Abstract
Goals
To determine whether the presence of dupA Helicobacter pylori (H. pylori) influences the cure rate of primary eradication therapy.
Background
Several virulence factors of H. pylori have been reported to affect the efficacy of the eradication rate. However, no study has investigated whether the presence of dupA affects eradication failure.
Study
The presence of dupA was evaluated in 142 H. pylori strains isolated from 142 patients with gastrointestinal diseases. Of these patients, 104 received primary eradication therapy for 1 week. The risk factors for eradication failure were determined using univariate and multivariate analyses.
Results
Among 142 strains, 44 (31.0%) were dupA-positive. There was no association between dupA status and gastroduodenal diseases (P > 0.05). The clarithromycin (CLR) resistance rate was generally lower in the dupA-positive than in the dupA-negative group (20.4 vs. 35.7%, P = 0.06). However, dupA prevalence was higher in the eradication failure group than in the success group (36.3 vs. 21.9%). Among the CLR-resistant H. pylori infected group, the successful eradication rate was significantly lower in patients infected with dupA-positive H. pylori than -negative H. pylori (P = 0.04). In multivariate analysis adjusted for age, gender, and type of disease, not only CLR resistance but also dupA presence was independent risk factors for eradication failure (adjusted odds ratio = 3.71, 95% confidence interval = 1.07–12.83).
Conclusions
Although CLR resistant was more reliable predictor, the presence of dupA may also be an independent risk factor for eradication failure.
Keywords: Helicobacter pylori, dupA, eradication rate
Introduction
Eradication therapy of Helicobacter pylori (H. pylori) infection is the first-line treatment for patients with gastroduodenal disorders, such as peptic ulcer diseases, gastric mucosa associated-lymphoid tissue (MALT) lymphoma, atrophic gastritis, hyperplastic polyp, and post-endoscopic resection of early gastric cancer (GC), as well as for patients with extra-gastrointestinal disorders, such as idiopathic thrombocytopenic purpura and chronic idiopathic urticaria 1. One week eradication therapy using 3 drugs (amoxicillin [AMX], clarithromycin [CLR], and a proton pump inhibitor [PPI]) is widely practiced in Japan 1. However, bacterial resistance to these drugs, which is common in infectious diseases, has been encountered. Particularly, a sustained increase in the CLR resistance rate is known to correlate with the increased usage in Japan 2, 3. CLR resistance of H. pylori is one of the primary reasons for eradication failure.
Virulence factors of H. pylori (e.g., cagA and vacA) play important roles in gastric mucosal injuries, such as gastric inflammation, peptic ulcer, atrophy, intestinal metaplasia, dysplasia, and malignancy 4. Furthermore, the importance of H. pylori virulence markers in the efficacy of cure rates has been reported 5–8. Lu et al. 9 described a novel virulence factor, the duodenal ulcer promoting (dupA) gene, which encompasses both jhp0917 and jhp0918 and is located in the plasticity region of the H. pylori genome. Interestingly, dupA is homologous to virB4, a gene encoding a component protein of the type IV secretion system (TFSS) in Agrobacterium tumefaciens. They reported that infections with the dupA-positive strains increased the risk for duodenal ulcer (DU) but were protective against gastric atrophy, intestinal metaplasia, and GC in Japanese, Korean, and Columbian subjects. Notably, dupA was the first genetic factor of H. pylori to be associated with differential susceptibility to DU and GC, and thus, it could be considered as a disease-specific virulence marker. The pathogenic mechanism of dupA appears to involve the induction of interleukin (IL)-8 production in the antrum, leading to the development of antrum-predominant gastritis, a well-recognized characteristic of DU 9. Recently, we conducted a meta-analysis and showed the high prevalence of dupA-positive H. pylori in patients with DU 10. However, no study has investigated whether the presence of dupA affects eradication failure.
In this study, we aimed to determine whether the presence of dupA influences the cure rate of primary eradication therapy.
Materials and Methods
Patients
Patients were considered to be H. pylori-infected when at least one of rapid urease test, culture, and microscopic examination showed positive results. Of the 244 patients included in our previous retrospective cohort study 11, 102 were excluded because they had already received eradication treatment for H. pylori. Therefore, 142 Japanese patients (74 males, 67 females, aged 22–91 years [mean, 58.1 years]) were recruited. Patients with drug allergies and those with serious complications, such as cardiac disease, renal disease, and hepatic disease, were excluded from the study. Four biopsy samples (2 from the antrum and 2 from the corpus) were endoscopically obtained from each patient and used for H. pylori culture and histopathologic examination. Gastric ulcer (GU) and DU were determined using endoscopic observation, and non-cardia GC and chronic gastritis were diagnosed histologically. Written informed consent was obtained from all participants, and the protocol was approved by the Ethics Committee of Oita University (P-07-04).
Study protocol
Of the 142 patients, 104 were treated for 1 week with primary eradication therapy (proton pump inhibitors [PPIs] +AMX 1500mg + CLR 400mg per day). One of PPIs was selected from lansoprazole (LPZ) 60mg (n = 88), omeprazole (OPZ) 40mg (n = 8), rabeprazole (RPZ) 20mg (n = 8). PPI was prohibited at least 4 weeks from completion of eradication therapy until evaluation of H. pylori eradication. When eradication was observed for at least 4 weeks following the 1-week treatment, it was considered to be successful if culture, microscopic examination, and urea breath tests showed negative results. Compliance was checked by the interview from doctor.
Drug sensitivity testing was conducted using an epsilometer test (E-test). A sterile swab was dipped into the bacterial suspension equivalent to a McFarland standard of 2. After swabbing the entire plate surface with inoculums, E-test strips impregnated with CLR (concentration ranging from 0.016 to 256μg/mL) were placed on the agar surface. After an incubation period of 48 to 72 h (37°C, 98 % humidity in microaerobic atmosphere), the minimum inhibitory concentrations (MICs) of CLR were determined. When the MIC of CLR was 1 μg/mL or higher, the strain was considered as resistant, in accordance with a report by the National Committee for Clinical Laboratory Standards 12.
Detection of dupA and serum gastrin levels
All isolates had been previously characterized with regard to dupA status (positive or negative) 11. Antral biopsy specimens were obtained for the isolation of H. pylori using standard culture methods as described previously 13. Chromosomal DNA was extracted from confluent plate cultures expanded from a single colony using a commercially available kit (QIAGEN, Valencia, CA). The dupA status was determined using polymerase chain reaction (PCR) methods as described previously 11. Amplified fragments were detected using 1.5% agarose gel electrophoresis and an ultraviolet transilluminator. To avoid false-negative PCR results resulting from variations in primer annealing sites, a dot blot was performed in all the cases as described previously 11. The serum gastrin level was determined under fasting conditions using a radioimmunoassay technique (Gastrin-RIA Kit II, TFB Co. Ltd., Tokyo, Japan).
Statistical Analyses
All statistical analyses were performed by SPSS version 18 (SPSS Inc., Chicago, IL, USA). The univariate association between each group was quantified using the unpaired t-test, Mann-Whitney U-test, Fisher’s exact test, and chi-square test. Multiple backward stepwise logistic regression analyses were used to examine the association of eradication failure with primary predictor variables. Predictor variables included age (continuous variable), gender (dichotomous variable), CLR resistance (dichotomous variable), type of diseases (dichotomous variable), and dupA status (dichotomous variable). For each variable, an odds ratio (OR) and 95% Confidence Interval (CI) were calculated. A two-tailed P value of < 0.05 was considered as statistically significant.
Results
Association between dupA and clinical outcomes
We analyzed 142 patients divided into 4 disease groups: chronic gastritis (n = 51), GU (n = 29), DU (n = 35), and non-cardia GC (n = 27). Of these 142 patients, 44 (31.0%) were infected with dupA-positive strains.
The dupA-positive strains were distributed nearly equally among the 4 disease groups (gastritis, 29.4%; GU, 27.6%; DU, 28.6%; and GC, 40.7%), indicating that there is no association between dupA status and gastroduodenal diseases (P > 0.05). This finding is in agreement with that of a previous study in Japan 11.
Characteristics of patients infected with dupA-positive or dupA-negative H. pylori
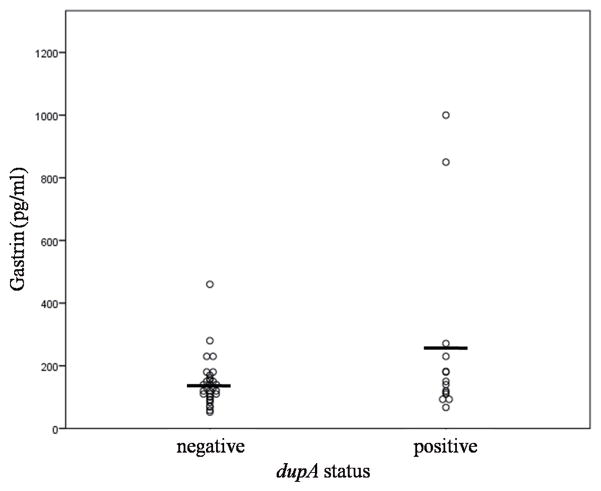
A comparison of characteristics of patients infected with dupA-positive or dupA-negative H. pylori is shown in Table 1. There was no difference in age and gender between the 2 groups. In all, the CLR resistant rate was 30.9% (44/142). The CLR resistance rate was generally lower in the dupA-positive than in the dupA-negative group (20.4% vs. 35.7%; P = 0.06). The gastrin level was evaluated in the sera of 45 of the 51 patients with gastritis (14 from the dupA-positive and 31 from the dupA-negative groups). Gastrin levels were higher in the dupA-positive group than in the negative group (257.2± 289.7 vs. 142.8 ± 77.9 pg/mL), although statistical significance was not obtained probably because of the wide range and the small sample size. In the gastritis group, histological scores according to Update Sydney System including neutrophil infiltration, mononuclear cell infiltration, atrophy, and intestinal metaplasia, were not significantly different between the dupA-positive and the dupA-negative group (data not shown). In addition, the rate of endoscopic atrophy did not differ between the 2 groups (data not shown).
Table 1.
Characteristics of the patients with dupA-positive or dupA-negative H. pylori
| dupA-positive | dupA-negative | P value | |
|---|---|---|---|
| n | 44 | 98 | |
| age | 58.0±15.4 | 58.1±13.3 | 0.78 |
| male | 23 (52.5%) | 52 (53.0%) | 0.93 |
| CLR resistant | 9 (20.4%) | 35 (35.7%) | 0.06 |
Factors for primary eradication failure
Among 142 patients, 38 patients refused the eradication therapy. Therefore, 104 subjects (37 chronic gastritis, 24 GU, 31 DU, and 12 non-cardia GC) received primary eradication therapy. The dupA-positive strains were distributed nearly equally among the 4 disease groups (gastritis, 24.3%; GU, 25.0%; DU, 25.8%; and GC, 25.0%). The compliance by the interview was 100%. The bacterium was not eradicated in 22 subjects (21.1%), the eradication therapy in per protocol analysis was 78.9%. Table 2 shows the characteristic differences between the eradication success and failure groups. Average age was significantly higher in the success group than in the failure group (59.3 ± 12.7 vs. 52.4 ± 14.6 years, P = 0.03). There was no gender difference between the 2 groups. The CLR resistant rate was 34.6% (36/104). The CLR resistance rate was significantly higher in the failure group than in the success group (63.6% vs. 26.8%, P = 0.001). The successful eradication rate was significantly higher in CLR susceptible strains than resistant strains (88.2% vs.61.1%, P = 0.001). Interestingly, the prevalence of dupA was higher in the failure group than in the success group (36.3% vs. 21.9%), despite the high CLR resistance rate in the dupA-negative group. However, the difference was not statistically significant (P = 0.16). Of the 36 CLR-resistant H. pylori infected group, 28.6% (2/7) of dupA-positive patients and 69.0% (20/29) of dupA-negative patients showed successful eradication (P = 0.04). The successful eradication rate was 70.2% (26/37) in chronic gastritis, 87.5% (21/24) in GU, 83.8% (26/31) in DU and 75% (9/12) in GC. There were no significant differences in univariate analysis although the successful eradication rate was tended to be higher in GU than gastritis (P = 0.10). With combining GU and DU as peptic ulcer disease (PUD), the successful eradication rate was significantly higher in PUD than gastritis (85.4% vs.70.2%, P = 0.045). The prevalence of PUD was tended to be higher in the success group than the failure group although this did not reach the statistical significance (57.3% vs.36.3%, P = 0.06).
Table 2.
Differences in the background of patients who experienced success or failure of eradication
| Success | Failure | P value | |
|---|---|---|---|
| n | 82 | 22 | |
| Age | 59.3±12.7 | 52.4±14.6 | 0.03 |
| Male | 48 (58.5%) | 9 (40.9%) | 0.14 |
| CLR resistant | 22 (26.8%) | 14 (63.6%) | 0.001 |
| dupA positive | 18 (21.9%) | 8 (36.3%) | 0.16 |
| Peptic ulcer diseases | 47 (57.3%) | 8 (36.3%) | 0.06 |
Multivariate analysis adjusted by age, gender, CLR resistance, and type of disease was performed to evaluate the influence of dupA on eradication failure. Due to the inverse relationship between dupA and CLR resistance, the presence of dupA was included in the final model, although the presence of dupA did not show a statistically significant association in the univariate analysis. Table 3 shows the association of the presence of dupA and clinical outcomes in logistical analysis. Not only CLR resistance but also the presence of dupA was found to be an independent risk factor for eradication failure (adjusted OR = 3.71, 95% CI = 1.07–12.83).
Table 3.
Multivariate analyses of H. pylori eradication failure risk by age, gender, CLR resistant, type of disease, and dupA status
| Adjusted OR | 95% CI | P value | |
|---|---|---|---|
| Age (per one year) | 0.95 | 0.91–0.98 | 0.013 |
| Gender (male) | 0.35 | 0.11–1.07 | 0.066 |
| CLR resistant | 7.96 | 2.45–25.8 | 0.001 |
| dupA positive | 3.71 | 1.07–12.83 | 0.038 |
| Peptic ulcer diseases | 0.54 | 0.16–1.80 | 0.319 |
Discussion
To our knowledge, this is the first study showing that the presence of dupA influences primary eradication failure. The dupA-positive status was an independent risk factor for eradication failure despite a lower CLR resistance rate than that of the dupA-negative status.
Several studies have reported a relationship between H. pylori antibiotic resistance patterns and virulence factor genotypes. Elviss et al. 14 reported that isolates susceptible to CLR are strongly associated with the vacA s1m2 genotype, but not with either the highest virulence vacA s1m1 genotype or the lowest virulence vacA s2 m2 genotype. A more recent report showed that CLR-resistant strains more frequently possess the vacA s2m2 and are more likely to be cagA-negative 15. However, the prevalence of cagA and vacA alleles did not significantly differ between CLR-susceptible and CLR-resistant strains in Italy 16. Suzuki et al. 7 conducted a meta-analysis and found that the risk ratio of eradication failure in patients with the cagA-negative strain (cure rate, 84%) relative to those with the cagA-positive strain (73%) was 2.0 (95% CI: 1.6–2.4, P < 0.01). We found that nearly all H. pylori strains were cagA positive and vacA s1m1, and there was no relationship between CLR-resistance and cagA or vacA status (data not shown).
The relationship of cagA with the success and failure of H. pylori eradication therapy has been explained by the enhanced gastric mucosal inflammation. A good correlation between cagA positivity and severe gastric inflammation has been confirmed 17, 18. Patients with severe inflammatory cell infiltrations in the antral mucosa experience significantly higher cure rates than those with milder inflammation 8. Since gastric inflammation increases mucosal blood blow, increased blood flow may probably facilitate the diffusion of antibiotics 19. However, the histological scores did not differ between the dupA-positive and dupA-negative group in this study, which suggests that the mechanism of the lower eradication rate in the dupA-positive group was not due to the histological differences.
Insufficient gastric acid inhibition during treatment also causes eradication failure by making antibiotics, particularly CLR and AMX, more unstable and more easily degraded in the stomach, minimizing the antimicrobial effects of the antibiotics 20, 21. Therefore, gastric acid secretion can be potently inhibited during treatment by using acid-inhibitory drugs such as PPIs22. Approximately 10 to 15% of patients chronically infected with H. pylori exhibit antral predominant inflammation. These patients, who are predisposed to DU, produce increased amounts of acid because of the elevated basal and stimulated gastrin secretion 23. A recent study showed that the gastric acid output is significantly higher in the dupA-positive than in the dupA-negative patients 24. In the present study, we found that serum gastrin levels were higher in the dupA-positive group than in the dupA-negative group, suggesting that gastric acid secretion might be higher in the dupA-positive than in the dupA-negative group. Lu et al. reported that the presence of dupA was associated with increased resistance to low pH in vitro study 9. dupA-positive strains may induce the high level of gastrin, and high gastric acid secretion . High gastric acid secretion in the dupA-positive group might be related to the lower eradication rate. However, the prevalence of dupA was not difference between DU and gastritis. If dupA is associated with high acid output, the prevalence of dupA needs to be higher in DU than gastritis. Other mechanism may relate with the low eradication rate in the patients with dupA-positive H. pylori. Further study is necessary to find the mechanism of the lower eradication rate in the dupA-positive group.
However, our study has several limitations. First, the number of patients infected with CLR-resistant and dupA-positive H. pylori was small due to the retrospective study. Although the number of subjects was enough to conduct multivariate analysis, larger number of subjects is necessary to confirm our findings. In addition, the prospective study based on the status of dupA is also necessary to confirm our results. Second, the successful eradication rates of H. pylori can also be affected by the type of PPIs due to the CYP2C19 polymorphism 25. RPZ does not underly this polymorphism, while the other 2 PPI’s do. There was no difference of successful eradication therapy among these 3 PPIs in this study (data not shown). This is due to the low number of patients received triple therapy including RPZ. Third, other known factors of failure of eradication treatment were not enough evaluated. The compliance, type of disease, and presence of atrophy and intestinal metaplasia may affect the treatment outcome 6. The successful eradication rate in our study was significantly higher in PUD than gastritis consistent with previous report 26, this factor was not associated with the successful eradication in multivariate analysis. The smoking habit is also important factor for the outcome of successful eradication therapy 6. However, unfortunately, the information for the smoking was not enough in this study. The information of smoking habit is required for the further study. However, CLR resistance is the most important factor for the treatment outcome. When we include other factors, such as the presence of atrophy and intestinal metaplasia, adjusting the conditions for statistical analysis was difficult. Finally, although dupA was an independent risk factor for eradication failure in multivariate analysis, the predictor was weak compared with CLR resistant. Furthermore, the high CLR resistant rate (30.9%) was found in our study. This suggests that the triple therapy included CLR might not be suitable for the first line eradication regimen in our country. In addition, it is difficult to assess the dupA status before eradication in clinical. This means that our findings are not enough to change the clinical practice. Eradication regimen included the antibiotics other than CLR may be suitable for better eradication rate rather than the examination of the status of dupA.
In conclusion, we first evaluated the influence of dupA on primary eradication. Although CLR resistant was more reliable predictor, the presence of dupA may also be an independent risk factor for eradication failure. Further studies are necessary to confirm these results.
Figure 1.
The distribution of serum gastrin level between the dupA-positive and -negative group.
Acknowledgments
This report is based on work supported in part by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (22390085 and 22659087), the Special Coordination Funds for Promoting Science and Technology from the MEXT of Japan and National Institutes of Health grant, DK 62813. We thank Ms. Yoko Kudo for excellent technical assistance.
Financial support: None
Footnotes
Potential competing interests: The authors declare that they have no competing interests.
References
- 1.Asaka M, Kato M, Takahashi S, et al. Guidelines for the Management of Helicobacter pylori Infection in Japan: 2009 Revised Edition. Helicobacter. 2010 Feb;15(1):1–20. doi: 10.1111/j.1523-5378.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 2.Horiki N, Omata F, Uemura M, et al. Annual change of primary resistance to clarithromycin among Helicobacter pylori isolates from 1996 through 2008 in Japan. Helicobacter. 2009 Oct;14(5):86–90. doi: 10.1111/j.1523-5378.2009.00714.x. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi I, Murakami K, Kato M, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007 Dec;45(12):4006–4010. doi: 10.1128/JCM.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010 Nov;7(11):629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo F, Berloco P, Cuomo R, et al. Helicobacter pylori strains and histologically-related lesions affect the outcome of triple eradication therapy: a study from southern Italy. Aliment Pharmacol Ther. 2003 Feb;17(3):421–428. doi: 10.1046/j.1365-2036.2003.01443.x. [DOI] [PubMed] [Google Scholar]
- 6.Sugimoto M, Yamaoka Y. Virulence factor genotypes of Helicobacter pylori affect cure rates of eradication therapy. Arch Immunol Ther Exp (Warsz) 2009 Jan–Feb;57(1):45–56. doi: 10.1007/s00005-009-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki T, Matsuo K, Sawaki A, et al. Systematic review and meta-analysis: importance of CagA status for successful eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2006 Jul;24(2):273–280. doi: 10.1111/j.1365-2036.2006.02994.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, Wang J, Yang L, et al. Influence of Helicobacter pylori genotype on triple eradication therapy. J Gastroenterol Hepatol. 2007 Dec;22(12):2251–2255. doi: 10.1111/j.1440-1746.2007.04836.x. [DOI] [PubMed] [Google Scholar]
- 9.Lu H, Hsu P, Graham D, et al. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology. 2005 Apr;128(4):833–848. doi: 10.1053/j.gastro.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiota S, Matsunari O, Watada M, et al. Systematic review and meta-analysis: the relationship between the Helicobacter pylori dupA gene and clinical outcomes. Gut Pathog. 2010;2(1):13. doi: 10.1186/1757-4749-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen L, Uchida T, Tsukamoto Y, et al. Helicobacter pylori dupA gene is not associated with clinical outcomes in the Japanese population. Clin Microbiol Infect. 2010 Aug;16(8):1264–1269. doi: 10.1111/j.1469-0691.2009.03081.x. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute; 2008. pp. M100–S18. 18th informational supplement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaoka Y, Kodama T, Kita M, et al. Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter. 1998 Dec;3(4):241–253. doi: 10.1046/j.1523-5378.1998.08056.x. [DOI] [PubMed] [Google Scholar]
- 14.Elviss N, Owen R, Xerry J, et al. Helicobacter pylori antibiotic resistance patterns and genotypes in adult dyspeptic patients from a regional population in North Wales. J Antimicrob Chemother. 2004 Aug;54(2):435–440. doi: 10.1093/jac/dkh343. [DOI] [PubMed] [Google Scholar]
- 15.Agudo S, Pérez-Pérez G, Alarcón T, et al. High prevalence of clarithromycin-resistant Helicobacter pylori strains and risk factors associated with resistance in Madrid, Spain. J Clin Microbiol. 2010 Oct;48(10):3703–3707. doi: 10.1128/JCM.00144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Francesco V, Margiotta M, Zullo A, et al. Claritromycin resistance and Helicobacter pylori genotypes in Italy. J Microbiol. 2006 Dec;44(6):660–664. [PubMed] [Google Scholar]
- 17.De Francesco V, Zullo A, Margiotta M, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther. 2004 Feb;19(4):407–414. doi: 10.1046/j.1365-2036.2004.01818.x. [DOI] [PubMed] [Google Scholar]
- 18.van der Hulst R, van der Ende A, Dekker F, et al. Effect of Helicobacter pylori eradication on gastritis in relation to cagA: a prospective 1-year follow-up study. Gastroenterology. 1997 Jul;113(1):25–30. doi: 10.1016/s0016-5085(97)70076-3. [DOI] [PubMed] [Google Scholar]
- 19.Maeda S, Yoshida H, Ikenoue T, et al. Structure of cag pathogenicity island in Japanese Helicobacter pylori isolates. Gut. 1999 Mar;44(3):336–341. doi: 10.1136/gut.44.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grayson M, Eliopoulos G, Ferraro M, et al. Effect of varying pH on the susceptibility of Campylobacter pylori to antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1989 Oct;8(10):888–889. doi: 10.1007/BF01963775. [DOI] [PubMed] [Google Scholar]
- 21.Hunt R. pH and Hp--gastric acid secretion and Helicobacter pylori: implications for ulcer healing and eradication of the organism. Am J Gastroenterol. 1993 Apr;88(4):481–483. [PubMed] [Google Scholar]
- 22.Peterson W. The role of antisecretory drugs in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1997 Apr;11( Suppl 1):21–25. doi: 10.1046/j.1365-2036.11.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 23.Schubert M, Peura D. Control of gastric acid secretion in health and disease. Gastroenterology. 2008 Jun;134(7):1842–1860. doi: 10.1053/j.gastro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Imagawa S, Ito M, Yoshihara M, et al. Helicobacter pylori dupA and gastric acid secretion are negatively associated with gastric cancer development. J Med Microbiol. 2010 Dec;59(Pt 12):1484–1489. doi: 10.1099/jmm.0.021816-0. [DOI] [PubMed] [Google Scholar]
- 25.Sugimoto M, Furuta T, Shirai N, et al. Treatment strategy to eradicate Helicobacter pylori infection: impact of pharmacogenomics-based acid inhibition regimen and alternative antibiotics. Expert Opin Pharmacother. 2007 Nov;8(16):2701–2717. doi: 10.1517/14656566.8.16.2701. [DOI] [PubMed] [Google Scholar]
- 26.Huang JQ, Zheng GF, Hunt RH, et al. Do patients with non-ulcer dyspepsia respond differently to Helicobacter pylori eradication treatments from those with peptic ulcer disease? A systematic review. World J Gastroenterol. 2005 May;11(18):2726–2732. doi: 10.3748/wjg.v11.i18.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]