Abstract
The purpose of this study was to investigate the role of interleukin-1 (IL-1) in modulating myofibroblast viability in mouse corneas with stromal opacity. Twenty-four female B6;129S1-Il1r1tm1Roml/J homozygous IL-1RI knockout mice and 24 control B6129SF2/J mice were included in this study. Each mouse had opacity-generating irregular phototherapeutic keratectomy (PTK) performed with an excimer laser in one eye. Groups of 8 mice from each group were euthanized at one month, three months and six months after surgery and the eyes cryo-preserved. The contralateral eye served as unwounded control. Immunohistochemistry was performed for α-smooth muscle actin (SMA) in central sections of all corneas. The TUNEL assay for apoptosis was performed on 8 sections of four eyes from each group. No SMA+ cells were detected in the stroma of unwounded control or knockout corneas. SMA+ myofibroblast density was significantly higher (p < 0.001) in the IL-1RI knockout group than in the control group at one month, three and six months after irregular PTK. Mean TUNEL+ stromal cells in the anterior 50 µm of stroma was significantly lower in the IL-1RI knockout group compared to the control group at six months after irregular PTK (p = 0.04). These results corroborate the findings of recent in vitro work that demonstrated an antagonistic effect of TGFβ and IL-1 on myofibroblast viability, and found that IL-1-triggered myofibroblast apoptosis was suppressed by TGFβ. Thus, IL-1 is an important modulator of myofibroblast viability during corneal wound healing.
Keywords: Cornea, stroma, wound healing, interleukin-1, interleukin-1 receptor, α-smooth muscle actin, myofibroblasts, apoptosis, phototherapeutic keratectomy
1. Introduction
After photorefractive keratectomy (PRK) and other corneal injuries, the organization of the stromal extracellular matrix is altered. Changes in environmental conditions modulate keratocyte phenotype, resulting in altered gene expression, contractility and other characteristics that contribute to the wound healing response (Jester et al., 2003; Soriano et al., 2001). Corneal myofibroblasts are the primary differentiated cells responsible for the generation of corneal haze (Jester et al., 1999a, 2003; Maltseva et al., 2001; Netto, et al., 2006) and have been shown to develop from keratocyte- (Masur, et al., 1996; Jester et al., 1999a) or bone marrow-derived (Barbosa et. al., 2010a) precursor cells. Regression of haze over time is associated with disappearance of myofibroblasts mediated by apoptosis (Wilson, Chaurasia, and Medeiros, 2007) or trans-differentiation (Maltseva et al., 2001), and remodeling of disorganized stromal matrix materials (Hassell and Birk, 2010).
Interleukin-1 (IL-1) is an important modulator of the cellular functions of stromal cells that contribute to the corneal wound healing response (Wilson, Liu, and Mohan, 1999). IL-1α and IL-1β regulate key functions of keratocytes, corneal fibroblasts and myofibroblasts during corneal wound healing (Wilson et. al., 2001), and also modulate the inflammatory cellular response to corneal injury (Dana et. al., 2007; Stapleton et. al., 2008). In vitro work has suggested that IL-1 and transforming growth factor beta (TGFβ) have opposing effects on the viability and persistence corneal myofibroblasts (Kaur et al., 2009). The purpose of this study was to investigate this role of IL-1 in modulating myofibroblast development and persistence in situ in mice homozygous for the Il1r1tm1Roml mutation that leaves the animals unresponsive to either IL-1α or IL-1β Labow et al., 1997).
2. Materials and methods
2.1. Animals and surgery
All animals were treated in accordance with the tenets of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the Animal Control Committee at the Cleveland Clinic approved the animal studies included in this work. Twenty-four female B6;129S1-Il1r1tm1Roml/J homozygous IL-1RI knockout mice and 24 control B6129SF2/J mice (The Jackson Laboratory, Bar Harbor, Maine) were included in this study. Anesthesia was obtained with an intraperitoneal injection of 130 µg ketamine and 8.8 µg xylazine per gram of body weight and 1 drop of 1% proparacaine HCl (Alcon, Ft. Worth, TX, USA) was applied to each eye at the time of phototherapeutic keratectomy (PTK).
An irregular PTK model (Mohan et al., 2008) was used because mice are otherwise resistant to haze generation after PTK or photorefractive keratectomy (PRK). Haze-generating irregular PTK was performed on the knockout and control mice in one eye selected at random with a VISX S4IR excimer laser (Abbott Laboratories, Irvine, CA) as previously reported (Mohan et al., 2008). Briefly, the corneal epithelium was scraped with a #64 Beaver blade (Becton-Dickinson, Chicago, IL) and PTK was performed by delivering 45 pulses of laser (ablation depth approximately 10 um) with a beam diameter of 2 mm on the central cornea. Irregularity was generated by positioning a fine mesh screen (Fig. 1) in the path of laser for the final 50% of the pulses (Mohan et al., 2008). Analgesia was provided for 3 days after surgery with meloxicam 0.5mg in 250ml of drinking water. Ciprofloxacin 0.3% (Alcon, Ft. Worth, TX, USA) was given topically twice a day to prevent infection. Euthanasia was performed with an inhaled overdose of 5% isofluorane gas.
Fig. 1.
Irregular PTK was performed by positioning a fine mesh screen in the path of laser over the mouse cornea for the final 50% of the pulses.
2.2. Cornea tissue preparation and immunohistochemistry
Eight knockout and 8 control mice were euthanized at 1, 3, and 6 months after PTK. After euthanasia, the whole eyes were removed with 0.12 forceps and Westcott scissors, embedded in liquid OCT compound (Sakura FineTek, Torrance, CA) within a 15 mm × 15 mm × 5 mm mold (Fisher Scientific, Pittsburgh, PA) and frozen. The tissue blocks were maintained at −85°C. Tissue sections (7 micron thickness) were cut with a cryostat (HM 505M, Micron GmbH, Walldorf, Germany) and maintained at −85°C until staining was performed.
Immunofluorescence staining was performed on tissue sections to study the expression of α-smooth muscle actin (α-SMA) in stromal myofibroblasts using monoclonal mouse anti-human antibody clone 1A4 (Cat. # M0851, Dako, Carpentaria, CA) that detects mouse α-SMA. The antibody was diluted 1:50 in physiologic saline solution. The M.O.M Immunodetection KIT (Cat. #2201, Vector Laboratories Inc., Burlingame, CA) was used according to the manufacturer’s instructions to detect the bound primary antibody. The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed with the fluorescence-based ApopTag assay according to the manufacturer’s instructions (Intergen Co, Purchase, NY, Cat. No. S7165).
Coverslips were mounted with Vectashield (Vector Laboratories Inc., Burlingame, CA) containing DAPI to allow visualization of all nuclei in the tissue sections. The sections were viewed and photographed with a Leica DM5000 microscope equipped with Q-Imaging Retiga 4000RV (Surrey, BC, Canada) camera and ImagePro software.
2.3 Quantification of cells and Statistical analysis
SMA+ stromal cells in the central cornea were counted real time under the microscope in three randomly selected, full thickness 400X microscopic as previously described (Mohan et al., 2003). The results for the three fields for each cornea were averaged to determine the value for that cornea and then the values for the eight corneas in each group were averaged and the standard error calculated.
Since stromal cell apoptosis at one month or more after an injury such as PTK is a rare event (Mohan, et al., 2003), quantitation of anterior stromal apoptosis for comparisons between the two groups were performed using a method that included a large number of central corneal sections from each eye evaluated. Thus, four eyes from both the knockout and control groups were randomly selected for each time point and eight central corneal 7 µm thick sections from each eye were stained for apoptotic cells using the TUNEL assay. The total number of TUNEL+ cells was counted in the anterior 50 µm of subepithelial stroma (the zone where myofibroblasts are localized) across the full diameter of each cornea. The average number of TUNEL+ cells in this stromal zone per slide was determined for each eye in each group at each time point. The mean and standard error for each group at each time point was calculated.
Data were analyzed comparing the knockout and control groups at each time point using ANOVA. p values less than 0.05 were considered statistically significant.
3. Results
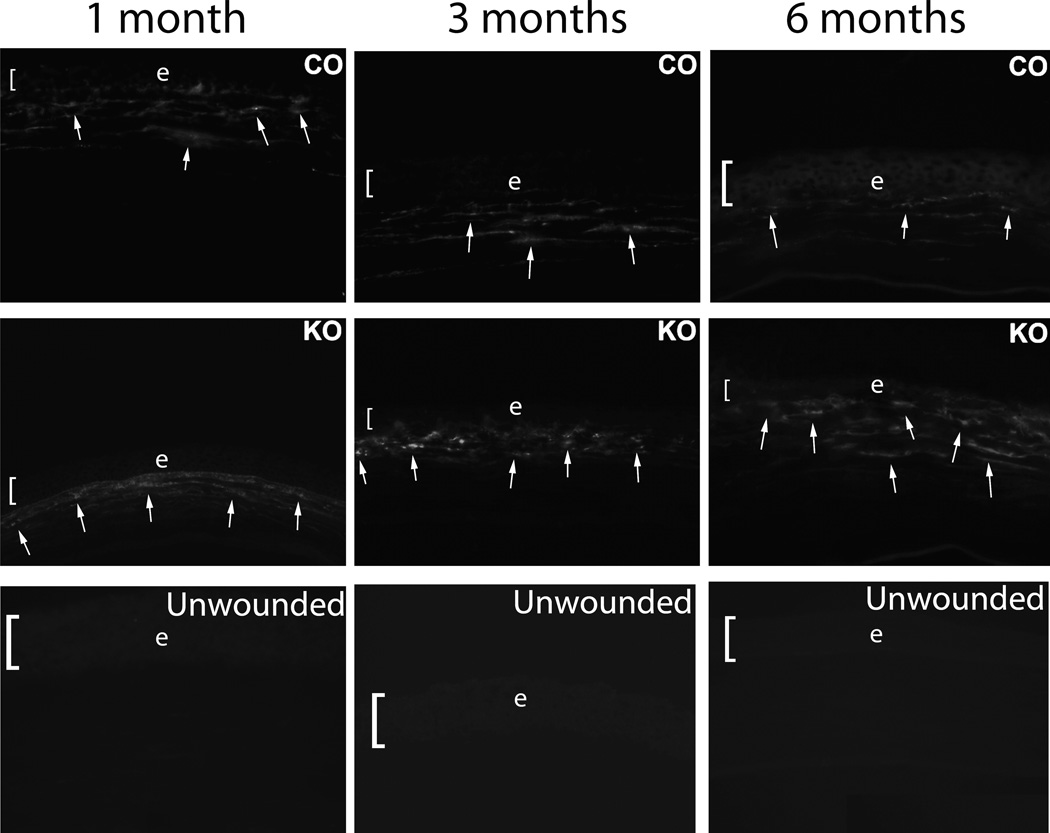
There were no morphologic differences noted between the IL-1 receptor knockout or control mice unwounded corneas at the slit lamp (corneal clarity, diameter, etc.) or upon sectioning the central cornea (epithelial thickness, total corneal thickness, etc.) (not shown). There were no SMA+ cells detected in unwounded corneas from either group at any time point (Figs. 2).
Fig. 2.
Immunohistochemistry for α-smooth muscle actin (SMA) in the central corneal stroma of control (CO) and IL-1 receptor knockout (KO) mouse corneas with haze at one month, three months or six months following irregular PTK. SMA+ myofibroblast density was significantly higher in the knockout than control mice, especially at the six-month time point, where myofibroblast density had markedly declined in the control cornea compared to three-month time point after irregular PTK. In the IL-1 receptor knockout mice, the SMA+ myofibroblast density has not declined and actually appears increased in this individual section. In unwounded corneas, SMA+ cells were not detected in either unwounded IL-1 receptor knockout (unwounded) or unwounded control (not shown) corneas. Representative SMA+ myofibroblasts are indicated by arrows. The bracket and e indicates the corneal epithelium in each panel. Magnifications 400X.
At one month following irregular PTK the immunohistochemistry revealed significantly higher density of SMA+ cells in the anterior stroma in the knockout groups than the control groups (Fig. 2). At three months and six months after irregular PTK, the control corneas continued to have significantly lower density of SMA+ cells than the IL-1 receptor knockout group (Fig. 2).
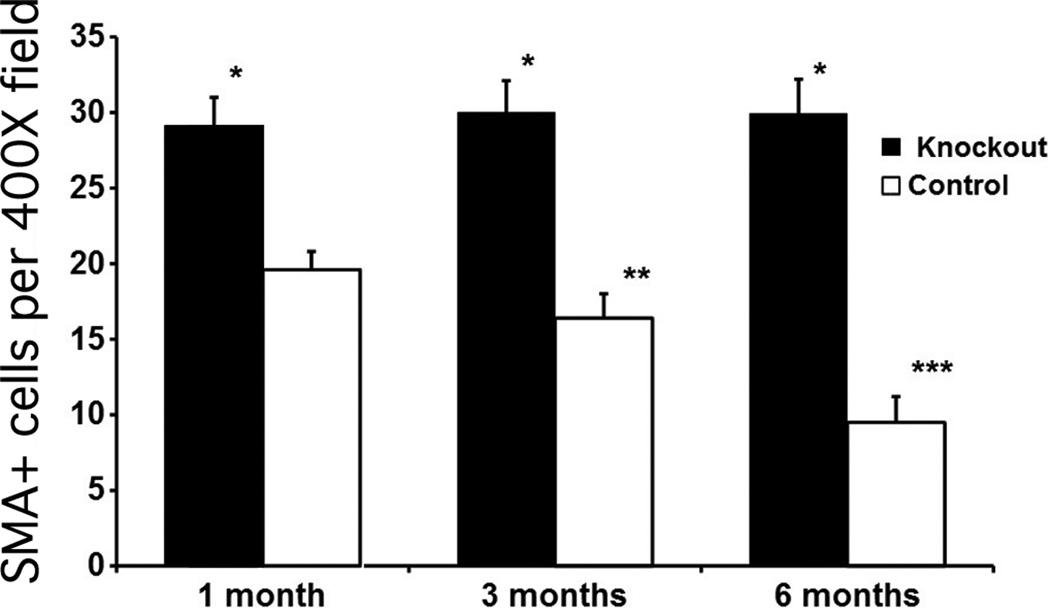
Quantitative analysis of the SMA+ density in both groups at the three time points is shown in Fig. 3. In control corneas, there was a statistically significant decrease in mean stromal myofibroblast density from one month to three months (P <0.001) and from three months to six months (p <0.001) after irregular PTK. Conversely, in the IL-1 receptor knockout corneas, there was no significant decrease in mean stromal myofibroblast density from one month to three months (p = 0.25) or from three months to six months (p = 0.88) after irregular PTK, although there was a trend towards an increase over the time course from one to six months after surgery. At each time point, the mean stromal myofibroblast density was significantly (p < 0.001) greater in the IL-1 receptor knockout group than the control group (Fig. 3).
Fig. 3.
Quantitative analysis of α-smooth muscle actin-positive myofibroblasts in central cornea stromal cells/400× microscope field at one month, three months, or six months after irregular PTK in the IL-1 receptor knockout and control groups. Error bars represent SEM. * indicates that the density of SMA+ cells was significantly different (p < 0.001) in the knockout that had irregular PTK compared to the control that had irregular PTK at one month, three months or six months after surgery. SMA+ cell density in the central corneal stroma did not significantly decrease over time in the KO corneas from one month to three months after surgery or from three months to six months after irregular PTK. Conversely, SMA+ myofibroblast density in the central corneal stroma significantly decreased between one and three months (**) or between three and six months (***) after irregular PTK in control corneas.
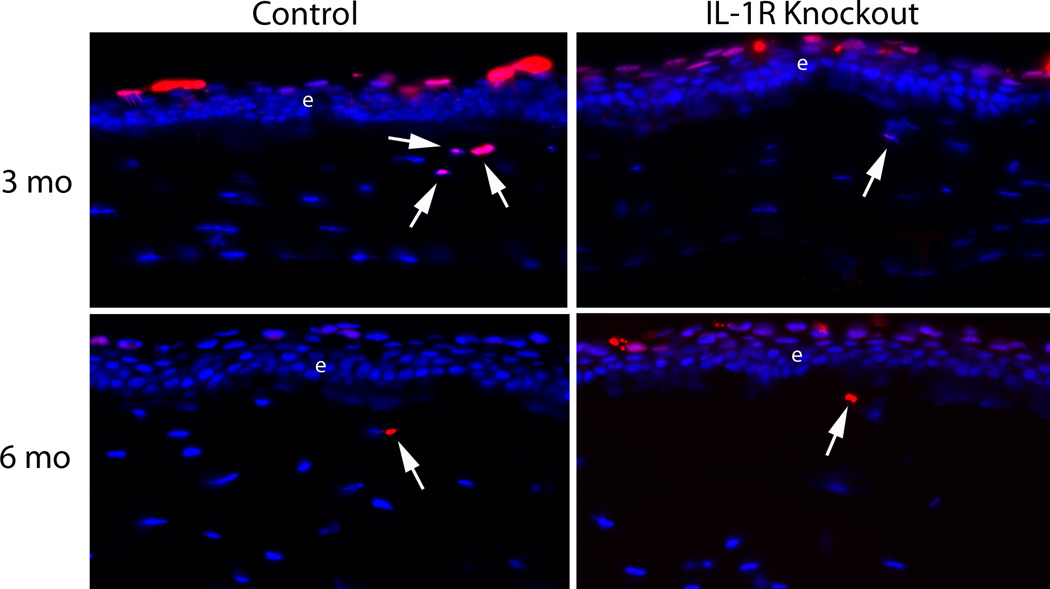
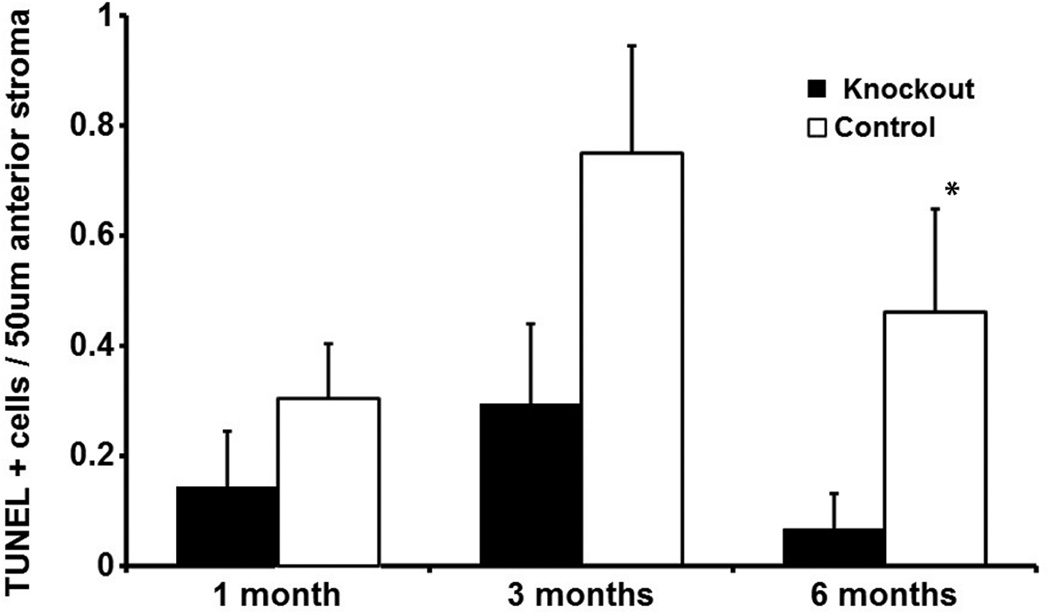
The number of apoptotic cells in the anterior 50 µm of stroma was low in both the IL-1 receptor knockout and control groups at each of the time points. It was rare to find more than one TUNEL+ cell in that zone across the entire corneal diameter in either group at any time point, and many slides had no TUNEL+ cells in this zone—depending on the group and time point. This necessitated using the large number of slides (eight) from each eye at each time point for quantitation. Representative TUNEL+ anterior stromal cells in the IL-1 receptor knockout group and the control group are shown in Figure 4. Quantitative analysis for TUNEL+ cells in the anterior 50 µm of stroma is shown in Figure 5. The average number of TUNEL+ cells per slide was greater in the control group than the IL-1 receptor knockout group at all three time points. The difference was statistically significant at the six-month time point (p = 0.042) and nearly reached significance at the three-month time point (p = 0.078).
Fig. 4.
Representative TUNEL+ stromal cells (arrows) in the anterior 50 µm of stroma in the IL-1 receptor knockout group (A) and control group (B) at three and six months after irregular PTK. Arrows indicate rare TUNEL+ cells in the anterior 50 µm of stroma in each group. e indicates the epithelium. Note there are also normal TUNEL+ cells in the apical epithelium of each cornea. Magnifications 400X.
Fig. 5.
Quantitative analysis of TUNEL+ stromal cells per slide in the anterior 50 µm of stroma at one month, three months, and six months after irregular PTK in the IL-1 receptor knockout and control groups. Error bars represent SEM. Note that mean apoptotic cells in the anterior stroma was less in the IL-1 receptor knockout group than the control group at each time point, although the difference was only statistically significant (asterisk, p = 0.04) at six months after irregular PTK.
4. Discussion
The corneal wound healing response is a complex cascade mediated by many growth factors and cytokines. IL-1 may be considered a master regulator of the corneal wound healing response because of its numerous effects regulating cellular processes such as the expression of other growth factors, expression of metalloproteinases, inflammatory cell infiltration and apoptosis (Wilson et al., 2001).
Myofibroblasts are key contributors to the stromal opacity (haze) that often occurs after corneal injury or surgery (Masur et al., 1996; Jester, Petroll, and Cavanagh, 1999; Mohan et al., 2003). These stromal cells disappear spontaneously and slowly over time measured in month to years after surgery, Myofibroblast apoptosis has been shown to have a role in this disappearance in situ (Wilson, Chaurasia, and Medeiros, 2007).
In vitro studies have suggested that IL-1α and IL-1β are important modulators of myofibroblast viability and cell death in non-ocular tissues (Shephard et. al., 2004; Zhang et. al., 1999). In vitro studies with corneal cells have supported the hypothesis that TGFβ and IL-1 have opposing effects on corneal myofibroblast viability (Kaur et al., 2009). Thus, TGFβ is the critical modulator involved in myofibroblast development from progenitor cells and myofibroblast persistence in the corneal stroma (Kaur et. al., 2009; V. Singh and S.E. Wilson, unpublished data, 2011). Conversely, IL-1α or IL-1β triggers myofibroblast apoptosis, but only when TGFβ falls to critically low levels (Kaur et. al., 2009). Epithelium-derived TGFβ levels in the stroma in situ likely fall when structural and functional integrity of the epithelial basement membrane is restored following injury and this layer resumes its TGFβ barrier function between the epithelium and stroma (Netto et al., 2006). In normal corneas, basement membrane integrity is restored early after photorefractive keratectomy (PRK) or PTK surgery and, therefore, most corneas do not develop significant numbers of myofibroblasts, or the opacity associated with these cells (Netto, Mohan, and Wilson, 2005). However, in some corneas, especially after higher levels of correction with PRK, irregular PRK or irregular PTK, basement membrane defects persist and high numbers of myofibroblasts develop and survive in the anterior stroma (Netto et al., 2006; Mohan et al., 2008).
We hypothesized that if IL-1 has an important role in myofibroblast disappearance in vivo, then the levels of myofibroblasts should be increased in mice that are missing the IL-1 receptor and, therefore, have lost the capacity for cells that normally express the receptor to respond to the cytokine, compared to control mice that retain the IL-1 receptor. This study supports that hypothesis. Stromal cell apoptosis in the anterior 50 µm of stroma was reduced in the IL-1 receptor knockout mice compared to control mice at one, three and six months after irregular PTK, although the result only became statistically significant at six months after surgery—likely due to the slow rate at which myofibroblasts disappear from the stoma by late apoptosis in corneas with haze (Wilson, Chaurasia and Medeiros, 2007; Mohan, et al., 2003). Consistent with this finding, myofibroblasts were present at higher density in IL-1 receptor knockout mice compared to control mice at one, three and six months after irregular PTK (Fig. 3).
The possible sources of stromal IL-1 to regulate myofibroblast viability have been investigated in a rabbit model (Barbosa et al., 2010b). That study demonstrated that SMA-cells such as corneal fibroblasts, keratocytes, or inflammatory cells produce IL-1α and/or IL-1β that could act in paracrine fashion to regulate myofibroblast apoptosis. However, some SMA+ myofibroblasts also produce IL-1α and/or IL-1β, suggesting that autocrine suicide could also have a role in triggering myofibroblast apoptosis when TGFβ levels fall to a critical level in the corneal stroma. Whatever the specific mechanism is through which myofibroblast viability is modulated, the present study demonstrates that IL-1 receptor plays an important role in that viability an in situ mouse model.
Highlights.
IL-1 receptor is involved in regulating stromal cell apoptosis and myofibroblast viability.
Stromal myofibroblasts significantly decrease over time after surgery in control mice.
Stromal myofibroblasts did not decrease over time after surgery in IL-1 receptor knockout mice.
Acknowledgements
This study was supported by EY010056 and EY015638, and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proprietary interest statement: The authors have no proprietary or financial interest in this manuscript.
References
- Barbosa FL, Chaurasia SS, Cutler A, Asosingh K, Kaur H, Medeiros FW, Agrawal V, Wilson SE. Corneal myofibroblast generation from bone marrow-derived cells. Exp. Eye Res. 2010a;91:92–96. doi: 10.1016/j.exer.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FL, Chaurasia S, Kaur H, de Medeiros FW, Agrawal V, Wilson SE. Stromal interleukin-1 expression in the cornea after haze-associated injury. Exp. Eye Res. 2010b;91:456–461. doi: 10.1016/j.exer.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana R. Comparision of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival. Trans. Am. Ophthalmolo. Soc. 2007;105:330–343. [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Birk DE. The molecular basis of transparency. Exp. Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor (beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest. Ophthalmol. Vis. Sci. 1999;40:1959–1967. [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog. Retinal Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytockines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Agrawal V, Suto C, Wilson SE. Corneal myofibroblast viability: opposing effects of IL-1 and TGFβ1. Exp. Eye Res. 2009;89:152–158. doi: 10.1016/j.exer.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan EB, Bartfai T, Solorzano C, Moldawer LL, Chizzonite R, McIntyre KW. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest. Ophthalmol. Vis. Sci. 2001;42:2490–2495. [PubMed] [Google Scholar]
- Masur S, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc. Natl. Acad. Sci. USA. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Stapleton WM, Sinha S, Netto MV, Wilson SE. A novel method for generating corneal haze in anterior stroma of the mouse eye with the excimer laser. Exp. Eye Res. 2008;86:235–240. doi: 10.1016/j.exer.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp. Eye Res. 2006;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Wilson SE. Wound healing in the cornea: A review of refractive surgery complications and new prospects for therary. Cornea. 2005;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Medeiros FW, Dupps WJ, Sinha S, Krueger RR, Stapleton WM, Rayborn M, Suto C, Wilson SE. Femtosecond lase and microkeratome LASIK flaps: comparative effects on wound healing and inflammatory cells infiltration in the cornea. J. Ref. Surg. 2007;23:667–676. doi: 10.3928/1081-597x-20070901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard P, Martin G, Smola-Hess S, Brunner G, Krieg T, Smola H. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-beta and interleukin-1. Am. J. Pathol. 2004;164:2055–2066. doi: 10.1016/s0002-9440(10)63764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano ES, Campos MS, Aguiar JA. Effect of epithelial debridement on human cornea proteoglycans. Braz. J Med. Biol. Res. 2001;34:325–331. doi: 10.1590/s0100-879x2001000300005. [DOI] [PubMed] [Google Scholar]
- Stapleton WM, Chaurasia S, Medeiros FW, Mohan RR, Sinha S, Wilson SE. Topical interleukin-1 receptor antagonist inhibits inflammatory cell infiltration into the cornea. Exp. Eye Res. 2008;86:753–757. doi: 10.1016/j.exer.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response. Exp. Eye Res. 2007;85:305–311. doi: 10.1016/j.exer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog. Retin. Eye Res. 1999;18:293–309. doi: 10.1016/s1350-9462(98)00017-2. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrósio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin, Eye Res. 2001;20:625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor beta 1. Am. J. Respir. Cell Mol. Biol. 1999;21:658–665. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]