Abstract
The choroid plexus (CP) epithelium develops from the ependyma that lines the ventricular system, and plays a critical role in the development and function of the brain. In addition to being the primary site of CSF production, the CP maintains the blood-CSF barrier via apical tight junctions between epithelial cells. Here we show that the 22-member γ-Protocadherin (γ-Pcdh) family of cell adhesion molecules, which we have implicated previously in synaptogenesis and neuronal survival, is highly expressed by both CP epithelial and ependymal cells, in which γ-Pcdh protein localization is, surprisingly, tightly restricted to the apical membrane. Multi-label immunostaining demonstrates that γ-Pcdhs are excluded from tight junctions, basolateral adherens junctions, and apical cilia tufts. RT-PCR analysis indicates that, as a whole, the CP expresses most members of the Pcdh-γ gene family. Immunostaining using novel monoclonal antibodies specific for single γ-Pcdh proteins shows that individual epithelial cells differ in their apically-localized γ-Pcdh repertoire. Restricted mutation of the Pcdh-γ locus in the choroid plexus and ependyma leads to significant reductions in ventricular volume, without obvious disruptions of epithelial apical-basal polarity. Together, these results suggest an unsuspected role for the γ-Pcdhs in CSF production and demonstrate a surprising molecular heterogeneity in the CP epithelium.
Keywords: ependyma, cell adhesion molecule, blood-CSF barrier, synaptogenesis, ventricles
INTRODUCTION
The choroid plexus (CP) epithelium is of central importance to the development and maintenance of brain function. The CP, which evaginates during embryonic development from the ependymal cells that line the cerebral ventricles, is composed of a single continuous layer of polarized epithelial cells, folded around a basal stroma rich in fenestrated capillaries. Each of the brain’s ventricles (the two lateral ventricles, the third ventricle, and the fourth ventricle) contains a similar, but morphologically distinct, CP: in mouse, the lateral ventricle CP is sheet-like, while that of the third and, especially, fourth ventricles is extensively folded and branched (reviewed by Wolburg and Paulus, 2010).
The CP performs two primary functions in the maintenance of brain homeostasis. First, it is responsible for the production of the majority of CSF, which is derived from blood (Cserr, 1971; Welch, 1963). Distinct populations of ion channels, aquaporin water channels, and transporters are tightly localized to the distinct apical and basal membranes of the polarized CP epithelium. This asymmetric localization of channels actively facilitates the unidirectional transport of ions and water through the cell, resulting in the distinct composition of CSF. The production and reabsorption of CSF is tightly regulated in order to provide the brain with the proper nutrients, maintain proper intracranial pressure, and rid the brain of wastes, such as excess peptides, neurotransmitters, electrolytes, and debris (reviewed by Brown et al., 2004; Wolburg and Paulus, 2010). A second major function of the CP epithelium is to maintain the blood-CSF barrier, which is formed by the apical tight junctions that hold the epithelial cells together (Engelhardt and Sorokin, 2009). The blood-CSF barrier not only prevents the passage of toxins from the blood to the CSF, but also helps to attenuate the effects of ion and metabolite fluctuations in the blood, protecting the brain from chemical insults (reviewed in Brown et al., 2004; Speake et al., 2001; Wolburg and Paulus, 2010). The CP is also a site of interaction with immune cells, which can gain entry to the brain via the CP epithelium (Engelhardt, 2006; Reboldi et al., 2009). Intriguingly, several immunologically relevant adhesion molecules of the Ig superfamily are localized to the apical surface of CP cells, and their expression is upregulated upon induction of experimental autoimmune encephalomyelitis (EAE) (Engelhardt, Wolburg-Buchholz, and Wolburg, 2001; Schulz and Engelhardt, 2005; Steffen et al., 1996; Wolburg et al., 1999). This suggests that molecular changes in adhesive signaling at the apical surface of the CP could be involved in regulating immune cell dissemination from the CSF throughout the brain during inflammatory disease states.
The diverse cadherin superfamily of cell adhesion molecules is important for a multitude of biological functions in both the brain and other organ systems (Halbleib and Nelson, 2006; Morishita and Yagi, 2007; Salinas and Price, 2005; Takeichi, 2007). The defining feature of the cadherin superfamily is the presence of a variable number of repetitive extracellular domains, termed cadherin repeats, that mediate cell-cell adhesion in the canonical “classical” cadherins. In addition to the ~20 “classical” cadherins, there are over 80 related protocadherins (Pcdhs), making this the largest subgroup of the cadherin superfamily (Garrett, Schreiner, and Weiner, 2009; Morishita and Yagi, 2007; Weiner, 2006). Nearly 60 Pcdh genes are arrayed in three contiguous gene clusters, termed Pcdh-α, -β, and -γ, encompassing ~1 megabase on human chromosome 5q31, with similar arrangements in other vertebrates (Wu and Maniatis, 1999; Wu, 2005). The Pcdh-γ gene cluster contains 22 “variable” (V) exons expressed from their own upstream promoters and spliced to three short, invariant “constant” (C) exons (Tasic et al., 2002; Wang, Su, and Bradley, 2002; Wu and Maniatis, 1999; Wu et al., 2001). Each V exon encodes six cadherin repeats, a transmembrane domain, and a proximal cytoplasmic domain of a single isoform, with the C-terminal ~125 amino acids of all γ-Pcdhs encoded by the C exons (see Fig. 4A). The Pcdh-γ genes are expressed throughout the developing nervous system, with each neuron expressing a subset (Kaneko et al., 2006; Wang et al., 2002). The 22 individual γ-Pcdh isoforms form cis-tetramers that interact in a strictly homophilic manner in trans, which indicates that this family could specify at least 104 distinct adhesive interfaces (Schreiner and Weiner, 2010; Zipursky and Sanes, 2010). Given that γ-Pcdhs can form complexes that include α- and β-Pcdhs as well (Han et al., 2010; Murata et al., 2004), the clustered Pcdhs could provide a molecular code underlying the vast diversity of neurons and the specificity of their circuitry.
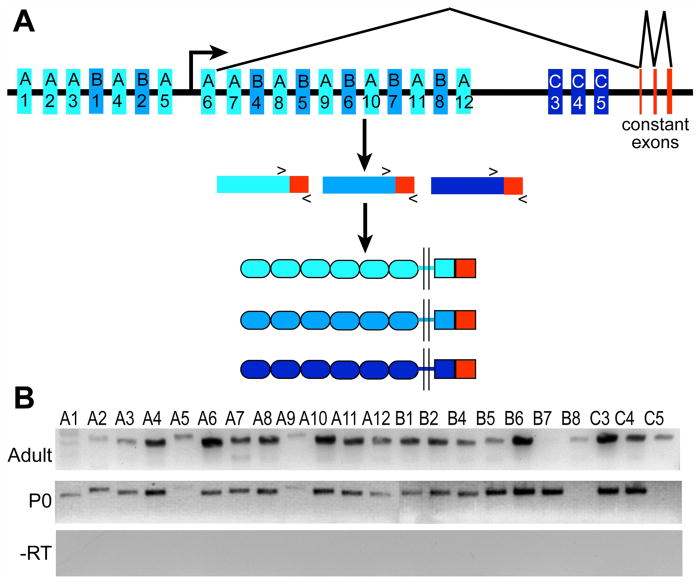
Figure 4. Most of the 22 Pcdh-γ genes are expressed in neonatal and adult choroid plexus.
(A) A schematic of the Pcdh-γ locus showing the organization of the A, B, and C Pcdh-γ subfamilies (V exons in different shades of blue; constant exons in red). After promoter choice and splicing (the promoter location and splicing pattern is diagrammed for A6 as an example) the Pcdh-γ gene cluster yields 22 proteins with six extracelluar cadherin repeats, a transmembrane domain followed by a variable cytoplasmic domain, and a constant domain (red). (B) RT-PCR analysis demonstrates the presence of nearly all 22 Pcdh-γ transcripts in adult and neonatal (P0) choroid plexus. –RT is a negative control in which reverse transcriptase was not added to the reaction. Gels are representative of at least three separate experiments.
Our previous analyses of mice in which all 22 Pcdh-γ genes have been deleted either constitutively (Pcdh-γdel/del) or in a tissue-restricted fashion (Pcdh-γfcon3/fcon3) have demonstrated a critical role for these adhesion molecules in CNS synaptogenesis and neuronal survival (Prasad et al., 2008; Wang et al., 2002; Garrett and Weiner, 2009; Weiner et al., 2005). During our studies, we have discovered that the γ-Pcdhs are expressed by ependymal and CP epithelial cells, at levels surprisingly greater than those in neurons and astrocytes. Here we demonstrate this expression as early as E13 and show that multiple γ-Pcdh proteins are localized to the apical membrane of CP epithelial cells. Multi-label immunostaining demonstrates that γ-Pcdhs are excluded from basolateral adherens junctions, tight junctions, and apical cilia tufts. Using RT-PCR, we show that all 22 Pcdh-γ genes can be expressed in the CP epithelium as a whole. Using newly-generated monoclonal antibodies specific for individual γ-Pcdh isoforms, we show that CP epithelial cells differ in their apical γ-Pcdh repertoire. Mice with CP- and ependyma-restricted γ-Pcdh disruption (FoxJ1-Cre; Pcdh-γfcon3/fcon3) exhibit a striking reduction in ventricular volume, despite grossly normal apical-basal polarity and morphology of CP epithelial cells. Together, our results demonstrate a previously unsuspected molecular diversity at the CP-CSF interface, and implicate apical γ-Pcdh adhesion and/or signaling in CSF dynamics and maintenance of ventricular volume.
MATERIALS AND METHODS
Mouse strains
Pcdh-γdel, Pcdh-γfusg (Wang et al., 2002), Pcdh-γfcon3 (Prasad et al., 2008), and FoxJ1-Cre mice (line 14) (Zhang et al., 2007) have been described elsewhere. Z/EG mice (Novak et al.,, 2000) were obtained from The Jackson Laboratory (Bar Harbor, ME). Both male and female mice were analyzed in all cases. All animal procedures were performed according to NIH and University of Iowa guidelines, and were approved by the IACUC. All animal facilities at the University of Iowa are AAALAC accredited.
Preparation of tissues
For the various procedures, either whole brains or isolated fourth ventricle CP were isolated from mice at E13 to adult. Embryonic tissues and isolated CP were either snap frozen in OCT using dry ice/EtOH-cooled isopentane, or immersion fixed in 4% PFA followed by sinking in 30% sucrose. Adult mice were anesthetized with Avertin and perfused intracardially with phosphate buffered saline (PBS) to flush the blood. In some cases, CP was then isolated and snap frozen as above. In other cases, PBS perfusion was followed by perfusion with 4% PFA. For immunofluorescence, these tissues were removed and post-fixed overnight in 4% PFA, followed by sinking in 30% sucrose. For electron microscopy, tissues were post-fixed in 2.5% glutaraldehyde overnight.
Ventricle quantifications
Following perfusion fixation, brains were embedded in 2% agarose in PBS and sectioned in the coronal plane at 100 μm using a Vibratome (Leica Microsystems). Sections were permeabilized for 2–4 h in a solution containing 2.5% bovine serum albumin (BSA) and 1% Triton X-100 in PBS. Sections were then incubated with the fluorescent Nissl stain NeuroTrace 500 (Invitrogen). Vibratome sections were scanned by using the “tiling” function of a Zeiss 510 confocal microscope (Central Microscopy Research Facility, The University of Iowa) with a 5x objective lens. Images were imported into Adobe Photoshop CS4 and the ventricles and entire brains traced. The areas of the casts rendered from these tracings were quantified in Image/J, and volume estimated by summing each area multiplied by the section thickness.
Immunohistochemistry
Whole mounts
Isolated and fixed fourth ventricle CP samples were immersed for 2–4 h at room temperature in blocking solution (2.5% BSA and 0.2% Triton X-100 in PBS), incubated with primary antibodies in blocking solution at 4°C overnight, washed, and incubated with secondary antibodies in PBS at room temperature for several hours.
Cryosections
Fixed or snap-frozen tissues were sectioned at 14μm in a Leica 1850 cryostat. Fresh-frozen sections were subsequently fixed at −20°C in 100% methanol for 10 minutes. Slide mounted sections were incubated in blocking solution for 1 h, followed by overnight incubation with primary antibodies, extensive washing, and a 1 h incubation with secondary antibodies in PBS at room temperature. Nuclei were counterstained by adding 4′6-diamidino-2-phenylindole (DAPI) to the final PBS wash.
Small Animal Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) was performed at the University of Iowa Small Animal Imaging Center on a 4.7 Tesla Varian Unity/INOVA small-bore MRI scanner (Varian Inc., Palo Alto, CA) equipped with a 25mm diameter transmit-receive volume RF coil. After an initial set of scout images, three sets of three-dimensional volumes of the mouse brain were acquired (one in each of the axial, sagittal, and coronal orientations) using a gradient-recalled echo (GRE) pulse sequence with TR=22ms, TE=4.5ms, and flip angle 25 degrees. For each volume, a matrix size of 512 × 256 × 32 covered a field of view of 50mm × 25mm × 12mm yielding contiguous slices of 0.1mm × 0.1mm × 0.3mm resolution.
Electron Microscopy
Scanning EM
Following overnight fixation in 2.5% glutaraldehyde, samples were thoroughly rinsed in 0.1 M phosphate buffer and fixed for 1 hour in 1% OsO4 in 0.1 M phosphate buffer. Samples were rinsed thoroughly in phosphate buffer and then in water. The samples were then dehydrated through graded EtOh solutions (25%, 50%, 75%, 95%, to 100%). After changing the samples to fresh 100% ethanol, they were dried by critical point drying. Samples were then mounted on aluminum stubs and sputter coated with Pt/Pd at 10 mA for 3 min prior to being imaged on a Hitachi S-4800 SEM.
Transmission EM
Following overnight fixation in 2.5% glutaraldehyde, samples were thoroughly rinsed in 0.1 M phosphate buffer and fixed for 1 hour in 1% OsO4 and 1.5% potassium ferrocyanide in 0.1 M phosphate buffer. Samples were again rinsed thoroughly in phosphate buffer and briefly rinsed with water. Samples were then stained with 2.5% uranyl acetate for 30 min before dehydration by successively increasing ethanol concentrations from 25%, 50%, 75%, 95%, to 100%. The samples were then embedded in Epon-12. Ultra-thin sections were cut at ~90 nm and examined on a JEOL 1230 TEM.
Reverse-transcriptase PCR
Reverse-transcriptase (RT)-PCR was performed on RNA isolated from the CP using the RNAqueous kit (Ambion). First-strand cDNA synthesis using M-MLV RT (Invitrogen) used standard conditions. To detect the 22 Pcdh-γ genes, a previously published primer set was used (Prasad et al., 2008) consisting of a common reverse primer in the 3′ UTR of constant exon 3 and 22 distinct forward primers specific to each variable exon. Cycling parameters were 94°C, 1 min; 55°C, 1 min; 72°C, 3 min for 30 cycles.
Monoclonal antibody production and testing
Three antigens were produced as GST-fusion proteins: 1) the 125-amino acid γ-Pcdh constant domain (γC); 2) the variable cytoplasmic domain (VCD) of γ-Pcdh-A3; and 3) the VCD of γ-Pcdh-B2. Both VCD antigens were produced as 3x concatemers of the ~90 amino acid domain to increase antigen molecular weight. Antigens were utilized for mouse monoclonal antibody (mAb) production at the UC Davis/NIH NeuroMab Facility, supported by NIH grant U24NS050606 and maintained by the Department of Neurobiology, Physiology and Behavior, College of Biological Sciences, University of California, Davis, CA 95616. All novel mAbs characterized here (as well as all other NeuroMabs) are available to researchers for a nominal fee (http://neuromab.ucdavis.edu). Antibodies were characterized for specificity in two assays: 1) HEK293 cells were separately transfected with plasmids encoding one of 20 full-length Pcdh-γ cDNAs, tagged at the C-terminus with GFP. The lysates were then dot-blotted using a vacuum apparatus, and the blots probed with individual mAbs to determine their isoform specificity; 2) Lysates from control and Pcdh-γdel/del neonatal brains were analyzed by western blot using each novel mAb, to ensure that they recognized only their appropriate γ-Pcdh antigen proteins. Protein blotting methods were as previously described (Schreiner and Weiner, 2010).
Statistics
In all cases differences between control and mutant animals were determined from each data set using ANOVA followed by Bonferroni-corrected post-hoc tests using Prism software. A p value less than 0.05 was the standard of statistical significance.
RESULTS
The γ-Pcdhs are highly expressed by ependymal and choroid plexus epithelial cells
In the Pcdh-γfusg knock-in mouse line (Prasad et al., 2008; Wang et al., 2002), the coding sequence for the green fluorescent protein (GFP) is fused in-frame to the end of the third Pcdh-γ constant exon. Thus, in Pcdh-γfusg tissues, immunostaining with robust, well-characterized anti-GFP antibodies can be used to examine the localization of all 22 γ-Pcdh proteins, which share a common C-terminal domain encoded by the three constant exons. In the course of characterizing adult brain regions with enriched γ-Pcdh neuropil staining (e.g., the hippocampal mossy fiber bundle; Fig. 1A, arrowhead), we noted, surprisingly, that the highest local protein concentrations appeared not amongst neurons and glia, but in the ependyma and choroid plexus (CP) (Fig. 1). Expression levels in CP epithelial cells appeared to be several-fold higher than those observed in the neuropil (Fig. 1A–C) and were similarly uniform in the CP of the lateral (Fig. 1A), third (Fig. 1B, E, F), and fourth ventricles (Fig. 1C). Intensity of ependymal staining was somewhat lower than that of CP, but was similarly uniform (Fig. 1E). Immunostaining of γ-Pcdh-GFP fusion proteins was entirely specific, as the anti-GFP antibodies produced no signal on wild-type brain tissues (Fig. 1D), and patterns were identical to those obtained with antibodies directed against the γ-Pcdh constant domain (see below for mAbs, Fig. 6; similar results were also obtained with rabbit polyclonal antibodies described in Wang et al., 2002 and Phillips et al., 2003).
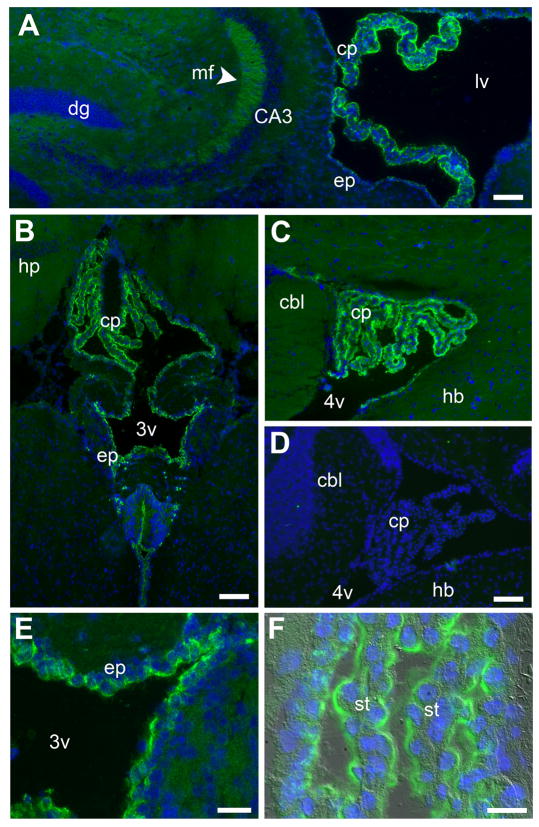
Figure 1. γ-Pcdh proteins are expressed at high levels by choroid plexus and ependymal epithelia.
Immnostaining of γ-Pcdh-GFP fusion proteins (green) in Pcdh-γfus brain cryosections reveals high levels of γ-Pcdh expression in the CP and ependyma (nuclei are counterstained with DAPI, blue). The CP of the lateral ventricle (A), the third ventricle (B), and the fourth ventricle (C) stains more strongly for γ-Pcdhs than the surrounding neuropil, including the mossy fibers of the hippocampus (A, arrowhead). (D) is a negative control, showing lack of any anti-GFP staining in wild-type mouse brain. Higher magnification images showγ-Pcdh staining in the ependyma surrounding the third ventricle (E) and in the fourth ventricle CP epithelium (F, fluorescence overlaid on a DIC image). Scale bars for A–D are 100 μm and for E-F are 25 μm. cp, choroid plexus; dg, dentate gyrus; mf, mossy fiber; ep, ependymal; lv, lateral ventricle; 3v, third ventricle; 4v, fourth ventricle; cbl, cerebellum; hb, hindbrain; st, stroma
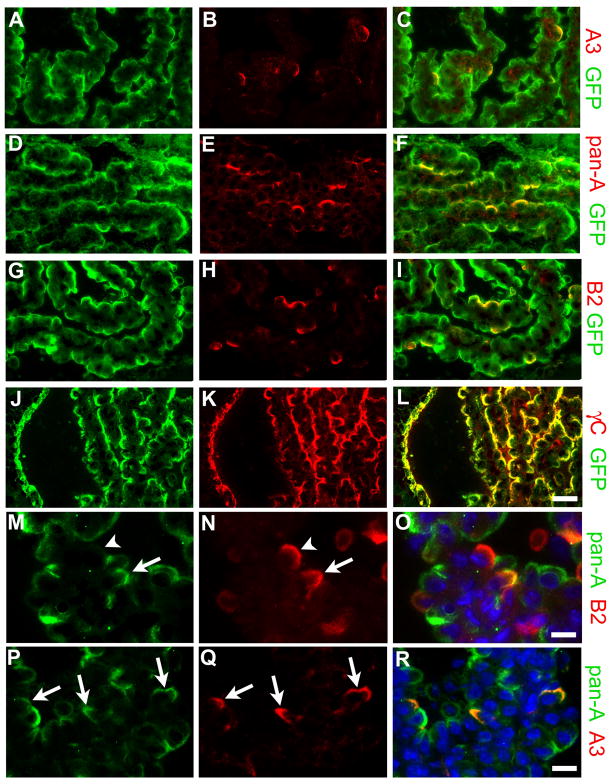
Figure 6. Individual γ-Pcdhs are differentially expressed among choroid plexus epithelial cells.
Cryostat sections of fourth ventricle adult Pcdh-γfus choroid plexus, immunostained using the indicated antibodies in each row of panels; right panel in each row shows a merged image. While GFP antibody, which detects all γ-Pcdhs in the Pcdh-γfus mouse, labels the apical surface of every epithelial cell, monospecific antibodies against A3 (B) or B2 (H) label only a small, apparently random, subset of cells. Pan-A antibody, which recognizes 11 of the 22 γ-Pcdhs, labels a larger subset of the cells (E). As expected, anti-γ-Pcdh constant domain antibody (γC) staining completely overlaps that of GFP (J–L). Double-labeling with the pan-A antibody and anti-A3 (P–R) shows that A3-expressing cells make up a small subset of those cells that express any of the A subfamily proteins. Double-labeling with the pan-A antibody and anti-B2 (M–O) demonstrates that some CP epithelial cells express both B2 and some A subfamily γ-Pcdhs (arrow), while some express B2 in the absence any A subfamily γ-Pcdhs (arrowhead); conversely, many cells that express A subfamily members (green) do not express B2. Together, these results show that different subsets of γ-Pcdh proteins localize to the apical surface in individual CP epithelial cells. Scale bar is 25 μm for (A–L), 12.5 μm for (M–R).
γ-Pcdh proteins are apically localized in developing and mature choroid plexus epithelial cells
The CP consists of a convoluted, folded sheet of polarized epithelial cells enclosing a stromal layer rich in blood vessels. The apical surface of the epithelial sheet faces the ventricular space, into which cerebrospinal fluid is secreted, while the basolateral surface is in contact with the stroma. In immunostained cryostat sections, it appeared that the γ-Pcdhs were enriched at, and possibly restricted to, the apical surface of both ependymal (Fig. 1E) and CP (Fig. 1F) epithelia, with little, if any, staining observed basolaterally or in stromal blood vessels. To more precisely determine the subcellular localization of γ-Pcdhs, we performed whole mount immunostaining on excised adult or neonatal Pcdh-γfusg CP tissues, and examined them using confocal microscopy.
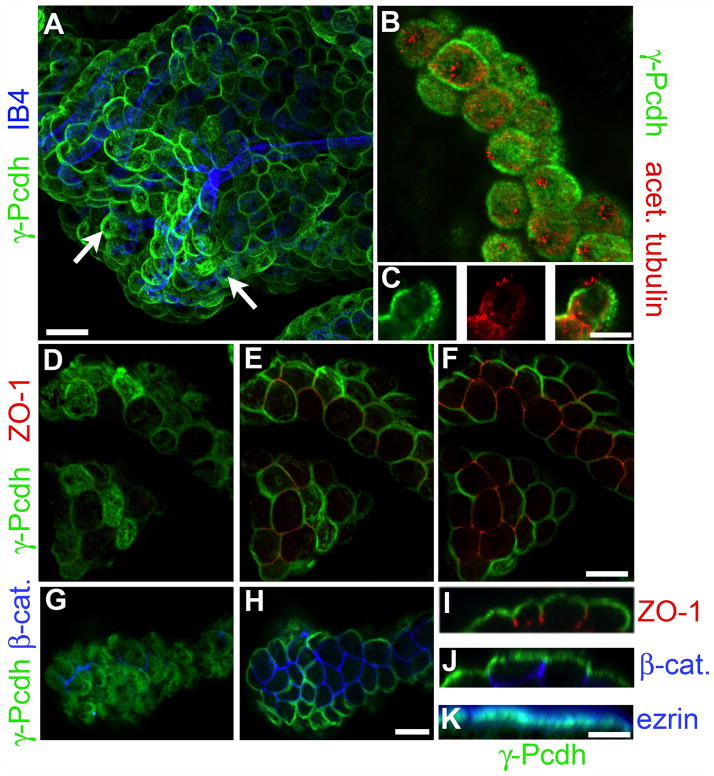
In confocal stacks through a portion of the CP, γ-Pcdh proteins appeared to localize in an apical “cap” on many cells, with reduced or absent staining in those cells for which only basolateral surfaces were captured due to the convolution of the epithelial sheet (Fig. 2A). No co-localization with the blood vessel marker isolectin B4 (IB4) was observed within the deep stromal layer (Fig. 2A and data not shown). The tight restriction of γ-Pcdhs to the apical membrane was confirmed by double-immunostaining with definitive markers of apical (ezrin) and basolateral (β-catenin) membranes, as well as of the tight junctions that demarcate these two compartments (ZO-1) (Masseguin et al., 2001). In progressively deeper optical sections through confocal stacks, abrupt loss of γ-Pcdh staining was coincident with ZO-1 staining becoming visible in each epithelial cell (Fig. 2D–F), and reconstructed orthogonal optical sections clearly indicated the restriction of γ-Pcdhs to the membrane apical to the tight junctions (Fig. 2I). Similar analyses demonstrated the complete lack of overlap between apical γ-Pcdhs and the basolateral marker β-catenin (Fig. 2G, H, J), while extensive overlap of γ-Pcdhs with ezrin was observed at the apical surface (Fig. 2K). Although light-level resolution is not sufficient to resolve it clearly, we did note that γ-Pcdh staining appeared just slightly basal to ezrin signal (Fig. 2K), which could indicate γ-Pcdh localization to the base of microvilli; unfortunately we were not able to obtain immuno-EM data with the antibodies currently available (data not shown). In many cells a near-circular “hole” in the apical γ-Pcdh staining could be observed in wholemount preparations (Fig. 2A, arrows). Double staining for γ-Pcdhs and the cilia marker acetylated α-tubulin demonstrated that this “hole” represented the membrane region from which cilia protruded (Fig. 2B). Single optical planes through the middle of CP epithelial cells showed no staining for γ-Pcdh proteins in the cilia themselves or in the immediately adjacent patch of plasma membrane (Fig. 2C).
Figure 2. γ-Pcdh proteins are apically localized in CP epithelial cells.
Whole-mount preparations of Pcdh-γfus CP were immunostained with the indicated antibodies or lectins: GFP, for γ-Pcdh-GFP fusion proteins; isolectin B4 (IB4), a blood vessel marker (A); acetylated tubulin, a cilia marker (B,C); ZO-1, a tight junction marker (D–F,I); β-catenin, a basolateral adherens junction marker (G,H,J); and ezrin, an apical sub-membrane marker (K). A and B are maximal projections through a confocal z-stack; D–F and G–H show selected planes within stacks, moving deeper into the tissue. C and I–K show 90° rotated cross-sections through a portion of a z-stack. The γ-Pcdhs are restricted to the apical surface and absent from stromal blood vessels (A). They are also excluded from the patch of membrane from which the cilia protrude (B,C). As expected for an apical protein, there is no overlap with ZO-1 (D–F,I), which marks tight junctions that lie just basal to the apical surface, or with β-catenin, a basolateral marker (G,H,J), but extensive overlap with ezrin (K). Scale bars are 30 μm A, 12μm B–H, and 10μm I–K.
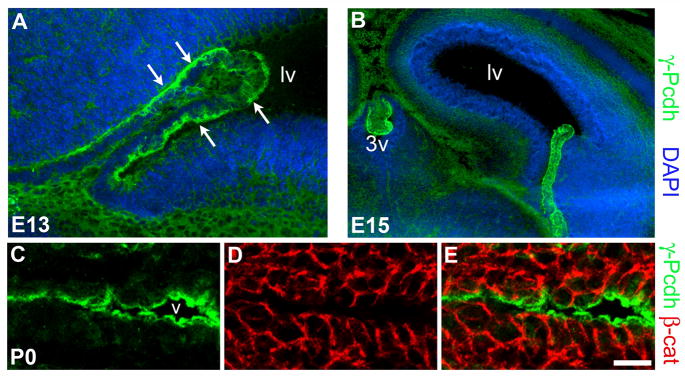
The CP first evaginates from the ependymal layer around embryonic day (E) 12–13 in mice, and grows substantially over the next several days (Sturrock, 1979). To ascertain whether the γ-Pcdhs were expressed in the CP during its development, we immunostained cryostat sections through the cerebral hemispheres at E13, E15, and P0. At E13, γ-Pcdh proteins were already highly expressed in the budding CP of the lateral ventricles (Fig. 3A). As in the adult, levels of expression in the choroid plexus appeared several-fold greater than those in the surrounding neural tissue. Already at this early stage, most γ-Pcdh immunolabeling was confined to the apical side of the epithelia (Fig. 3A, arrows). Similar staining was observed at E15, with high expression and preferentially apical localization of the γ-Pcdhs observed in the CP of the lateral, third, and fourth ventricles (Fig. 3B and data not shown). By P0, the complete restriction of γ-Pcdh proteins to the apical surface of the ependyma (Fig. 3C–E) and the CP (data not shown) observed in confocal stacks was evident in cryostat sections double-stained for β-catenin. Together, these data indicate that the γ-Pcdhs are highly expressed by, and apically localized in, epithelial cells of the CP from the time it grows out of the ependymal lining, through adulthood.
Figure 3. γ-Pcdhs are expressed in the CP epithelium soon after it evaginates.
(A,B) coronal cryosections through the lateral ventricle of Pcdh-γfusg embryos at E13 (A) and E15 (B) stained for γ-Pcdh-GFP proteins. Arrows indicate apical γ-Pcdh staining in the newly developed CP. (C–E) P0 ependyma, showing strong γ-Pcdh staining restricted to the apical surface, with no overlap with the basolateral markerβ-catenin. Scale bar is 50 μm in A, 200 μm in B, 25 μm in C–E.
Differential and combinatorial expression of individual γ-Pcdhs in the choroid plexus epithelium
Having demonstrated expression of the γ-Pcdh family as a whole, we next addressed the expression of individualγ-Pcdhs in the choroid plexus. The Pcdh-γ gene cluster consists of 22 large “variable” (V) exons, each of which encodes 6 extracellular cadherin domains, a transmembrane domain, and a variable cytoplasmic domain (VCD) of ~90 amino acids, and 3 small “constant” (C) exons, which encode a 125 amino acid common C-terminal domain (Fig. 4A) (Wu and Maniatis, 1999). Transcripts can be initiated from individual promoter regions upstream of each V exon, which can be grouped into “A”, “B”, or “C” subfamilies based on sequence similarity; following transcription through the rest of the gene cluster, the 5′ cap-proximal V exon is spliced to the 3 constant exons to produce a mature transcript (Tasic et al., 2002; Wang, Su, and Bradley, 2002). The Pcdh-γ cluster thus encodes 22 distinct adhesion molecules with unique ectodomains and VCDs but with a common C-terminus (Fig. 4A).
We used reverse transcriptase (RT) PCR to examine the expression of the 22 individual Pcdh-γ transcripts, using RNA isolated from both P0 and adult CP. Twenty-two separate PCR reactions were performed, utilizing distinct forward primers near the 3′ end of each V exon and a common reverse primer in the 3′ UTR of constant exon 3 (Fig. 4A). In both P0 and adult samples, almost all (18–20 of 22) of the individual transcripts could be detected clearly. Results were similar at the two ages tested, with three exceptions: Pcdh-γ-B7 was detected in P0, but not adult, samples; Pcdh-γ-B8 was barely detectable in adult, but not P0 samples; and Pcdh-γ-C5 was detected only in adult samples, consistent with a prior report that this isoform, in contrast to all others, is only produced after the first postnatal week (Frank et al., 2005) (Fig. 4B). Thus, the staining observed using antibodies against the constant domain likely reflects the aggregate expression of many individual γ-Pcdh proteins in the CP.
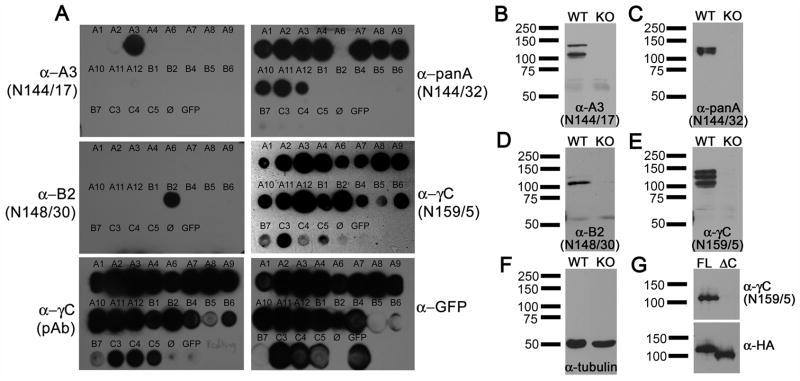
To determine whether individual γ-Pcdh proteins are differentially expressed among CP epithelial cells, we partnered with NeuroMab, a joint facility of the University of California-Davis and the NIH (www.neuromab.org), to raise a series of monoclonal antibodies (mAbs) against γ-Pcdhs. As antigens, we chose individual VCDs, as this is the most divergent region amongst the 22 γ-Pcdh proteins: for example, within the A subfamily, the degree of sequence similarity within the first cadherin domain (EC1) ranges from 79–94%, but within the VCD the range it is somewhat less, at 68–88%; within the B subfamily, EC1 similarity ranges from 68–98%, but only 39–74% within the VCD. We also used the constant domain as an antigen, hoping that the resultant hybridoma would provide a renewable source of antibodies that recognize the entire γ-Pcdh family of proteins (previously published antibodies [Phillips et al., 2003; Wang et al., 2002] have been polyclonal and of limited supply). Mice were inoculated with this constant domain antigen or with VCD antigens derived from γ-Pcdh-A3 or -B2, hybridoma fusions performed, and the resultant mAbs screened initially at NeuroMab to confirm that they detected the appropriate full-length γ-Pcdh overexpressed by transfection in cell lines. We then subjected positive mAbs to rigorous screening tests in our laboratory, as described next.
First, we determined whether each mAb was truly selective for the individual γ-Pcdh targeted with each VCD antigen. We amplified full-length cDNAs encoding 20 out of the 22 murine γ-Pcdhs and cloned each into a mammalian expression vector with GFP fused at the C-terminal end of the constant domain. We then transfected HEK293 cells with each construct, spotted protein lysates onto nitrocellulose to create dot blot arrays, and probed these blots with each mAb. Results of these dot blot analyses are presented in Figure 5A. Two mAbs were produced from the A3 VCD fusion: N144/17 (anti-A3), which is specific for A3, and N144/32 (anti-panA), which detects 11 of the 12 A subfamily proteins but none of the B or C subfamily proteins (Fig. 5A). The recovery of this pan-A subfamily clone was not unexpected given the particularly high conservation amongst these proteins, as compared to the B or C subfamilies. One mAb was produced from the B2 VCD fusion (N148/30, anti-B2), which is specific for B2 (Fig. 5A). The mAb produced using the constant domain (N159/5, anti-γC) detected all of the overexpressed γ-Pcdhs, as expected, similar to an existing polyclonal antibody (pAb) produced previously in our laboratory utilizing the same antigen protein reported by Phillips et al. (2003).
Figure 5. Characterization of novel monoclonal antibodies against γ-Pcdhs.
(A) Dot blot analysis of lysates from HEK293 cells, separately transfected with the indicated individual γ-Pcdh-GFP fusion construct. N144/17 is monospecific for γ-Pcdh-A3. N144/32 detects 11 of the 12 proteins in the A subfamily, but none in the B or C subfamilies. N148/30 is monospecific forγ-Pcdh-B2. N159/5, raised against the constant exon, detects all γ-Pcdhs as expected, similar to a polyclonal antibody raised against the same antigen. GFP antibody detects all transfected cell lysates. (B–E) Western blots of whole brain lysates from either wild-type (WT) or Pcdh-γdel/del (KO) neonates. All of the monoclonal antibodies recognize appropriately sized bands in WT, but not KO, brain, confirming the their specificity. (F) Tubulin loading control blot. (G) Western blot of HEK293 cell lysates transfected with N-terminal HA-tagged full-length (FL) or truncated (ΔC) γ-Pcdh construct lacking the constant domain. N159/5 detects the full-length γ-Pcdh, but not the truncated construct, as expected.
Next, we tested these mAbs by western blot to determine whether: 1) bands of the correct size(s) were detected in wild-type neonatal brain samples; and 2) these bands were absent from neonatal brain samples of Pcdh-γdel/del null mice. This was the case for all of the mAbs tested (Fig. 5B–F). Each VCD mAb labeled one or two bands of ~120–140 kDa (Fig. 5B–D), while the anti-γC mAb labeled three or four bands within this size range (Fig. 5E). The multiple bands are seen with all anti-constant region antibodies reported (Frank et al., 2005; Phillips et al., 2003; Wang et al., 2002), and presumably represent multiple glycosylation states and slightly different sizes among family members. None of these bands were detected in samples from neonatal Pcdh-γdel/del null brains, despite abundant labeling with an anti-tubulin antibody to demonstrate similar lane loading (Fig. 5F). We further confirmed the specificity of the anti-γC mAb by using western blots of cell lines transfected with constructs encoding either full-length HA-Pcdh-γ-A3 or HA-Pcdh-γ-A3ΔC, which lacks the coding sequence for the constant domain. Only full-lengthγ-Pcdh-A3 was detected using the anti-γC mAb (Fig. 5G), as expected.
Having generated these novel reagents and demonstrated their specificity, we used them, along with antibodies against GFP, to stain cryostat sections of CP from adult Pcdh-γfusg mice. As expected, the anti-γC mAb intensely stained the apical surface of all CP epithelial cells, a pattern essentially identical to that obtained with anti-GFP on the same section (Fig. 6J–L). Remarkably, the monospecific mAbs against γ-Pcdh-A3 or γ-Pcdh-B2 each labeled the apical surface of only a small subset of CP epithelial cells (Fig. A–C and G–I), demonstrating a surprising molecular heterogeneity of cells within the epithelium. Consistent with the differential expression of individual γ-Pcdh proteins among choroid plexus epithelial cells, the pan-A subfamily mAb labeled a larger proportion of epithelial cells than did the monospecific anti-γ-Pcdh-A3 mAb (Fig. 6D–F).
Because the pan-γ-Pcdh-A mAb is of the IgG2a isotype, we were able to perform double-labeling experiments with this mAb and with each of the (IgG1) monospecific mAbs. These experiments yielded two important results. First, we found that all epithelial cells that were positive for γ-Pcdh-A3 were also stained with the pan-γ-Pcdh-A mAb; conversely, the γ-Pcdh-A3-positive cells represented only a small portion of those that were pan-γ-Pcdh-A-positive (Fig. 6P–R). Second, we found that some, but far from all, epithelial cells were positive for both the pan-γ-Pcdh-A and γ-Pcdh-B2-specific mAbs (Fig. 6M–O), showing that a given cell can co-express γ-Pcdh isoforms from multiple subfamilies. Together, these results provide further confirmation of the specificity of our new mAbs, and represent proof-of-principle that individual choroid plexus epithelial cells express a variable repertoire of the 22 γ-Pcdh proteins.
Restricted mutation of the Pcdh-γcluster in the ependyma and choroid plexus of FoxJ1-Cre;Pcdh-γfcon3/fcon3 mice
Because Pcdh-γdel/del null mice die on the day of birth (Wang et al., 2002), we sought to develop a new genetic model in which we could examine the consequences of Pcdh-γ disruption specifically in the CP and ependyma. For this, we utilized Pcdh-γfcon3 conditional mutants, which harbor a floxed constant exon 3-GFP fusion (similar to the Pcdh-γfusg line), which allows us to monitor Cre excision by loss of immunostaining for GFP. We showed previously that excision of the Pcdh-γfcon3 allele results in a null, since no protein can be detected using a polyclonal antibody targeting constant exons 1 and 2, and since ubiquitous excision phenocopies Pcdh-γdel/del mice (Garrett and Weiner, 2009; Prasad et al., 2008). We crossed Pcdh-γfcon3 mice to a transgenic line in which Cre recombinase is driven by the promoter for the FoxJ1 homeobox transcription factor gene. In FoxJ1-Cre mice, Cre is expressed in ciliated epithelial cells, including those of the ependyma and CP (Zhang et al., 2007).
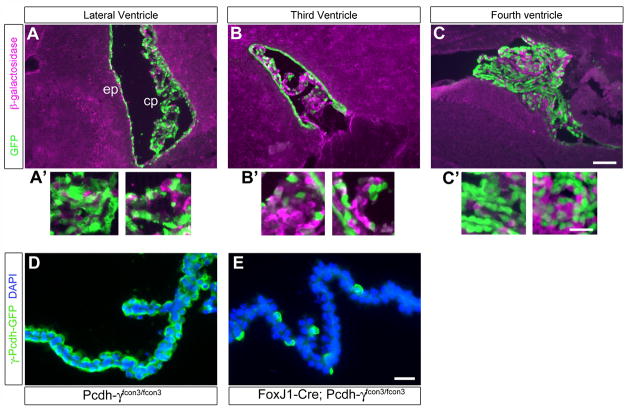
In order to confirm the efficacy and restriction of Cre recombinase expression to the CP and ependyma in the brain, we crossed the FoxJ1-Cre line to the Z/EG reporter mouse (Novak et al., 2000) (Fig. 7). In Z/EG mice, cells ubiquitously express a floxed β-galactosidase (β-gal) gene prior to Cre excision; following excision, an adjacent GFP gene is expressed. We stained cryosections from FoxJ1; Z/EG mice with antibodies against β-gal and GFP, and found that all brain tissue other than the ependyma and CP was β-gal+/GFP-, confirming the specificity of Cre transgene expression (Fig. 7). Excision of the floxed β-gal cassette and expression of GFP was nearly complete in the ependyma surrounding all ventricles, but varied in the CP epithelia: Most (~75–80%) lateral and fourth ventricle CP cells were GFP+ (Fig. 7A, C), but only ~25–30% of third ventricle CP cells were (Fig. 7B). In subsequent analyses of FoxJ1-Cre;Pcdh-γfcon3/fcon3 restricted mutants, we thus focused on the CP of the fourth and lateral ventricles, in which the excision of the floxed contant exon 3/GFP fusion was also confirmed to be widespread (Fig. 7D,E).
Figure 7. FoxJ1-Cre restricts mutation of the Pcdh-γ gene cluster to the choroid plexus and ependyma within the brain.
A FoxJ1-Cre transgenic line was crossed the Z/EG reporter mouse in order to visualize the spatial pattern of Cre recombinase activity. In Z/EG, β-galactosidase is ubiquitously expressed until Cre excises the β-gal cassette, leading to subsequent expression of EGFP. Widespread Cre activity is detected by GFP staining in the lateral and fourth ventricle choroid plexus and ependyma (A, A′, C, C′); Cre activity is very patchy, however, in the third ventricle choroid plexus (B, B′). Insets (A′–C′) are magnified views of the choroid plexus from each of the panels. Cre activity can also be demonstrated in FoxJ1-Cre; Pcdh-γfcon3/fcon3 choroid plexus as loss of the floxed GFP-tagged constant exon 3 (D, E). The vast majority of choroid plexus epithelial cells are knocked-out in this restricted mutant. Scale bar is 150 μm in A–C, 50 μm in A′–C′, and 30 μm in D–E.
Loss of γ-Pcdhs in the choroid plexus and ependyma results in reduced ventricular volume
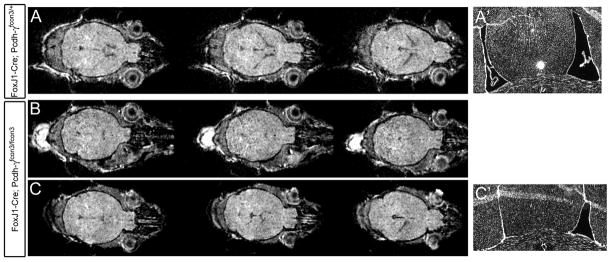
FoxJ1-Cre;Pcdh-γfcon3/fcon3 mutants were viable and lived to adulthood with no obvious external abnormalities. When preparing cryostat sections of FoxJ1-Cre;Pcdh-γfcon3/fcon3 brains, however, we immediately noticed that mutants had smaller ventricles compared to littermate controls. In order to verify this initial observation, and to eliminate the possibility that it could be due to any variances in fixation or sectioning, we imaged FoxJ1-Cre;Pcdh-γfcon3/fcon3 and both FoxJ1-Cre;Pcdh-γfcon3/+ and Pcdh-γfcon3/fcon3 control animals using small animal magnetic resonance imaging (MRI). In several axial image series, we found that ventricles were clearly smaller in mutants compared to controls (Fig. 8). The extent of this defect was somewhat variable: in some mutants, ventricles appeared very small or absent bilaterally (Fig. 8B), while in others, a more normal lateral ventricle was observed on one side while none could be observed contralaterally (Fig. 8C). In several cases we confirmed this difference between controls and mutants by perfusion fixing the imaged animals and examining cryosections (Fig. 8A′, C′).
Figure 8. MRI imaging of FoxJ1-Cre; Pcdh-γfcon3/fcon3 mice indicates a reduction in ventricle size.
(A–C) Consecutive axial MRI image planes through the brains of live adult FoxJ1-Cre; Pcdh-γfcon3/+ (A) and FoxJ1-Cre; Pcdh-γfcon3/fcon3 mice. The ventricles in these images appear gray/black, whereas brain tissue is white. Ventricles are barely visible at all in the mutant shown in (B), in these or any other planes; for the mutant shown in (C), the lateral ventricle on the right side of the brain can be seen, but not on the left. To confirm the results of the MRI imaging, a subset of imaged mice were killed and their brains examined by coronal cryosectioning (stained here for DAPI). Control animals always showed normal bilateral lateral ventricles (A′), while these were either small or absent in mutants, sometimes differing between hemispheres. The sections of the mutant shown in (C) confirm the MRI imaging, showing a closed ventricle in the left hemisphere, and a smaller-than-normal ventricle in the right hemisphere (C′).
Because of the limited resolution obtained from MRI images, we utilized a different methodology for quantitative analysis of the ventricles in FoxJ1-Cre;Pcdh-γfcon3/fcon3 mutant and control animals. Serial 100 μm coronal vibratome sections were collected throughout the forebrain, labeled with a fluorescent Nissl stain (NeuroTrace) and imaged using confocal microscopy (Fig. 9A,B). The outline of the brain itself and of the ventricles (lateral and third) were then traced in Adobe Photoshop and the area quantified using NIH Image/J; volumes were estimated by multiplying each area by the section thickness (Fig. 9C–F). This analysis revealed a striking 60% reduction in ventricular volume in mutant mice compared to controls (Fig. 9F). Plotting the area per section from most rostral to most caudal (Fig. 9E) showed that mutant ventricles were reduced in size throughout the forebrain. No significant differences in total brain volume (Fig. 9D), area per section (Fig. 9C), or cortical thickness (data not shown) were observed between FoxJ1-Cre;Pcdh-γfcon3/fcon3 mutants and controls, confirming that the ventricular volume reduction was a specific consequence of γ-Pcdh loss in the ependyma and CP (Note that there is a small reduction in total brain volume in the mutants, almost exactly equal in magnitude to the reduction of ventricular volume).
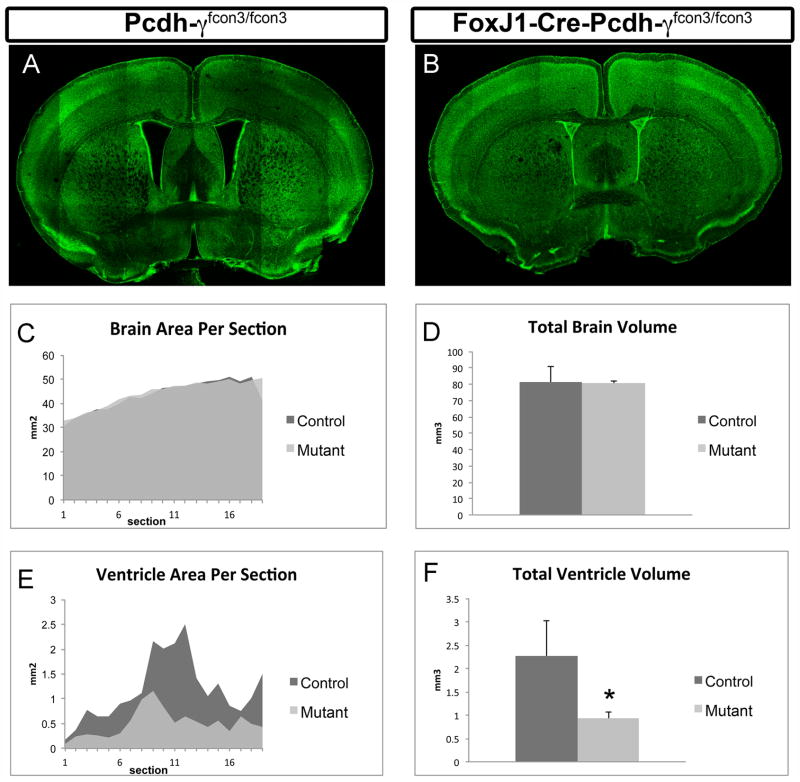
Figure 9. FoxJ1-Cre; Pcdh-γfcon3/fcon3 mutant mice exhibit reduced ventricular volume.
(A) and (B) are examples of tiled confocal micrographs of vibratome sections, stained with the fluorescent Nissl stain NeuroTrace. These images show approximately the same coronal level, revealing the severe decrease in the size of the lateral ventricles in FoxJ1-Cre; Pcdh-γfcon3/fcon3 mouse (B). Graphs (C–F) show quantifications of brain and ventricular area per section (C,E) or total estimated volume (D, F). Overall brain size is not significantly reduced in FoxJ1-Cre; Pcdh-γfcon3/fcon3 mutants compared to controls (C & D), but ventricle volume is reduced by ~60% (F). *p < 0.05 by t-test; bars show mean +/− S.D. of three animals per genotype.
Loss of γ-Pcdhs does not grossly disrupt morphology or apical-basal polarity of choroid plexus epithelial cells
There are several ways in which the loss of γ-Pcdhs in the ependyma and CP might result in a reduction of ventricular volume. One possibility is that ependymal γ-Pcdhs negatively regulate the production of neurons from stem cells known to reside in a niche adjacent to the ventricular surface (Chojnacki, Mak, and Weiss, 2009), such that loss of the γ-Pcdh results in overproduction of cells near the ventricular walls. Analysis of neuron and overall cell density in regions medial and lateral to the lateral ventricles, however, found no difference between FoxJ1-Cre;Pcdh-γfcon3/fcon3 mutants and controls (Fig. S1), consistent with normal overall forebrain volume as noted above (Fig. 9D). We also found no gross differences in cortical thickness or layer composition (data not shown). A more likely possibility is that loss of the γ-Pcdhs from the ependyma and CP results in a disruption of epithelial morphology, integrity, or apical-basal polarity, leading to defects of CSF production. We therefore utilized well-characterized polarity markers in whole mount immunostaining analyses of FoxJ1-Cre;Pcdh-γfcon3/fcon3 and control CP, and performed both transmission and scanning electron microscopy (EM) on CP and ependymal tissues.
Confocal analysis did not reveal any defects in the localization or expression level of the apical markers Na+/K+ ATPase and aquaporin, both critical proteins in the production of CSF (Praetorius, 2007) (Fig. S2), or of ezrin (data not shown). The tight junction marker ZO-1 was properly positioned just basal to these apical markers (Fig. S2A,B), and β-catenin localization was correctly polarized basolaterally (Fig. S2C,D). Scanning EM analysis showed that FoxJ1-Cre;Pcdh-γfcon3/fcon3 mutant CP epithelial cells had abundant apical microvilli and cilia tufts similar to control cells (Fig. S3A–D); mutant ependymal cells also had long cilia that were similar in appearance to, though somewhat less organized than, those of controls (Fig. S3E,F). Finally, because CSF secretion might be affected by disruptions to microvilli surface area at the apical membrane of FoxJ1-Cre;Pcdh-γfcon3/fcon3 mutant CP cells, we performed transmission EM analyses of thin sections and measured both average microvillus length and microvilli area. No significant differences were found between microvilli in control and mutant CP cells (Fig. S4), and we confirmed that mutant cells had intact tight junctions and grossly normal cytoplasmic appearance. Together, our analyses show that FoxJ1-Cre;Pcdh-γfcon3/fcon3 mutants exhibit severe reductions in ventricular volume, and thus apparently disrupted CSF regulation, in the absence of any gross structural abnormalities in ependyma and CP epithelial cells.
DISCUSSION
Despite its critical role in the development, homeostasis, and immune surveillance of the brain, the CP remains a somewhat understudied tissue. The biophysical and biochemical processes involved in CSF production are largely understood, and many of the critical ion channels, water channels, and transporters have been identified and their apical-basal polarity mapped (Brown et al., 2004; Praetorius, 2007; Wolburg and Paulus, 2010). Less is known about the receptors and signaling pathways that may regulate the differentiation and function of the CP epithelium and that could mediate interactions between the CP and other cells of the nervous and immune systems. Here, we have identified the 22 members of the γ-Pcdh adhesion molecule family as apically-localized proteins of the CP epithelium. Individual γ-Pcdh members are differentially expressed among cells of the CP epithelium, indicating a surprising molecular diversity of these cells. Restricted mutation of the Pcdh-γ gene cluster in CP and ependyma leads to a reduction in ventricular volume, consistent with a disruption of CSF production, without any obvious disruption of epithelial polarity or cell morphology. Together, these data identify a novel family of proteins important for CP biology while demonstrating intriguing new roles for the γ-Pcdhs, which we have previously shown to be critical neuronal and glial regulators of apoptosis and synaptogenesis (Garrett and Weiner, 2009; Prasad et al., 2008; Wang et al., 2002; Weiner et al., 2005; Weiner, 2006).
A previous study detected expression of one member of the Pcdh-γ gene family, C3, in neonatal and adult mouse ependymal cells (Hirano, Wang, and Suzuki, 2002). Similarly, Bass et al., (2007) reported that transcription of the genes that make up one of two Pcdh-γ clusters in zebrafish, DrPcdh2γ, was detectable in ependymal cells and other non-neural epithelia. Our results confirm these preliminary findings and expand them to show that most, if not all, of the 22 Pcdh-γ genes are expressed in the CP and ependyma. Focusing on the γ-Pcdh proteins, we found two surprising aspects of their expression: the differential expression of γ-Pcdh isoforms among CP epithelial cells, and their restricted subcellular localization to the apical membrane.
Although we were only able to assay a portion of the 22 γ-Pcdh proteins using available antibodies (Fig. 6), it is clear that each CP epithelial cell expresses only a subset of the γ-Pcdh isoforms. This appears to be the case with neurons as well: RT-PCR analysis of Pcdh-α and -γ gene expression in single, isolated cerebellar Purkinje cells shows that each neuron expresses perhaps 2–4 members of each gene cluster (Esumi et al., 2005; Kaneko et al., 2006), in an apparently stochastic fashion. This is consistent with the largely non-overlapping appearance of labeled cells in brain sections subjected to in situ hybridization with isoform-specific Pcdh-α or -γ probes (Frank et al., 2005; Kohmura et al., 1998; Wang et al., 2002; Zou et al., 2007). Due to the limited antibodies we have available, we cannot as yet say with confidence how many γ-Pcdhs each CP epithelial cell expresses. Ideally, this could be assessed using single-cell RT-PCR as for Purkinje cells (Kaneko et al., 2006). In neurons, it has suggested that the diversity of the protocadherins, coupled with their differential expression and assembly into homophilically trans-interacting cis-multimers, could underlie synaptic specificity (Morishita and Yagi, 2007; Schreiner and Weiner, 2010; Zipursky and Sanes, 2010). The importance of similar molecular diversity at the apical surface of CP epithelial cells, however, remains unclear. Interestingly, ultrastructural studies have previously noted apical morphological diversity within the CP epithelium (Mathew, 2007), but it is not clear whether or how this relates to any molecular repertoire. Choroid plexus epithelial cells interact apically with epiplexus (Kolmer) cells, which resemble macrophages; however, we did not detect γ-Pcdh expression (using GFP staining in Pcdh-γfusg mice) in F4/80 (pan-macrophage marker)-positive epiplexus cells (data not shown). A more intriguing possibility is that CP γ-Pcdhs may facilitate interactions with immune cells such as T cells, which can be trafficked through the CP epithelium (Engelhardt, 2006; Reboldi et al., 2009). If lymphocytes, either constitutively or upon activation, express the γ-Pcdhs (currently unknown), then γ-Pcdh-mediated interactions might regulate the entry of these cells into the CNS. Several adhesion molecules are found to be upregulated on the surface of the CP epithelium following the induction of EAE, including immunoglobulin superfamily molecules that bind to integrins on lymphocytes (Engelhardt, Wolburg-Buchholz, and Wolburg, 2001; Steffen et al., 1996; Wolburg et al., 1999). It will be important in the future to examine if the γ-Pcdhs are upregulated in EAE, and whether CP-restricted Pcdh-γ mutants have altered disease progression.
Because the γ-Pcdhs can interact in trans as homophilic adhesion molecules (Schreiner and Weiner, 2010; Obata et al., 1995; Reiss et al., 2006), their restriction to the apical surface of CP epithelial cells is puzzling: this membrane faces outward into the CSF, and is not constitutively in contact with other cells. Interestingly, a number of other cadherin superfamily molecules are expressed in a similar apically-restricted fashion. Cad99, the Drosophila homolog of the hair cell stereocilia protein and Usher syndrome disease gene PCDH15 (Kazmierczak et al., 2007), is strictly localized to the apical microvilli in follicular cells, where it regulates microvillus length (D’Alterio et al., 2005; Schlichting et al., 2006). Though this function does not appear to involve adhesion between adjacent microvilli, D’Alterio et al. (2005) speculate that Cad99 may interact homophilically within each microvillus to form an extracellular scaffold that stabilizes the membrane. Protocadherin of the liver, kidney and colon (PLKC/PCDH24) localizes to the apical surface of polarized MDCK cells (Krahn et al., 2010), while mu-protocadherin is found at the apical brush-border of kidney proximal tubule cells (Goldberg et al., 2002). In the absence of an obvious partner cell for homo- or heterophilic adhesion, these protocadherins might act as regulators of a number of signaling pathways; for example, we recently found that the γ-Pcdhs negatively regulate a PKC signaling pathway in neurons (A.M. Garrett, D. Schreiner, M.A. Lobas, and J.A. Weiner, submitted), and it has been reported that the γ-Pcdhs can inhibit Wnt signaling and suppress growth of tumor cell lines (Dallosso et al., 2009). Interestingly, 10 genes within the Pcdh clusters exhibit copy number amplification in human ependymoma samples (Johnson et al., 2010), though the contribution of this to the disease is unknown.
Although we found no obvious disruption of apical-basal polarity in the CP of FoxJ1-Cre;Pcdh-γfcon3/fcon3 mutants, several atypical protocadherins (e.g., the seven-transmembrane Pcdhs Flamingo and Celsr1–3) are known to regulate planar cell polarity (PCP), which is important for the proper movement of CSF by directed ependymal cilia beating (Mirzadeh et al., 2010; Saburi and McNeill, 2005; Tissir et al., 2010). Mice lacking Celsr2 and Celsr3, which are expressed by ependymal cells but not by CP epithelia, exhibit loss of ependymal cilia and severe hydrocephalus, essentially the opposite phenotype to the reduced ventricles we have observed in Pcdh-γ mutants (Tissir et al., 2010). The contribution of PCP to CP epithelial function, or any role that the γ-Pcdhs may play in this process, should be a focus of future studies.
The ventricular volume reduction observed in Pcdh-γ mutant mice implies that the γ-Pcdhs have significant effect on CSF dynamics, though at present the mechanism by which they may do this is unclear. Future analyses aimed at elucidating this novel role for the γ-Pcdhs will be focused on the composition of CSF in Pcdh-γ mutant mice, the possible dysregulation of the many channels and transporters implicated in CSF production, and uncovering transcriptomic and proteomic changes in the CP in the absence of γ-Pcdhs.
Supplementary Material
Acknowledgments
We thank Leah Fuller and Courtney Tuegel for expert assistance with the animal colony, and Austin Keeler, Karry Jannie, and Dr. Andrew Garrett for helpful suggestions. This work was supported by NIH grant R01 NS055272 to J.A.W. The authors report no conflicts of interest regarding this work.
References
- Bass T, Ebert M, Hammerschmidt M, Frank M. Differential expression of four protocadherin alpha and gamma clusters in the developing and adult zebrafish: DrPcdh2gamma but not DrPcdh1gamma is expressed in neuronal precursor cells, ependymal cells and non-neural epithelia. Dev Genes Evol. 2007;217(5):337–351. doi: 10.1007/s00427-007-0145-4. [DOI] [PubMed] [Google Scholar]
- Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129(4):957–970. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacki AK, Mak GK, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat. Rev Neurosci. 2009;10(2):153–163. doi: 10.1038/nrn2571. [DOI] [PubMed] [Google Scholar]
- Cserr HF. Physiology of the choroid plexus. Physiol Rev. 1971;51(2):273–311. doi: 10.1152/physrev.1971.51.2.273. [DOI] [PubMed] [Google Scholar]
- Dallosso AR, Hancock AL, Szemes M, et al. Frequent long-range epigenetic silencing of protocadherin gene clusters on chromosome 5q31 in Wilms’ tumor. PLoS Genet. 2009;5(11):e1000745. doi: 10.1371/journal.pgen.1000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alterio C, Tran DD, Yeung MW, et al. Drosophila melanogaster Cad99C, the orthologue of human Usher cadherin PCDH15, regulates the length of microvilli. J Cell Biol. 2005;171(3):549–558. doi: 10.1083/jcb.200507072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B. Regulation of immune cell entry into the central nervous system. Results Probl Cell Differ. 2006;43:259–280. doi: 10.1007/400_020. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31(4):497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Wolburg-Buchholz K, Wolburg H. Involvement of the choroid plexus in central nervous system inflammation. Microsc Res Tech. 2001;52(1):112–129. doi: 10.1002/1097-0029(20010101)52:1<112::AID-JEMT13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Esumi S, Kakazu N, Taguchi Y, Hirayama T, Sasaki A, Hirabayashi T, Koide T, Kitsukawa T, Hamada S, Yagi T. Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat Genet. 2005;37(2):171–6. doi: 10.1038/ng1500. [DOI] [PubMed] [Google Scholar]
- Frank M, Ebert M, Shan W, Phillips GR, Arndt K, Colman DR, Kemler R. Differential expression of individual gamma-protocadherins during mouse brain development. Mol Cell Neurosci. 2005;29(4):603–16. doi: 10.1016/j.mcn.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Garrett AM, Weiner JA. Control of CNS synapse development by {gamma}-protocadherin-mediated astrocyte-neuron contact. J Neurosci. 2009;29(38):11723–11731. doi: 10.1523/JNEUROSCI.2818-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AM, Schreiner D, Weiner JA. The Cadherin Superfamily in Synapse Formation and Function. In: Hortsch Michael, Umemori Hisashi., editors. The Sticky Synapse. Springer Science; New York, NY: 2009. pp. 159–184. [Google Scholar]
- Goldberg M, Wei M, Tycko B, Falikovich I, Warburton D. Identification and expression analysis of the human mu-protocadherin gene in fetal and adult kidneys. Am J Physiol Renal Physiol. 2002;283(3):F454–63. doi: 10.1152/ajprenal.00012.2002. [DOI] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20(23):3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Han MH, Lin C, Meng S, Wang X. Proteomics analysis reveals overlapping functions of clustered protocadherins. Mol Cell Proteomics. 2010;9(1):71–83. doi: 10.1074/mcp.M900343-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Wang X, Suzuki ST. Restricted expression of protocadherin 2A in the developing mouse brain. Brain Res Mol Brain Res. 2002;98(1–2):119–23. doi: 10.1016/s0169-328x(01)00317-5. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Wright KD, Poppleton H, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466(7306):632–636. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko R, Kato H, Kawamura Y, Esumi S, Hirayama T, Hirabayashi T, Yagi T. Allelic gene regulation of Pcdh-alpha and Pcdh-gamma clusters involving both monoallelic and biallelic expression in single Purkinje cells. J Biol Chem. 2006;281(41):30551–30560. doi: 10.1074/jbc.M605677200. [DOI] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449(7158):87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- Kohmura N, Senzaki K, Hamada S, Kai N, Yasuda R, Watanabe M, Ishii H, Yasuda M, Mishina M, Yagi T. Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron. 1998;20(6):1137–51. doi: 10.1016/s0896-6273(00)80495-x. [DOI] [PubMed] [Google Scholar]
- Krahn MP, Rizk S, Alfalah M, Behrendt M, Naim HY. Protocadherin of the liver, kidney, and colon associates with detergent-resistant membranes during cellular differentiation. J Biol Chem. 2010;285(17):13193–13200. doi: 10.1074/jbc.M109.080051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Ebert M, Shan W, Phillips G, Arndt K, Colman D, Kemler R.Anonymous. Differential expression of individual gamma-protocadherins during mouse brain development. Mol Cell Neurosci. 2005;29(4):603. doi: 10.1016/j.mcn.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Masseguin C, Mani-Ponset L, Herbute S, Tixier-Vidal A, Gabrion J. Persistence of tight junctions and changes in apical structures and protein expression in choroid plexus epithelium of rats after short-term head-down tilt. J Neurocytol. 2001;30(5):365–377. doi: 10.1023/a:1015008308515. [DOI] [PubMed] [Google Scholar]
- Mathew TC. Diversity in the surface morphology of adjacent epithelial cells of the choroid plexus: an ultrastructural analysis. Mol Cell Biochem. 2007;301(1–2):235–239. doi: 10.1007/s11010-007-9416-7. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Han YG, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Cilia organize ependymal planar polarity. J Neurosci. 2010;30(7):2600–2610. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Yagi T. Protocadherin family: diversity, structure, and function. Curr Opin Cell Biol. 2007;19(5):584–592. doi: 10.1016/j.ceb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Murata Y, Hamada S, Morishita H, Mutoh T, Yagi T. Interaction with protocadherin-gamma regulates the cell surface expression of protocadherin-alpha. J Biol Chem. 2004;279(47):49508–16. doi: 10.1074/jbc.M408771200. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28(3–4):147–155. [PubMed] [Google Scholar]
- Obata S, Sago H, Mori N, Rochelle JM, Seldin MF, Davidson M, St John T, Taketani S, Suzuki ST. Protocadherin Pcdh2 shows properties similar to, but distinct from, those of classical cadherins. J Cell Sci. 1995;108(Pt 12):3765–73. doi: 10.1242/jcs.108.12.3765. [DOI] [PubMed] [Google Scholar]
- Phillips GR, Tanaka H, Frank M, Elste A, Fidler L, Benson DL, Colman DR. Gamma-protocadherins are targeted to subsets of synapses and intracellular organelles in neurons. J Neurosci. 2003;23(12):5096–104. doi: 10.1523/JNEUROSCI.23-12-05096.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius J. Water and solute secretion by the choroid plexus. Pflugers Arch. 2007;454(1):1–18. doi: 10.1007/s00424-006-0170-6. [DOI] [PubMed] [Google Scholar]
- Prasad T, Wang X, Gray PA, Weiner JA. A differential developmental pattern of spinal interneuron apoptosis during synaptogenesis: insights from genetic analyses of the protocadherin-{gamma} gene cluster. Development. 2008;135(24):4153–4164. doi: 10.1242/dev.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10(5):514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Haas IG, Schulte M, Ludwig A, Frank M, Saftig P. Regulated ADAM10-dependent ectodomain shedding of gamma-protocadherin C3 modulates cell-cell adhesion. J Biol Chem. 2006;281(31):21735–21744. doi: 10.1074/jbc.M602663200. [DOI] [PubMed] [Google Scholar]
- Saburi S, McNeill H. Organising cells into tissues: new roles for cell adhesion molecules in planar cell polarity. Curr Opin Cell Biol. 2005;17(5):482–488. doi: 10.1016/j.ceb.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Salinas PC, Price SR. Cadherins and catenins in synapse development. Curr Opin Neurobiol. 2005;15(1):73–80. doi: 10.1016/j.conb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Schlichting K, Wilsch-Brauninger M, Demontis F, Dahmann C. Cadherin Cad99C is required for normal microvilli morphology in Drosophila follicle cells. J Cell Sci. 2006;119(Pt 6):1184–1195. doi: 10.1242/jcs.02831. [DOI] [PubMed] [Google Scholar]
- Schreiner D, Weiner JA. Combinatorial homophilic interaction between gamma-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proc Natl Acad Sci U S A. 2010;107(33):14893–14898. doi: 10.1073/pnas.1004526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M, Engelhardt B. The circumventricular organs participate in the immunopathogenesis of experimental autoimmune encephalomyelitis. Cerebrospinal Fluid Res. 2005;2:8. doi: 10.1186/1743-8454-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speake T, Whitwell C, Kajita H, Majid A, Brown PD. Mechanisms of CSF secretion by the choroid plexus. Microsc Res Tech. 2001;52(1):49–59. doi: 10.1002/1097-0029(20010101)52:1<49::AID-JEMT7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Steffen BJ, Breier G, Butcher EC, Schulz M, Engelhardt B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol. 1996;148(6):1819–1838. [PMC free article] [PubMed] [Google Scholar]
- Sturrock RR. A morphological study of the development of the mouse choroid plexus. J Anat. 1979;129(Pt 4):777–793. [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8(1):11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- Tasic B, Nabholz CE, Baldwin KK, Kim Y, Rueckert EH, Ribich SA, Cramer P, Wu Q, Axel R, Maniatis T. Promoter choice determines splice site selection in protocadherin alpha and gamma pre-mRNA splicing. Mol Cell. 2002;10(1):21–33. doi: 10.1016/s1097-2765(02)00578-6. [DOI] [PubMed] [Google Scholar]
- Tissir F, Qu Y, Montcouquiol M, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13(6):700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- Wang X, Su H, Bradley A. Molecular mechanisms governing Pcdh-gamma gene expression: evidence for a multiple promoter and cis-alternative splicing model. Genes Dev. 2002;16(15):1890–905. doi: 10.1101/gad.1004802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Weiner JA, Levi S, Craig AM, Bradley A, Sanes JR. Gamma protocadherins are required for survival of spinal interneurons. Neuron. 2002;36(5):843–54. doi: 10.1016/s0896-6273(02)01090-5. [DOI] [PubMed] [Google Scholar]
- Weiner JA. Protocadherins and Synapse Development. In: Dityatev A, El-Husseini A, editors. Molecular Mechanisms of Synaptogenesis. Springer; New York: 2006. [Google Scholar]
- Weiner JA, Wang X, Tapia JC, Sanes JR. Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci U S A. 2005;102:8–14. doi: 10.1073/pnas.0407931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K. Secretion of Cerebrospinal Fluid by Choroid Plexus of the Rabbit. Am J Physiol. 1963;205:617–624. doi: 10.1152/ajplegacy.1963.205.3.617. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Paulus W. Choroid plexus: biology and pathology. Acta Neuropathol. 2010;119(1):75–88. doi: 10.1007/s00401-009-0627-8. [DOI] [PubMed] [Google Scholar]
- Wolburg K, Gerhardt H, Schulz M, Wolburg H, Engelhardt B. Ultrastructural localization of adhesion molecules in the healthy and inflamed choroid plexus of the mouse. Cell Tissue Res. 1999;296(2):259–269. doi: 10.1007/s004410051287. [DOI] [PubMed] [Google Scholar]
- Wu Q. Comparative Genomics and Diversifying Selection of the Clustered Vertebrate Protocadherin Genes. Genetics. 2005 doi: 10.1534/genetics.104.037606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97(6):779–90. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhang T, Cheng JF, et al. Comparative DNA sequence analysis of mouse and human protocadherin gene clusters. Genome Res. 2001;11(3):389–404. doi: 10.1101/gr.167301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Huang G, Shornick LP, Roswit WT, Shipley JM, Brody SL, Holtzman MJ. A transgenic FOXJ1-Cre system for gene inactivation in ciliated epithelial cells. Am J Respir Cell Mol Biol. 2007;36(5):515–519. doi: 10.1165/rcmb.2006-0475RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell. 2010;143(3):343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Zou C, Huang W, Ying G, Wu Q. Sequence analysis and expression mapping of the rat clustered protocadherin gene repertoires. Neuroscience. 2007;144(2):579–603. doi: 10.1016/j.neuroscience.2006.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.