Abstract
Aims/hypothesis
We sought to evaluate if the cellular localisation and molecular species of diacylglycerol (DAG) were related to insulin sensitivity in human skeletal muscle.
Methods
Healthy sedentary obese controls (Ob; n=6; mean±SEM age 39.5±2.3 years; mean±SEM BMI 33.3±1.4 kg/m2), individuals with type 2 diabetes (T2D; n=6; age 44±1.8 years; BMI 30.1±2.3 kg/m2), and lean endurance-trained athletes (Ath; n=10; age 35.4±3.1 years; BMI 23.3±0.8 kg/m2) were studied. Insulin sensitivity was determined using an IVGTT. Muscle biopsy specimens were taken after an overnight fast, fractionated using ultracentrifugation, and DAG species measured using liquid chromatography/MS/MS.
Results
Total muscle DAG concentration was higher in the Ob (mean±SEM 13.3±1.0 pmol/μg protein) and T2D (15.2±1.0 pmol/μg protein) groups than the Ath group (10.0±0.78 pmol/μg protein, p=0.002). The majority (76-86%) DAG was localised in the membrane fraction for all groups, but was lowest in the Ath group (Ob, 86.2±0.98%; T2D, 84.2±1.2%; Ath, 75.9±2.7%; p=0.008). There were no differences in cytoplasmic DAG species (p>0.12). Membrane DAG species C18:0/C20:4, Di-C16:0 and Di-C18:0 were significantly more abundant in the T2D group. Cytosolic DAG species were negatively related to activation of protein kinase C (PKC)ε but not PKCθ, whereas membrane DAG species were positively related to activation of PKCε, but not PKCθ. Only total membrane DAG (r=−0.624, p=0.003) and Di-C18:0 (r=−0.595, p=0.004) correlated with insulin sensitivity. Disaturated DAG species were significantly lower in the Ath group (p=0.001), and significantly related to insulin sensitivity (r=−0.642, p=0.002).
Conclusions/interpretation
These data indicate that both cellular localisation and composition of DAG influence the relationship to insulin sensitivity. Our results suggest that only saturated DAG in skeletal muscle membranes are related to insulin resistance in humans.
Keywords: Athlete’s paradox, Diacylglycerol, Intramuscular lipid, Lipid composition, Protein kinase C
Introduction
As the epidemic of type 2 diabetes continues to grow, exploitation of new targets for diabetes prevention and treatment has become critical. Targets in skeletal muscle may hold particular promise in this regard. Skeletal muscle is the major tissue responsible for insulin action on peripheral glucose uptake, and therefore has been implicated as a primary site for the development of insulin resistance and type 2 diabetes [1]. Nevertheless, known mechanisms of insulin resistance in muscle have failed to render new therapeutic targets. For example, considerable attention has been paid to the association between intramuscular triacylglycerol (IMTG) concentration and muscle insulin resistance despite consensus that IMTG itself probably does not cause insulin resistance. Further, increasing evidence suggests that IMTG concentration and insulin action may be dissociated [2, 3]. Importantly, however, emerging data from our laboratory and others suggest that intramuscular lipid composition, rather than concentration, may play an important role [4-7].
There are several lipid molecules that may influence muscle insulin sensitivity, including long-chain acyl-CoA [8], ceramides [9, 10] and diacylglycerol (DAG) [11-14]. This study focused on intramuscular DAG, which is created in the synthesis and degradation of IMTG as well as from degradation of phospholipids. DAG contributes only a small fraction of the total intramuscular lipid pool, but has potent biological effects. Specifically, increased skeletal muscle DAG concentration and insulin resistance have been reported in animal models [11-13] and humans [14], presumably from its ability to activate protein kinase C (PKC) [11, 14, 15]. To date, DAG has been universally viewed as having a negative effect on muscle insulin action. If one considers that DAG consists of two fatty acids bound mainly on the first and second carbons of glycerol, many possible species of DAG exist, each with potential for unique biological action. Previous data from our laboratory showed that DAGs comprised of saturated fatty acids were related to insulin resistance in younger people [4], and this was particularly evident in highly insulin-sensitive endurance-trained athletes, who had lower DAG saturation [5]. Although not universally observed [10], corroborative data in both cells [16] and humans [6, 7] support this notion. Altogether, there is reason to believe that DAG composition in skeletal muscle deserves closer attention. Our aim was to determine if all DAG molecular species are equally deleterious to insulin sensitivity.
Notably, in addition to composition, the insulin-desensitising effects of DAG may be governed by its subcellular location [17]. The vast majority of studies published in humans report total skeletal muscle DAG concentration, without measuring compartmentalisation within the cell. Since the adverse effects of PKC are confined to cell membranes, it is likely that only DAGs located in certain compartments influence PKC activation and insulin sensitivity. We hypothesised that changes in DAG molecular species and localisation influence PKC activation and insulin action, and may help explain differences in insulin sensitivity between individuals.
Methods
Participants
Six obese sedentary controls (Ob), six individuals with type 2 diabetes (T2D), and ten endurance-trained athletes (Ath) were recruited for this study. They gave written informed consent, and were excluded if they had a BMI < 20 kg/m2 or > 25 kg/m2 for Ath, and BMI <28 or >40 kg/m2 for Ob and T2D. Participants were excluded if they had fasting triacylglycerol concentration >1.7 mmol/l or liver, kidney, thyroid or lung disease. Sedentary controls were engaged in planned physical activity <2 h/week. Ath were competitive cyclists, triathletes and runners with mean±SEM lactate thresholds of 81±2.6% of maximal oxygen consumption (), and had been training on average 15.6±1.5 h/week for the past 9.6±2.2 years for the purpose of competition. The T2D individuals were included in the study if they did not take insulin and/or thiazoladinediones. All other glucose-lowering medications were washed out for 2 weeks before metabolic testing. Controls and athletes were not taking medications. Participants were weight stable in the 6 months before the study. This study was approved by the Colorado Multiple Institution Review Board at the University of Colorado Denver.
Preliminary testing
Participants reported to the General Clinical Research Center (GCRC) for screening procedures after a 12 h overnight fast, where they were given a health and physical examination, followed by a fasting blood draw. Body composition was determined using dual-energy x-ray absorptiometry (DEXA) analysis (Lunar DPX-IQ, Lunar Corporation, Madison, WI, USA).
Insulin sensitivity
Insulin sensitivity was determined via an IVGTT using standard methods after an overnight fast [18]. Briefly, after baseline samples had been taken, intravenous glucose (0.3 g/kg) was infused over 1 min, followed by insulin at 0.03 U/kg, 20 min after glucose administration. Blood samples were then frequently sampled over 3 h, and whole-body insulin sensitivity was calculated using the Bergman minimal model [18] (Millennium Version, MINMOD, Los Angeles, CA, USA).
Diet and exercise control
All participants were given a prescribed diet for 3 days before admission to the GCRC. Daily energy requirement was estimated from the DEXA measurement of fat free mass (FFM) using the equation: daily energy intake = 5.86 kJ (1.4 kcal)/day × [372 + (23.9 × FFM)], and analysis of dietary records. The composition of this diet was 55% carbohydrate, 30% fat and 15% protein. The fat content of the diet was controlled with the composition of saturated, monounsaturated and polyunsaturated fat in a 1:1:1 ratio. Participants were asked to refrain from planned physical activity for 48 h before the muscle biopsy study.
Muscle biopsy
Participants arrived in the morning after a 12 h overnight fast. After 4 h of rest, muscle biopsy samples were taken from midway between the greater trochanter of the femur and patella. The anatomic location and depth of the biopsy was as similar as possible between participants to minimise variance in muscle fibre composition, which varies with depth and length in the vastus lateralis. Muscle was immediately flash frozen in liquid nitrogen and stored at −80°C until dissection and analysis. Skeletal muscle samples were dissected free of extramuscular fat on ice as previously described [19].
Cell fractionation
The membrane and cytosolic fractions were isolated using a well-accepted ultracentrifugation protocol, which has already been described [11]. Briefly, muscle biopsy specimens (~40-50 mg wet weight) were extracted in ice-cold homogenising buffer A (20 mmol/l 3-(N-morpholino)propanesulfonic acid (MOPS), pH 7.5, 250 mmol/l mannitol, 1.2 mmol/l EGTA, 1 mmol/l dithiothreitol, 2 mmol/l phenylmethylsulphonyl fluoride [PMSF], protease [Roche Applied Science, Indianapolis, IN, USA] and phosphatase inhibitors [Sigma, St Louis, MO, USA]) at 4°C. The homogenate was centrifuged at 100,000 g for 45 min, and the supernatant fraction, representing the cytosolic fraction, removed and stored in liquid nitrogen. The 100,000 g pellet was washed once by resuspension in homogenising buffer A and re-centrifuged, and the protein pellet solubilised in homogenising buffer B (20 mmol/l MOPS, pH 7.5, 0.5% decanoyl-N-methyl-glucamide, 2 mmol/l EDTA, 5 mmol/l EGTA, 1 mmol/l dithiothreitol, 2 mmol/l PMSF, protease and phosphatase inhibitors). After 1 h at 4°C with constant gentle inversion, the extract was centrifuged again at 100,000 g for 45 min, and the supernatant fraction saved, representing the membrane fraction.
Liquid chromatography/tandem MS
Isolated cell fractions were shipped frozen on dry ice overnight to the Medical University of South Carolina lipidomics laboratory for analysis. In the lipidomics laboratory, samples were fortified with internal standards, extracted into a one-phase neutral organic solvent system, and analysed by a Thermo Finnegan TSQ 7000 triple quadrupole mass spectrometer as previously described [20]. Examination of DAG molecular species was performed by a parent ion scan of a common fragment ion characteristic of each class of lipid. Concentration was determined by comparing ratios of unknowns with internal standards, and referencing a standard curve.
Western blotting
To determine PKC activation and enrichment of membrane and cytosolic fractions, 15 μg of sample protein from membrane and cytosolic fractions were run on an SDS-PAGE 8% Bis-Tris gel (Invitrogen, Carlsbad, CA, USA), then transferred to a poly(vinylidene difluoride) membrane, and blocked with 5% BSA for 1 h at room temperature. Primary antibodies were from Cell Signaling (Danvers, MA, USA). Incubations were performed in 5% BSA overnight at 4°C, and a horseradish peroxidase-conjugated secondary antibody was incubated for 1 h at room temperature. Enhanced chemiluminescence was used to visualise protein bands of interest. Intensity of protein bands was captured using an AlphaImager 3300 and quantified using FluorChem software (Alpha Innotech Corp, San Leandro, CA, USA).
Statistical analysis
Data are presented as mean±SEM. Differences in normally distributed data between groups were analysed using a one-way ANOVA (SPSS, Chicago, IL, USA). Non-normally distributed data were log transformed before analysis using a one-way ANOVA. When significant differences were detected, individual means were compared using Student’s t tests to determine differences between groups. An alpha level of 0.05 was used for statistical significance other than comparisons with multiple DAG species. For evaluation of statistical significance with multiple DAG species, the Bonferroni method was used to correct for 16 multiple comparisons, leaving a significant p value that had to be <0.0031. Relationships between DAG molecular species, insulin sensitivity and PKC activation were determined using Pearson’s correlation coefficient.
Results
Demographic information for participants is shown in Table 1. As expected, the Ob and T2D groups had higher BMI and percentage body fat than the Ath group, and the of the Ath group was more than twice that of the Ob and T2D groups. As expected HbA1c, fasting glucose and insulin levels were significantly higher in the T2D group than the other two groups. Insulin sensitivity was significantly greater in the Ath group than the Ob and T2D groups.
Table 1.
Participants’ demographics
| Variable | Obese | T2D | Athletes | |
|---|---|---|---|---|
| Number (female/male) | 6 (2/4) | 6 (0/6) | 10 (2/8) | |
| Age (years) | 39.5±2.3 | 44±1.8 | 35.4±3.1 | |
| BMI (kg/m2) | 33.3±1.4 | 30.1±2.3 | 23.3±0.8*† | |
| Body fat (%) | 34.1±4.5 | 30.7±2.8 | 14.1±2.2*† | |
|
|
25.1±3.9 | 25.4±1.8 | 55.6±4.8*† | |
| HbA1c (proportion of total) | 0.055±0.001 | 0.071±0.005*‡ | 0.055±0.001 | |
| HbA1c (mmol/mol) | 36.2±0.9 | 54.3±3.6 | 36.6±0.4 | |
| Fasting glucose (mmol/l) | 5.4±0.2 | 7.7±1.3*‡ | 4.4±0.2 | |
| Fasting insulin (pmol/l) | 52.1±13.2 | 140.3±30.6*‡ | 24.3±2.8 | |
| Fasting NEFA (umol/l) | 716±68 | 589±87 | 678±47 | |
| Insulin sensitivity (10−4 μU−1 ml−1)a | 3.2±0.4 | 2.4±0.6 | 12.6±1.7*† |
Values are means±SEM
To convert values to SI units multiply by 0.167
Significantly different from obese, p<0.05
significantly different from T2D, p<0.05
significantly different from athletes, p<0.05
Skeletal muscle fractionation
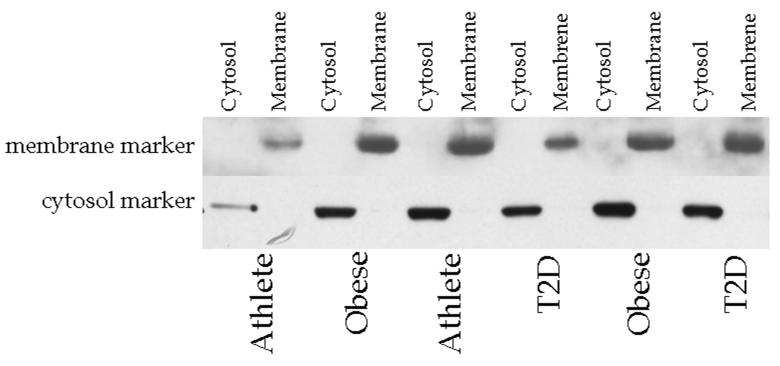
Enrichment of membrane and cytosolic markers in their respective subcellular compartments isolated by ultracentrifugation are shown in Fig. 1. These data show that the cellular fractionation methods were successful in enriching membrane and cytosolic proteins, and suggest minimal contamination between fractions.
Fig. 1.
Western blot showing enrichment of membrane and cytosolic proteins in human skeletal muscle biopsy specimens separated by ultracentrifugation. The membrane marker antibody is Na/K ATPase α1, and the cytosolic marker is glyceraldehyde-3-phosphate dehydrogenase. Cyto, cytosol; Mem, membrane; T2D, type 2 diabetes
Subcellular localisation of DAG
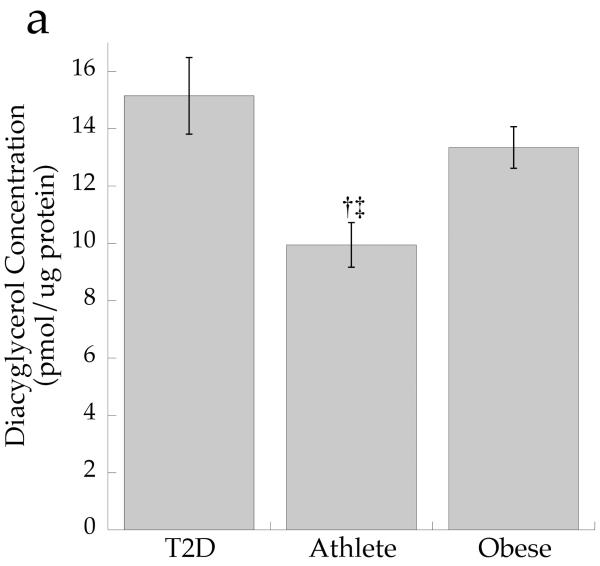
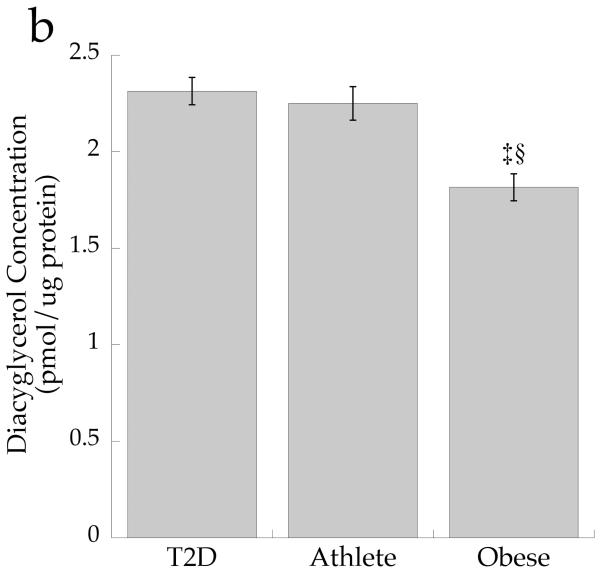
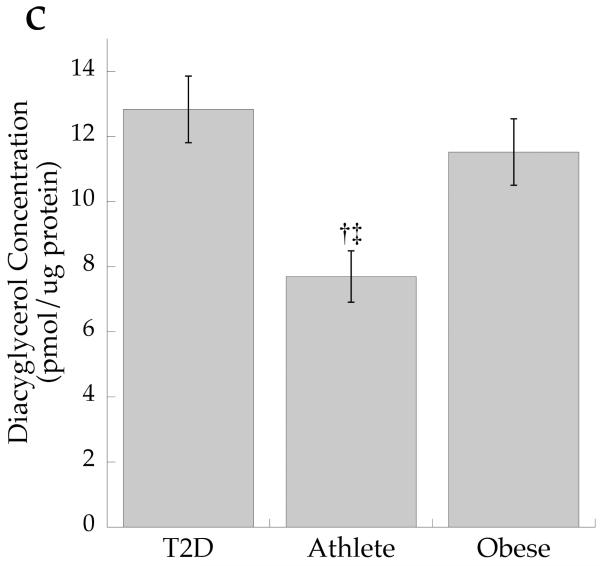
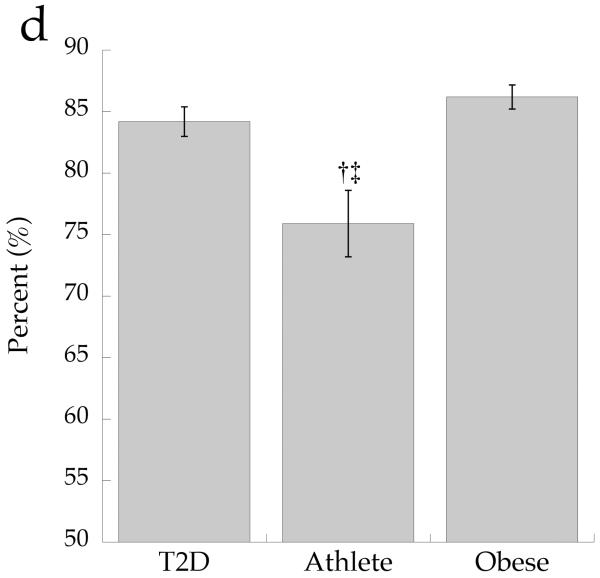
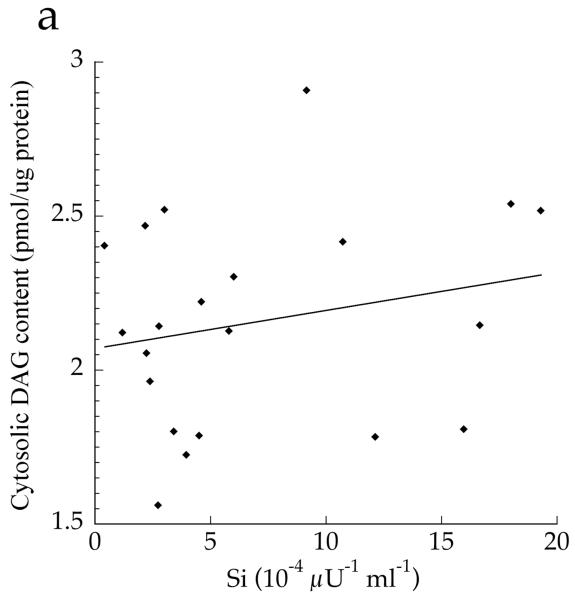
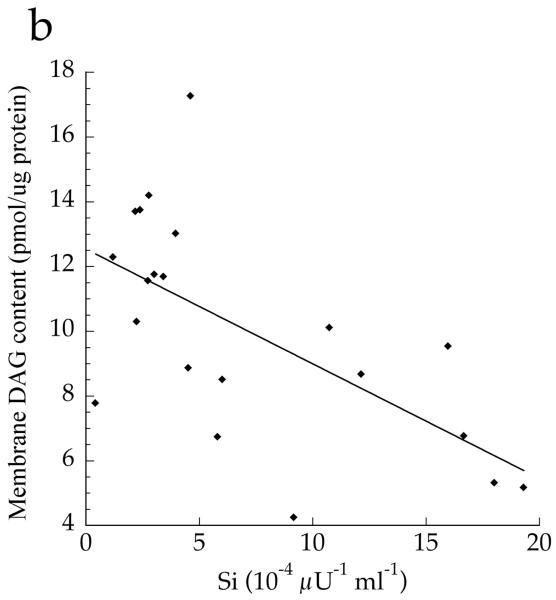
The cellular distribution of skeletal muscle DAG is shown in Fig. 2. Total DAG concentration was significantly higher in the Ob and T2D groups than in the Ath group (Fig. 2a, p=0.002), commensurate with their greater insulin resistance. We found a significant inverse relationship between total muscle DAG concentration and insulin sensitivity (r = −0.626, p=0.002). Cytosolic DAG concentration was significantly lower in the Ob group than the T2D and Ath groups (Fig. 2b, p=0.009). Most DAG was in the membrane in all groups, but both absolute (Fig. 2c, p=0.002) and percentage of total DAG concentration in membranes was lowest in the Ath group (Fig. 2d, p=0.01). Importantly, percentage of total DAG localised to membranes was significantly inversely related to insulin sensitivity (r = −0.691, p=0.0005). There was no relationship between cytosolic DAG and insulin sensitivity (Fig. 3a, p=0.34), but a significant relationship between membrane DAG and insulin sensitivity was observed in the cohort as a whole (Fig. 3b, r = −0.624, p=0.003).
Fig. 2.
DAG concentration by group in a total muscle homogenate, b cytosolic fraction and c membrane fraction and d percentage DAG in membrane fraction. Values are means±SEM. Significantly different from Ob, *p<0.05; significantly different from T2D, †p<0.05; significantly different from Ath, ‡p<0.05. T2D, type 2 diabetes
Fig. 3.
Relationship between insulin sensitivity (Si) and a total cytosolic DAG (r=0.217, p=0.34) and b total membrane DAG, r=−0.624, p=0.003 in T2D, Ath and Ob groups. To convert Si values to SI units multiply by 0.167
DAG molecular species
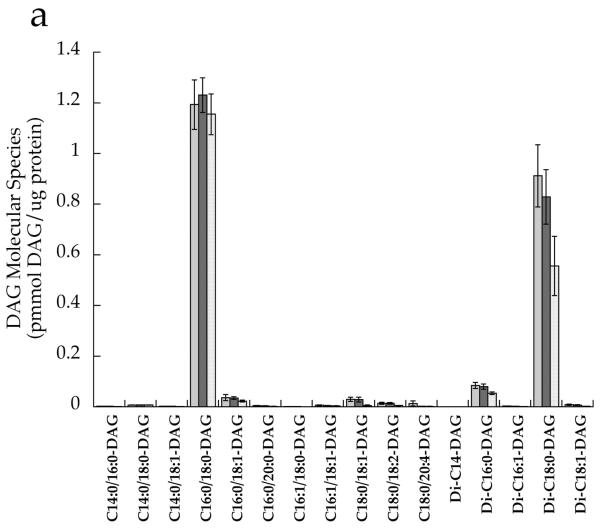
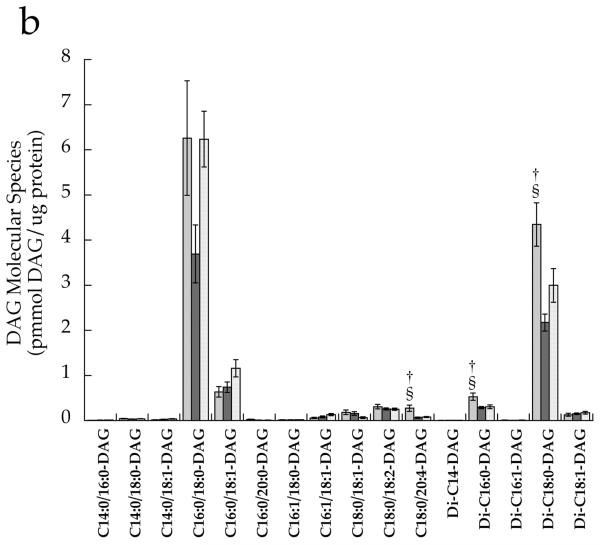
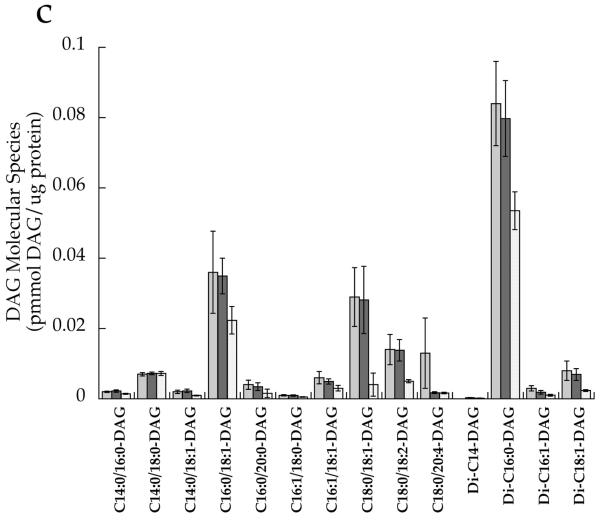
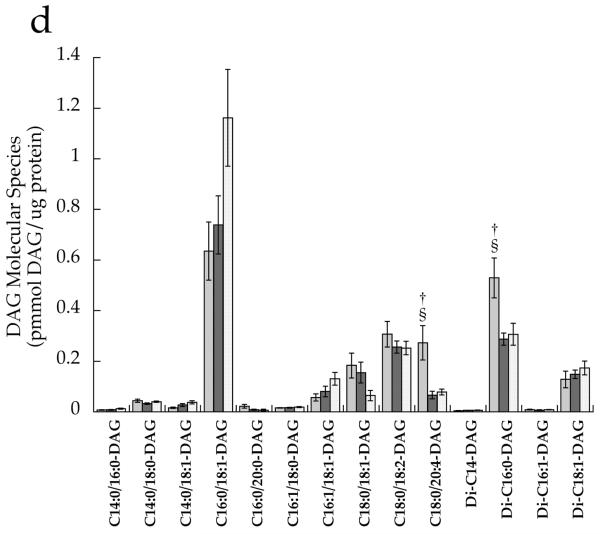
After correcting for multiple comparisons with Bonferroni, we found no significant relationships between individual DAG species in the whole cell and insulin sensitivity (p>0.02). There were no significant differences between groups for any cytosolic DAG species (Fig. 4a). However, the membrane DAG species, C18:0/C20:4 (p=0.0008), di-C16:0 (p=0.004) and di-C18:0 (p=0.0005), were significantly higher in the T2D group than the other two groups (Fig. 4b,d). Low-abundance cytosolic (Fig. 4c) and membrane (Fig. 4d) DAG species are shown in separate panels, with more abundant species removed in order to facilitate interpretation.
Fig. 4.
DAG molecular species in T2D (light grey), Ath (dark grey) and Ob (speckled) groups localised in the a cytosolic fraction and b membrane fraction, and low-abundance DAG species in the c cytosolic fraction and d membrane fraction. Values are means±SEM. Significantly different from Ob, *p<0.05; significantly different from Ath, †p<0.05
Data were combined from all groups and molecular species correlated by compartment with insulin sensitivity (Table 2). As with DAG species in the whole cell, there were no significant relationships between any DAG species in the cytosol and insulin sensitivity (p>0.02). The only membrane DAG species that correlated with insulin sensitivity was di-C18:0 (r = −0.595, p=0.003), and appeared to be the species responsible for the significant overall relationship between membrane DAG and insulin sensitivity (Table 2).
Table 2.
p values and Pearson’s correlation coefficients between DAG molecular species in cytosolic and membrane fractions and insulin sensitivity, and PKCε and θ activation
| DAG | Insulin sensitivity | PKCε activation | PKCθ activation | |||
|---|---|---|---|---|---|---|
| p value | Correlation | p value | Correlation |
p value |
Correlation | |
| Cytosolic | ||||||
| C14:0/16:0-DAG | 0.123 | 0.347 | 0.019 | −0.508 | 0.747 | −0.073 |
| C14:0/18:0-DAG | 0.389 | 0.198 | 0.549 | 0.139 | 0.047 | 0.429 |
| C14:0/18:1-DAG | 0.023 | 0.495 | 0.097 | −0.371 | 0.668 | −0.097 |
| C16:0/18:0-DAG | 0.953 | 0.014 | 0.165 | 0.314 | 0.191 | 0.290 |
| C16:0/18:1-DAG | 0.247 | 0.264 | 0.926 | −0.022 | 0.302 | 0.231 |
| C16:1/18:0-DAG | 0.207 | 0.287 | 0.027 | −0.483 | 0.563 | 0.130 |
| C16:1/18:1-DAG | 0.742 | 0.077 | 0.991 | −0.003 | 0.275 | 0.243 |
| C18:0/18:1-DAG | 0.124 | 0.347 | 0.0006a | −0.686 | 0.127 | −0.336 |
| C18:0/18:2-DAG | 0.078 | 0.393 | 0.140 | −0.334 | 0.974 | −0.007 |
| C18:0/20:4-DAG | 0.420 | −0.186 | 0.257 | 0.259 | 0.182 | 0.295 |
| Di-C14:0-DAG | 0.070 | 0.403 | 0.003a | −0.616 | 0.806 | −0.056 |
| Di-C16:0-DAG | 0.235 | 0.271 | 0.002a | −0.636 | 0.376 | −0.198 |
| Di-C16:1-DAG | 0.909 | −0.027 | 0.010 | −0.546 | 0.943 | −0.016 |
| Di-C18:0-DAG | 0.567 | 0.132 | 0.002a | −0.635 | 0.104 | −0.356 |
| Di-C18:1-DAG | 0.201 | 0.291 | 0.198 | −0.293 | 0.913 | 0.025 |
| Membrane | ||||||
| C14:0/16:0-DAG | 0.347 | −0.216 | 0.010 | 0.549 | 0.657 | −0.100 |
| C14:0/18:0-DAG | 0.089 | −0.380 | 0.060 | 0.417 | 0.367 | 0.202 |
| C14:0/18:1-DAG | 0.853 | −0.043 | 0.0187 | 0.508 | 0.680 | −0.093 |
| C16:0/18:0-DAG | 0.027 | −0.483 | 0.014 | 0.529 | 0.061 | 0.406 |
| C16:0/18:1-DAG | 0.578 | −0.129 | 0.002a | 0.628 | 0.311 | 0.226 |
| C16:0/20:0-DAG | 0.318 | −0.229 | 0.218 | −0.281 | 0.372 | −0.200 |
| C16:1/18:0-DAG | 0.932 | 0.020 | 0.131 | 0.341 | 0.416 | 0.183 |
| C16:1/18:1-DAG | 0.539 | −0.142 | 0.0005a | 0.692 | 0.400 | 0.189 |
| C18:0/18:1-DAG | 0.281 | 0.247 | 0.009 | −0.557 | 0.069 | −0.395 |
| C18:0/18:2-DAG | 0.656 | 0.103 | 0.853 | 0.043 | 0.990 | −0.003 |
| C18:0/20:4-DAG | 0.056 | −0.423 | 0.502 | 0.155 | 0.713 | 0.083 |
| Di-C14:0-DAG | 0.715 | 0.085 | 0.401 | 0.193 | 0.696 | −0.088 |
| Di-C16:0-DAG | 0.097 | −0.372 | 0.606 | −0.119 | 0.820 | −0.051 |
| Di-C16:1-DAG | 0.049 | −0.433 | 0.188 | 0.299 | 0.591 | −0.121 |
| Di-C18:0-DAG | 0.003a | −0.595 | 0.781 | 0.065 | 0.749 | 0.072 |
| Di-C18:1-DAG | 0.753 | 0.073 | 0.020 | 0.503 | 0.438 | 0.174 |
Significant relationship
Fasting glucose concentration was significantly positively related to both membrane DAG (r = 0.478, p=0.024) and total cell DAG (r = 0.496, p=0.019), but not cytosolic DAG (r = −0.023, p=0.92). There were no significant relationships between DAG and fasting insulin or NEFA concentrations.
DAG and PKC
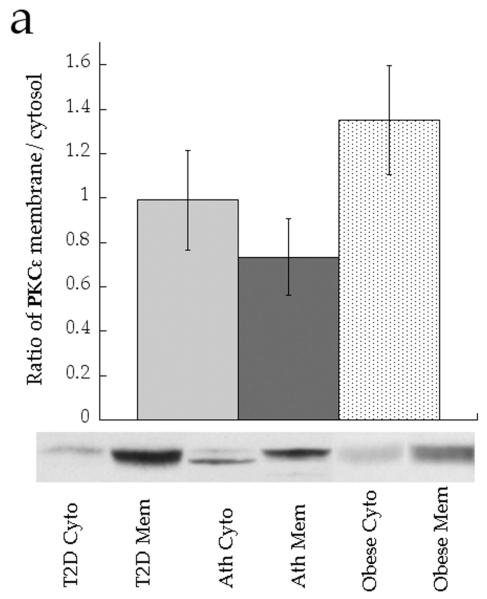
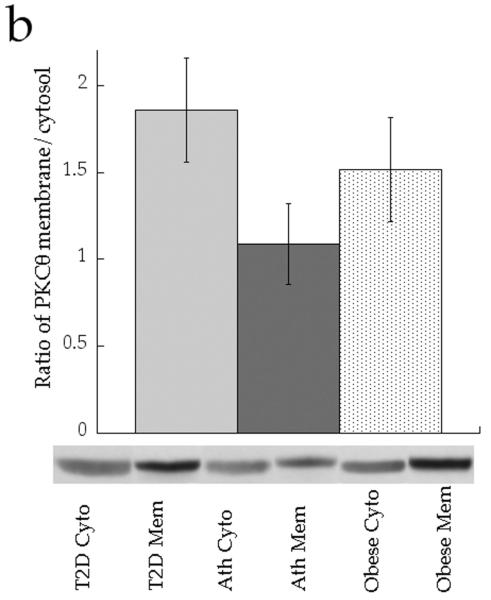
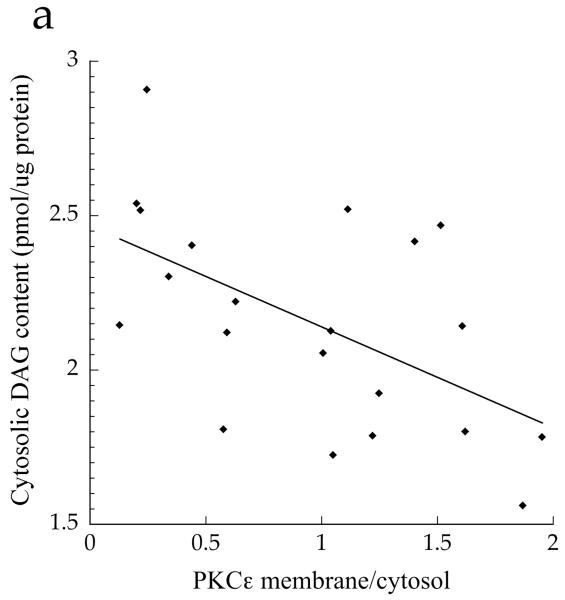
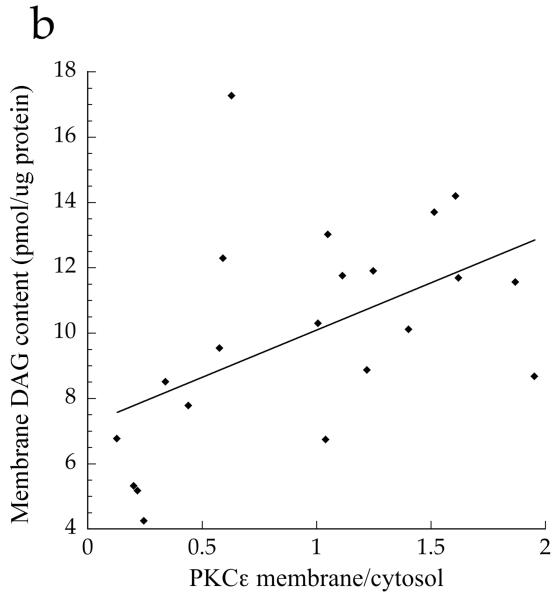
The ratio of membrane to cytosolic PKC content is a common surrogate for activation, since PKC is thought to be active in the membrane fraction only [21]. Although the data trended for lower PKCε and PKCθ activation in the Ath group compared with the T2D and Ob groups, the differences were not significant (Fig. 5a,b, p=0.15). When analysed as continuous variables, cytosolic DAG concentration was negatively related to PKCε activation (Fig. 6a, r = −0.552, p=0.009), whereas membrane DAG concentration was positively related to PKCε (Fig. 6b, r = 0.507, p=0.019). We found that, in the cytosol, C18:0/C18:1 (p=0.0006), di-C14:0 (p=0.003), di-C16:0 (p=0.002) and di-C18:0 (p=0.002) were significantly inversely related to PKCε activation (Table 2). No similar relationships were observed for PKCθ (p>0.05), suggesting PKC isoform specificity for particular DAG species. In the membrane, we found significant positive relationships between PKCε activation and C16:0/C18:1 (p=0.002) and C16:1/C18:1 (p=0.0005) (Table 2). No significant relationships were found for PKCθ (p>0.06).
Fig. 5.
Ratio of membrane (Mem) to cytosolic (Cyto) content of a PKCε and b PKCθ in T2D (light grey), Ath (dark grey), and Ob (speckled) groups. Values are means±SEM
Fig. 6.
Relationship between PKCε activation and a cytosolic DAG (r=−0.552, p=0.009) and b membrane DAG (r=0.507, p=0.019) in T2D, Ath and Ob groups
DAG saturation and insulin sensitivity
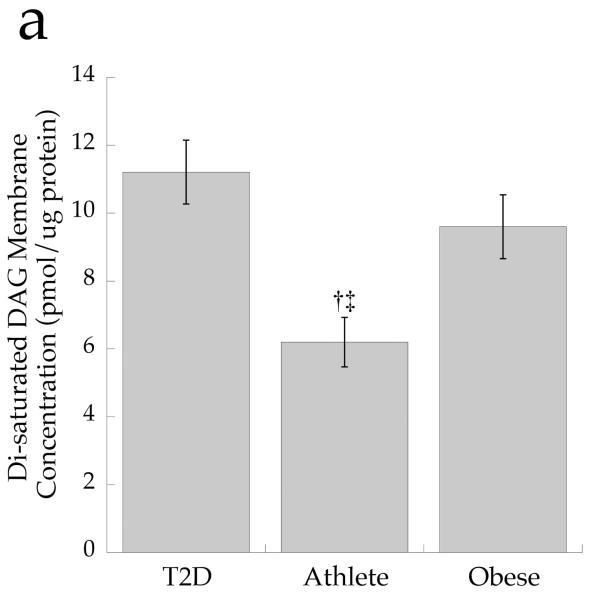
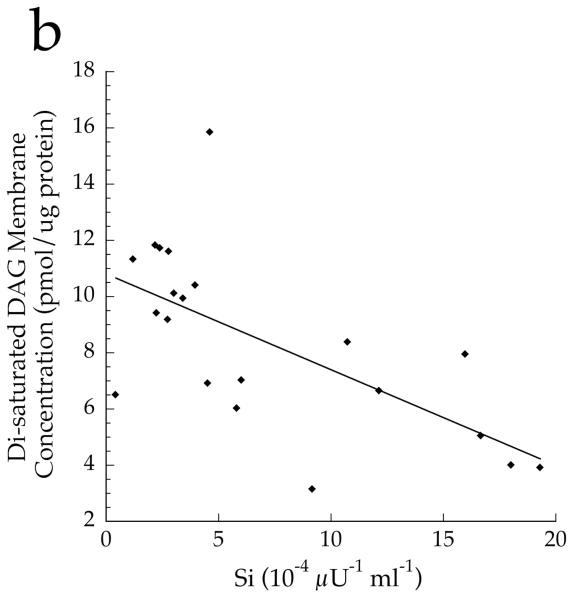
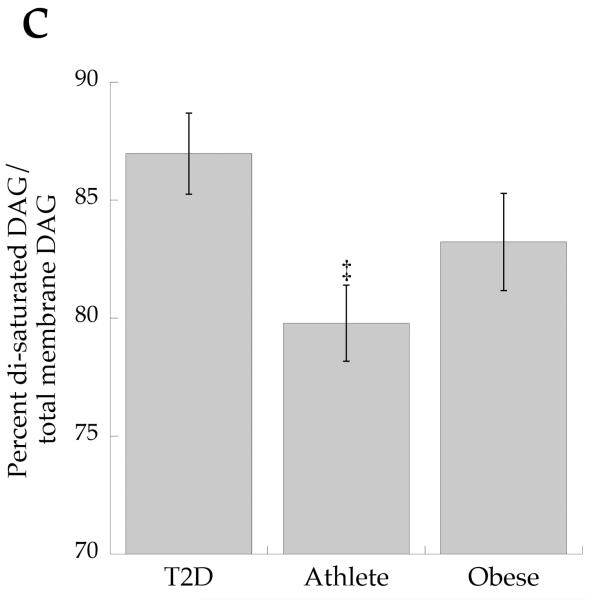
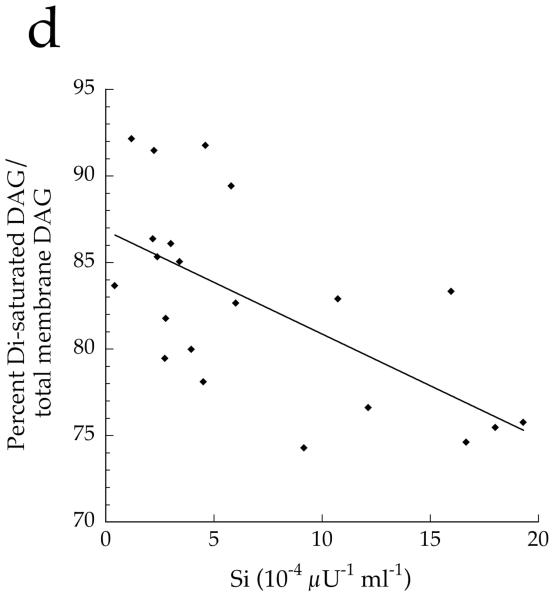
To determine if overall DAG saturation differed by group, we calculated the sum of DAG species with saturated acyl species on the first and second carbon. The concentration of total cell disaturated DAG (p=0.001) and absolute membrane disaturated DAG content (p=0.001, Fig. 7a) was significantly lower in the Ath group than the other two groups. The absolute content of membrane disaturated DAG was significantly negatively related to insulin sensitivity (r = −0.642, p=0.002, Fig. 7b). The relative membrane disaturated DAG content was significantly lower in the Ath than the T2D group (p=0.03, Fig. 7c), and was significantly negatively related to insulin sensitivity (r = −0.633, p=0.002, Fig. 7d).
Fig. 7.
a Content of disaturated DAG in membrane fraction in T2D, Ath and Ob groups. b Relationship between disaturated membrane DAG and insulin sensitivity (Si) (r=−0.642, p=0.002). c Percentage of disaturated DAG relative to total membrane DAG. d Relationship between percentage disaturated DAG relative to total membrane DAG and Si (r=−0.633, p=0.002). To convert Si values to SI units multiply by 0.167. Significantly different from Ob, *p<0.05; significantly different from T2D, †p<0.05. T2D, type 2 diabetes
Muscle protein expression
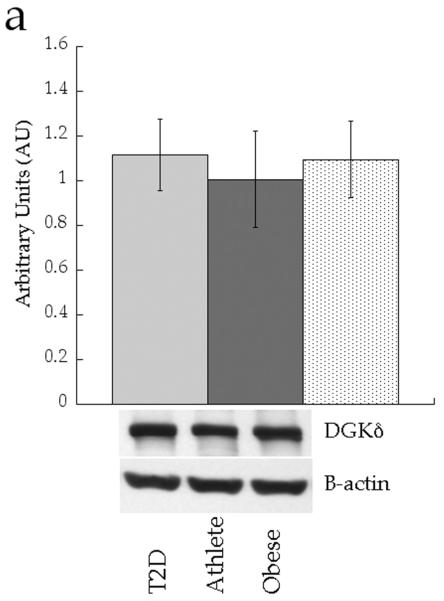
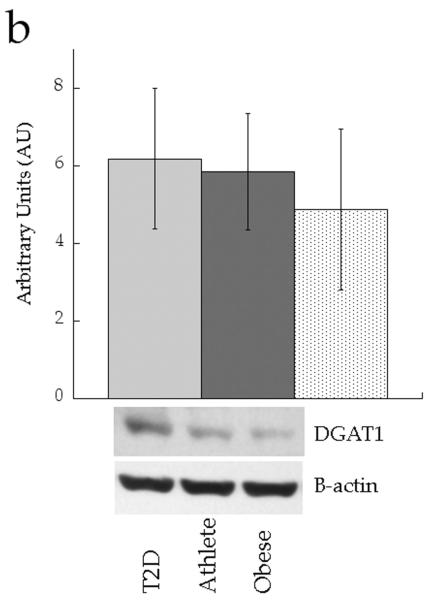
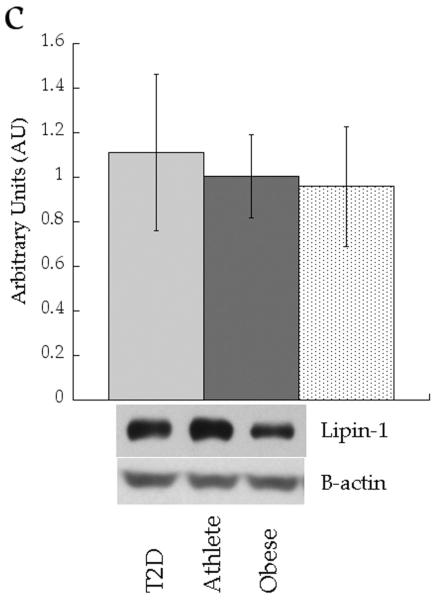
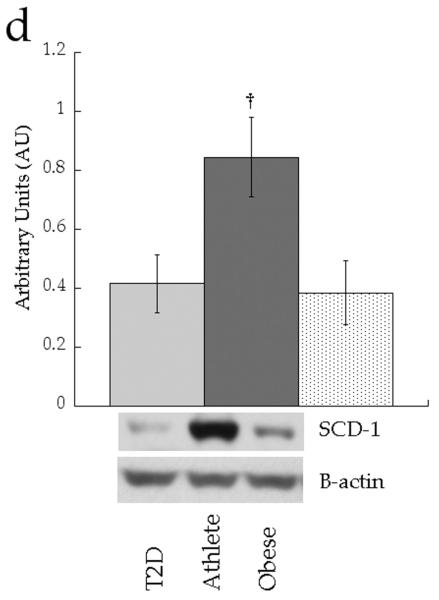
We analysed expression of enzymes involved in attenuating DAG signalling (diacylglycerol kinase δ [DGKδ]), triacylglycerol synthesis (diacylglycerol acyltransferase 1 [DGAT1] and lipin 1) and lipid desaturation (stearoyl-CoA desaturase 1 [SCD1]). Figure 8 shows no significant differences in protein content of whole cell DGKδ (p=0.71), DGAT1 (p=0.88) or lipin 1 (p=0.20) between groups. Only SCD1 content was different between groups, with significantly increased production in the Ath group compared with the Ob group (p=0.03).
Fig. 8.
Skeletal muscle protein levels of a DGKδ, b DGAT1, c lipin 1 and d SCD1 in T2D (light grey), Ath (dark grey) and Ob (speckled) groups. Values are means±SEM. Significantly different from Ob, *p<0.05. T2D, type 2 diabetes
Discussion
Strong evidence suggests that DAG may be an important link between tissue lipids and insulin resistance. Skeletal muscle DAG concentration is elevated in individuals with obesity and type 2 diabetes [22], and has been shown to be negatively related to insulin sensitivity [6, 11, 13, 14]. Most studies report whole cell DAG content, yet there are a large number of potential molecular species of DAG, as well as variability in intracellular localisation that could influence biological action. We hypothesised that both membrane DAG localisation and saturated molecular species would be independently related to skeletal muscle insulin action. Indeed, major findings from the present study confirm our hypotheses. Of the 16 measurable molecular species of DAG in human skeletal muscle, the majority are localised to membranes, and only membrane DAG were positively related to PKC activation and insulin resistance. Three particular species of DAG were significantly higher in individuals with type 2 diabetes than the other groups, but only di-C18:0 was significantly related to insulin resistance. Decreasing DAG localised to skeletal muscle membranes, or decreasing stearate-containing DAG may be a novel therapeutic target for the treatment and prevention of insulin resistance in humans.
Currently, all DAG in human skeletal muscle is thought to promote insulin resistance. Consistent with previous investigations, total intramuscular DAG concentration correlated inversely with insulin sensitivity in this study. Nevertheless, because DAG induces insulin resistance by activating PKC in membranes, we hypothesised that only membrane DAG would be related to PKC activation and insulin resistance in skeletal muscle. Supportive data were reported in a recent study demonstrating increased skeletal muscle membrane DAG content during aging, which was associated with increased PKC activation and insulin resistance in rodents [17]. Similarly, liver samples from morbidly obese participants also revealed compartmentalisation of DAG, with cytosolic, but not membrane, DAG correlated with PKCε activation [23]. Our data show the importance of DAG compartmentalisation in human skeletal muscle, as only membrane DAG was related to PKC activation and decreased insulin sensitivity. In contrast, cytosolic DAG was inversely related to insulin resistance and PKCε activation. Together, these data are the first to reveal that membrane localisation of DAG in skeletal muscle, rather than total intramuscular concentration, drives insulin resistance in humans.
Alterations in DAG production induced by obesity and/or type 2 diabetes may explain differences in DAG localisation. For example, hyperglycaemia increases phospholipase C activity [24], which degrades membrane phospholipids and would produce membrane DAG. Increased C18:0/C20:4 DAG in type 2 diabetes is consistent with phospholipase DAG generation. Hyperinsulinaemia and hyperglycaemia also increase de novo DAG synthesis [25], which occurs at the endoplasmic reticulum [26], and could be an important mechanism promoting membrane DAG accumulation in obesity and diabetes. In contrast, DAG formed during IMTG degradation [27] would promote cytosolic accumulation and may be less prominent in type 2 diabetes. The combination of enhanced de novo DAG synthesis driven by elevated plasma concentrations of glucose and insulin, high saturated fat intake [28], and less muscle lipid desaturation form a plausible explanation for how saturated membrane DAG accumulate in obesity and diabetes. Exploiting pathways dictating intracellular DAG localisation may prove a novel target for insulin sensitisation.
Whether decreasing membrane DAG increases insulin sensitivity is not known, but would be supported by these data. Altered abundance of DGKδ, an enzyme responsible for converting DAG into phosphatidic acid to terminate DAG signalling [29], may be one manner of doing so. DGKδ exists in many locations of the cell, including endoplasmic reticulum [30], neuromuscular junction [31], cytoskeletal compartments [32] and the nucleus, which confirms intracellular DAG compartmentalisation [29]. In a previous study, hyperglycaemia downregulated DGKδ and explained increased DAG concentration in individuals with type 2 diabetes [33]. We found no differences in whole cell DGKδ between groups, suggesting that changes in DAG content and/or localisation was not due to downregulation of this enzyme. Of note, hyperglycaemia in our participants with type 2 diabetes was ~2.2 mmol/l lower than in the previous report, and may contribute to the lack of differences in this study. Membrane DAG content may also be influenced by muscle oxidative capacity. Preventing mitochondrial oxidative damage preserved oxidative capacity and precluded membrane DAG accumulation and insulin resistance in aging mice [17]. Similarly, muscle oxidative capacity is increased in endurance-trained athletes, who also had decreased membrane DAG content in the present study. These studies suggest a possible link between oxidative capacity, membrane DAG localisation and insulin sensitivity. Nevertheless, further studies are needed to determine if membrane DAG content can be decreased, and if this change results in increased insulin sensitivity.
In addition to DAG localisation, DAG composition appears to discriminate DAG function, and therefore plays an important role linking muscle lipids to insulin resistance. Of the 16 measurable membrane DAG species, only di-C18:0 was significantly related to insulin sensitivity. Interestingly, these data corroborate previous data from our laboratory and others suggesting that saturated DAG has a particularly negative impact on insulin sensitivity [4, 5, 7]. This is exemplified by the observation that disaturated membrane DAGs were negatively related to insulin sensitivity in the cohort as a whole, and were also significantly lower in our insulin-sensitive endurance-trained athletes. Similar to other reports [5], SCD1 content was increased in athletes, which may be one mechanism explaining less saturated skeletal muscle DAG in this group. Less saturated membrane DAG in endurance-trained athletes may help explain how they maintain insulin sensitivity, despite a high IMTG concentration, the so-called ‘athletes paradox’ [34]. Further, these data highlight that DAG species are not homogeneous and probably have dissimilar impacts on insulin sensitivity. Similar data showing that unique DAG species correlated with PKC activation and insulin sensitivity were recently reported in human liver [23]. However, not all data agree, as two recent studies [10, 35] did not observe the relationship between skeletal muscle DAG molecular species and insulin sensitivity observed in the present study. This apparent discrepancy can be reconciled when considering the relative importance of DAG species differs by their subcellular location. Similar to the data of Coen et al [10] and Dube et al [35], we also did not find a significant relationship between insulin sensitivity and individual DAG species when DAG species from the whole cell were analysed. However, when only membrane species were examined, the relationship between di-C18:0 DAG and insulin sensitivity was revealed. Therefore our data agree with previous studies in this area, but, importantly, extend what is known, highlighting the importance of DAG based on location and species.
DAG is thought to decrease insulin sensitivity in skeletal muscle by promoting activity of conventional and novel PKC isoforms [11, 14]. Localisation of DAG species was important for PKC activation, as C16:1/C18:1 was positively related to PKCε activation in the membrane, with no relationship in the cytosol. The prevailing paradigm would contend that polyunsaturated fatty acid-containing DAG activate PKC [36, 37]; however, the literature contains reports suggesting that saturated DAGs are related to PKC activation as well [16, 38, 39]. Nevertheless, we found no significant relationships between polyunsaturated fatty acid containing-DAG and PKCθ and PKCε activation, but rather an effect of monounsaturated fatty acid containing-DAG in the membrane on PKCε. Divergence between membrane DAG molecular species related to insulin sensitivity and PKC activation may due to involvement of PKC isoforms PKCßII and PKCδ, which we did not measure [14], and/or implicate non-PKC-mediated mechanisms for the effect of DAG on insulin resistance in humans [40]. Alternatively, while there are data suggesting that PKC isoforms are involved in acute insulin resistance [14, 15, 41-43], other data suggest that they may not be related to chronic insulin resistance [44-46]. Our data can also be interpreted as placing less importance on DAG-induced PKC activation in chronic insulin resistance in humans. These data are also consistent with the interpretation that saturated DAG are only a marker of insulin resistance, and may reflect increased saturated lipid content of other species, such as ceramides [9, 10] and/or long-chain acyl-CoA [8], which may play a more direct role.
There are several limitations to this study. It is well known that dietary fat influences muscle lipid composition [47], and a recent study showed that a 1-week dietary intervention changed the composition of DAG in healthy lean men and women [28]. Therefore our 3-day dietary control, designed to ensure energy balance, may have minimised differences between groups. Although others have reported PKC activation using membrane/cytosol ratios [15, 21, 48], variability between participants and a small sample size in this study may have precluded finding relationships that exist between PKC isoforms and DAG species. We did not have a lean control group, which does not allow us to discern the influence of BMI from exercise training when comparing athletes with the other two groups. In addition, skeletal muscle was only separated into two fractions. Therefore alterations in DAG localised to endoplasmic reticulum from de novo synthesis cannot be delineated from plasma membrane DAG with our methods. Owing to the hydrophobic nature of DAG, the cytosolic fraction may also not truly represent ‘cytosolic’ distribution, but rather DAG localised to small membrane structures or in cytosolic lipid droplets pelleting during the first centrifugation step. In addition, we measured insulin sensitivity using an IVGTT, which does not isolate the influence of muscle on insulin sensitivity as well as a hyperinsulinaemic/euglycaemic clamp. Further, we are assuming that DAG promotes insulin resistance by binding to C1-containing domains of PKC [40]. However, other molecules with C1-containing domains may be stimulated by DAG, including chimaerins, protein kinase D, ras guanyl nucleotide-releasing proteins (RasGRPs), mammalian homologues of C. elegans Unc-13 (Munc13s) and DAG kinase γ [40].
In summary, these data are the first report of cellular localisation of molecular species of DAG in human skeletal muscle. Together, they challenge the existing paradigm that all DAG species negatively impact insulin action in skeletal muscle. Only membrane, not cytosolic, DAG were found to be related to insulin resistance in the present study. Further, this relationship was largely driven by the amount of di-C18:0 DAG. Therefore therapeutic strategies to alter DAG composition and/or decrease membrane DAG localisation may be a novel target to promote insulin sensitivity in humans.
Acknowledgments
Funding
This work was partially supported by the National Institutes of Health General Clinical Research Center (grant RR-00036) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants to L. Perreault [DK-064811] and B.C. Bergman [DK-059739 and R01DK089170]).
Abbreviations
- Ath
Endurance-trained athletes
- DAG
Diacylglycerol
- DEXA
Dual-energy x-ray absorptiometry
- DGAT1
Diacylglycerol acyltransferase 1
- DGKδ
Diacylglycerol kinase delta
- GCRC
General Clinical Research Center
- IMTG
Intramuscular triacylglycerol
- Ob
Obese controls
- PKC
Protein kinase C
- SCD1
Stearoyl-CoA desaturase 1
- T2D
Individuals with type 2 diabetes
Maximal oxygen consumption
Footnotes
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
BCB designed the study, performed participant testing, analysed data, and wrote the manuscript. DMH performed participant testing, analysed samples, interpreted data and edited the manuscript. AK performed participant testing, analysed samples, interpreted data and edited the manuscript. MCP performed participant testing, analysed samples, interpreted data, and edited the manuscript. LP helped design the study, provided medical oversight, performed all biopsies, and helped write the manuscript. All authors approved the final manuscript prior to publication.
References
- [1].DeFronzo RA, Ferrannini E, Hendler R, Felig P, Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983;32:35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- [2].Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679–1689. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. Journal of Clinical Investigation. 2007;117:1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. Intramuscular lipid metabolism in the insulin resistance of smoking. Diabetes. 2009;58:2220–2227. doi: 10.2337/db09-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. Increased intramuscular lipid synthesis and low saturation relate to insulin sensitivity in endurance-trained athletes. J Appl Physiol. 2010;108:1134–1141. doi: 10.1152/japplphysiol.00684.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bruce CR, Thrush AB, Mertz VA, et al. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab. 2006;291:E99–E107. doi: 10.1152/ajpendo.00587.2005. [DOI] [PubMed] [Google Scholar]
- [7].van Hees AM, Jans A, Hul GB, Roche HM, Saris WH, Blaak EE. Skeletal muscle fatty acid handling in insulin resistant men. Obesity. 2011;19:1350–1359. doi: 10.1038/oby.2011.10. [DOI] [PubMed] [Google Scholar]
- [8].Houmard JA, Tanner CJ, Yu C, et al. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty acyl-CoAs in morbidly obese subjects. Diabetes. 2002;51:2959–2963. doi: 10.2337/diabetes.51.10.2959. [DOI] [PubMed] [Google Scholar]
- [9].Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metabolism. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- [10].Coen PM, Dube JJ, Amati F, et al. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes. 2010;59:80–88. doi: 10.2337/db09-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schmitz-Peiffer C, Browne CL, Oakes ND, et al. Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes. 1997;46:169–178. doi: 10.2337/diab.46.2.169. [DOI] [PubMed] [Google Scholar]
- [12].Avignon A, Yamada K, Zhou X, et al. Chronic activation of protein kinase C in soleus muscles and other tissues of insulin-resistant type II diabetic Goto-Kakizaki (GK), obese/aged, and obese/Zucker rats. A mechanism for inhibiting glycogen synthesis. Diabetes. 1996;45:1396–1404. doi: 10.2337/diab.45.10.1396. [DOI] [PubMed] [Google Scholar]
- [13].Heydrick SJ, Ruderman NB, Kurowski TG, Adams HB, Chen KS. Enhanced stimulation of diacylglycerol and lipid synthesis by insulin in denervated muscle. Altered protein kinase C activity and possible link to insulin resistance. Diabetes. 1991;40:1707–1711. doi: 10.2337/diab.40.12.1707. [DOI] [PubMed] [Google Scholar]
- [14].Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- [15].Griffin ME, Marcucci MJ, Cline GW, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- [16].Montell E, Turini M, Marotta M, et al. DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am J Physiol Endocrinol Metab. 2001;280:E229–237. doi: 10.1152/ajpendo.2001.280.2.E229. [DOI] [PubMed] [Google Scholar]
- [17].Lee HY, Choi CS, Birkenfeld AL, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12:668–674. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- [19].Guo Z, Mishra P, Macura S. Sampling the intramyocellular triglycerides from skeletal muscle. J Lipid Res. 2001;42:1041–1048. [PubMed] [Google Scholar]
- [20].Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- [21].Schmitz-Peiffer C, Biden TJ. Protein kinase C function in muscle, liver, and beta-cells and its therapeutic implications for type 2 diabetes. Diabetes. 2008;57:1774–1783. doi: 10.2337/db07-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Moro C, Galgani JE, Luu L, et al. Influence of gender, obesity, and muscle lipase activity on intramyocellular lipids in sedentary individuals. J Clin Endocrinol Metab. 2009;94:3440–3447. doi: 10.1210/jc.2009-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kumashiro N, Erion DM, Zhang D, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yasunari K, Kohno M, Kano H, Yokokawa K, Horio T, Yoshikawa J. Possible involvement of phospholipase D and protein kinase C in vascular growth induced by elevated glucose concentration. Hypertension. 1996;28:159–168. doi: 10.1161/01.hyp.28.2.159. [DOI] [PubMed] [Google Scholar]
- [25].Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes. 1994;43:1122–1129. doi: 10.2337/diab.43.9.1122. [DOI] [PubMed] [Google Scholar]
- [26].Hodgkin MN, Pettitt TR, Martin A, Michell RH, Pemberton AJ, Wakelam MJ. Diacylglycerols and phosphatidates: Which molecular species are intracellular messengers? Trends Biochem Sci. 1998;23:200–204. doi: 10.1016/s0968-0004(98)01200-6. [DOI] [PubMed] [Google Scholar]
- [27].Timmers S, Schrauwen P, de Vogel J. Muscular diacylglycerol metabolism and insulin resistance. Physiol Behav. 2008;94:242–251. doi: 10.1016/j.physbeh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- [28].Kien CL, Everingham KI, R DS, Fukagawa NK, Muoio DM. Short-term effects of dietary fatty acids on muscle lipid composition and serum acylcarnitine profile in human subjects. Obesity. 2011;19:305–311. doi: 10.1038/oby.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Luo B, Regier DS, Prescott SM, Topham MK. Diacylglycerol kinases. Cell Signal. 2004;16:983–989. doi: 10.1016/j.cellsig.2004.03.016. [DOI] [PubMed] [Google Scholar]
- [30].Nagaya H, Wada I, Jia YJ, Kanoh H. Diacylglycerol kinase delta suppresses ER- to-Golgi traffic via its SAM and PH domains. Mol Biol Cell. 2002;13:302–316. doi: 10.1091/mbc.01-05-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Abramovici H, Hogan AB, Obagi C, Topham MK, Gee SH. Diacylglycerol kinase-zeta localization in skeletal muscle is regulated by phosphorylation and interaction with syntrophins. Mol Biol Cell. 2003;14:4499–4511. doi: 10.1091/mbc.E03-03-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tolias KF, Couvillon AD, Cantley LC, Carpenter CL. Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex. Mol Cell Biol. 1998;18:762–770. doi: 10.1128/mcb.18.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chibalin AV, Leng Y, Vieira E, et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 2008;132:375–386. doi: 10.1016/j.cell.2007.12.035. [DOI] [PubMed] [Google Scholar]
- [34].Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- [35].Dube JJ, Amati F, Toledo FG, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54:1147–1156. doi: 10.1007/s00125-011-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wakelam MJ. Diacylglycerol: when is it an intracellular messenger? Biochim Biophys Acta. 1998;1436:117–126. doi: 10.1016/s0005-2760(98)00123-4. [DOI] [PubMed] [Google Scholar]
- [37].Hinderliter AK, Dibble AR, Biltonen RL, Sando JJ. Activation of protein kinase C by coexisting diacylglycerol-enriched and diacylglycerol-poor lipid domains. Biochemistry. 1997;36:6141–6148. doi: 10.1021/bi962715d. [DOI] [PubMed] [Google Scholar]
- [38].Inoguchi T, Li P, Umeda F, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- [39].Yu HY, Inoguchi T, Kakimoto M, et al. Saturated non-esterified fatty acids stimulate de novo diacylglycerol synthesis and protein kinase c activity in cultured aortic smooth muscle cells. Diabetologia. 2001;44:614–620. doi: 10.1007/s001250051668. [DOI] [PubMed] [Google Scholar]
- [40].Brose N, Rosenmund C. Move over protein kinase C, you’ve got company: alternative cellular effectors of diacylglycerol and phorbol esters. J Cell Sci. 2002;115:4399–4411. doi: 10.1242/jcs.00122. [DOI] [PubMed] [Google Scholar]
- [41].Kim JK, Fillmore JJ, Sunshine MJ, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. Journal of Clinical Investigation. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Raddatz K, Turner N, Frangioudakis G, et al. Time-dependent effects of Prkce deletion on glucose homeostasis and hepatic lipid metabolism on dietary lipid oversupply in mice. Diabetologia. 2011;54:1447–1456. doi: 10.1007/s00125-011-2073-0. [DOI] [PubMed] [Google Scholar]
- [43].Samuel VT, Liu ZX, Wang A, et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. The Journal of clinical investigation. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Serra C, Federici M, Buongiorno A, et al. Transgenic mice with dominant negative PKC-theta in skeletal muscle: a new model of insulin resistance and obesity. J Cell Physiol. 2003;196:89–97. doi: 10.1002/jcp.10278. [DOI] [PubMed] [Google Scholar]
- [45].Gao Z, Wang Z, Zhang X, et al. Inactivation of PKCtheta leads to increased susceptibility to obesity and dietary insulin resistance in mice. American journal of physiology Endocrinology and metabolism. 2007;292:E84–91. doi: 10.1152/ajpendo.00178.2006. [DOI] [PubMed] [Google Scholar]
- [46].Schmitz-Peiffer C, Laybutt DR, Burchfield JG, et al. Inhibition of PKCepsilon improves glucose-stimulated insulin secretion and reduces insulin clearance. Cell metabolism. 2007;6:320–328. doi: 10.1016/j.cmet.2007.08.012. [DOI] [PubMed] [Google Scholar]
- [47].Andersson A, Nalsen C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am J Clin Nutr. 2002;76:1222–1229. doi: 10.1093/ajcn/76.6.1222. [DOI] [PubMed] [Google Scholar]
- [48].Itani SI, Zhou Q, Pories WJ, MacDonald KG, Dohm GL. Involvement of protein kinase C in human skeletal muscle insulin resistance and obesity. Diabetes. 2000;49:1353–1358. doi: 10.2337/diabetes.49.8.1353. [DOI] [PubMed] [Google Scholar]