Abstract
Lowering intraocular pressure (IOP) is currently the only strategy documented to slow the onset and progression of glaucomatous blindness. Ouabain, a cardiotonic glycoside inhibitor of Na+, K+-activated ATPase, was recently reported to enhance outflow facility in porcine anterior segments at concentrations as low as 30 nM for ≥ 4 hrs, suggesting a novel approach to lowering IOP. The underlying mechanism is unknown, but associated cytoskeletal changes were observed in porcine trabecular meshwork cells. We have previously found that changes in ATP release and subsequent ectoenzymatic conversion to adenosine may play a role in linking cytoskeletal remodeling with modulation of outflow resistance. We now tested whether altered ATP release might also be a mediator of ouabain’s effect on outflow facility. ATP release from transformed human TM5 and explant-derived human trabecular meshwork cells was measured by the luciferin-luciferase reaction. Matrix metalloproteinases (MMPs) were studied by zymography, cell Na+concentration by SBFI fluorometry, gene expression of ATP-release pathways by real-time PCR, cell volume by electronic cell sorting and cell viability by the LDH and MTT methods. Actin was examined by confocal microscopy of phalloidin-stained cells. Contrary to expectation, ouabain at concentrations ≥ 10 nM inhibited swelling-triggered ATP release from TM5 cells after ≥ 4 hrs of exposure. Inhibition was enhanced by increasing ouabain concentration and exposure time. Similar effects were produced by the reversible cardiac aglycone strophanthidin. Ouabain also inhibited swelling-activated ATP release from explant-derived native human TM cells. Ouabain (4 hrs, 30 nM and 100 nM) did not alter gene expression of the ATP-release pathways, and cell viability was unchanged by exposure to ouabain (30 nM to 1 μM). Preincubation with 30 nM ouabain for 4 hrs did not detectably change Na+ level, the regulatory volume decrease (RVD) or the actin cytoskeleton of TM5 cells, but did inhibit hypotonicity-elicited ATP release. Moreover, even when N-methyl-D-glucose replaced Na+in the extracellular fluid, ouabain still inhibited swelling-initiated ATP release at 100 nM. In the absence of ouabain, extracellular ATP stimulated MMP secretion, which was largely blocked by inhibiting conversion of ATP to adenosine, as expected. In contrast, ouabain reduced ATP release, but did not alter secretion of MMP-2 and MMP-9 from cells pretreated for ≤4 hrs. The results suggest that: (1) ouabain can trigger enhancement of outflow facility independent of its transport and actin-restructuring effects exerted at higher concentration and longer duration; (2) ouabain exerts parallel independent effects on ATP release and outflow facility; and (3) these effects likely reflect ouabain-induced changes in the scaffolding and/or signaling functions of Na+, K+-activated ATPase.
Keywords: ATP release; adenosine; matrix metalloproteinase; Na+, K+-activated ATPase; cytoskeleton
1. Introduction
Reduction of intraocular pressure (IOP) is currently the only intervention demonstrated to delay the onset and slow the progression of irreversible blindness associated with glaucoma, even in patients without elevated tension (Collaborative Normal-Tension Glaucoma Study Group, 1998a, b; Kass et al., 2002; Leske et al., 2003; The AGIS investigators, 2000). IOP can be reduced by lowering the rate of aqueous humor formation, increasing outflow facility through the pressure-sensitive trabecular outflow pathway, or diverting part of the trabecular outflow to exit the relatively pressure-insensitive uveoscleral pathway. Recently, the cardiotonic steroid ouabain, an inhibitor of Na+, K+-activated ATPase, was found to increase outflow facility in perfused porcine anterior segments, suggesting a novel approach for lowering IOP (Dismuke et al., 2009). Ouabain was effective at relatively low concentrations (≥30 nM) after perfusion for 4 hrs or more. The basis for the phenomenon is unknown, but parallel changes in the actin cytoskeleton were noted in ouabain-treated porcine trabecular meshwork (TM) cells.
Remodeling of the actin cytoskeleton is known to alter outflow facility, and cytoskeletal-disrupting drugs have been found to lower IOP in humans (Tanihara et al., 2008) and non-human primates (Tian et al., 2000). We have recently observed that changes in ATP release and subsequent ectoenzymatic conversion to adenosine may play a role in linking actin remodeling with modulation of outflow facility. In particular, cytoskeletal changes leading to increased ATP release might enhance ectoenzyme-mediated delivery of adenosine to A1adenosine receptors, thereby stimulating secretion of matrix metalloproteinases MMP-2 and MMP-9 by TM cells (Li et al., 2011). Enhanced MMP activity is known to increase outflow facility of human (Bradley et al., 2001) and bovine anterior segments (Crosson et al., 2005), and is thought to reflect changes in MMP secretion by TM cells.
In the present study, we have tested whether ouabain might alter outflow facility by modulating ATP release-initiated MMP secretion by trabecular meshwork cells. We have also examined whether ouabain’s effects on TM cells are mediated solely by inhibiting the ion-exchange activity of the Na+,K+-activated ATPase or possibly by the scaffolding and signaling functions of the enzyme (Schoner and Scheiner-Bobis, 2007; Xie and Askari, 2002).
2. Materials and Methods
2.1. Cellular Model
Transformed normal human trabecular meshwork cell line TM5 (Alcon Research Inc., Fort Worth, TX) was maintained in DMEM high-glucose media supplemented with 10% fetal bovine serum, 2 mM L-glutamine and 50 μg/mL of gentamicin at 37°C in a humidified atmosphere of 5% CO2and 95% air, as previously described (Li et al., 2010; Li et al., 2011; Li et al., 2012; Pang et al., 1994). Cells from passages 25 to 40 were used in the experiments. Primary human trabecular meshwork cells (HTM; Stamer et al., 1995) were maintained in DMEM low-glucose media containing the same supplements; cells from passages 3-5 were used in the experiments.
2.2. Solutions and Pharmacological Reagents
The isotonic solution (295-305 mosmol/kg) with 0.1 mM extracellular free Ca2+ contained (in mM): 110 NaCl, 4.7 KCl, 5.1 CaCl2, 1.2 MgCl2, 30 NaHCO3, 1.2 KH2PO4, 15 HEPES, 5 EGTA and 10 glucose, as previously described (Li et al., 2010; Li et al., 2011; Li et al., 2012). Selectively omitting NaCl decreased osmolality to ~100 mosmol/kg (67% hypotonicity). Intermediate osmolalities were generated by appropriate mixing of the iso- and hypotonic solutions. In some experiments, sodium and chloride were replaced with equimolar N-methyl-D-glucamine (NMDG) and gluconate, respectively, to maintain the desired ion strength and osmolality. The final osmolalities were verified and pH values adjusted to 7.4 before each experiment. Biochemical reagents were purchased from Sigma-Aldrich (St Louis, MO). Chemicals and media for cell culture were obtained from GIBCO (Invitrogen, Carlsbad, CA). DMSO (<0.5%) was used to solubilize hydrophobic drugs, exposing controls to the same concentration of vehicle. Unless otherwise stated, all experiments were performed at room temperature.
2.3. ATP Measurement
ATP release was determined by the bioluminescent luciferin-luciferase reaction with light emission recorded using the Synergy 2 microplate luminometer (BioTEK, Winooski, VT), as previously reported (Li et al., 2010; Li et al., 2011; Li et al., 2012). In brief, TM cells were seeded onto 96-well microplates (Corning Costar, Corning, NY) at 0.1 million per well, permitting confluence within 1-2 days. Drugs were added to the culture media at the final concentrations and for the periods specified. To minimize ATP release resulting from changing solutions, culture media were removed and replaced with 100 μL isotonic solutions with/without drugs 1 hr before experiment. Thereafter, 75 μL of isotonic solutions were replaced by an equal volume of test solution to establish the final drug concentrations and osmolalities. Measurements were begun instantaneously after dispensing 10 μL of the ATP assay solution into each well through the internal dispenser system, and recorded for 2 hrs at 2-min intervals, with an integration time of 0.2 sec/measurement. ATP levels were calculated at each time point from a standard curve converting arbitrary light units into ATP concentrations. Separate standard curves were utilized in experiments involving changes in ionic strength. No test substance interfered with the ATP assay at the specified concentration used in this study. Inhibition of the hypotonicity-triggered enhancement of ATP release was calculated from Eq. 1, as previously described (Li et al., 2010; Li et al., 2011; Li et al., 2012). Inhibition
| (Eq. 1) |
was the maximal ATP concentration after hypotonic treatment without inhibitor, Cconwas the control ATP concentration in the isotonic bath at the same time point, and Cexpwas the maximal ATP concentration after hypotonic treatment in the presence of inhibitor.
2.4. Gelatin Zymography for Matrix Metalloproteinases (MMPs)
Using previously reported methods (Hawkes et al., 2010; Li et al., 2011; Sanka et al., 2007), the secretion of MMP-2 and MMP-9 into the external media was measured by gelatin zymography. Briefly, TM5 cells were plated onto 48-well plates at a density of 0.2 million per well, and allowed to grow to confluence, followed by serum starvation for 24 hrs. Thereafter, 140 L of fresh DMEM media with or without drugs were added to each well to condition cells at 37°C for the period s specified. The conditioned media were completely collected and cleared by centrifugation (10,000 g) for 20 min. The supernatants were then mixed with the Zymogram Sample Buffer (Bio-Rad, Hercules, CA), and 30 L per sample were loaded onto each lane of the 10% Precast Zymogram Gels (Bio-Rad) for SDS-PAGE separation. After electrophoresis, gels were washed sequentially with the Zymogram Renaturing Buffer for 3 hrs, Zymography Developing Buffer for 24 hrs (at 37 °C), and Coomassie Brillia nt Blue R-250 Staining Solution for 8 hrs (all from Bio-Rad). Gels were destained in the Coomassie Brilliant Blue R-250 Destaining Solution (Bio-Rad) until clear bands were visible against the blue background. The gels were subsequently scanned (Scanjet 3570c, Hewlett-Packard, Palo Alto, CA), and band density was analyzed by Image J Software (Ver. 1.45, National Institutes of Health, Bethesda, MD).
2.5. Real-time Quantitative PCR
TM5 cells were treated with drugs under specified conditions before RNA extraction. Total RNA extraction, reverse transcription, and Taqman gene expression assays were performed as previously reported (Li et al., 2010; Li et al., 2011; Li et al., 2012). FAM-labeled MGB Taqman probes utilized in the assays are listed in Appendix Table A1. The expression levels of indicated genes were all normalized to that of PX1 in non-treated control cells after 2−ΔΔCtcalculation, with human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the endogenous control.
2.6. Cell Volume Measurement
Cell volume was measured by electronic cell sorting, using the ZBI-Channelyzer II Coulter Counter (Beckman Coulter Inc., Brea, CA) with a 100 m aperture as previously described (Li et al., 2011; Yantorno et al., 1989). TM5 cells were trypsinized and resuspended in isotonic solutions with or without drugs for the periods specified. Thereafter, 50% hypotonicity was applied, and cell volume changes were recorded at indicated time points. The obtained data were normalized to the average size of the same cell population bathed in isotonic solutions at the zero time point.
2.7. Cell Viability Assays
Cell viability after treatment was estimated by the lactose dehydrogenase (LDH) and the thiazolyl blue tetrazolium bromide (MTT) assays. As previously described (Li et al., 2010; Li et al., 2011; Li et al., 2012), activity of released LDH was measured with the Cytotoxicity Detection Kit (Roche Diagnostic, Indianapolis, IN) following the manufacturer’s instruction, and the cell metabolic states were determined by the MTT assay.
2.8. Intracellular Sodium Measurement
Cytosolic free Na+levels were determined following the protocol of Harootunian et al. (Harootunian et al., 1989). In brief, coverslips with confluent adherent TM5 cells were mounted in a perfusion chamber on the stage of an inverted fluorescence microscope (Nikon Eclipse Ti, Melville, NY). Background fluorescence was initially measured for 5 min using a photomultiplier (D-104, Photon Technology International, Birmingham, NJ). Thereafter, cells were loaded with the sodium ion indicator SBFI-AM (10 μM) and 0.05 % Pluronic F-127 (Molecular Probes, Invitrogen) for 1 hr, followed by washing away the dye with isotonic solutions for about 30 min. Ouabain was added to the media at appropriate time points for the desired incubation periods. After the experiments were started, the emission fluorescence intensity at 510 nm was acquired every 5 sec with the dye excited at the dual wavelengths (340 nm/380 nm). The concentration of intracellular Na+was converted from the fluorescence ratio according to the equation provided by the Felix 32 Software (Photo Technology International).
2.9. Confocal Microscopy of F-actins by Phalloidin Staining
Actin cytoskeleton was visualized by phalloidin staining as described before (Li et al., 2011). In brief, TM5 cells were trypsinized and plated on non-coated coverslips for 12 hrs to allow firm attachment to the underlying surface. Ouabain (30 nM and 100 nM) was added to the culture media for 4 hrs before fixation. The cells were then fixed with 4% paraformaldehyde for 10 min and permeabilized with 100 μM digitonin for 10 min, followed by blocking with 1% bovine serum albumin-PBS for 1 hr. Samples were probed with Alexa Fluor-488 Phalloidin (1/2,000, Invitrogen) for 2 hrs, and rinsed with PBS three times. DAPI (0.15 μg/mL) was added to counterstain the nuclei fluorescently. Coverslips mounted with Prolong Gold Antifade Reagent (Invitrogen) were observed by confocal laser scanning microscopy (Olympus FluoView 1000, Olympus America, Center Valley, PA). Single layers of 0.5-μm thickness were photographed.
2.10. Statistics
Student’s t-test, paired or unpaired as appropriate, was used to compare two sets of data, and one- or two-way ANOVA was applied in comparing three or more sets of data. Statistical analyses were performed with SigmaStat (Aspire Software International, Ashburn, VA). Unless otherwise stated, the results are presented as means ± SE, with n and N indicating the number of wells studied and the number of independent experiments performed, respectively. A probability (P) less than 0.05 was considered statistically significant.
3. Results
3.1. Effects of cardiotonic steroids on swelling-activated ATP release
Consistent with our prior reports (Li et al., 2011; Li et al., 2012), the ATP concentration was 13.1 ± 0.3 nM (n=752 wells) in the control isotonic sodium-containing solutions (ISCS) after incubation of human TM5 cells. This low baseline level was stable during 2 hrs of measurement, and not altered by preincubation with 1 μM ouabain (OUA) for 4 hrs (n=26, P=0.799 vs. ISCS Control). Swelling cells with 50% hypotonic sodium-containing solutions (HSCS) triggered ATP release, raising bath concentration by 4.4-fold to 70.4 ± 1.4 nM (n=913, P<0.001 vs. ISCS Control).
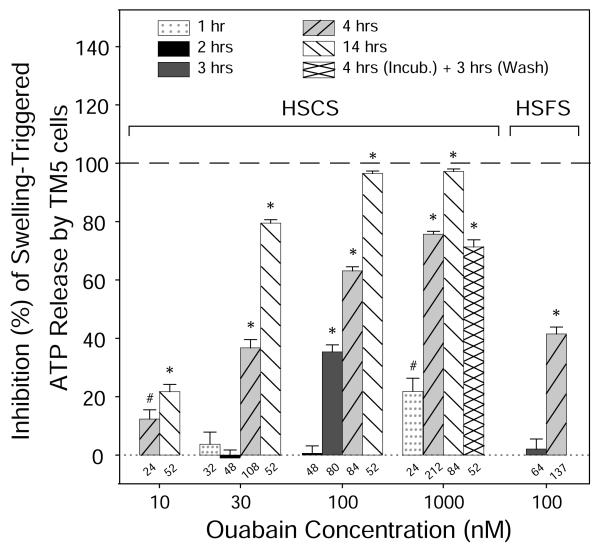
Swelling-induced ATP release was inhibited by the cardiotonic steroid OUA in a time-dependent, as well as concentration-dependent manner. As shown in Fig. 1, OUA at 30 nM blocked the release by −1% ± 3% (n=48, P>0.7 vs. HSCS), 37% ± 3% (n=108, P<0.001), and 79% ± 1% (n=52, P<0.001), after pretreatment for 2, 4 and 14 hrs, respectively. The inhibitory effects of OUA at other specified concentrations were similarly enhanced by lengthening the preincubation periods. For instance, OUA at 100 nM reduced the release by 1% ± 3% (n=48, P>0.8), 35% ± 2% (n=80, P<0.001), and 63% ± 1% (n=84, P<0.001), after 2, 3, and 4 hrs, respectively. For a given pretreatment duration, a higher concentration of OUA usually inhibited ATP release to a greater extent. Full inhibition was observed at ouabain concentrations ≥ 100 nM for 14 hrs (Fig. 1). Preincubation with 1 μM OUA for 1 hr reduced ATP release by 22% ± 5% (n=24, P<0.01 vs. HSCS), the identical inhibition produced by 10 nM OUA over 14 hrs. Thus, the same inhibition could be obtained by pretreatment with OUA for either a sufficiently long exposure, or at a sufficiently high concentration, or by an appropriate combination of the two parameters.
Fig. 1. Effects of ouabain on swelling-triggered ATP release from TM5 cells.
TM5 cells were treated with ouabain (OUA) in isotonic solution for specified periods, followed by exposure to 50% hypotonic solutions with (HSCS) or without Na+ (HSFS). OUA inhibited swelling-activated ATP release in a concentration-dependent manner, and a longer incubation generally resulted in a greater inhibition. Inhibition was irreversible since washout of the 1 μM ouabain over 3 hrs [“4 hrs (Incub.) + 3 hrs (Wash)”] did not alter the effect of 1 μM ouabain alone. Removing external Na+ reduced, but did not abolish, the effect of OUA. Numbers of wells analyzed are entered along the X-axis. In the absence of ouabain, the concentrations of ATP in isotonic sodium-containing solution (ISCS) and hypotonic HSCS were 13.1 ± 0.3 (n = 752) and 70.4 ± 1.4 (n = 913) nM, respectively. In isotonic and hypotonic sodium-free solutions, the control ATP concentrations were 15.4 ± 1.3 (n = 40) and 74.4 ± 2.7 (n = 305) nM, respectively. #P<0.01 and *P<0.001 vs. Hypotonic controls.
Ouabain’s inhibition of hypotonically-stimulated ATP release proved irreversible (Fig. 1). Reversibility was tested by washing TM5 cells with fresh ISCS for 3 hrs after preincubation TM5 cells with 1 μM OUA for 4 hrs [indicated as “4 hrs (Incub.) + 3 hrs (Wash)”, Fig. 1]. After washout, swelling-activated ATP release was still 71% ± 3% (n=24), not significantly different from that without washout (76% ± 1%, n=212, P>0.05). Because OUA has been proven cytotoxic when used at high concentrations, and/or for longer periods, the observed reduction in ATP release might potentially have reflected cell damage. We addressed this question in two ways, by applying the cardiotonic aglycone strophanthidin (STR) known to be more reversible than OUA in other cell preparations and by assaying cell viability after ouabain exposure.
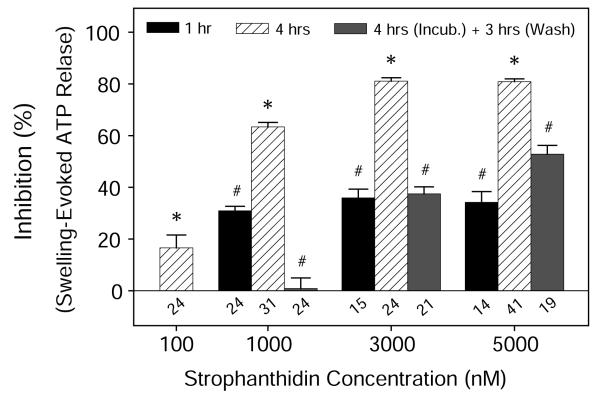
As expected, STR also decreased swelling-evoked ATP release, albeit with a lower potency (Fig. 2). However, unlike OUA, 3 hrs of washing substantially reduced inhibition, from 63% ± 2% (1 μM, n=31), 81% ± 1% (3 μM, n=24), and 80% ± 1% (5 μM, n=41), to 1% ± 4% (n=24), 37% ± 3% (n=21), and 53% ± 3% (n=19), respectively. In addition, there was no indication from the LDH assay that OUA decreased cell viability, or from the MTT assay that it altered the metabolic state at the specified concentration (Fig. 3). Collectively, the results gave no indication of compromised cell viability after treating cells with cardiotonic steroids under these experimental conditions.
Fig. 2. Reversible inhibition of hypotonicity-elicited ATP release by strophanthidin.
Like ouabain, strophanthidin (STR) inhibited hypotonically-evoked ATP release in a concentration- and time-dependent manner. However, the inhibitory effects were reversed by washout for 3 hr. The absolute control concentrations of ATP in isotonic and hypotonic extracellular sodium-containing fluid were 13.1 ± 0.3 (n = 752) and 70.4 ± 1.4 (n = 913) nM, respectively. Numbers above the abscissa are the numbers of wells studied. *P<0.001 vs. Hypotonic controls, and #P<0.05 vs. 4-hr STR Group at the same concentration without washing.
Fig. 3. Effects of osmolality and ouabain on viability of TM5 cells.
Viability was assayed by measuring lactose dehydrogenase release (LDH) or formazan formation (MTT). Triton X-100 reduced viability to 0, while neither hypotonicity [Hypo (50%)] nor ouabain [OUA (1 μM, 4 hrs)] affected cell viability. Numbers along the abscissa are numbers of wells tested. Data were normalized to the viability of the isotonic control (Iso). *P<0.001 vs. Iso Group by one-way ANOVA.
Insofar as transformed cell lines may behave differently from cells in primary culture, we tested whether ouabain also inhibited swelling-activated ATP release from explant-derived, non-transformed human TM (HTM) cells. The ATP concentrations in the ouabain-free isotonic and hypotonic bath solutions were 8.8 ± 0.7 nM (n=68) and 35.8 ± 1.9 nM (n=76), respectively. Ouabain inhibited swelling-elicited ATP release from the HTM cells, as well. The close quantitative similarity of the results is illustrated by Fig. 4, in which the data obtained with HTM cells are juxtaposed with TM5-cell results reproduced from Fig. 1. As with the TM5 cells, inhibition of ATP release from HTM cells was directly dependent on ouabain concentration and duration of preincubation.
Fig. 4. Comparison of ouabain’s effects on swelling-triggered ATP release from HTM and TM5 cells.
Similar to its effect on the TM5 cell line, ouabain inhibited swelling-activated ATP release by explant-derived HTM cells. In the absence of ouabain, the concentrations of ATP in isotonic and hypotonic HSCS were 8.8 ± 0.7 (n = 68) and 35.8 ± 1.9 (n = 76) nM, respectively. The data entered for TM5 cells in this figure have been replicated from Fig. 1 to facilitate comparison with the results obtained with the TM cells. *P<0.001 vs. Hypotonic controls.
3.2. Mechanism underlying ouabain-mediated inhibition to ATP release
The major ATP-releasing pathways in TM5 cells following hypotonic challenge have been recently identified (Li et al., 2011; Li et al., 2012), including the ubiquitously-expressed pannexin-1 (PX1) hemichannels, the longer-recognized connexin (Cx) hemichannels, and the purinergic P2X7ionotropic ATP receptors (P2RX7). Concurrent blocking of these three conduits nearly abolishes the release (Li et al., 2011; Li et al., 2012). OUA has been established as a potent blocker highly specific to Na+, K+-ATPases. Interestingly, it also largely inhibited swelling-initiated ATP release without damaging cells in this study. We accordingly investigated the possible underlying mechanism.
Attempts to dissect out the potential role of OUA in regulating the aforementioned conduits pharmacologically proved unsuccessful for two reasons: (1) the high potency and efficacy of OUA overshadowed the inhibitory effects of other drugs, and (2) ouabain’s inhibition was strongly dependent on the duration of preincubation. Nevertheless, treating TM5 cells with OUA (30 nM and 100 nM) for 4 hrs did not change the mRNA expression of PX1, Cxs, or P2RX7(Fig. 5), indicating that the cardiotonic steroid did not affect transcription of these ATP-release pathways.
Fig. 5. Gene expression profiles in TM5 cells after ouabain treatment.
TM5 cells with ouabain (OUA, 30 nM and 100 nM, 4 hrs) exposure displayed similar expression levels of PX1, Cx43, P2RX7, A1 adenosine receptors (ADOR1), and β-actin (ACTB) genes, compared with non-treated (NT) cells (P>0.05 by one-way ANOVA). Data obtained from 5 independent experiments were normalized to PX1 gene of NT Group, using GAPDH as loading control.
Remodeling the actin cytoskeleton modulates hypotonicity-elicited ATP release from TM5 cells (Li et al., 2011). We therefore examined whether OUA at sub-micromolar levels could also trigger such changes, using high-resolution single-layer confocal microscopy. As illustrated in Fig. 6, phalloidin-stained F-actins of similar quantity and distribution were observed in non-treated and OUA-treated (30 nM and 100 nM for 4 hrs) cells. Thus, the effect of sub-micromolar OUA on reducing ATP release was not mediated directly via cytoskeletal restructuring.
Fig. 6. Unchanged actin cytoskeleton after brief exposure to 30 nM and 100 nM ouabain.
TM5 cells were treated with ouabain (30 nM and 100 nM) for 4 hrs. F-actin filaments were visualized by phalloidin staining (green), and nuclei counterstained with DAPI (blue). Compared with non-treated controls (Figs. 6A and 6D), no significant cytoskeletal change was observed in cells treated with either 30 nM (Figs. 6B and 6E) or 100 nM ouabain (Figs. 6C and 6F) for 4 hrs. Scale bars: 20 μm.
Although Na+,K+-activated ATPase sustains the ionic composition of mammalian cells, the enzyme also exerts scaffolding and signaling actions (Schoner and Scheiner-Bobis, 2007; Xie and Askari, 2002). We next explored whether ouabain-elicited changes in ionic composition were necessary in order to produce the observed inhibitions in ATP release after 4 hrs of OUA preincubation. Three strategies were followed: (1) directly measuring intracellular Na+, (2) conducting experiments in the absence of external Na+, and (3) assaying changes in ionic composition indirectly by measuring the regulatory volume decrease (RVD).
The first strategy was to measure intracellular Na+concentration by SBFI fluorescence. Figure 7 presents fluorescence traces from representative experiments under non-treated conditions (NT, Fig. 7A) and following 4 hrs of preincubation with either 30 nM (Fig. 7B) or 100 nM (Fig. 7C) of ouabain. The non-treated cells displayed an initial Na+concentration of some 20 mM, which was stable during 30 min of perfusion with control ouabain-free isotonic solution (Fig. 7A). Subsequent perfusion with 1 μM ouabain for 30 min produced a slow rise in fluorescence. Thereafter, calibration solutions were applied, containing Na+concentrations of 0, 10, 20, 30, 50 and 100 mM. As expected, the intracellular Na+concentration was substantially elevated by preincubation with 100 nM ouabain (Fig. 7C). In contrast, perfusion with 30 nM OUA for 4 hrs did not alter the Na+concentration (Fig. 7B). The mean values ± SE for the non-treated cells and following 30 nM and 100 nM ouabain pretreatment were 22 ± 3 mM (N=4), 19 ± 3 mM (N=4) and 104 ± 1 mM (N=4), respectively. Preincubation with 100 nM ouabain significantly increased the mean intracellular Na+concentration (P<0.05), but pretreatment with 30 nM ouabain did not (one-way ANOVA of log-transformed data). Thus, inhibition of swelling-activated ATP release produced by 4-hr exposure to 30 nM ouabain was not mediated by a change in intracellular Na+.
Fig. 7. Intracellular Na+ concentration increased by 100 nM, but not by 30 nM, ouabain pretreatment for 4 hrs.
Intracellular Na+ levels were monitored by SBFI fluorometry. TM5 cells had been pretreated with ouabain (OUA) at indicated concentrations applied for 4 hrs. As indicated in the boxes at the top of each panel, the cells were perfused sequentially by isotonic solution with/without ouabain, followed by isotonic solution containing 1 M ouabain, and finally by calibration solutions containing six different increasingly-large Na+ concentrations. In the absence of pretreatment with ouabain (NT, Fig. 7A), the initial Na+ concentration was approximately 20 mM, and stable for 30 min. Addition of 1 M ouabain triggered a slow rise in Na+ concentration over the next 30 min. Perfusion with calibration solution containing no Na+ then reduced the fluorescence signal. Subsequent perfusions with increasing Na+ concentrations progressively increased the fluorescence trace. Compared with the non-treated control (NT, Fig. 7A), preincubation with 100 nM OUA increased the initial Na+ concentration measured at t=0 (Fig. 7C), whereas prior exposure to 30 nM ouabain did not (Fig. 7B). The similar responses to 1 μM OUA in all three groups documented that the cells were alive and responsive to a higher concentration of OUA.
A second strategy was to measure the effect of OUA on ATP release in isotonic sodium-free solutions, thereby precluding exchange of intracellular K+for external Na+. Bathing the cells in isotonic sodium-free solution (ISFS) did not alter the isotonic baseline level of ATP release observed in sodium-containing solution (ISCS) (n=40, P>0.05 vs. ISCS). Four-hour preincubation with 30 nM (n=24) or 100 nM (n=32) ouabain (OUA) in ISFS did not significantly shift the baseline (P>0.05 vs. ISCS by one-way ANOVA), either. Nevertheless, swelling-activated ATP release in 50% hypotonic sodium-free solutions (HSFS) was still inhibited by preincubation with 100 nM OUA for 4 hrs. The mean inhibition was substantial, 41% ± 2% (n=137, P<0.001 vs. HSFS Control), albeit lower than that observed under sodium-containing conditions (Fig. 1).
A third strategy was to assess indirectly whether exposure to 30 nM ouabain for 4 hrs significantly altered the TM-cell ionic composition. The regulatory volume decrease (RVD) of explant-derived human TM cells is strongly dependent on K+and Cl− release (Mitchell et al., 2002). Figure 8 presents the time course of cell volumes following application of 50% hypotonicity. The control and two experimental groups treated with 30 nM and 100 nM OUA for 4 hrs swelled rapidly, and reached the same peak at the same time point (N=5 individual experiments, P>0.05 vs. HSCS Control). Thereafter, all three groups displayed a regulatory volume decrease (RVD) characterized by spontaneous and progressive cell shrinkage towards the initial baseline volume, even in the continued presence of hypotonicity. As expected, preincubation with 100 nM OUA for 4 hrs strongly slowed the RVD (P<0.001 vs. HSCS Control by two-way ANOVA). However, exposure to 30 nM OUA over the same period did not affect the RVD (P>0.05 vs. HSCS Control by two-way ANOVA). OUA treatments also did not change the baseline cell volume within the same period (N=4, P>0.4). The measurements of RVD complement the direct measurements of Na+ concentration, indicating that 30 nM ouabain did not significantly alter the ion composition after 4 hrs of exposure.
Fig. 8. Effects of ouabain on the regulatory volume decrease of TM5 cells after hypotonic exposure.
TM5 Cells were pretreated with ouabain (OUA, 30 nM and 100 nM) for 4 hrs before exposure to hypotonicity (50%). The trajectories are 3-parameter exponential fits from 4 min to 30 min. Compared with the non-treated control (NT), the regulatory volume decrease (RVD) was slower and incomplete after 100 nM OUA (P<0.05), but not significantly altered after 30 nM OUA (P>0.05, two-way ANOVA). Data for each group are means ± SE obtained from 5 independent experiments.
3.3 Test of ouabain’s effects on MMP secretion
We have previously documented that multiple ectoenzymes are expressed in the plasma membrane of TM5 cells (Li et al., 2011), and activating A1 adenosine receptors (AR) triggers the release of matrix metalloproteinases (MMPs) (Shearer and Crosson, 2002; Li et al., 2011), potentially clearing the outflow pathway and reducing resistance. In the current study, exogenous ATP (1 mM) stimulated the secretion of both MMP-9 and MMP-2 to 1.8 ± 0.1 fold (N= 21, P<0.001 vs. Non-treated Group) and 1.5 ± 0.1 fold (N=21, P<0.001), respectively, in comparison to non-treated control (NC) (Fig. 9A). APCP (0.2 mM), a blocker of CD73 that converts AMP to adenosine, had little effect on the baseline level (N=14, P>0.05 vs. NC). However, compared with NC, it reduced the ATP-stimulated secretion down to 1.3 ± 0.1 fold (N= 21, P<0.05 vs. ATP Group) and 1.0 ± 0.1 fold (N= 21, P<0.05) for MMP-9 and MMP-2, respectively. These data provided the first evidence that ATP released into the extracellular milieu could be physiologically converted into adenosine by the ectoenzymes in vivo, followed by activating A1 ARs and releasing MMPs from TM5 cells.
Fig. 9. MMP-2 and MMP-9 secretion from TM5 cells modulated by purinergic signaling (A) but not by brief ouabain challenge (B).
(9A) Incubating TM5 cells with ATP (1 mM, 20 hrs) significantly increased the secretion of both MMP-2 and MMP-9. Such stimulation was blocked by concurrent application of a blocker of CD73 (APCP, 0.2 mM), indicating that conversion of ATP into adenosine is a critical step in mediating ATP-triggered enhancement of MMP secretion. Numbers of independent experiments are indicated along the abscissa. *P<0.001 vs. non-treated controls (NT), and #P<0.05 vs. ATP Groups by one-way ANOVA. (9B) Four-hour treatments with ouabain at either 30 nM or 100 nM did not alter the relative secretory rates of MMP-2 and MMP-9 from TM5 cells (P>0.05 vs. NT by one-way ANOVA).
Our results showed that ouabain reduces swelling-activated ATP release (Fig. 1). We wondered whether ouabain might stimulate secretion of MMPs in an ATP-independent manner, thereby accounting for ouabain’s enhancement of outflow facility (Dismuke et al., 2009). However, ouabain at 30 nM and 100 nM did not influence MMP-2 or MMP-9 secretion within 4 hrs (Fig. 9B). Additionally, OUA neither altered activity of the MMPs after their secretion into the external environment (data not shown), nor interfered with the expression of A1 ARs (ADOR1, Fig. 5).
4. Discussion
Ouabain has been observed to increase the outflow facility of the porcine anterior segment. This observation by Dismuke et al. (Dismuke et al., 2009) is of great interest, both pharmacologically and physiologically. Pharmacologically, the observation suggests the novel possibility of lowering IOP with cardiotonic steroids. The observation also raises the possibility that ouabain or other circulating endogenous cardiotonic steroids (Schoner and Scheiner-Bobis, 2007) might physiologically modulate outflow facility, particularly since the blood-aqueous barrier is incomplete (Bert et al., 2006).
4.1. Potential mediating role of ATP release and MMP secretion in enhancing outflow facility
The mechanism of ouabain’s action on outflow is unknown, but cytoskeletal changes were noted in the porcine TM cells (Dismuke et al., 2009). Cytoskeletal remodeling has been associated with alterations in secretion and activity of MMPs (Sanka et al., 2007). Increases in MMP secretion and activity are documented to raise outflow facility (Bradley et al., 2001; Crosson et al., 2005). One pathway linking actin depolymerization and MMP secretion appears to be enhancement of ATP release sequentially stimulating ectoenzymatic adenosine formation, activating A1 ARs, and thereby increasing MMP secretion (Li et al., 2011). This view is further supported by the current observation that extracellular ATP stimulates MMP-2 and MMP-9 secretion by TM5 cells, and that this stimulation depends on ectoenzymatic formation of adenosine by ecto-5′-nucleotidase (CD73).
In the present study, we addressed two questions. First, might the same sequence of events posited to link pharmacologically-induced cytoskeletal changes in TM cells and outflow facility play a role in mediating ouabain’s actions? Second, are ouabain’s effects on the TM cells and outflow facility necessarily produced by inhibiting the ion-transport functions of the target, Na+,K+-activated ATPase?
In addressing the first question, we found that ouabain decreases swelling-elicited ATP release, contrary to expectation. This reduction in ATP release is opposite to the effect of cytochalasin D which increases outflow facility, and is similar to the effect of dexamethasone which likely reduces outflow facility (Li et al., 2011). Furthermore, in contrast to the cytoskeletal-remodeling agents, ouabain had little effect on MMP-2 or MMP-9 secretion. Thus, the events triggered by ouabain do not conform to the signaling steps induced by actin depolymerization of TM cells.
The physiologic implications of this central observation are potentially limited by two caveats. First, the experiments have been conducted with isolated cells. We have previously found that the regulatory volume responses, and possibly other responses, of other cells were highly region-dependent within the intact rabbit ciliary epithelium (McLaughlin et al., 2007). Furthermore, the great bulk of the current work was performed with the transformed TM5 cell line (Pang et al., 1994), which has been enormously useful to investigators, but whose responses to ouabain may differ from those of native cells. Because of this possibility, we verified that ouabain inhibits swelling-activated ATP release similarly in explant-derived HTM and transformed TM5 cells.
The second potential caveat is that we have taken swelling-activated ATP release to provide an index of ATP release from TM cells in vivo. In life, TM cells are expected to be stretched and displaced by IOP oscillations of 2.4 ± 0.7 mmHg arising from mechanical events of the cardiac cycle in normal human subjects (Evans et al., 2002). We have previously reported that stretch and cell swelling of human TM cells elicit ATP release through the same pathways (Li et al., 2011). Thus, the observation that ouabain reduces swelling-activated ATP release by TM cells in vitro appears relevant to the in vivo condition.
4.2. Possible alternative mechanisms underlying ouabain’s enhancement of outflow facility
The actin cytoskeleton has been recognized as a modulator of plasma-membrane ion-channel activity for at least two decades (Cantiello et al., 1991), and is also known to regulate trafficking of Na+,K+-activated ATPase (Namekata et al., 2008). Nonetheless, 4-hr exposure to ouabain at concentrations of 30 and 100 nM produced no discernible change in the actin cytoskeleton of human TM cells (Fig. 6), while yet inhibiting swelling-activated ATP release (Fig. 1). In contrast, cytoskeletal changes were observed in porcine TM cells following exposure to 30 nM ouabain (Dismuke et al., 2009). The basis for the different results is unclear, but may reflect species differences.
Ouabain might have reduced gene expression of the major ATP-releasing pathways, which comprise PX1, connexins and P2RX7 in TM cells (Li et al., 2010; Li et al., 2011; Li et al., 2012). However, 4 hrs of exposure to 30 or 100 nM ouabain did not affect expression of PX1, Cx43 or P2RX7 (Fig. 5), as assessed by real-time PCR.
Cell damage was noted in TM cells from porcine anterior segments at high ouabain concentration (300 M) (Dismuke et al., 2009). Nevertheless, even at lower ouabain concentrations, a correlation was noted between the time course of changes in outflow facility and in changes of cell shape and actin stress fibers. In the current work, 1 M ouabain did not alter viability or mitochondrial function of TM5 cells evaluated by LDH release and the MTT assay, respectively (Fig. 3).
4.3. Role of ionic redistribution in ouabain-triggered enhancement of outflow facility
Na+/K+ exchange activity of Na+,K+-activated ATPase is fundamentally required for maintaining the ionic composition of mammalian cells (Tosteson and Hoffman, 1960; Leaf, 1956; Wilson, 1954). Notwithstanding, in addition to its ion-transfering activity, Na+,K+-activated ATPase subserves scaffolding and signaling functions (Schoner and Scheiner-Bobis, 2007; Xie and Askari, 2002). We wondered whether the increase in cell Na+ and fall in cell K+ that can result from ouabain treatment were an indispensible mediating step in blocking ATP release by TM5 cells observed in the present study, and in enhancing outflow facility observed by Dismuke et al. (Dismuke et al., 2009). To address this question, we both measured intracellular Na+ concentration and conducted experiments in the absence of external Na+. The latter strategy was guided by the observation that the rate of cell uptake of external Na+ entry can limit the rate of ouabain-triggered changes in the ionic composition (Macknight et al., 1980). Blocking Na+/K+ exchange activity of intact human trabecular outflow tissue with 100 μM ouabain for 30 min initially increases Na+ and reduces K+ contents before any measurable increase in Cl− content (McLaughlin et al., 2008). With continued exposure to ouabain, membrane depolarization will permit Cl− to enter together with Na+, leading to cell swelling, as we have observed in ciliary epithelial cells (McLaughlin et al., 2004).
The current data demonstrate that inhibition of Na+,K+-activated ATPase by 4 hrs of exposure to 100 nM ouabain indeed increases TM-cell Na+ concentration (Fig. 7C). However, exposure to 30 nM ouabain for 4 hrs did not measurably alter Na+ level, and yet reduced swelling-activated ATP release in parallel experiments. We also indirectly confirmed that little K+/Na+ exchange could have resulted from 4 hrs of exposure to 30 nM ouabain. The RVD of explant-derived human TM cells strongly depends on release of K+ and Cl− (Mitchell et al., 2002), so that ouabain-dependent loss of cell K+ should reduce the rate and magnitude of the regulatory shrinkage. This was indeed observed following exposure to 100 nM ouabain for 4 hrs, but not with 30 nM ouabain (Fig. 8). These two sets of experiments dissociated ouabain’s effects on ATP release and on intracellular ionic composition.
We utilized an additional strategy in testing whether ouabain’s actions on ATP release and ionic content could be dissociated. By bathing the TM5 cells with Na+-free solution, we precluded exchange of intracellular K+ for extracellular Na+. Even under these circumstances, application of 100 nM ouabain for 4 hrs inhibited swelling-activated ATP release (Fig. 1).
4.4. Conclusions
Contrary to expectation, inhibiting Na+,K+-activated ATPase with ouabain or strophanthidin reduced swelling-activated ATP release, and ouabain did not alter MMP secretion. These observations indicate that the ouabain-induced increase in outflow facility is not mediated by the ATP-MMP signaling pathway posited to link actin depolymerization and increased outflow facility. Experimental searches for alternative pathways indicated that cytoskeletal remodeling, reduced cell viability or down-regulation of gene expression of ATP-release pathways are unlikely to link ouabain to increased outflow facility. Although Na+,K+-activated ATPase fulfills an indispensable function in maintaining the ionic composition of mammalian cells (Leaf, 1956; Tosteson and Hoffman, 1960; Wilson, 1954), ouabain’s effects on ATP release and ion transfer could be dissociated. We conclude that ouabain’s effects on ATP release are mediated by inhibiting the signaling and/or scaffolding functions of Na+,K+-activated ATPase (Schoner and Scheiner-Bobis, 2007; Xie and Askari, 2002). The importance of scaffolding proteins is increasingly recognized in regulating selectivity in pathways and modifying output behaviors (Good et al., 2011). We suggest that the ouabain-induced enhancement of outflow facility may also be mediated by inhibiting non-transport functions of Na+,K+-activated ATPase in TM cells.
Highlights.
Ouabain exerts several parallel effects on human TM5 cells
These include inhibitions of ATP release and the RVD
ATP release inhibited even before changes in cell Na+ or cytoskeleton
Without ouabain, ATP increases MMP release dependent on ATP conversion to adenosine
Ouabain likely raises outflow facility via scaffolding/signaling activity of Na+ Pump
Acknowledgments
Supported by NIH Research Grant EY13624 (to M.M. Civan) and a Core Grant EY01583 (to the University of Pennsylvania). We thank Dr. Iok-hou Pang and Alcon Laboratories Inc. for generously providing the TM5 cell line, and Yuting Zhao for zymography assistance.
Appendix
Table A1.
Inventoried Taqman Gene Expression Assays for Real-Time PCR
| Target | Assay ID of FAM-labeled MGB Probe |
|---|---|
| PX1 | Hs00209791_m1 |
| Cx43 | Hs00748445_s1 |
| P2RX7 | Hs00175721_m1 |
| ADOR1 | Hs00379752_m1 |
| β-Actin | 4333762F |
| GAPDH | 4333764F |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bert RJ, Caruthers SD, Jara H, Krejza J, Melhem ER, Kolodny NH, Patz S, Freddo TF. Demonstration of an anterior diffusional pathway for solutes in the normal human eye with high spatial resolution contrast-enhanced dynamic MR imaging. Invest Ophthalmol Vis Sci. 2006;47:5153–5162. doi: 10.1167/iovs.05-0372. [DOI] [PubMed] [Google Scholar]
- Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42:1505–1513. [PubMed] [Google Scholar]
- Cantiello HF, Stow JL, Prat AG, Ausiello DA. Actin filaments regulate epithelial Na+ channel activity. Am J Physiol. 1991;261:C882–888. doi: 10.1152/ajpcell.1991.261.5.C882. [DOI] [PubMed] [Google Scholar]
- Collaborative Normal-Tension Glaucoma Study Group Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998a;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- Collaborative Normal-Tension Glaucoma Study Group The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998b;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- Crosson CE, Sloan CF, Yates PW. Modulation of conventional outflow facility by the adenosine A1 agonist N6-cyclohexyladenosine. Invest Ophthalmol Vis Sci. 2005;46:3795–3799. doi: 10.1167/iovs.05-0421. [DOI] [PubMed] [Google Scholar]
- Dismuke WM, Mbadugha CC, Faison D, Ellis DZ. Ouabain-induced changes in aqueous humour outflow facility and trabecular meshwork cytoskeleton. Br J Ophthalmol. 2009;93:104–109. doi: 10.1136/bjo.2008.142133. [DOI] [PubMed] [Google Scholar]
- Evans DW, Hosking SL, Embleton SJ, Morgan AJ, Bartlett JD. Spectral content of the intraocular pressure pulse wave: glaucoma patients versus normal subjects. Graefes Arch Clin Exp Ophthalmol. 2002;240:475–480. doi: 10.1007/s00417-002-0460-4. [DOI] [PubMed] [Google Scholar]
- Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332:680–686. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harootunian AT, Kao JP, Eckert BK, Tsien RY. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J Biol Chem. 1989;264:19458–19467. [PubMed] [Google Scholar]
- Hawkes SP, Li H, Taniguchi GT. Zymography and reverse zymography for detecting MMPs and TIMPs. Methods Mol Biol. 2010;622:257–269. doi: 10.1007/978-1-60327-299-5_16. [DOI] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829-730. [DOI] [PubMed] [Google Scholar]
- Leaf A. On the mechanism of fluid exchange of tissues in vitro. Biochem J. 1956;62:241–248. doi: 10.1042/bj0620241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- Li A, Leung CT, Peterson-Yantorno K, Mitchell CH, Civan MM. Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am J Physiol Cell Physiol. 2010;299:C1308–1317. doi: 10.1152/ajpcell.00333.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Civan MM. Cytoskeletal dependence of adenosine triphosphate release by human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:7996–8005. doi: 10.1167/iovs.11-8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Mitchell CH, Civan MM. Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow. J Cell Physiol. 2012;227:172–182. doi: 10.1002/jcp.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight AD, DiBona DR, Leaf A. Sodium transport across toad urinary bladder: a model “tight” epithelium. Physiol Rev. 1980;60:615–715. doi: 10.1152/physrev.1980.60.3.615. [DOI] [PubMed] [Google Scholar]
- McLaughlin CW, Karl MO, Zellhuber-McMillan S, Wang Z, Do CW, Leung CT, Li A, Stone RA, Macknight AD, Civan MM. Electron probe X-ray microanalysis of intact pathway for human aqueous humor outflow. Am J Physiol Cell Physiol. 2008;295:C1083–1091. doi: 10.1152/ajpcell.340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin CW, Zellhuber-McMillan S, Macknight AD, Civan MM. Electron microprobe analysis of ouabain-exposed ciliary epithelium: PE-NPE cell couplets form the functional units. Am J Physiol Cell Physiol. 2004;286:C1376–C1389. doi: 10.1152/ajpcell.00248.2003. [DOI] [PubMed] [Google Scholar]
- McLaughlin CW, Zellhuber-McMillan S, Macknight AD, Civan MM. Electron microprobe analysis of rabbit ciliary epithelium indicates enhanced secretion posteriorly and enhanced absorption anteriorly. Am J Physiol Cell Physiol. 2007;293:C1455–1466. doi: 10.1152/ajpcell.00205.2007. [DOI] [PubMed] [Google Scholar]
- Mitchell CH, Fleischhauer JC, Stamer WD, Peterson-Yantorno K, Civan MM. Human trabecular meshwork cell volume regulation. Am J Physiol Cell Physiol. 2002;283:C315–326. doi: 10.1152/ajpcell.00544.2001. [DOI] [PubMed] [Google Scholar]
- Namekata K, Harada C, Kohyama K, Matsumoto Y, Harada T. Interleukin-1 stimulates glutamate uptake in glial cells by accelerating membrane trafficking of Na+/K+-ATPase via actin depolymerization. Mol Cell Biol. 2008;28:3273–3280. doi: 10.1128/MCB.02159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang IH, Shade DL, Clark AF, Steely HT, DeSantis L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Curr Eye Res. 1994;13:51–63. doi: 10.3109/02713689409042398. [DOI] [PubMed] [Google Scholar]
- Sanka K, Maddala R, Epstein DL, Rao PV. Influence of actin cytoskeletal integrity on matrix metalloproteinase-2 activation in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:2105–2114. doi: 10.1167/iovs.06-1089. [DOI] [PubMed] [Google Scholar]
- Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs. 2007;7:173–189. doi: 10.2165/00129784-200707030-00004. [DOI] [PubMed] [Google Scholar]
- Shearer TW, Crosson CE. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002;43:3016–3020. [PubMed] [Google Scholar]
- Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995;14:611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- Tanihara H, Inatani M, Honjo M, Tokushige H, Azuma J, Araie M. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch Ophthalmol. 2008;126:309–315. doi: 10.1001/archophthalmol.2007.76. [DOI] [PubMed] [Google Scholar]
- The AGIS investigators The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–623. [PubMed] [Google Scholar]
- Tosteson DC, Hoffman JF. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TH. Ionic permeability and osmotic swelling of cells. Science. 1954;120:104–105. doi: 10.1126/science.120.3107.104. [DOI] [PubMed] [Google Scholar]
- Xie Z, Askari A. Na(+)/K(+)-ATPase as a signal transducer. Eur J Biochem. 2002;269:2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- Yantorno RE, Coca-Prados M, Krupin T, Civan MM. Volume regulation of cultured, transformed, non-pigmented epithelial cells from human ciliary body. Exp Eye Res. 1989;49:423–437. doi: 10.1016/0014-4835(89)90051-1. [DOI] [PubMed] [Google Scholar]