Dear Editor,
Cyclin-dependent kinase 4 (CDK4) is a key protein in G1 transition during cell-cycle progression [1]. Two different groups have postulated that CDK4 may be considered a pancreatic tumor marker. These groups have not detected CDK4 expression in healthy pancreas, but they have detected CDK4 in pancreatic endocrine tumors [2], intraepithelial neoplasia and ductal adenocarcinoma of the pancreas [3]. These results clearly contrast with those obtained from murine models, in which CDK4 is the main G1 transition kinase in the pancreatic tissue. CDK4-null mice exhibit severe beta-cell mass hypoplasia, whereas islet CDK4 hyperactivity induces hyperplasia without causing hypoglycemia [4]. The explanation for this divergence was discussed at a symposium [5], where cyclin-dependent kinase 6 (CDK6) was proposed as the protein responsible for regulating G1 transition in the healthy pancreas of humans because only CDK6, not CDK4, has been detected in healthy pancreases. However, in a subsequent study, these authors suggested that healthy isolated human pancreatic islets express CDK6 and CDK4 [6].
To assess the usefulness of CDK4 as a biomarker of pancreatic cancer, we extensively examined its presence using several techniques in normal pancreas (n=24) and islets (n=11), pancreatic adenocarcinomas (n=125) and pancreatic neuroendocrine tumors (n=5). The detailed material and methods in the supplementary information are available at https://sites.google.com/site/conchimoralabs/cdk4-pancreas-supplementary-information.
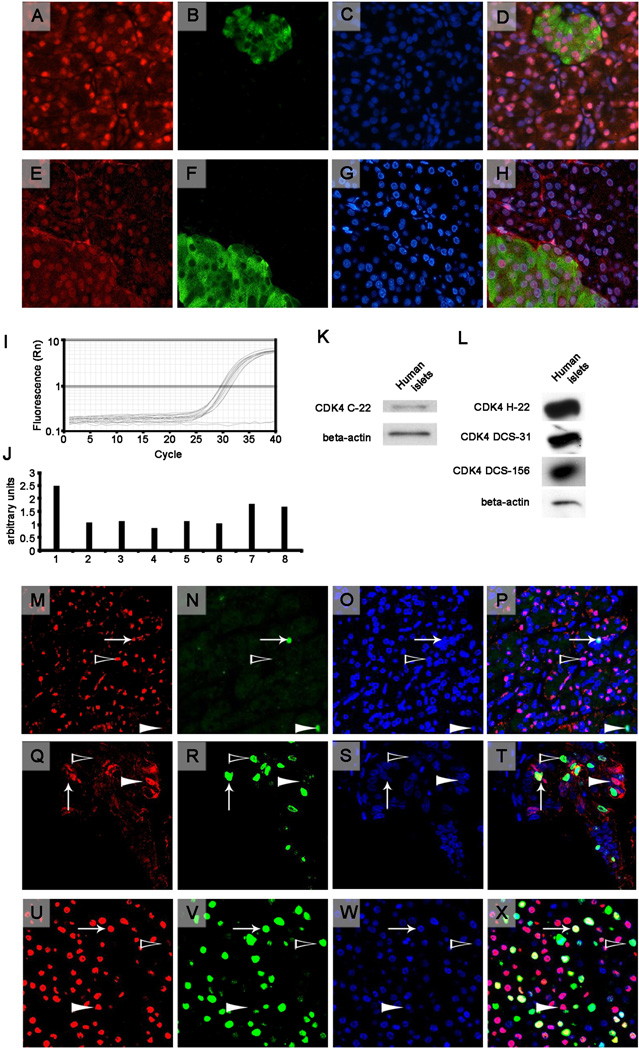
To observe the presence of CDK4 using immunohistochemistry, we used two similar antibodies against CDK4 (H-22 and C-22; Santa Cruz Technology, Santa Cruz, CA, USA), which were raised against the human or mouse C-terminal portion of CDK4, respectively. It is noteworthy that one of the research groups mentioned above failed to detect CDK4 by immunohistochemistry and western blot analysis in healthy human pancreas using the H-22 antibody [2]. We obtained similar results with both antibodies (see representative images in Fig. 1, A-H) and observed that 58% of the healthy samples (14 of 24), 67% of the adenocarcinomas (84 of 125) and 80% of the neuroendocrine tumors (4 of 5) were positive for CDK4 staining (detailed results are available in the supplementary information). To ensure staining specificity, we performed several quality controls. First, some human pancreatic sections were stained only with the secondary antibodies, which did not produce any staining signals. Second, Cdk4 staining of murine pancreatic sections showed a clear nuclear staining in the endocrine and exocrine pancreatic tissue. Third, pancreatic tissue sections of Cdk4-deficient mice were immunonegative for Cdk4 after the sections were incubated with the anti-Cdk4 antibody. Finally, CDK4 staining in human samples that were blocked with the CDK4-blocking peptide (Santa Cruz Technology) showed no signal. After performing all of these controls (representative images are available in the supplementary information), we concluded that the staining observed using the anti-CDK4 antibody in human pancreatic sections was specific.
Figure 1.
Top Panels (A-H). Two representative sections of healthy human pancreas from the UTIP healthy series (A-H) immunostained for CDK4 (A, E) and co-stained with insulin (B, F) and the nuclear marker Hoechst (Sigma) (C, G) for nuclear detection. Each row corresponds, from left to right, to the same section that was stained for CDK4 using the C-22 antibody (A) or the H-22 antibody (E) in red (Cy3), insulin in green (B, F) (Cy2) and nuclear staining with Hoechst in blue (C, G). The images were then merged (D, H). Images are at 40× magnification using an epifluorescence microscope. Middle panels (I-L). Real-time PCR (I, J) and western blot analysis (K, L) for CDK4 in human islets. I: Graphic representation of the fluorescence vs. the cycles of real-time PCR for CDK4 for 8 different samples of human islets (in triplicate) and a negative control (no sample, plain line, in duplicate). J: Quantification of the intensity that was obtained using real-time PCR and normalized to a housekeeping gene (TBP). K: Representative western blots of CDK4 (C-22 antibody) and beta-actin in human islets. L: Representative western blots of CDK4, which was performed with the H-22 antibody, the DCS-31 antibody or the DCS-156 antibody, and beta-actin in human islets. Bottom panels (M-X). Representative CDK4-positive cores from the PAC481 array showing a healthy human pancreas (M-P) and two different adenocarcinoma cores (Q-T, U-X). From left to right, images of the same section that were stained for CDK4 (C-22 antibody) in red (Cy3), the proliferation marker Ki-67 in green (Cy2), nuclear staining with Hoechst in blue, and a merge of all images are shown. An arrow indicates a CDK4- and Ki-67-positive nucleus, a white arrowhead indicates a CDK4-positive and Ki-67-negative nucleus, and a black arrowhead indicates a CDK4-negative and Ki-67-positive nucleus. Images were taken at 40× magnification using a confocal microscope.
The lack of homogeneity in healthy pancreatic tissue -only 58% of the human healthy pancreatic samples showed CDK4 staining- may be caused by the expected heterogeneity within the human species or differences in processing of samples before performing the immunohistochemistry technique (time before organ extraction, fixation time, etc.), which remains to be explored in depth.
We evaluated the presence of CDK4 in human healthy islets using real-time PCR and western blot analysis (Fig. 1, I-L). The presence of CDK4 was detected using western blot analysis with four different anti-CDK4 antibodies: DCS-31 (Sigma, St. Louis, MO, USA), DCS-156 (Becton Dickinson, Franklin Lakes, NJ, USA), C-22 and H-22 (Santa Cruz Technology). To ensure the specificity of the results, we performed several quality controls. First, we observed a clear western blot band using mouse pancreas lysates. Second, this band was not present in the lysates of the Cdk4 knockout mouse pancreas. Third, the incubation of the antibody with the corresponding blocking peptide did not render any signal in human healthy islets (representative images are available in the supplementary information).
The role of CDK4 in cell-cycle progression has been clearly demonstrated [1]. Therefore, we co-stained for CDK4 and the proliferation marker Ki-67. We found no correlation between Ki-67 and CDK4 staining. These results suggest that the sole presence of CDK4 is not indicative of proliferation (Fig. 1, M-X),
All of these results indicate that CDK4 is not useful as a tumor marker. We showed that many healthy pancreatic samples were positive for this protein using different techniques. Moreover, the in-depth analysis of the immunohistochemistry results from the SEER array (Surveillance Epidemiology and End Results (SEER) Residual Tissue Repository tissue array (Bethesda, MD, USA) [7]) reveal that, after adjusting for demographic and clinical attributes, the survival analysis for 50 cases of pancreatic ductal adenocarcinoma-resection tumors (41 cases with nuclear CDK4 staining versus 9 cases without nuclear CDK4 staining), showed a marginally improved survival time for the cases that exhibited nuclear CDK4 staining versus those that did not (log-rank test, p=0.016; hazard ratio, 2.2; 95% confidence interval, 1.0–4.8).
In conclusion, our results suggest that the presence of CDK4 alone cannot be used as a pancreatic tumor marker to distinguish between normal and tumor pancreas. In addition, we hypothesize that the role of CDK4 in pancreatic tissue may extend beyond cell-cycle progression, as CDK4 is also involved in other processes as the regulation of insulin secretion in beta-cells [8].
Yours sincerely,
Supplementary Material
Acknowledgments
This study was supported by IDIBAPS fellowships (J.A. and L.H.), Ministerio de Ciencia y Tecnología (Ramon y Cajal Program, C.M.; SAF 2003-06139, A.G; SAF2006-07382, R.G. and SAF2004-02666, T.S), EFSD/Paul Langerhans/Amylin Pharmaceuticals Award 2004 (C.M.), the EFSD/Lilly Research Fund grant (C.M.) and Ministerio de Sanidad y Consumo (PI040587, T.S). CIBERDEM is an initiative of ISCIII (Ministerio de Ciencia e Innovacion). Our thanks to E. Muntanya and M. Nacher for providing us with healthy human pancreatic sections (UTIP healthy series); the Statistics, Epidemiology, and End Results (SEER) Residual Tissue Repository (RTR) for the tissue SEER Array; and P. Fernández, E. Gonzalvo and M. Mainar for technical assistance. We thank E. Llagostera for HeLa extract lysates as well as C.F. Lynch, W. Cozen, B. Hernandez and M. Goodman for helpful discussions. The National Cancer Institute contracts for the participating SEER registries were N01-PC-35143 (Iowa), N01-PC-35137 (Hawaii), and N01-PC-35139 (Los Angeles).
References
- 1.Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta. 2002;1602:73–87. doi: 10.1016/s0304-419x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 2.Lindberg D, Hessman O, Akerstrom G, Westin G. Cyclin-dependent kinase 4 (CDK4) expression in pancreatic endocrine tumors. Neuroendocrinology. 2007;86:112–118. doi: 10.1159/000106762. [DOI] [PubMed] [Google Scholar]
- 3.Al-Aynati MM, Radulovich N, Ho J, Tsao MS. Overexpression of G1-S cyclins and cyclin-dependent kinases during multistage human pancreatic duct cell carcinogenesis. Clin Cancer Res. 2004;10:6598–6605. doi: 10.1158/1078-0432.CCR-04-0524. [DOI] [PubMed] [Google Scholar]
- 4.Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat Gene. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- 5.Bigatel TA, Cozar-Castellano I, Velásquez-García S, Harb G, Fiachi-Taesch N, Selk K, Takane KK, Stewart AF. American Diabetes Association 2007. Chicago, IL: 2007. Comprehensive comparative cell cycle analysis reveals critical differences in human vs. murine cell regulation: Human islets contain Cdk-6, but lack Cdk-4. Abstract number: 1636-P. [Google Scholar]
- 6.Fiaschi-Taesch NM, Salim F, Kleinberger J, Troxell R, Cozar-Castellano I, Selk K, Cherok E, Takane KK, Scott DK, Stewart AF. Induction of human beta-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6. Diabetes. 2010;59:1926–1936. doi: 10.2337/db09-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takikita M, Altekruse S, Lynch CF, Goodman MT, Hernandez BY, Green M, Cozen W, Cockburn M, Sibug Saber M, Topor M, Zeruto C, Abedi-Ardekani B, et al. Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res. 2009;69:2950–2955. doi: 10.1158/0008-5472.CAN-08-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annicotte JS, Blanchet E, Chavey C, Iankova I, Costes S, Assou S, Teyssier J, Dalle S, Sardet C, Fajas L. The CDK4-pRB-E2F1 pathway controls insulin secretion. Nat Cell Biol. 2009;11:1017–1023. doi: 10.1038/ncb1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.