CJ, Fender D, Labrom RD, Askin GN. Recovery of pulmonary function following endoscopic anterior scoliosis correction: evaluation at 3, 6, 12, and 24 months after surgery. Spine. 2006;31:2469–2477. doi: 10.1097/01.brs.0000238659.12918.b5. [DOI] [PubMed] [Google Scholar]
- 46.Vedantam R, Lenke LG, Bridwell KH, Haas J, Linville DA. A prospective evaluation of pulmonary function in patients with adolescent idiopathic scoliosis relative to the surgical approach used for spinal arthrodesis. Spine. 2000;25:82–90. doi: 10.1097/00007632-200001010-00015. [DOI] [PubMed] [Google Scholar]
- 47.Koller H, Zenner J, Hitzl W, Meier O, Ferraris L, Acosta F, Hempfing A. The morbidity of open transthoracic approach for anterior scoliosis correction. Spine. 2010;35:1586–1592. doi: 10.1097/BRS.0b013e3181f07a90. [DOI] [PubMed] [Google Scholar]
- 48.Kadoury S, Cheriet F, Beasuejour M, Stokes IA, Parent S, Labelle H. A three-dimensional retrospective analysis of the evolution of spinal instrumentation for the correction of adolescent idiopathic scoliosis. Eur Spine J. 2009;18:23–37. doi: 10.1007/s00586-008-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nepple JJ, Lenke LG. Severe idiopathic scoliosis with respiratory insufficiency treated with preoperative traction and staged anteroposterior spinal fusion with a 2-level apical vertebrectomy. Spine J. 2009;9:E9–E13. doi: 10.1016/j.spinee.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Lonner BS, Murthy SK, Boachie-Adjei O. Single-staged double anterior and posterior spinal reconstruction for rigid adult spinal deformity: a report of four cases. Spine J. 2005;5:104–108. doi: 10.1016/j.spinee.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Hamed AM (2010) Effect of multi rib osteootmy on pulmonary functions after correction of adolescent scoliosis. In: 45th annual meeting of the SRS, Kyoto, Japan
- 52.Olgun ZD, Demirkiran HG, Ayvaz M, Alanay A, Yazici M (2009) Is vertebral column resection the only effective treatment option for correction in adolescent patients with complex congenital thoracic kyphoscoliosis: the safety and efficacy of posterior all pedicle screw instrumentation combined with multiple chevron and concave rib osteotomies. In: 17th IMAST, Toronto, Canada
- 53.Park DK, Braaksma B, Hammerberg K, Sturm P (2009) The effect of long-term halo traction in pediatric deformity. In: 16th IMAST, Vienna, Austria
- 54.Seller K, Haas S, Raab P, Krauspe R, Wild A. Preoperative halo-traction in severe paralytic scoliosis. Z Orthop Ihre Grenzgeb. 2005;143:539–543. doi: 10.1055/s-2005-836750. [DOI] [PubMed] [Google Scholar]
- 55.Flierl S, Carstens C. The effect of halo-gravity traction in the preoperative treatment of neuromuscular scoliosis. Z Orthop Ihre Grenzgeb. 1997;135:162–170. doi: 10.1055/s-2008-1039574. [DOI] [PubMed] [Google Scholar]
- 56.Tokunaga M, Minami S, Kitahara H, Isobe K, Nakata Y, Moriya H. Vertebral decancellation for severe scoliosis. Spine. 2000;25:469–474. doi: 10.1097/00007632-200002150-00013. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Zhang Y, Zhang X, Huang P, Xiao S, Wang Z, Liu Z, Liu B, Lu N, Mao K. A single posterior approach for multilevel modified vertebral column resection in adults with severe rigid congenital kyphoscoliosis: a retrospective study of 13 cases. Eur Spine J. 2008;17:361–372. doi: 10.1007/s00586-007-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M, Ni J, Li Y, Fang X, Gu S, Zhang Z, Zhu X. Single-staged anterior and posterior spinal fusion: a safe and effective alternative for severe and rigid adolescent idiopathic scoliosis in China. J Paediatr Child Health. 2009;45:246–253. doi: 10.1111/j.1440-1754.2009.01491.x. [DOI] [PubMed] [Google Scholar]
- 59.Ayvaz M, Akalan N, Yazici M, Alanay A, Acaroglu E. Is it necessary to operate all split cord malformations before corrective surgery for patients with congenital spinal deformities. Spine. 2009;34:2413–2418. doi: 10.1097/BRS.0b013e3181b9c61b. [DOI] [PubMed] [Google Scholar]
- 60.Suken A, Shah MD, Sucato D, Newton PO, Shufflebarger HL, Emans JB, Sponseller PD, Neiss G, Yorgova P, Lenke LG (2010) Perioperative neurologic events from a multicenter consecutive series of pediatric vertebral column resection: nature, frequency and outcomes. In: 17th IMAST, Toronto, Canada
- 61.Takaso M, Nakazawa T, Imura T, Takahira N, Itoman M, Takahashi K, Yamazaki M, Otori S, Akazawa T, Minami S, Kotani T. Surgical management of severe scoliosis with high-risk pulmonary dysfunction in Duchenne muscular dystrophy. Int Orthop. 2010;34:401–406. doi: 10.1007/s00264-009-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamzaoglu A, Ozturk C, Aydogan M, Tezer M, Aksu N, Bruno MB. Posterior only pedicle screw instrumentation with intraoperative halo-femoral traction in the surgical treatment of severe scoliosis (>100°) Spine. 2008;33:979–983. doi: 10.1097/BRS.0b013e31816c8b17. [DOI] [PubMed] [Google Scholar]
- 63.Pizones J, Izquierdo E, Sanchez-Marscal F, Alvarez P, Zuniga L, Gomez A. Does wide posterior multiple level release improve the correction of adolescent idiopathic scoliosis curves? J Spinal Disord Tech. 2010;23(7):e24–e30. doi: 10.1097/BSD.0b013e3181c29d16. [DOI] [PubMed] [Google Scholar]
- 64.Lenke LG, O’Leary PT, Bridwell KH, Sides BA, Koester LA, Blanke KM. Posterior vertebral column resection for severe pediatric deformity. Spine. 2009;34:2213–2221. doi: 10.1097/BRS.0b013e3181b53cba. [DOI] [PubMed] [Google Scholar]
- 65.Bullmann V, Halm H, Schulte T, Lerner T, Weber TP, Liljenqvist UR. Combined anterior and posterior instrumentation in severe and rigid idiopathic scoliosis. Eur Spine J. 2006;15:440–448. doi: 10.1007/s00586-005-1016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saeed MU, Dacuycuy MAC, Kennedy DJ. Halo pin insertion-associated brain abscess. Case report and review of literature. Spine. 2007;32:E271–E274. doi: 10.1097/01.brs.0000259976.46403.8a. [DOI] [PubMed] [Google Scholar]
- 67.Limpaphayom N, Skaggs DL, McComb G, Krieger M, Tolo VT (2007) Complications of halo use in children. In: 42nd annual meeting of the SRS, Edinburgh, Scotland [DOI] [PubMed]
- 68.Liang J, Qiu G, Shen J, Zhang J, Wang Y, Li S, Zhao H. Predictive factors of postoperative pulmonary complications in scoliotic patients with moderate or severe pulmonary dysfunction. J Spinal Disord. 2010;23:388–392. doi: 10.1097/BSD.0b013e3181b55ff4. [DOI] [PubMed] [Google Scholar]
- 69.Udink ten Cate FEA, van Royen BJ, Heerde M, Roerdink D, Plötz FB. Incidence and risk factors of prolonged mechanical ventilation in neuromuscular scoliosis surgery. J Pediatr Orthop B. 2008;17:203–206. doi: 10.1097/BPB.0b013e328301e962. [DOI] [PubMed] [Google Scholar]