Abstract
Liposomes (phospholipid bilayer vesicles) are versatile and robust delivery systems for induction of antibody and T lymphocyte responses to associated subunit antigens. In the last 15 years, liposome vaccine technology has matured and now several vaccines containing liposome-based adjuvants have been approved for human use or have reached late stages of clinical evaluation. Given the intensifying interest in liposome-based vaccines, it is important to understand precisely how liposomes interact with the immune system and stimulate immunity. It has become clear that the physicochemical properties of liposomal vaccines – method of antigen attachment, lipid composition, bilayer fluidity, particle charge, and other properties – exert dramatic effects on the resulting immune response. Here, we present a comprehensive review of the physicochemical properties of liposomal vaccines and how they influence immune responses. A discussion of novel and emerging immunomodulators that are suitable for inclusion in liposomal vaccines is also presented. Through a comprehensive analysis of the body of liposomal vaccine literature, we enumerate a series of principles that can guide the rational design of liposomal vaccines to elicit immune responses of a desired magnitude and quality. We also identify major unanswered questions in the field, pointing the direction for future study.
Keywords: liposome, adjuvant, vaccine delivery, subunit vaccine
1. Introduction
Subunit vaccines are increasingly sought because they offer superior safety profiles and can be manufactured with minimal risk of contamination [1, 2]. When coupled with appropriate adjuvants, they can also focus the immune response on protective or highly conserved antigenic determinants that may not elicit a potent response during natural infection or vaccination with an intact pathogen [3, 4]. Currently marketed hepatitis B and human papillomavirus vaccines are two successful examples of protein subunit vaccines [5, 6], while glycoconjugate subunit vaccines have dramatically impacted prevention of bacterial pneumonia and meningitis in pediatric populations [7, 8]. Despite their obvious advantages, subunit antigens alone are poorly immunogenic and must be formulated with particulate adjuvants to elicit robust humoral and cell-mediated immunity [9, 10]. These particulate systems mediate efficient delivery to antigen presenting cells and may induce inflammation through activation of innate immunity [11–13]. The continued development of particulate antigen delivery systems is an essential element of the pursuit of safe, effective subunit vaccines.
Liposomal vaccines used in humans
Aluminum salts have been the most widely used vaccine adjuvants since their use was first reported in 1926 [14], and prior to 2009, alum was the only adjuvant approved for use in the United States [15]. However, because alum-based adjuvants elicit sub-optimal TH1 responses and weak cell-mediated immunity, alternatives are being evaluated. Of the numerous particulate delivery systems that have been developed to replace alum – gels, emulsions, polymeric particles – phospholipid bilayer vesicles (liposomes) are among the most promising. Gregoriadis and Allison first reported the use of liposomes as immunological adjuvants in 1974 [16, 17]. Since that time, liposomes and related vesicular carriers have been established as robust systems for induction of humoral and cell-mediated immunity to a broad spectrum of infectious diseases and cancers [18–20].
Currently, at least 8 liposome-based adjuvant systems are approved for human use or undergoing clinical evaluation (Table 1). Virosomes, composed of reconstituted influenza virus membranes (lipids and envelope glycoproteins) supplemented with phosphatidylcholine (PC), have been a component of licensed influenza and hepatitis A vaccines since 1997 [21–24]. Inflexal® V, the virosomal influenza vaccine, is marketed in 43 countries with over 60 million doses distributed. Liposomal vaccines comprised of more traditional constituents (PC; phosphatidylglycerol, PG; cholesterol, Chol) are also progressing toward regulatory approval for therapy of non-small cell lung cancer (Stimuvax®, Oncotheryon; PC, PG, Chol, MPL) and prevention of malaria (RTS, S/AS01, GlaxoSmithKline; PC, PG, Chol, MPL, QS-21) [25–28]. Liposome vaccine research and development has greatly intensified in the last 10 years; of the 1316 published investigations of liposome-based vaccines in the years 1974–2010 (according to PubMed), half have appeared in the last 8 years and one quarter have been published the last 3 years.
Table 1.
Selected liposome and lipid-based vaccines approved for human use or in clinical trials
| Name | Company | Disease | Description | Status | Refs. |
|---|---|---|---|---|---|
| Inflexal ® V | Crucell | Influenza | Virosomes – reconstituted influenza viral membranes (phospholipids, haemagglutinin, and neuraminidase) supplemented with PC | Marketed | [21, 22] |
| Epaxal ® | Crucell | Hepatitis A | Formalin-inactivated Hepatitis A virus adsorbed to virosomes | Marketed | [23, 24] |
| Stimuvax | Merck KGaA, Oncothyreon | Non-small cell lung cancer | BLP25 (palmitoylated MUC1), MPL, DPPC, DMPG, Chol | Phase 3 | [25, 26] |
| RTS,S/AS01a | GlaxoSmithKline | Malaria | Recombinant fusion of P. falciparum circumsporozoite protein and Hepatitis B surface antigen, PC, Chol, MPL, QS21 | Phase 3 | [27, 28] |
| Vaxisome | NasVax | Influenza | Inactivated influenza vaccine, CCS, Chol | Phase 2 | [174, 228] |
| JVRS-100 | Juvaris BioTherapeutics | Influenza | Inactivated influenza vaccine, DOTIM, Chol, non-coding plasmid DNA | Phase 2 | [229] |
| Vaxfectin | Vical | Influenza | Plasmid DNA-encoded influenza proteins, GAP-DMORIE, DPyPE | Phase 1 | [230, 231] |
| CAF01 | Statens Serum Institut | Tuberculosis | Subunit protein antigen Ag85B-ESAT, DDA, TDB | Phase 1 | [133] |
Advantages of liposome-based adjuvant systems
The success of liposomal vaccines and increasing interest in their development can be attributed to several key advantages that they offer over other particulate systems. Most importantly, liposomes are known to be safe and well tolerated, as shown through the extensive use of approved liposome-based anti-cancer and anti-infective drugs such as DoxiI® (Johnson & Johnson) and AmBisome® (Gilead Sciences) [29–31]. In addition, numerous human trials of liposomal vaccine candidates have demonstrated acceptably low reactogenicity [21–28, 32, 33]. Also, because liposomes are often composed of lipids that occur naturally in cell membranes, such as PC and cholesterol, these formulations are completely biodegradable. As this review will emphasize, another key advantage of liposome-based vaccine delivery systems is their versatility. Lipid constituents and methods of vesicle preparation can be tailored to achieve particular desired physicochemical properties of the liposome formulation [34]. Hydrophilic molecules can be encapsulated in the aqueous interior or conjugated to the vesicle surface, whereas hydrophobic compounds can be intercalated into the lipid bilayer [35]. This versatility allows antigens of all types, including peptides, proteins, carbohydrates, nucleic acids, and small molecule haptens, to be incorporated in liposome formulations with appropriate modifications in vesicle properties to accommodate antigen size and charge. Combination of immunomodulators, such as Toll-like receptor (TLR) agonists or other pattern recognition receptor (PRR) agonists, can be readily co-formulated as well.
Rationale for this article
After 35 years and over thirteen hundred publications, it has become clear that the physicochemical properties of lipid vesicles exert a dramatic influence on the nature of the resultant immune response to associated antigens. However, the relationship between physicochemical properties and immunogenicity has been challenging to define because of the difficulty in modifying one physicochemical property without perturbing others. Thus, we have carefully reviewed the evidence concerning this relationship in an attempt to identify key correlations between physicochemical properties of liposome-based vaccines (depicted in Figure 1) and the immune responses they elicit. Because of the difficulty in comparing results from studies that use different vesicle compositions, model antigen systems, and species, we have constrained our review to emphasize those studies that directly compare one or more physicochemical properties within a single experimental system in vivo. We have attempted to focus on the issues particular to liposomes and related lipid-based systems, and for information on more general topics we refer the readers to more focused reviews on those specific areas. The design principles presented herein may inform the future design of improved liposome adjuvant systems.
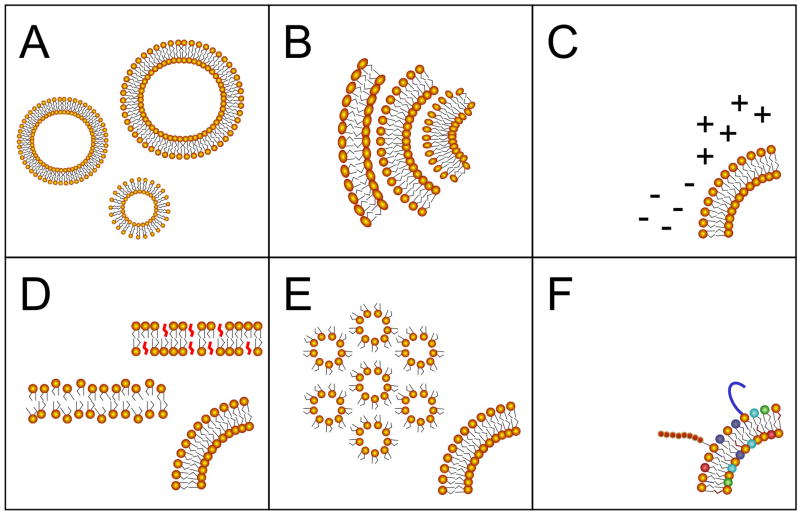
Figure 1. Liposome composition parameters that influence immune responses.
Biophysical formulation parameters that influence adjuvanticity of liposomal vaccines include (A) vesicle size, (B) lamellarity, (C) membrane surface charge, (D) bilayer fluidity (as examples, cholesterol-rich liquid ordered and cholesterol-free liquid crystal phases are shown), (E) propensity to undergo lamellar-hexagonal bilayer phase transition, and (F) presence of immunosimulatory lipids.
2. Physicochemical properties of liposomal vaccines evaluated in vivo
Properties reviewed elsewhere
Many of the properties of a successful subunit vaccine are not specific to liposomes or any particular delivery system. For example, route of administration can be critically important when seeking to induce mucosal immunity, which is remarkably compartmentalized [36]. Oral immunization elicits potent IgA secretion in the small intestine and ascending colon, but weak responses in the distal colon and female genital tract; however, nasal immunization elicits IgA secretion in the respiratory tract and female genital tract with virtually no response in the gut [37, 38]. Antigen dose is another important consideration that is determined by the age, nutritional status, and immune status of the target population [39, 40]; for example, increased doses have been found to generate significantly higher antibody responses to a trivalent inactivated influenza vaccine in adults over 65 years of age [41]. The nature of the antigen itself is critically important; larger or repetitively displayed antigens are more immunogenic, and for protein antigens T cell epitope density is critical [42–45]. In addition, vaccines can now be efficiently targeted to specific receptors on antigen presenting cells, such as DC-SIGN or DEC-205 on dendritic cells [46, 47]. Finally, particle size and shape are important determinants of the in vivo fate of a vaccine formulation, including clearance from the injection site and distribution to the lymphatic system [48]. Particle elasticity may also be important in determining trafficking of parenterally injected particulates to lymph nodes [49]. Although particle size is considered in this review, we have constrained our discussion to specific investigations of liposomes. For all of these more general topics, we refer the reader to appropriate articles (Table 2). Our intent is to focus only on those issues that are particular to lipid-based particulate formulations.
Table 2.
Formulation factors reviewed elsewhere.
| Topic | Mechanism of action | Key Refs. |
|---|---|---|
| “Traditional” molecular adjuvants | Activation of innate immunity via traditional ligands of pattern recognition receptors (Toll-like receptors, NOD-like receptors, RIG-like receptors, C-type lectin receptors) | [50–54] |
| Route of administration | Targeting a specific mucosal compartment | [36, 234] |
| Dose | Heterogeneous requirements for protective immunity of target population determined by age, immune status, nutrition | [235, 236] |
| Nature of antigen | Molecular size, T cell epitope density, amino acid composition, degradability, lipidation, conformation, phylogenetic distance | [42–45, 237] |
| Receptor targeting | Enhanced uptake by a specific subset of relevant antigen presenting cells | [46, 47] |
| Particle size, shape, and charge | Clearance from site of injection and accumulation in draining lymph nodes | [48, 238] |
In addition to the properties discussed above, agonists for pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), NOD-like receptors (NLRs), and C-type lectin receptors (CLRs), are extremely important candidate adjuvants for subunit vaccines and have been extensively investigated [50–54]. TLR and NLR agonists such as lipopolysaccharide (LPS) derivative monophosphoryl lipid A (MPL), unmethylated cytosine-phosphate-guanine motifs (CpG), polyinosinic:polycytidylic acid (poly(I:C)), and muramyl dipeptide (MDP) are commonly incorporated into liposome-based vaccines [20, 55–57]. However, because these molecules have been reviewed extensively elsewhere, we will not discuss them here. Instead, our discussion of molecular immunopotentiators is limited to molecules that are novel or particular to liposome-based systems.
Archetypal liposomal vaccines
Liposome and lipid-based nanoparticle formulations used for vaccination can be broadly grouped into several archetypal classes (summarized in Table 3). Conventional and cationic liposomes, composed of neutral or cationic lipids, respectively, and cholesterol, have been the most widely studied and afford the greatest versatility – desired formulation parameters can be achieved through modification of the lipid composition or vesicle preparation method [18, 20, 58, 59]. For delivery of plasmid DNA, which can act both as antigen (through encoded proteins) and adjuvant (through stimulation of TLR9 via CpG motifs), the nucleic acid is typically mixed with a cationic lipid to form an electrostatic complex (lipoplex) [60]. Greater formulation control may be achieved by condensing the DNA with a polycation prior to addition of pre-formed vesicles, which in some cases results in formation of a bilayer structure surrounding the DNA-polycation complex [61]. Virosomes, as mentioned in the previous section, are a special class of vesicles prepared from reconstituted influenza virus membranes supplemented with PC [62, 63]. The physicochemical features of virosomes are constrained by their well-defined composition and method of preparation, but these vesicles benefit greatly from the inherent delivery properties (efficient cell binding, internalization, and cytosolic release) and immunogenicity of the influenza virus. Other specialized formulations include archaeosomes (prepared from polar glycerolipids extracted from Archaea), which are inherently immunostimulatory, and niosomes (nonionic surfactants with cholesterol), which have been investigated for topical delivery [64–66]. This review will focus mainly on conventional and cationic liposomes, as well as lipid-DNA particles of various types.
Table 3.
Archetypal liposome vaccine formulations
| Vesicle type | Composition | Key attributes | Refs. |
|---|---|---|---|
| Archaeosome | Polar glycerolipids obtained from Archaea via CHCl3/MeOH/H2O extraction. Typical archaeal lipids contain two ether-linked phytanyl groups, with or without one of many different possible head groups. | Induction of TH1 and cell-mediated responses without addition of TLR agonists. Ether lipid backbone confers stability but biodegradability is not well understood. | [64] |
| Cationic liposome | A cationic lipid (e.g. DOTAP, DDA, DC-Chol), typically with a neutral helper lipid (e.g. Chol, PC) and/or an immunomodulator (e.g. MPL, TDB). An exemplary formulation is CAF01 (DDA and TDB). | Binding and uptake by APCs strongly favored due to electrostatic interactions. Retained at the injection site longer than uncharged formulations. Some cationic lipids are inherently immunostimulatory (e.g. DOTAP). | [58, 59] |
| Conventional liposome | One or more neutral or anionic lipids (PC, PG, Chol), typically with an immunomodulator (e.g. MPL). | Greatest versatility in formulation parameters and modes of antigen incorporation. Immunogenicity is typically weak without specific addition of immunomodulators. | [18, 20] |
| Lipoplex | A cationic lipid electrostatically complexed with plasmid DNA, often with a helper lipid (e.g. DOPE, Chol). | DNA component may encode the antigen or act as an adjuvant through TLR9 activation. Standard formulation for delivery of lipid-based nucleic acid vaccines. | [60] |
| Lipopolyplex | Cationic liposomes mixed with pDNA that has been pre-condensed with a polycation (e.g. protamine). An exemplary formulation is LPD (DOTAP, protamine, pDNA). | As compared to conventional lipoplexes, the double bilayer of LPD confers greater stability and structural homogeneity. | [61] |
| Niosome | Cholesterol and a single-alkyl chain nonionic surfactant (e.g. GMS, SMS, T20) | Suggested as inexpensive, simple alternatives to conventional liposomes. Like liposomes, generally immunologically inert without added immunomodulators. Widely investigated for topical vaccination. | [65, 66] |
| Virosome | Reconstituted influenza virus membranes (phospholipids, HA, NA) supplemented with PC. | Efficient cell binding, internalization, and endosomal release due to presence of HA. Highly immunogenic due to presence of HA and NA. | [62, 63] |
Antigen attachment method
One of the most critical parameters influencing the immunogenicity of liposomal vaccines is the method by which the antigen is physically or chemically associated with the formulation. The most common modes of association include covalent lipid conjugation (either pre- or post-vesicle formation), non-covalent surface attachment (via biotin, NTA-Ni(II)-His6, or antibody-epitope interactions), encapsulation, and surface adsorption (Figure 2). Many of the early investigations of liposomal peptide and protein antigenicity in mice compared encapsulated antigens to those conjugated to the surface of pre-formed liposomes. Collectively, these studies by Alving, Gregoriadis, Therien, and others confirmed that both methods are generally effective for inducing antibody and T cell responses to associated protein antigens such as albumin and tetanus toxoid [67–69]. In some cases, covalent antigen conjugation results in superior antibody induction [70–74], which is not surprising because B cell receptors can recognize intact antigen on the liposome surface [74]. As the size and complexity of the antigen decreases, the benefit of surface conjugation for antibody induction becomes more pronounced. Synthetic peptides for which surface conjugation provides superior immune responses to encapsulation include those derived from viral antigens (HIV-1 gp120, HSV glycoprotein D) and tumor antigens (MUC1) [70, 74–77].
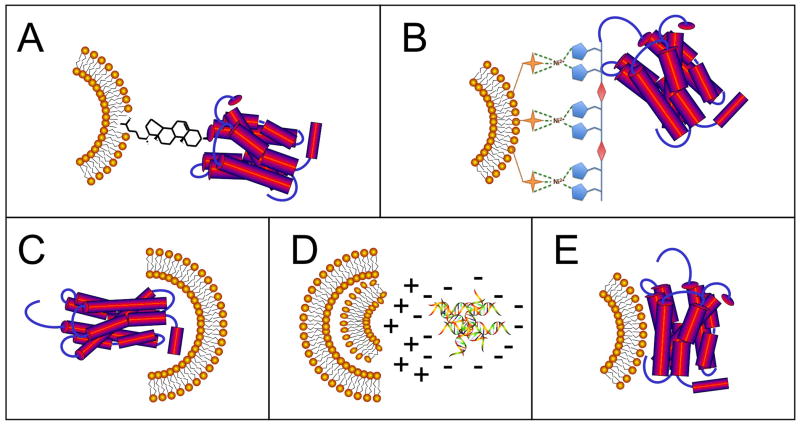
Figure 2. Modes of antigen attachment to lipid nanoparticles.
Antigens may be (A) covalently conjugated to the liposome surface via a lipid moiety (as an example, cholesterol is shown), (B) non-covalently attached to the liposome surface (as an example, NTA-Ni(II)-His6 is shown), (C) encapsulated in the aqueous interior, (D) electrostatically complexed with lipids of opposing charge, or (E) adsorbed to the liposome surface or reconstituted in the liposomal membrane.
Helper and cytotoxic T cell responses to liposome-associated antigens are also highest when the antigen is conjugated to the vesicle surface as compared to encapsulated in the aqueous interior, but the benefit is not as great as that observed for antibody induction. For example, Therien and coworkers demonstrated that surface-linked conalbumin (via SPDP) elicited 50% greater proliferation of restimulated splenocytes than encapsulated conalbumin, despite immunization with 5-fold higher dose of encapsulated antigen [72]. In a follow-on study, the surface-conjugated formulation also increased splenocyte IFN-γ production [69]. Chen and Huang also observed a benefit when a peptide derived from human papilloma virus protein E7 was palmitoylated at the N-terminus and incorporated into cationic liposomes [78]. Palmitoylation increased E7-specific CTL responses 2-fold over unconjugated peptide. This study also emphasized the importance of the antigen-lipid linkage; addition of a Lys-Ser-Ser linker to E7 resulted in enhanced affinity for MHC I. In contrast, other studies have demonstrated parity between surface conjugation and encapsulation for elicitation of antigen-specific splenocyte proliferation, IFN-γ secretion, and CTL cytotoxicity for peptide and protein antigens [68, 74, 75, 79].
For covalently conjugated peptide and protein antigens, the conjugation chemistry and structure of the lipid anchor can also influence the magnitude and TH balance of the response. Nakano and colleagues observed that ovalbumin (OVA) conjugated to liposomes via amino groups (via glutaraldehyde or DSS) elicited anti-OVA IgG, but not IgE, in sera of mice [80]. By contrast, OVA conjugated to liposomes via thiols or amines derivatized to thiols (via EMCS or SPDP) elicited serum anti-OVA IgE, which correlated with splenocyte IL-4 production. In addition, orthogonal chemistries can be used to conjugate multiple peptide or protein antigens to a single formulation. As demonstrated by Garçon and Six, liposomes provide “conjugation by proxy” in the sense that chemical conjugation of a haptenic antigen to a T helper epitope is not required to elicit a T-dependent immune response if both the hapten and the T helper epitope are co-formulated in the same liposome preparation [81, 82]. In one example, Boeckler et al. sequentially conjugated two thiol-containing peptides to a single liposome; a TH epitope was coupled to a maleimide-functionalized lipid at pH 6.5, and a B cell epitope was attached to a bromoacetylated DPPE lipid after adjusting the pH of the formulation to 9.0 [83]. In that study, changing the TH epitope anchor from Pam3Cys-Ala-Gly to DPPE-PEG3 resulted in antibody titers that were significantly lower at peak and declined more rapidly over time, underscoring the importance of the TLR2-activating Pam3Cys moiety. For self-adjuvanting lipopeptide anchors such as Pam3Cys, care must be taken to ensure that the conjugation scheme does not disrupt the immunostimulatory properties of the anchor [84]. It should be noted that the need for orthogonal chemistry is eliminated if lipopeptide conjugates are prepared prior to liposome formation.
The structure of the covalent lipid anchor is a critical parameter for induction of maximal antibody responses to peptide antigens. White et al. compared cholesterol and palmitate anchors for elicitation of antibodies and CTL responses in mice to a peptide derived from the V3 loop of HIV-1 gp120 [74]. Covalent anchorage (via either palmitate or cholesterol) was superior to antigen encapsulation for antibody induction in that study, generating a 2-fold increase in antibody titer. Further, the cholesterol linkage elicited slightly higher antibody titers than N-terminal palmitoylation. The site of lipid conjugation was important; C-terminally palmitoylated peptide failed to elicit an antibody response. To clarify the role of lipid structure, Watson and Szoka recently measured the anti-peptide serum IgG response in mice to hydrophilic and hydrophobic peptides derived from the membrane proximal external region of HIV-1 gp41, conjugated to a series of sterols, fatty acids, and phospholipids [85]. All conjugates were retained in a conventional MPL-adjuvanted liposome formulation in an ultracentrifugation sedimentation assay. However, some lipid anchors, in particular cholesterol hemisuccinate and phosphatidylethanolamine, elicited far higher antibody responses than others in sera of mice. Notably, palmitate, which is historically the most common lipid anchor for peptide antigens, gave the weakest response.
More recently, several groups have investigated stable non-covalent linkages, such as avidin-biotin and nitrilotriacetic acid (NTA)-hexahistidine, for attachment of antigens to pre-formed vesicles. If successful, these strategies could decrease the complexity and cost of producing liposomal vaccines by eliminating chemistry and purification steps. For example, attachment of HIV-1 gag p24 to the surface of solid lipid nanoparticles via an NTA lipid elicited greater antigen-specific serum IgG responses than a formulation in which the antigen was adsorbed to the particle surface [86]. van Broekhaven and Altin reported that a trivalent NTA lipid could be used to attach His-tagged targeting ligands and co-stimulatory molecules (B7.1 and CD40) to a liposomal tumor vaccine, resulting in a measurable improvement in antitumor effect in a murine model [87]. More recently, His-tagged Candida albicans hsp90 was attached to the surface of NTA-containing liposomes adjuvanted with MDP. When administered to mice, these formulations elicited anti-hsp90 serum IgG and splenocyte IFN-γ production comparable to hsp90 emulsified in Freund’s adjuvant [88]. However, further studies are needed to determine if vaccine formulations containing non-covalently linked antigens can match or exceed the immunogenicity of preparations in which the antigen is covalently attached to a lipid anchor. For example, Watson et al. showed that covalent linkage to a lipid anchor was superior to NTA-Ni(II)-His6 attachment for elicitation of antibodies to OVA or to synthetic peptides derived from the MPER of HIV-1 gp41 [89].
Surface adsorption, via electrostatic interactions, is another useful method for liposomal association of antigens, primarily for proteins. The recent work of Christensen, Perrie, and others with proteins antigens adsorbed to cationic DDA/TDB liposomes has demonstrated that formulations with surface-adsorbed antigens can be highly stable and elicit robust antibody and cell-mediated responses in mice and ferrets [18, 90–93]. However, adsorption has not been comprehensively compared to other modes of association. Two underexploited strategies for antigen-liposome conjugation are avidin-biotin and post-translational lipidation of recombinant proteins (via glycophosphatidylinositol, for example). Phillips et al. reported that avidin-biotin interactions could be used to aggregate antigen-loaded liposomes in draining lymph nodes by sequential injection of biotinylated liposomes followed by avidin [94]. This procedure increased retention of liposome material in the draining lymph node by up to 10-fold following subcutaneous administration. McConville and colleagues observed that the predominant cell surface glycogonjugate antigen of Leishmania major contained a GPI-like membrane anchor. When they reconstituted this antigen in multilamellar vesicles and immunized mice, the animals were protected from cutaneous leishmaniasis [95]. Similarly, detergent-soluble membrane protein antigens can be reconstituted in phospholipid vesicles by detergent dialysis methods [96]. This approach can sometimes be used to renature purified membrane proteins to achieve a vaccine-competent conformation, as has been shown for Neisseria meningitides PorA [97, 98].
Size and lamellarity
Vesicle size and bilayer structure are important factors in liposomal vaccine design. As mentioned earlier, general considerations for the role of particle size in uptake and trafficking of particulate subunit vaccines are extensively reviewed elsewhere [48]. However, several studies have been performed that specifically focus on liposomal vaccine size and these merit discussion. Brewer et al. evaluated the effect of vesicle size (100 ± 10 nm, 155 ± 10 nm, 225 ± 25 nm, and 560 ± 60 nm diameter) on the serum anti-OVA antibody response in mice immunized subcutaneously with OVA encapsulated in MPG:DCP:Chol liposomes [99]. All formulations elicited equivalent anti-OVA IgG1, but larger vesicles elicited significantly higher IgG2a, as well as greater IFN-γ production by restimulated lymph node cells, in an IL-12-dependent manner. Differential uptake by B cells or macrophages did not account for the difference between 155 nm and 225 nm vesicles. In vitro, only large vesicles (diameter ≥ 225 nm) elicited IL-12 production in peritoneal macrophages, whereas smaller vesicles elicited greater IL-1β. Similar results were reported in another study in which influenza A hemagglutinin was entrapped in liposomes of either 250 nm or 980 nm in diameter [100]. Henriksen-Lacey et al. investigated the immune response to DDA:TDB vesicles of <200 nm, 700 nm, and 1.5 μm in diameter with an adsorbed protein antigen derived from M. tuberculosis antigen [101]. When these formulations were administered intramuscularly to mice, vesicles of increasing size trended toward greater accumulation in the draining lymph node. Of the formulations studied, 700 nm diameter vesicles elicited the greatest IFN-γ secretion by restimulated splenocytes. Collectively, these studies suggest that vesicles 250–700 nm in diameter skew the TH profile of the response toward TH1 and increase both persistence at the injection site and transit to draining lymph nodes.
Vesicle lamellarity may also influence the immune response against liposome-associated antigens. Shek and coworkers compared multilamellar and unilamellar vesicles of equivalent size composed of lecithin:DCP:Chol in which bovine serum albumin (BSA) was encapsulated as a model antigen [102]. BSA-specific IgG plaque-forming cells were approximately 2-fold greater in spleens of mice receiving unilamellar vesicles as compared to those receiving multilamellar preparations. More recently, Bhowmick et al. compared the immune response in mice to leishmanial membrane antigen encapsulated in multilamellar vesicles (MLVs), dehydration-rehydration vesicles (DRVs), and reverse phase-evaporation vesicles (REVs) [103]. MLVs elicited higher antigen-specific serum IgG1, whereas DRVs and REVs elicited higher IgG2a and splenocyte IFN-γ. However, because the unilamellar DRVs and REVs were twice the diameter of the MLVs (900nm and 400 nm, respectively), vesicle size may have also influenced the outcome of the study. In addition, MLV preparations may vary with regard to the number of lamellae per vesicle, typically as many as 10 [104]; unappreciated differences may exist in the immune response potentiated by MLVs of varying lamellarity.
Charge
Several studies have directly compared antigen-specific immune responses elicited by positively charged, negatively charged, and neutral liposome formulations with similar lipid compositions. Kraaijeveld measured virus-neutralizing antibodies elicited in serum by UV-inactivated encephalomyocarditis and Semliki Forest viruses admixed with positively charged (DPPC:ODA:Chol), negatively charged (DPPC:PA:Chol), or neutral (DPPC:Chol) liposomes and administered intraperitoneally to mice [105]. Formulations that contained charged vesicles (either positive or negative) elicited greater neutralizing antibody responses than neutral vesicles, but the difference between positive and negative formulations was minimal. Nakanishi assessed antigen-specific antibody and cytotoxic T cell responses in spleens of mice following administration of OVA or diphtheria toxin encapsulated in positively charged (PC:Chol:SA), negatively charged (PC:Chol:PA), or neutral (PC:Chol) liposomes [106]. In the OVA study, only positively charged liposomes elicited an OVA-specific CTL response. The positively charged formulation also elicited the greatest anti-OVA serum IgG1 response, followed by negatively charged liposomes, with neutral liposomes eliciting the weakest response. In a follow-on study, only the positively charged formulation elicited a specific CTL response to a second model antigen, β-galactosidase [107]. The authors attributed this observation to greater cytoplasmic release of proteins encapsulated in cationic formulations, which was confirmed by cell culture studies. In an unrelated model system, Badiee and colleagues compared the ability of neutral (DPPC:Chol), negatively charged (DPPC:Chol:DCP), and positively charged (DPPC:Chol:DDAB) large multilamellar vesicles to elicit serum antibodies to L. major rgp63 protein in mice [108]. The cationic formulation elicited the greatest antigen-specific IgG1 and IgG2, whereas neutral liposomes elicited the weakest antibody response. Interestingly, the neutral vesicles elicited the greatest IFN-γ production by restimulated splenocytes, and animals receiving the neutral formulation had the lowest parasite burden and least swelling following a footpad challenge. Collectively, these studies support the conclusion that cationic vesicles promote stronger antigen-specific serum antibody responses than otherwise equivalent neutral or anionic formulations, but antibody and cell-mediated response are not always correlated.
Membrane fluidity
The effects of lipid gel-liquid crystal transition temperature and membrane phase behavior on immune responses to liposome-associated antigens have been investigated extensively in several model systems. In the earliest studies, Yasuda et al. compared the ability of liposomes composed of PCs with various transition temperatures (DOPC, −19 °C; DLPC, −2 °C; DMPC, 23 °C; DSPC, 54 °C) to elicit antibodies to a lipid-linked dinitrophenyl hapten (DNP-Cap-PE), as measured by quantification of spleen antibody-secreting cells in a plaque assay [109]. All formulations contained cholesterol (2:1.5:0.2:0.1 PC:Chol:DCP:DNP-Cap-PE). In that study, it was determined that higher transition lipids gave higher anti-DNP antibody response over a 4-fold range. This pattern was maintained when the formulations contained lipid A (although overall response magnitudes were 10-fold higher), emphasizing the importance of bilayer phase behavior even in the presence of a potent immunostimulator. In the same experimental system, the group later compared formulations with mixed compositions of low transition temperature (DOPC) and high transition temperature (DSPC) lipids [110]. Titration of high transition temperature lipids into the formulation increased the antibody response over a 4-fold range beginning at ~50% DSPC. Again, a clear correlation between lipid transition temperature and antibody response was observed for DSPC, DMPC, DLPC, and DOPC; the presence of cholesterol up to 50% did not abolish this relationship. A more recent study by a different group also observed an effect of lipid transition temperature on anti-DNP antibody response on mice, but the correlation was not quite as clear, with lipids of an intermediate transition temperature (DPPC, 42 °C) being optimal [111]. In contrast, T cell responses against another lipid-linked hapten (p-azobenzenearsonate), as measured by footpad DTH response, was unaffected by varying either the PC transition temperature or the presence of cholesterol. The influence of lipid transition temperature on antibody responses to liposome-associated protein antigens has also been investigated. Bakouche et al. encapsulated Gross cell surface antigen (GCSA) in liposomes and measured GCSA-specific serum antibody responses following immunization of rats [112]. Liposomes composed solely of DSPC failed to elicit a GCSA-specific response, but inclusion of cholesterol at varying levels enabled immunogenicity over a 10-fold range with an optimum at 20 mol% cholesterol. Inclusion of 12.5 mol% negative charge, whether DCP or DPPG, was optimal. A final optimal formulation of 7:2:1 PC:Chol:DCP was selected, influenced also by the precedent of the 7:2:1 EPC:Chol:DCP formulation used by Allison and Gregoriadis [16]. In the 7:2:1 molar ratio format, a convincing correlation between PC transition temperature and antibody response was observed, with DSPC eliciting the strongest response (DSPC > DPPC > PLiPC > DOPC > DLiPC). For PCs with saturated acyl chains, chain length correlated well with serum antibody responses (C18 > C17 > C16 > C14 > C12). Titration of greater than 25 mol% shorter chain PC into a DSPC formulation significantly inhibited the antibody response. A similar study by Kersten compared the ability of vesicles composed of EPC, DPPC, or DSPC to elicit antibody responses to a membrane protein antigen from N. gonorrhoeae [113]. In that study, DSPC-containing formulations were found to be superior. In addition, the authors evaluated a matrix of formulations containing varying amounts of cholesterol and established an inverse relationship between membrane fluidity (as measured by fluorescence polarization) and serum antibody responses. Studied by Kahl, Garnier, and others have also reported the superiority of DSPC-containing formulations for elicitation of CTL responses to a variety of membrane-associated and entrapped soluble protein antigens [114–117].
Several noteworthy exceptions to this trend have been reported. A series of studies by Gregoriadis and colleagues reported a minimal effect of transition temperature of constituent phospholipids (in the range of −32 °C to 41.5 °C) on the serum antibody response to tetanus toxoid entrapped in dehydration-rehydration vesicles or covalently conjugated to the surface of multilamellar vesicles [67, 118]. The cause of this discrepancy is unknown, but could be explained in part by antigen-specific effects arising from the potent intrinsic adjuvanticity of tetanus toxoid [119]. Also, Nakano et al. reported that liposomes composed of lower phase transition lipids elicited higher serum IgG in mice to covalently attached OVA when compared to formulations containing higher phase transition lipids [120, 121]. However, the results of the studies were inconsistent; an inverse relationship between antibody response and membrane fluidity was observed when cholesterol was titrated into DPPC liposomes. Setting aside these exceptions, the totality of the literature clearly indicates that liposomes of greater rigidity and higher gel-liquid crystal transition temperature elicit higher antibody and cell-mediated responses to a variety of encapsulated and surface-associated antigens.
Fusogenicity
The incorporation of certain constituent lipids endows a liposome formulation with the ability to fuse with the plasma membrane or endosomal membrane, releasing associated cargo into the cytosol. Liposome-virus hybrid particles may fuse with the plasma membrane directly (virosomes are a well-characterized example), whereas pH-sensitive formulations disrupt endosomal compartments as they acidify [62, 122, 123]. Fusogenicity has been widely exploited to increase the efficiency of carrier-mediated gene delivery, and vaccinologists have hypothesized that increased cytosolic delivery may also increase the magnitude of immune responses, particularly CTL responses, to liposome associated antigens. When liposome-encapsulated OVA was injected intravenously into mice, Nair et al. observed that pH-sensitive liposomes (containing DOPE) stimulated CTL responses up to 5-fold more efficiently than non-pH-sensitive formulations [124]. Cationic liposomes are also well known to disrupt endosomal membranes and promote cytosolic release [125, 126], which likely contributes (in addition to cell and tissue binding) to the superior immunogenicity of cationic formulations as compared to anionic or uncharged formulations, as discussed earlier. In some cases fusogenicity is conflated with other important factors. For example, Ahmad et al. reported superior antibody and CTL responses against encapsulated Salmonella antigens elicited by a fusogenic formulation prepared from an E. coli lipid extract as compared to a non-fusogenic PC formulation [127]. However, the potent immunostimulatory potential of LPS and other E. coli membrane components was not accounted for. Other studies have attributed the immunostimulatory potential of lipid extracts from viruses [128] or fungi [129] to fusogenicity without sufficient consideration of other factors. Fusogenicity is particularly critical for nucleic acid vaccines because access to the cytosol (and for DNA, the nucleus) is required for expression of the encoded antigen. In one specific example, addition of fusogenic PE also increased the antibody response in mice to hepatitis B surface antigen encoded by a liposomal DNA vaccine [130]. Collectively, the studies suggest that fusogenicity does increase the capacity of liposomes to promote immunity to associated antigens but conflating factors must be taken into account.
Novel immunostimulatory lipids
Classical TLR and NLR agonists such as MPL, MDP, CpG, and poly(I:C) have been incorporated into liposomal vaccines for decades. These molecules have been studied extensively; for more information on these, the reader is referred to pertinent reviews [50–54]. Rather, this review will highlight some of the other lipophilic molecules that have gained attention recently as candidate immunomodulators for inclusion in liposomal vaccines and immune-modulating therapies (Table 4). Many such lipids are derived from bacteria or other natural sources; a principal example is trehalose dibehenate, a synthetic analog of mycobacterial cord factor [131, 132]. This molecule, which was recently identified as an agonist of the C-type lectin receptor Mincle, is a component of the potent cationic liposomal adjuvant ‘CAF01’ [133]. CAF01 is reviewed in detail elsewhere [18]. Other naturally derived lipid adjuvants include glycolipids that bind CD1d and activate invariant NKT cells, such as α-galactosylceramide, a glycosphingolipid first isolated from the marine sponge A. mauritianus [134–136].
Table 4.
Novel and emerging immunostimulatory lipids
| Name | Origin | Mechanism | Refs. |
|---|---|---|---|
| All-trans retinoic acid | Vitamin A metabolite | RAR agonist | [150, 239] |
| DiC14-amidine | Synthetic cationic transfection reagent | TLR4 agonist | [142, 143] |
| Docosahexaenoic acid | Polyunsaturated ω-3 fatty acid | GPR120 agonist | [151, 155] |
| DOTAP | Synthetic cationic transfection reagent | Induction of reactive oxygen species, subsequent activation of ERK and p38 | [139–141] |
| α-galactosylceramide | Glycosphingolipid first isolated from A. mauritianus | CD1d agonist | [134, 135] |
| Lauric acid | Saturated fatty acid | TLR4 agonist | [151] |
| Lysophosphatidylcholine | Endogenous lysophospholipid | Upregulation of MHC II, CD86; secretion of chemokines | [169] |
| Palmitic acid | Saturated fatty acid | Activation of NLRP3-ASC inflammasome | [153] |
| Phosphatidylserine | Phospholipid found in cytoplasmic leaflet of plasma membrane | Induction of TGF-β, possibly via PI3K-ERK | [158, 159] |
| Sphingosine-1-phosphate | Endogenous | Agonist of S1PR receptor family | [163–165] |
| Trehalose dibehenate | Synthetic analogue of mycobacterial cord factor | Mincle agonist | [131–133] |
One exciting recent development is the discovery of specific adjuvant activity of certain cationic lipids originally synthesized as transfection reagents. Lipid-protamine-DNA complexes, initially conceived as non-viral gene delivery vectors, were observed to induce robust IFN-γ and TNF-α responses in serum and anti-tumor immunity in murine models of pulmonary metastasis and fibrosarcoma [137, 138]. Aside from the presence of unmethylated CpG motifs within associated plasmid DNA, it was also observed that DOTAP, a cationic lipid used for many years as a staple of non-viral gene delivery [139], exerted an enantiospecific adjuvant effect in dendritic cells through induction of reactive oxygen species and downstream activation of ERK and p38 pathways [140, 141]. Another cationic transfection reagent, diC14-amidine, was recently reported to be an agonist for TLR4 in mouse and human dendritic cells in vitro [142, 143], though in a mouse model diC14-amidine appears to interact with serum lipoproteins to antagonize CpG- and LPS-induced inflammation [144, 145].
Vitamin A and its metabolites, most notably all-trans retinoic acid (ATRA), are unique molecules that exert a diverse array of pleiotropic immunologic effects, both stimulatory and suppressive. ATRA plays a key role in immunosuppression through induction of regulatory T cells [146], but also primes gut immunity by promoting lymphocyte gut homing and mucosal IgA secretion [147]. This dichotomy may be explained through cooperation of ATRA with certain pro-inflammatory cytokines to shift the balance of gut immunity from tolerance to inflammation [148]. Liposomal formulations of ATRA have long been used for therapy of acute promyeolocytic leukemia [149]. More recently, Watson et al. reported that a liposomal vaccine containing ATRA, MPL, and a lipopeptide antigen elicited 3-fold greater antigen-specific antibody response in mice as compared to a formulation lacking ATRA [150], and further studies are needed to sort out the intracellular signaling that dictates the influence of ATRA on the balance of tolerance and immunity.
The ability of endogenous lipids to modulate immunity is being increasingly appreciated. Saturated and polyunsaturated fatty acids exert reciprocal effects on dendritic cells in vitro, with saturated fatty acids seemingly promoting pro-inflammatory signaling through several mechanisms. Weatherill and coworkers reported that lauric acid (dodecanoic acid) induced cytokine secretion and costimulatory molecule expression of dendritic cells in a manner dependent on TLR4 signaling [151], although subsequent studies indicate that saturated fatty acids do not activate TLRs directly [152]. More recently, Wen et al. demonstrated that saturated palmitic acid (hexadecanoic acid), but not unsaturated oleic acid, activates the NLRP3-ASC inflammasome through induction of reactive oxygen species and MAP kinase signaling [153]. Conversely, polyunsaturated ω-3 fatty acids antagonize many of the same pathways in vitro and in vivo. For example, Weatherill and others have found that the ω-3 fatty acid docosahexaenoic acid (DHA) inhibits LPS-induced dendritic cell activation in vitro [151, 154], perhaps in part through attenuation of NF-κB signaling [155], and reduces the ability of macrophages to control M. tuberculosis infection through impaired IFN-γ signaling [156]. In vivo, dietary DHA suppresses inflammation and autoimmunity in murine models of colitis and experimental autoimmune encephalomyelitis [154, 157]
Other endogenous lipids with immunomodulation activity include phosphatidylserine and lysophospholipids. Phosphatidylserine was widely used in liposomal vaccine formulations before its anti-inflammatory properties were well known. PS is a component of the cytoplasmic leaflet of the plasma membrane and is exposed on the cell surface during apoptosis. PS is recognized by a specific receptor on macrophages as part of the process of clearance of apoptotic cells, leading to production of TGF-β and induction of an anti-inflammatory state in macrophages [158, 159]. Nonetheless, in some cases PS exhibited a beneficial adjuvant effect; in one instance PS liposomes elicited a higher OVA-specific serum IgG titer against surface-associated OVA when compared an analogous PC-containing formulation [160]. Lysophospholipids such as lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) are also important regulators of innate and adaptive immunity [161, 162]. Although S1P has long been known to regulate T lymphocyte trafficking [163], it has more recently been found to modulate the phenotypes of dendritic cells and macrophages [164, 165]. LPA has chemotactic effects on T cells and dendritic cells [166, 167], and also may promote dendritic cell maturation [168]. Lysophosphatidylcholine (LPC) has also been reported to induce DC maturation in vitro and elicit cytotoxic T cell responses to co-administered antigens in vivo [169]. The adjuvant actitivities of these lysophospholipids have not been thoroughly studied, and the mechanistic basis for immune modulation by fatty acids, phospholipids, and other endogenous lipids are only beginning to be understood.
3. Mechanistic basis for liposome-mediated adjuvanticity
The influence of liposome formulation parameters on immunogenicity can be viewed in the context of the steps necessary for antigen uptake, trafficking, processing, and presentation. In the sections below, the mechanistic contributions of vesicle properties to these processes are discussed.
Trafficking of liposomal antigens from the injection site
Formulation parameters play an important role in the retention of liposomes and associated antigens at the site of injection and subsequent trafficking to draining lymph nodes, which in turn influences the magnitude of the antigen-specific immune response (Figure 3). A series of studies by Oussoren, Storm, and colleagues described the biodistribution of radiolabeled liposomes (labeled with [3H]-cholesteryloleylether) following subcutaneous administration to rats. They observed that small (100 nm) anionic liposomes comprised of EPC:EPG:Chol accumulated in regional lymph nodes following subcutaneous administration in the footpad or dorsal side foot, with approximately 2% of the injected lipid dose detected in lymph nodes after 52h [170]. Strikingly, 20–30% of the injected dose was observed in the blood at peak concentration (2–4h post injection), while 5% and 15% of the injected dose was recovered in the spleen and liver, respectively. Liposome retention at the injection site showed clear size dependence; only 20% of a 40 nm liposome dose remained at a subcutaneous injection site after 52h, whereas over 80% of a 400 nm liposome dose remained, with 70 nm and 170 nm liposomes exhibiting an intermediate level of retention [171]. Interestingly, vesicle size had essentially no influence on the fraction of the radioactive lipid dose recovered in regional lymph nodes. An important caveat to these studies is that they do not determine what fraction of the radioactivity is associated with intact liposomes and what fraction is degraded or metabolized. However, in comparing the distribution of liposome encapsulated and free radiolabel from a subcutaneous injection site, Harrington et al. found that pegylated liposomes containing encapsulated 111In-DTPA retained significantly more radioactivity than unencapsulated 111In-DTPA at both the injection site (518-fold increase in total exposure) and in local lymph nodes (88-fold increase at 24 h and 564-fold increase at 72 h), suggesting little retention of unencapsulated radiolabel at these sites [172].
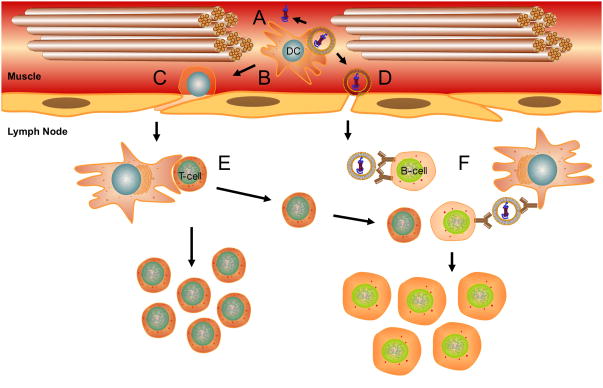
Figure 3. Uptake and trafficking of liposomal antigens.
Antigen uptake and trafficking events influenced by liposome properties include (A) persistence at the injection site and release of associated antigen, (B) internalization by resident or infiltrating APCs, (C) migration of APCs with phagocytosed antigen to LNs, (D) drainage of free vaccine particles to LNs, (E) processing of phagocytosed antigen in APCs and presentation to T cells, and (F) presentation of intact antigen to B cells.
Oussoren et al. later reported that substitution of PS, DPPC, or DPPG into the formulation did not influence extent of retention at the injection site (with a lone exception – liposomes composed solely of DPPC exhibited a slight increase in retention at the injection site). Also, ‘DPPC only’ liposomes and PS-containing formulations exhibited an approximately 3-fold increase in lymph node accumulation. When macrophages were depleted prior to injection of radiolabeled liposomes, lymph node accumulation was significantly decreased, particularly for large vesicles, emphasizing the importance of phagocytic cells in facilitating liposome trafficking to lymph nodes [173]. More recent studies in the CCS/C and DDAB/TDB systems have confirmed that persistence at the injection site and accumulation in lymph nodes are important determinants of immunogenicity [91, 92, 101, 174]. Henriksen-Lacey et al. also observed that cationic charge and large vesicle diameter dramatically increase retention at the injection site, and the benefits were greater for some cationic lipids (DDA, DC-Chol) than others (DOTAP) [91, 92, 101].
Several additional factors could affect the stability of liposomal vaccines in vivo. The size and charge dependence of liposome immunogenicity could be influenced by complement-mediated vesicle destabilization. Richards, Bradley, and others have demonstrated complement-dependent disruption of liposomal membranes, with lysis being greatest for small, anionic vesicles [175, 176]. Also, cholesterol is known to exchange readily between liposomes and biomembranes in biological fluids [177]. Depletion of cholesterol could cause vesicles to aggregate in vivo [178], effectively increasing their size and thus their retention at the injection site. Conversely, steric stabilization of vesicles through incorporation of polyethylene glycol-functionalized lipids could inhibit vesicle aggregation and tissue binding, causing more rapid drainage to lymph nodes. Kaur and colleagues reported that the addition of 25 mol% PEG lipid to a DDA/TDB formulation reduced antigen retention at the injection site approximately 10-fold when measured 24 h after injection [179].
Uptake and processing of liposomal antigens
Although encapsulated and surface-associated liposomal antigens induce T cell responses equivalently, many studies have shown increased antibody induction mediated by surface-associated antigen [70–74]. This may be because surface-conjugated antigen is available on the particle surface for antibody or B cell receptor (BCR) recognition, whereas encapsulated antigen requires some measure of processing or vesicle disruption to be accessible [74, 180]. For surface-associated antigens, B cells may recognize intact liposomal antigen directly or via opsonized liposomes bound to Fc receptors or complement receptors on antigen presenting cells [181].
Liposome formulation and antigen conjugation also influence the intracellular processing and presentation of T cell epitopes within antigen presenting cells (Figure 4). Cationic liposomes are internalized by macrophages and dendritic cells (and virtually all other cell types) more efficiently than neutral or anionic liposomes due electrostatic interactions with the anionic plasma membrane [182, 183]. This effect may be enhanced by immunostimulatory properties of some cationic lipids that promote phagocytosis [140, 143]. At high charge densities, anionic vesicles are also internalized to a greater extent than neutral vesicles, an effect sometimes attributed to scavenger receptor-mediated uptake [184].
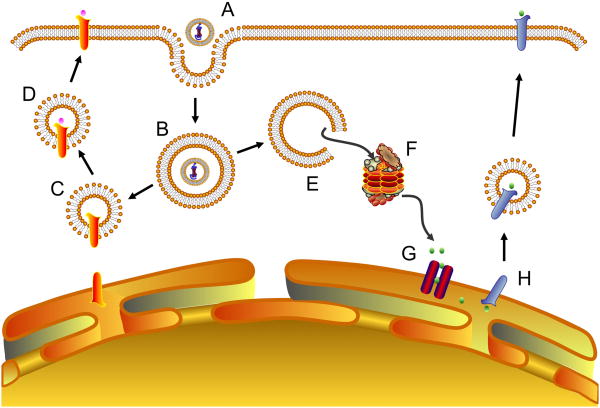
Figure 4. Intracellular processing of liposomal antigens.
Intracellular antigen processing events influenced by liposome properties include (A) cell binding, (B) internalization, (C) fusion with MHC II-containing organelles, (D) loading onto MHC II molecules followed by trafficking to the cell surface for antigen presentation, (E) escape from endosomes to the cytosol, (F) proteasomal degradation, (G) transit to the ER, and (H) loading onto MHC I molecules followed by trafficking to the cell surface for antigen presentation.
An additional benefit of cationic liposomes is increased cytosolic delivery through disruption of endosomal and phagosomal membranes. Membrane disruption is proposed to occur by lateral diffusion of anionic lipids from the lumenal leaflet of the endosomal membrane into the contact zone with the liposome bilayer, forming charge-neutralized ion pairs [185]. Cationic lipids with headgroups containing titratable amines may also buffer protons during endosomal acidification, leading to Cl− accumulation and osmolysis [186]. Cytoplasmic release may also be accomplished through inclusion of pH-sensitive lipids such as DOPE that promote lamellar-to-hexagonal phase transition as the endosome acidifies [187].
Cytosolic delivery has been shown to augment MHC I presentation of associated antigens in vitro [188, 189] and increase antigen-specific T cell responses in vivo [124, 130] as compared to non-fusogenic formulations. A series of studies by Alving and coworkers has illuminated the pathways by which liposomal carriers direct associated antigens to the MHC I pathway. Peptide fragments derived from liposome-encapsulated proteins were detected in the trans-Golgi of macrophages in a TAP-dependent manner [190]. Transport of peptides to the trans-Golgi was dependent on TAP, functional microtubules, and proteasomal activity [190–192], suggesting liposome-associated antigens are processed by a classical MHC I pathway. Liposomes were more effective in promoting MHC I presentation of associated antigens in macrophages as compared to dendritic cells [193], perhaps due to the greater intrinsic capacity of DCs for cross-presentation of exogenous antigens [194].
Covalent lipid conjugation of T cell epitopes also appears to direct intracellular antigen trafficking in a manner that increases MHC I presentation. N-terminally palmitoylated MHC I epitopes are efficiently endocytosed by DCs, processed, and presented to CD8 T cells [195–197]. Palmitoylated peptides differ in their intracellular routes, with some proteolytically degraded in the cytosol [197] and others in the endosome [196]. Lipidation also increases peptide accumulation in the Golgi [197], similar to the effect Alving and colleagues showed for liposome-encapsulated protein [190].
4. Design principles for liposomal vaccines
A synthesis of the body of liposome vaccine literature suggests several underlying guidelines that should be considered in the design of liposomal vaccine formulations, as summarized in Table 5. Firstly, this review clearly establishes that biophysical formulation parameters are important determinants of antigen-specific immune responses elicited by liposomal vaccines, even in the presence of potent immunostimulants such as MPL. Moreover, an antigen must be physically associated with the liposomal matrix in some form, typically through covalent conjugation, encapsulation, or adsorption, to achieve an optimal immune response. The various methods of antigen attachment influence the magnitude of the immune response to liposome-associated antigens but do not generally affect the quality of the response (e.g. TH1 versus TH2). Surface attached and encapsulated antigens stimulate CTL responses equivalently, whereas surface conjugated antigens induce more potent antibody responses. However, as the molecular size and antigenic diversity of an antigen increases, the influence of antigen attachment method on the resulting immune response decreases.
Table 5.
Design principles for liposomal vaccines
| Parameter | Guideline | Supporting Refs. |
|---|---|---|
| Antigen attachment | Physical association in some form is required. Surface conjugated Ag generally elicits greater antibody responses than encapsulated Ag, whereas CTL responses are equivalent. | [67–79] |
| Vesicle size | Larger vesicles (~250–700 nm diameter) promote a more robust TH1 response. | [99–101] |
| Lamellarity | Multilamellar vesicles may promote a TH2 response. | [102–104] |
| Vesicle charge | Charged formulations are more potent adjuvants than neutral ones, with cationic being more effective than anionic. | [105–108] |
| Membranefluidity | Vesicles composed of lipids with higher gel-liquid crystal transition temperatures elicit stronger responses. | [109–117] |
| Fusogenicity | Fusogenicity increases CTL responses, particularly for peptide and DNA vaccines. | [124, 130] |
| Lipid composition | In addition to classical TLR and NLR agonists, a versatile and expanding toolkit is available to tailor the adjuvanticity of liposomal vaccines. | [131, 141, 151, 168] |
Regarding other formulation parameters, vesicles comprised of lipids with higher gel-liquid crystal transition temperatures elicit stronger antigen-specific immunity than those comprised of lipids with lower transition temperatures. Charged vesicles (either positive or negative) elicit more potent responses than uncharged vesicles, with cationic formulations generally being the most effective. In addition, prolonged retention at the injection site, mediated through either cationic vesicle charge or larger vesicle size, skews the elicited immune response toward TH1 (as indicated by increased antigen-specific IgG2a and CTL responses). Finally, careful consideration must be given to the choice of lipids in the formulation; the large number of endogenous lipids with recently described immunomodulatory properties suggests that others have not yet been discovered. Inclusion of fusogenic lipids, such as DOPE, may facilitate enhanced antigen-specific CTL responses, but this effect may be understated for formulations that include large, immunogenic antigens or potent immunomodulators. PRR agonists, such as MPL, MDP, and other molecular adjuvants, can be easily included in liposome formulations to modulate both the magnitude and the TH bias of the immune response.
5. Confounding factors
Though clear trends are observable from the studies discussed here, they must be carefully interpreted to avoid conflating multiple interrelated variables. For example, comparisons of negatively and positively charged vesicles should consider a possible role of monocytic scavenger receptors, some of which are reported to have broad specificity for polyanionic molecules [198]. Similarly, some studies have included PS as an anionic lipid to impart a negative liposomal charge, but have not accounted for receptor mediated clearance of PS and anti-inflammatory properties of PS-containing liposomes [171, 199]. Conversely, many cationic lipids commonly used in liposomal vaccines are now known to be immunostimulatory [140–143]. Also, liposome formulations are typically compared at molar equivalence, but in some cases this may not be an ideal basis for comparison. For example, formulations that vary in vesicle size or lamellarity will also vary in the number of vesicles per molar unit of lipid [34] and the encapsulated aqueous volume [200]. Antigen surface density may also vary. Future studies should incorporate additional controls to address these variables.
Limitations of the choice of antigen must also be taken into account, as there will always be exceptions to the general guidelines we prescribe resulting from the intrinsic physicochemical and immunologic properties of the antigen itself. Some protein antigens may be more readily adsorbed to liposomes due to their hydrophobicity and charge. Alternatively, some proteins have a higher density of B or T cell epitopes than others, while some antigens, such as the B subunit of cholera toxin, have intrinsic immunostimulatory properties [201]. With regard to model antigens, source and purity must be considered. For example, many of the studies discussed in this review use OVA as a model antigen [80, 99, 106, 120, 121, 124]. Endotoxin contamination of commercial OVA preparations has been widely reported [202, 203]. Many studies that use OVA do not describe any purification method used to reduce endotoxin content.
Immunologic variability between animal strains and species should be considered. Nearly all of the studies discussed here have been performed in rats and mice, with the majority of studies being done in two inbred mouse strains – BALB/C and C57BL/6. Several groups have proposed that a TH1 or TH2 “bias” exists in specific inbred mouse strains, with BALB/C mice having a propensity toward TH2 responses and C57BL/6 mice being more prone to TH1 responses [204, 205]. This paradigm has recently been disputed, and may be confined to immune responses directed against specific pathogens [206], but animal species and strain should nonetheless remain a consideration in design of experiments and interpretation of results.
In addition, the data from these studies should be evaluated with regard to their potential applicability to human vaccines. Although the mouse is an excellent model for human physiology, differences between the two species should be acknowledged. For example, the effects of liposome vesicle size on retention at the injection site and trafficking to lymph nodes, and the subsequent ability to elicit an immune response, may differ between the two species because of the physical differences in interstitial spaces, lymph vessels, nodes, and other relevant tissues. Human lymph nodes are nearly 1000-fold more massive than murine lymph nodes (1 g in human and only 15 mg in mouse) and the mass of a lymph node relative to total body mass is approximately 70-fold greater in mouse as compared to human [207, 208]. Thus, mouse studies investigating the relationship between vesicle size, lymph node trafficking, and adjuvanticity should be interpreted carefully. In addition, differences between the mouse and human immune systems should also be considered. These differences, including proportions of lymphocytes, polarization of helper T cells, expression of PRRs, immunoglobulin subsets, cytokines, and cytokine receptors, have been extensively reviewed elsewhere [209]. Thus, as is the case with all subunit vaccine delivery systems, rodent studies of liposomal vaccines are not necessarily predictive of immunogenicity in humans. For example, the cationic lipid DC-Chol elicited potent humoral and cellular responses to co-administered antigens in mice [210], but a DC-Chol-adjuvanted HIV-1 vaccine candidate (gp160) failed to elicit measurable antigen-specific immunity in a Phase I trial [211].
Finally, epitope density and geometry of surface-displayed antigens are important parameters for induction of B cell responses. This is because multivalent, highly repetitive antigens can crosslink BCRs, increasing the level and duration of BCR signaling [212]. As a result, polyvalent antigens can elicit antigen-specific B cell responses in the absence of CD4 T cell help [213]. In a particulate vaccine delivery system, modifications to antigen dose may change the effective density of antigen present on the particle surface unless the antigen/adjuvant mass ratio is preserved. Indeed, increased antigen density on the surface of particulate vaccine systems has been shown to augment antigen-specific antibody responses, particularly IgG [214, 215]. Antigen surface density is rarely considered in liposomal vaccine studies and the influence of antigen density on the T cell-independent antibody response is not known.
The design guidelines presented here are synthesized from convergent evidence representing a large number of diverse studies, and thus should apply to a majority of antigens. However, it is critical to remember that exceptions will arise, owing to the intrinsic properties of the antigens themselves or to the interdependence of the many factors involved in liposomal vaccine formulation. Thus, although these guidelines provide a framework for liposomal vaccine development, one must always expect to optimize formulation parameters carefully for each new liposomal vaccine candidate. By considering these and other potentially confounding factors, we hope to encourage the understanding of liposome vaccine studies in full context, thereby enhancing future research and applicability in advancing the rational design of human vaccines.
6. Unanswered questions and opportunities for future study
Although a vast body of literature exists describing the importance of biophysical formulation parameters in the immunogenicity of liposomal vaccines, many important unanswered questions remain. At the cellular level, how does lipid conjugation influence antigen processing and presentation? Little is known regarding the cellular distribution of lipid-modified peptides. Lipid conjugation is reported to target T cell epitopes to the Golgi [216] – how significantly does that increase the efficiency of loading and presentation on MHC molecules, if at all? Do lipid-modified peptides bind to MHC, and if so, how do their affinities compare with unmodified peptides? Some studies have suggested that fatty acylation increases loading and presentation of MHC I epitopes but inhibits direct binding to MHC [195, 197]; more work is needed to generalize these observations. Once a system is established to answer these questions, it will be important to determine the importance of lipid structure (fatty acid, phospholipid, sterol, etc.). In turn, these modifications may also influence antigen trafficking and disposition in vivo.
Another question concerns the in vivo fate of constituent lipids in liposomal vaccines. Radiolabeling studies have shown divergent biodistribution of liposomal lipid and protein [92]. Some liposomal lipids, particularly sterols such as cholesterol, readily exchange with host cell membranes in biological fluids [177]; to what extent does this occur at subcutaneous injection sites and in lymph nodes? Immunogenicity experiments comparing lipids of various gel-liquid crystal transition temperatures have indicated lipid transition temperature influences the immune response, even when sufficient cholesterol is included to eliminate this thermotropic phase transition and force formulations into an intermediate liquid ordered phase (approximately 30 mol% cholesterol with synthetic diacylphospholipids) [217]. Does cholesterol exchange in vivo play a role in the context of liposomal vaccines? Non-exchangeable cholesterol-phospholipid chimeras may be useful tools to address these questions [218].
Further research is needed to elucidate the mechanistic basis of adjuvanticity of cationic lipids and liposome formulations generally. Cationic lipids such as DOTAP activate pro-inflammatory signaling in dendritic cells; do they bind PRRs, and what signaling pathways do they initiate? Is the enantiospecific activity of DOTAP [141] mediated by a specific receptor interaction? Do liposomes activate the inflammasome, as other particulate adjuvants do [219, 220]? If so, what is the relationship between physicochemical vesicle properties and inflammasome activation? Transcription profiling, genome-wide siRNA screens, and other high throughput approaches will be useful in addressing these issues. Similarly, what is the mechanistic basis for the role of lipids in maintaining the balance of inflammation and immunity, and what structural relationships can be derived? For example, saturated fatty acids are pro-inflammatory but polyunsaturated fatty acids are immunosuppressive. This effect is partly mediated by specific receptor interactions (e.g. GPR40 and GPR120 [221, 222]). Do biophysical effects of lipids on host cell membrane properties act as danger signals to activate innate immunity? This has been suggested as a mechanism to explain the apparent activation of TLR4 by saturated fatty acids [223]. If so, how can this be exploited for both pro-inflammatory (vaccination) and anti-inflammatory (autoimmune therapy) applications?
As described in this review, several studies have described the influence of liposome parameters on the TH1/TH2 balance of the immune response. For example, vesicles within a particular size range (roughly 250–750 nm diameter) appear to elicit more potent TH1 responses than smaller or larger vesicles in some systems [99–101]. In contrast, multilamellar vesicles may skew immune responses toward TH2 [103]. Thus, the possibility exists that formulation parameters may also promote, or inhibit, induction of more recently described T cell subsets. The importance of Treg and TH17 responses is well established [224]; a TH9 CD4 lymphocyte population has also been described [225]. Can liposomal vaccines be designed to selectively induce or inhibit these T cell subsets?
Finally, recent studies have demonstrated dramatic synergy when multiple PRR agonists are included in a single vaccine formulation. For example, Kasturi et al. showed that nanoparticle formulation containing both TLR4 and TLR7 agonists significantly increased antigen-specific serum IgG titer in mice against influenza virus hemagglutinin as compared to formulations containing a single TLR agonist [226]. Virus neutralization titer, antibody avidity, and duration of the antibody response were also markedly improved. In another example, a combination adjuvant including ligands for TLRs 2, 3, and 9 significantly increased the protective efficacy of a vaccine containing HIV envelope-derived peptide antigens in a mouse pseudovirus challenge model when compared to single TLR ligand adjuvants [227]. Given the versatility of liposomal carriers and their amenability to simultaneous incorporation of multiple adjuvant molecules, the liposome would seem an ideal model system to study the synergy phenomenon in great detail in vitro and in vivo.
7. Conclusions
Liposomal vaccine delivery technology is currently enjoying a renaissance. Given the recent explosion of interest, we have undertaken a systematic review of the physicochemical factors that should be considered when designing a liposomal vaccine formulation. The collective body of literature shows conclusively that these factors – size, charge, composition, antigen attachment method – have meaningful impacts on immunogenicity and should be carefully chosen. Though we have identified trends in the relationship between formulation biophysics and immunogenicity, the interconnectedness of the various biophysical factors underscores the need to optimize individual formulations for specific applications. Lastly, we have identified important questions regarding liposome immunogenicity that remain unanswered, pointing toward future directions in the field.
Highlights.
We have comprehensively reviewed the current state of liposomal vaccine technology.
We describe the relationship between formulation parameters and immunogenicity.
Biophysical properties dictate the immunogenicity of liposomal vaccines.
Many lipids are immunomodulatory, so formulations must be selected carefully.
We also highlight unanswered questions and opportunities for further study.
Acknowledgments
SRI International’s Center for Advanced Drug Research was established with funding support from the Commonwealth of Virginia to SRI International. This work was also supported by NIH CA129421 (L.H.). The interlibrary loan staff at the James Madison University Library are gratefully acknowledged for kind assistance in obtaining many research articles not available online. The authors wish to acknowledge the encouragement and guidance of the following members of SRI Biosciences: Vice President Walter Moos, Executive Director Krishna Kodukula, and Director Amit Galande.
Abbreviations
- 111In-DTPA
111In-labeled diethylenetriaminepentaacetic acid
- APC
Antigen presenting cell
- ATRA
all-trans retinoic acid
- BCR
B cell receptor
- BSA
Bovine serum albumin
- CCS
Ceramide carbamoyl-spermine
- Chol
Cholesterol
- CLR
C-type lectin receptor
- CpG
Unmethylated cytosine-phosphate-guanine motifs
- CTL
Cytotoxic T lymphocyte
- DC-Chol
3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl]cholesterol
- DC-SIGN
Dendritic cell-specific intracellular adhesion molecule-3-grabbing non-integrin
- DCP
Dicetylphosphate
- DDA
Dimethyldioctadecylammonium
- DLiPC
1,2-dilinoleoyl-sn-glycero-3-phosphocholine
- DLPC
1,2-dilauryl-sn-glycero-3-phosphocholine
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DNP
Dinitrophenyl
- DNP-Cap-PE
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[6-[(2,4-dinitrophenyl)amino]hexanoyl]
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOTAP
1,2-dioleoyl-3-trimethylammonium propane
- DOTIM
1-[2-(oleoyloxy)ethyl]-2-oleyl-3-(2-hydroxyethyl)imidazolinium
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine
- DPPE
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine
- DPyPE
1,2-diphytanoyl-sn-glycero-3-phosphoethanolamine
- DRV
Dehydration-rehydration vesicle
- DSPC
1,2-distearoyl-sn-glycero-3-phosphocholine
- DSS
Disuccinimidyl suberate
- DTH
Delayed-type hypersensitivity
- EMCS
N-(ε-Maleimidocaproyloxy)succinimide
- EPC
1,2-diacyl-sn-glycero-3-phosphocholine from egg
- EPG
1,2-diacyl-sn-glycero-3-phosphoglycerol from egg
- ER
Endoplasmic reticulum
- GAP-DMORIE
N-(3-aminopropyl)-N,N-dimethyl-2,3-bis(cis-9- tetradeceneyloxy)-1-propanaminium
- GCSA
Gross cell surface antigen
- GMS
Glyceryl monostearate
- IFN-γ
Interferon gamma
- IL-12
Interleukin 12
- LPA
Lysophosphatidic acid
- LPC
Lysophosphatidylcholine
- LPD
Lipid-protamine-DNA nanoparticle
- LPS
Lipopolysaccharide
- MDP
Muramyl dipeptide
- MHC
Major histocompatibility complex
- MLV
Multilamellar vesicle
- MPG
2-monopalmitoylglycerol
- MPL
Monophosphoryl lipid A
- NLR
Nod-like receptor
- NTA
Nitrilotriacetic acid
- ODA
Octadecanoic acid
- OVA
Ovalbumin
- PA
Palmitic acid
- PC
1,2-diacyl-sn-glycero-3-phosphocholine
- PEG
Poly(ethylene glycol)
- PG
1,2-diacyl-sn-glycero-3-phosphoglycerol
- PLiPC
1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine
- poly(I:C)
polyinosinic:polycytidylic acid
- PRR
Pattern recognition receptor
- PS
1,2-diacyl-sn-glycero-3-phosphoserine
- QS21
Quillaja saponaria Molina saponin
- REV
Reverse phase evaporation vesicle
- SA
Stearylamine
- SMS
Sorbitan monostearate
- SP1
Sphingosine-1-phosphate
- T20
Polyoxyethylene(20) sorbitan monolaurate
- SPDP
(N-Succinimidyl-3-(2-pyridyldithio)-propionate)
- TAP
Transporter associated with antigen processing
- TDB
α,α-trehalose-6,6′-dibehenate
- TGF-β
Transforming growth factor beta
- TH1
T helper type 1
- TH17
T helper type 17
- TH2
T helper type 2
- TH9
T helper type 9
- TLR
Toll-like receptor
- Treg
T regulatory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008 Nov 18;26(49):6266–73. doi: 10.1016/j.vaccine.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 2.Munoz N, Manalastas R, Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009 Jun 6;373(9679):1949–57. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 3.O’Hagan D, Valiante N. Recent advances in the discovery and delivery of vaccine adjuvants. Nature Reviews Drug Discovery. 2003;2:727–35. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrie Y, Mohammed A, Kirby D, McNeil S, Bramwell V. Vaccine adjuvant systems: Enhancing the efficacy of sub-unit protein antigens. Int J Pharm. 2008;364:272–80. doi: 10.1016/j.ijpharm.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 5.Koff R. Immunogenicity of hepatitis B vaccines: implications of immune memory. Vaccine. 2002;20(31–32):3695–701. doi: 10.1016/s0264-410x(02)00405-x. [DOI] [PubMed] [Google Scholar]
- 6.Lowy D, Schiller J. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006;116(5):1167–73. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004 Jul 24–30;364(9431):365–7. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 8.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000 Mar;19(3):187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Gupta R, Siber G. Adjuvants for human vaccines - current status, problems and future prospects. Vaccine. 1995;13(14):1263–76. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 10.Singh M, O’Hagan D. Advances in vaccine adjuvants. Nat Biotechnol. 1999 Nov;17(11):1075–81. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]
- 11.Storni T, Kundig T, Senti G, Johansen P. Immunity in response to particulate antigen-delivery systems. Advanced Drug Delivery Reviews. 2005;57:333–55. doi: 10.1016/j.addr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Harris J, Sharp FA, Lavelle EC. The role of inflammasomes in the immunostimulatory effects of particulate vaccine adjuvants. Eur J Immunol. 2010 Mar;40(3):634–8. doi: 10.1002/eji.200940172. [DOI] [PubMed] [Google Scholar]
- 13.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009 Apr;9(4):287–93. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glenny A, Pope C, Waddington H, Wallace V. The antigenic value of toxoid precipitated by potassium-alum. J Pathol Bacteriol. 1926;29:38–45. [Google Scholar]
- 15.Harper DM. Currently approved prophylactic HPV vaccines. Expert Rev Vaccines. 2009 Dec;8(12):1663–79. doi: 10.1586/erv.09.123. [DOI] [PubMed] [Google Scholar]
- 16.Allison AG, Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974 Nov 15;252(5480):252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- 17.Gregoriadis G, Allison AC. Entrapment of proteins in liposomes prevents allergic reactions in pre-immunised mice. FEBS Lett. 1974 Sep 1;45(1):71–4. doi: 10.1016/0014-5793(74)80813-6. [DOI] [PubMed] [Google Scholar]
- 18.Henriksen-Lacey M, Korsholm KS, Andersen P, Perrie Y, Christensen D. Liposomal vaccine delivery systems. Expert Opin Drug Deliv. 2011 Apr;8(4):505–19. doi: 10.1517/17425247.2011.558081. [DOI] [PubMed] [Google Scholar]
- 19.Gregoriadis G, Gursel I, Gursel M, McCormack B. Liposomes as immunological adjuvants and vaccine carriers. J Control Release. 1996;41(1–2):49–56. [Google Scholar]
- 20.Alving C, Rao M. Lipid A and liposomes containing lipid A as antigens and adjuvants. Vaccine. 2008;26(24):3036–45. doi: 10.1016/j.vaccine.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Herzog C, Hartmann K, Kunzi V, Kursteiner O, Mischler R, Lazar H, et al. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine. 2009 Jul 16;27(33):4381–7. doi: 10.1016/j.vaccine.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Mischler R, Metcalfe IC. Inflexal V a trivalent virosome subunit influenza vaccine: production. Vaccine. 2002 Dec 20;20( Suppl 5):B17–23. doi: 10.1016/s0264-410x(02)00512-1. [DOI] [PubMed] [Google Scholar]
- 23.Bovier PA. Epaxal: a virosomal vaccine to prevent hepatitis A infection. Expert Rev Vaccines. 2008 Oct;7(8):1141–50. doi: 10.1586/14760584.7.8.1141. [DOI] [PubMed] [Google Scholar]
- 24.Usonis V, Bakasenas V, Valentelis R, Katiliene G, Vidzeniene D, Herzog C. Antibody titres after primary and booster vaccination of infants and young children with a virosomal hepatitis A vaccine (Epaxal) Vaccine. 2003 Nov 7;21(31):4588–92. doi: 10.1016/s0264-410x(03)00509-7. [DOI] [PubMed] [Google Scholar]
- 25.Butts C, Murray N, Maksymiuk A, Goss G, Marshall E, Soulieres D, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005 Sep 20;23(27):6674–81. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 26.North S, Butts C. Vaccination with BLP25 liposome vaccine to treat non-small cell lung and prostate cancers. Expert Rev Vaccines. 2005 Jun;4(3):249–57. doi: 10.1586/14760584.4.3.249. [DOI] [PubMed] [Google Scholar]
- 27.Regules JA, Cummings JF, Ockenhouse CF. The RTS,S vaccine candidate for malaria. Expert Rev Vaccines. 2011 May;10(5):589–99. doi: 10.1586/erv.11.57. [DOI] [PubMed] [Google Scholar]
- 28.Agnandji ST, Asante KP, Lyimo J, Vekemans J, Soulanoudjingar SS, Owusu R, et al. Evaluation of the safety and immunogenicity of the RTS,S/AS01E malaria candidate vaccine when integrated in the expanded program of immunization. J Infect Dis. 2010 Oct 1;202(7):1076–87. doi: 10.1086/656190. [DOI] [PubMed] [Google Scholar]
- 29.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005 Feb;4(2):145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 30.Tollemar J, Klingspor L, Ringden O. Liposomal amphotericin B (AmBisome) for fungal infections in immunocompromised adults and children. Clin Microbiol Infect. 2001;7( Suppl 2):68–79. doi: 10.1111/j.1469-0691.2001.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 31.Stewart S, Jablonowski H, Goebel FD, Arasteh K, Spittle M, Rios A, et al. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J of Clinical Oncology. 1998;16(2):683–91. doi: 10.1200/JCO.1998.16.2.683. [DOI] [PubMed] [Google Scholar]
- 32.Fries LF, Gordon DM, Richards RL, Egan JE, Hollingdale MR, Gross M, et al. Liposomal malaria vaccine in humans: a safe and potent adjuvant strategy. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):358–62. doi: 10.1073/pnas.89.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011 Nov 17;365(20):1863–75. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 34.Szoka F, Jr, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes) Annu Rev Biophys Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- 35.Sharma AK, Sharma US. Liposomes in drug delivery: Progress and limitations. Int J Pharm. 1997;154(2):123–40. [Google Scholar]
- 36.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005 Apr;11(4 Suppl):S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 37.Johansson EL, Bergquist C, Edebo A, Johansson C, Svennerholm AM. Comparison of different routes of vaccination for eliciting antibody responses in the human stomach. Vaccine. 2004 Feb 25;22(8):984–90. doi: 10.1016/j.vaccine.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997 Apr;65(4):1387–94. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Loveren H, Van Amsterdam JG, Vandebriel RJ, Kimman TG, Rumke HC, Steerenberg PS, et al. Vaccine-induced antibody responses as parameters of the influence of endogenous and environmental factors. Environ Health Perspect. 2001 Aug;109(8):757–64. doi: 10.1289/ehp.01109757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monto AS, Ansaldi F, Aspinall R, McElhaney JE, Montano LF, Nichol KL, et al. Influenza control in the 21st century: Optimizing protection of older adults. Vaccine. 2009 Aug 13;27(37):5043–53. doi: 10.1016/j.vaccine.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 41.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009 Jul 15;200(2):172–80. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 42.Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B. A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol. 2008 Apr;4(4):e1000048. doi: 10.1371/journal.pcbi.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haste Andersen P, Nielsen M, Lund O. Prediction of residues in discontinuous B-cell epitopes using protein 3D structures. Protein Sci. 2006 Nov;15(11):2558–67. doi: 10.1110/ps.062405906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsen JE, Lund O, Nielsen M. Improved method for predicting linear B-cell epitopes. Immunome Res. 2006;2:2. doi: 10.1186/1745-7580-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–92. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 46.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002 Dec 16;196(12):1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007 Oct;7(10):790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 48.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010 Nov;10(11):787–96. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 49.Merkel TJ, Jones SW, Herlihy KP, Kersey FR, Shields AR, Napier M, et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):586–91. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009 Jul;9(7):465–79. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010 May;11(5):373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 52.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006 Jul 6;442(7098):39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 53.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007 Oct;27(4):549–59. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev. 2009 Jan;227(1):54–65. doi: 10.1111/j.1600-065X.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 55.Alving C. Liposomes as carriers of antigens and adjuvants. J Immunol Methods. 1991;140(1):1–13. doi: 10.1016/0022-1759(91)90120-5. [DOI] [PubMed] [Google Scholar]
- 56.Wilson KD, de Jong SD, Tam YK. Lipid-based delivery of CpG oligonucleotides enhances immunotherapeutic efficacy. Adv Drug Deliv Rev. 2009 Mar 28;61(3):233–42. doi: 10.1016/j.addr.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 57.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176:7335–45. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 58.Christensen D, Agger EM, Andreasen LV, Kirby D, Andersen P, Perrie Y. Liposome-based cationic adjuvant formulations (CAF): past, present, and future. J Liposome Res. 2009;19(1):2–11. doi: 10.1080/08982100902726820. [DOI] [PubMed] [Google Scholar]
- 59.Christensen D, Korsholm KS, Rosenkrands I, Lindenstrom T, Andersen P, Agger EM. Cationic liposomes as vaccine adjuvants. Expert Rev Vaccines. 2007 Oct;6(5):785–96. doi: 10.1586/14760584.6.5.785. [DOI] [PubMed] [Google Scholar]
- 60.Chen WC, Huang L. Non-viral vector as vaccine carrier. Adv Genet. 2005;54:315–37. doi: 10.1016/S0065-2660(05)54013-6. [DOI] [PubMed] [Google Scholar]
- 61.Vangasseri DP, Han SJ, Huang L. Lipid-protamine-DNA-mediated antigen delivery. Curr Drug Deliv. 2005 Oct;2(4):401–6. doi: 10.2174/156720105774370168. [DOI] [PubMed] [Google Scholar]
- 62.Daemen T, de Mare A, Bungener L, de Jonge J, Huckriede A, Wilschut J. Virosomes for antigen and DNA delivery. Adv Drug Deliv Rev. 2005 Jan 10;57(3):451–63. doi: 10.1016/j.addr.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Huckriede A, Bungener L, Stegmann T, Daemen T, Medema J, Palache AM, et al. The virosome concept for influenza vaccines. Vaccine. 2005 Jul 8;23( Suppl 1):S26–38. doi: 10.1016/j.vaccine.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 64.Krishnan L, Sprott GD. Archaeosome adjuvants: immunological capabilities and mechanism(s) of action. Vaccine. 2008 Apr 16;26(17):2043–55. doi: 10.1016/j.vaccine.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 65.Azeem A, Anwer MK, Talegaonkar S. Niosomes in sustained and targeted drug delivery: some recent advances. J Drug Target. 2009 Nov;17(9):671–89. doi: 10.3109/10611860903079454. [DOI] [PubMed] [Google Scholar]
- 66.Vyas SP, Khatri K, Mishra V. Vesicular carrier constructs for topical immunisation. Expert Opin Drug Deliv. 2007 Jul;4(4):341–8. doi: 10.1517/17425247.4.4.341. [DOI] [PubMed] [Google Scholar]
- 67.Davis D, Gregoriadis G. Liposomes as adjuvants with immunopurified tetanus toxoid: influence of liposomal characteristics. Immunology. 1987 Jun;61(2):229–34. [PMC free article] [PubMed] [Google Scholar]
- 68.Shahum E, Therien HM. Immunopotentiation of the humoral response by liposomes: encapsulation versus covalent linkage. Immunology. 1988 Oct;65(2):315–7. [PMC free article] [PubMed] [Google Scholar]
- 69.Shahum E, Therien HM. Liposomal adjuvanticity: effect of encapsulation and surface-linkage on antibody production and proliferative response. Int J Immunopharmacol. 1995 Jan;17(1):9–20. doi: 10.1016/0192-0561(94)00082-y. [DOI] [PubMed] [Google Scholar]
- 70.Tan L, Weissig V, Gregoriadis G. Comparison of the immune response against polio peptides covalently-surface-linked to and internally-entrapped in liposomes. Asian Pac J Allergy Immunol. 1991 Jun;9(1):25–30. [PubMed] [Google Scholar]
- 71.Therien HM, Lair D, Shahum E. Liposomal vaccine: influence of antigen association on the kinetics of the humoral response. Vaccine. 1990 Dec;8(6):558–62. doi: 10.1016/0264-410x(90)90008-a. [DOI] [PubMed] [Google Scholar]
- 72.Shahum E, Therien HM. Correlation between in vitro and in vivo behaviour of liposomal antigens. Vaccine. 1994 Sep;12(12):1125–31. doi: 10.1016/0264-410x(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 73.Vannier WE, Snyder SL. Antibody responses to liposome-associated antigen. Immunol Lett. 1988 Sep;19(1):59–64. doi: 10.1016/0165-2478(88)90120-4. [DOI] [PubMed] [Google Scholar]
- 74.White WI, Cassatt DR, Madsen J, Burke SJ, Woods RM, Wassef NM, et al. Antibody and cytotoxic T-lymphocyte responses to a single liposome-associated peptide antigen. Vaccine. 1995;13(12):1111–22. doi: 10.1016/0264-410x(94)00058-u. [DOI] [PubMed] [Google Scholar]
- 75.Guan HH, Budzynski W, Koganty RR, Krantz MJ, Reddish MA, Rogers JA, et al. Liposomal formulations of synthetic MUC1 peptides: effects of encapsulation versus surface display of peptides on immune responses. Bioconjug Chem. 1998 Jul-Aug;9(4):451–8. doi: 10.1021/bc970183n. [DOI] [PubMed] [Google Scholar]
- 76.Brynestad K, Babbit B, Huang L, Rouse BT. Influence of peptide acylation, liposome incorporation, and synthetic immunomodulators on the immunogenicity of a 1–23 peptide of glycoprotein D of herpes simplex virus: implications for subunit vaccines. J Virol. 1990 Feb;64(2):680–5. doi: 10.1128/jvi.64.2.680-685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frisch B, Muller S, Briand J, Van Regenmortel M, Schuber F. Parameters affecting the immunogenicity of a liposome-associated synthetic hexapeptide antigen. Eur J Immunol. 1991;21(1):185–93. doi: 10.1002/eji.1830210128. [DOI] [PubMed] [Google Scholar]
- 78.Chen W, Huang L. Induction of cytotoxic T-lymphocytes and antitumor activity by a liposomal lipopeptide vaccine. Mol Pharm. 2008 May-Jun;5(3):464–71. doi: 10.1021/mp700126c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Engler O, Schwendener R, Dai W, Wolk B, Pichler W, Moradpour D, et al. A liposomal peptide vaccine inducing CD8+ T cells in HLA-A2.1 transgenic mice, which recognise human cells encoding hepatitis C virus (HCV) proteins. Vaccine. 2004;23(1):58–68. doi: 10.1016/j.vaccine.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 80.Nakano Y, Mori M, Nishinohara S, Takita Y, Naito S, Horino A, et al. Antigen-specific, IgE-selective unresponsiveness induced by antigen-liposome conjugates. Comparison of four different conjugation methods for the coupling of antigen to liposome. Int Arch Allergy Immunol. 1999 Nov;120(3):199–208. doi: 10.1159/000024268. [DOI] [PubMed] [Google Scholar]
- 81.Garcon NM, Six HR. Universal vaccine carrier. Liposomes that provide T-dependent help to weak antigens. J Immunol. 1991 Jun 1;146(11):3697–702. [PubMed] [Google Scholar]
- 82.Pietrobon PJ, Garcon N, Lee CH, Six HR. Liposomes that provide T-dependent help to weak antigens (T-independent antigens) Immunomethods. 1994 Jun;4(3):236–43. doi: 10.1006/immu.1994.1026. [DOI] [PubMed] [Google Scholar]
- 83.Boeckler C, Dautel D, Schelte P, Frisch B, Wachsmann D, Klein JP, et al. Design of highly immunogenic liposomal constructs combining structurally independent B cell and T helper cell peptide epitopes. Eur J Immunol. 1999 Jul;29(7):2297–308. doi: 10.1002/(SICI)1521-4141(199907)29:07<2297::AID-IMMU2297>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 84.Roth A, Espuelas S, Thumann C, Frisch B, Schuber F. Synthesis of thiol-reactive lipopeptide adjuvants. Incorporation into liposomes and study of their mitogenic effect on mouse splenocytes. Bioconjug Chem. 2004 May-Jun;15(3):541–53. doi: 10.1021/bc034184t. [DOI] [PubMed] [Google Scholar]
- 85.Watson D, Szoka FC. Role of lipid structure in the humoral immune response in mice to covalent lipid-peptides from the membrane proximal region of HIV-1 gp41. Vaccine. 2009;27:4672–83. doi: 10.1016/j.vaccine.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patel J, O’Carra R, Jones J, Woodward J, Mumper R. Preparation and characterization of nickel nanoparticles for binding to His-tag proteins and antigens. Pharm Res. 2007;24(2):343–52. doi: 10.1007/s11095-006-9154-7. [DOI] [PubMed] [Google Scholar]
- 87.Van Broekhoven C, Altin J. The novel chelator lipid 3(nitrilotriacetic acid)-ditetradecylamine (NTA3-DTDA) promotes stable binding of His-tagged proteins to liposomal membranes: Potent anti-tumor responses induced by simultaneously targeting antigen, cytokine and costimulatory signals to T cells. Biochim Biophys Acta. 2005;1716:104–16. doi: 10.1016/j.bbamem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 88.Masek J, Bartheldyova E, Turanek-Knotigova P, Skrabalova M, Korvasova Z, Plockova J, et al. Metallochelating liposomes with associated lipophilised norAbuMDP as biocompatible platform for construction of vaccines with recombinant His-tagged antigens: preparation, structural study and immune response towards rHsp90. J Control Release. 2011 Apr 30;151(2):193–201. doi: 10.1016/j.jconrel.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 89.Watson DS, Platt VM, Cao L, Venditto VJ, Francis C, Szoka J. Antibody response in mice to polyhistidine-tagged peptide and protein antigens attached to liposomes via lipid-linked nitrilotriacetic acid. Clin Vaccine Immunol. 2011;18(2):289–97. doi: 10.1128/CVI.00425-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henriksen-Lacey M, Bramwell VW, Christensen D, Agger EM, Andersen P, Perrie Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J Control Release. 2010 Mar 3;142(2):180–6. doi: 10.1016/j.jconrel.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 91.Henriksen-Lacey M, Christensen D, Bramwell VW, Lindenstrom T, Agger EM, Andersen P, et al. Comparison of the depot effect and immunogenicity of liposomes based on dimethyldioctadecylammonium (DDA), 3beta-[N-(N′,N′-Dimethylaminoethane)carbomyl] cholesterol (DC-Chol), and 1,2-Dioleoyl-3-trimethylammonium propane (DOTAP): prolonged liposome retention mediates stronger Th1 responses. Mol Pharm. 2011 Feb 7;8(1):153–61. doi: 10.1021/mp100208f. [DOI] [PubMed] [Google Scholar]
- 92.Henriksen-Lacey M, Christensen D, Bramwell VW, Lindenstrom T, Agger EM, Andersen P, et al. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J Control Release. 2010 Jul 14;145(2):102–8. doi: 10.1016/j.jconrel.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 93.Martel CJ, Agger EM, Poulsen JJ, Hammer Jensen T, Andresen L, Christensen D, et al. CAF01 potentiates immune responses and efficacy of an inactivated influenza vaccine in ferrets. PLoS ONE. 2011;6(8):e22891. doi: 10.1371/journal.pone.0022891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phillips WT, Klipper R, Goins B. Novel method of greatly enhanced delivery of liposomes to lymph nodes. J Pharmacol Exp Ther. 2000 Oct;295(1):309–13. [PubMed] [Google Scholar]
- 95.McConville MJ, Bacic A, Mitchell GF, Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8941–5. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Engelhard VH, Guild BC, Helenius A, Terhorst C, Strominger JL. Reconstitution of purified detergent-soluble HLA-A and HLA-B antigens into phospholipid vesicles. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3230–4. doi: 10.1073/pnas.75.7.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Humphries HE, Williams JN, Blackstone R, Jolley KA, Yuen HM, Christodoulides M, et al. Multivalent liposome-based vaccines containing different serosubtypes of PorA protein induce cross-protective bactericidal immune responses against Neisseria meningitidis. Vaccine. 2006 Jan 9;24(1):36–44. doi: 10.1016/j.vaccine.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 98.Arigita C, Kersten GF, Hazendonk T, Hennink WE, Crommelin DJ, Jiskoot W. Restored functional immunogenicity of purified meningococcal PorA by incorporation into liposomes. Vaccine. 2003 Feb 14;21(9–10):950–60. doi: 10.1016/s0264-410x(02)00546-7. [DOI] [PubMed] [Google Scholar]
- 99.Brewer JM, Tetley L, Richmond J, Liew FY, Alexander J. Lipid vesicle size determines the Th1 or Th2 response to entrapped antigen. J Immunol. 1998 Oct 15;161(8):4000–7. [PubMed] [Google Scholar]
- 100.Mann JF, Shakir E, Carter KC, Mullen AB, Alexander J, Ferro VA. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine. 2009 Jun 2;27(27):3643–9. doi: 10.1016/j.vaccine.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 101.Henriksen-Lacey M, Devitt A, Perrie Y. The vesicle size of DDA:TDB liposomal adjuvants plays a role in the cell-mediated immune response but has no significant effect on antibody production. J Control Release. 2011;154(2):131–7. doi: 10.1016/j.jconrel.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 102.Shek P, Heath T. Immune response mediated by liposome-associated protein antigens. III. Immunogenicity of bovine serum albumin covalently coupled to vesicle surface. Immunology. 1983;50(1):101–6. [PMC free article] [PubMed] [Google Scholar]
- 103.Bhowmick S, Mazumdar T, Sinha R, Ali N. Comparison of liposome based antigen delivery systems for protection against Leishmania donovani. J Control Release. 2010 Jan 25;141(2):199–207. doi: 10.1016/j.jconrel.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 104.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011 Mar;10(3):243–51. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kraaijeveld CA, Schilham M, Jansen J, Benaissa-Trouw B, Harmsen M, van Houte AJ, et al. The effect of liposomal charge on the neutralizing antibody response against inactivated encephalomyocarditis and Semliki Forest viruses. Clin Exp Immunol. 1984 Jun;56(3):509–14. [PMC free article] [PubMed] [Google Scholar]
- 106.Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, et al. Positively charged liposome functions as an efficient immunoadjuvant in inducing immune responses to soluble proteins. Biochem Biophys Res Commun. 1997 Nov 26;240(3):793–7. doi: 10.1006/bbrc.1997.7749. [DOI] [PubMed] [Google Scholar]
- 107.Nakanishi T, Kunisawa J, Hayashi A, Tsutsumi Y, Kubo K, Nakagawa S, et al. Positively charged liposome functions as an efficient immunoadjuvant in inducing cell-mediated immune response to soluble proteins. J Control Release. 1999 Aug 27;61(1–2):233–40. doi: 10.1016/s0168-3659(99)00097-8. [DOI] [PubMed] [Google Scholar]
- 108.Badiee A, Jaafari MR, Khamesipour A, Samiei A, Soroush D, Kheiri MT, et al. The role of liposome charge on immune response generated in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63) Exp Parasitol. 2009 Apr;121(4):362–9. doi: 10.1016/j.exppara.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 109.Yasuda T, Dancey GF, Kinsky SC. Immunogenicity of liposomal model membranes in mice: dependence on phospholipid composition. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1234–6. doi: 10.1073/pnas.74.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dancey GF, Yasuda T, Kinsky SC. Effect of liposomal model membrane composition on immunogenicity. J Immunol. 1978 Apr;120(4):1109–13. [PubMed] [Google Scholar]
- 111.van Houte AJ, Snippe H, Schmitz MG, Willers JM. Characterization of immunogenic properties of haptenated liposomal model membranes in mice. V. Effect of membrane composition on humoral and cellular immunogenicity. Immunology. 1981 Nov;44(3):561–8. [PMC free article] [PubMed] [Google Scholar]
- 112.Bakouche O, Gerlier D. Enhancement of immunogenicity of tumour virus antigen by liposomes: the effect of lipid composition. Immunology. 1986 Jul;58(3):507–13. [PMC free article] [PubMed] [Google Scholar]
- 113.Kersten GF, van de Put AM, Teerlink T, Beuvery EC, Crommelin DJ. Immunogenicity of liposomes and iscoms containing the major outer membrane protein of Neisseria gonorrhoeae: influence of protein content and liposomal bilayer composition. Infect Immun. 1988 Jun;56(6):1661–4. doi: 10.1128/iai.56.6.1661-1664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kahl LP, Scott CA, Lelchuk R, Gregoriadis G, Liew FY. Vaccination against murine cutaneous leishmaniasis by using Leishmania major antigen/liposomes. Optimization and assessment of the requirement for intravenous immunization. J Immunol. 1989 Jun 15;142(12):4441–9. [PubMed] [Google Scholar]
- 115.Garnier F, Forquet F, Bertolino P, Gerlier D. Enhancement of in vivo and in vitro T cell response against measles virus haemagglutinin after its incorporation into liposomes: effect of the phospholipid composition. Vaccine. 1991;9:340–5. doi: 10.1016/0264-410x(91)90061-a. [DOI] [PubMed] [Google Scholar]
- 116.Mazumdar T, Anam K, Ali N. Influence of phospholipid composition on the adjuvanticity and protective efficacy of liposome-encapsulated Leishmania donovani antigens. J Parasitol. 2005 Apr;91(2):269–74. doi: 10.1645/GE-356R1. [DOI] [PubMed] [Google Scholar]
- 117.Badiee A, Jaafari MR, Khamesipour A, Samiei A, Soroush D, Kheiri MT, et al. Enhancement of immune response and protection in BALB/c mice immunized with liposomal recombinant major surface glycoprotein of Leishmania (rgp63): the role of bilayer composition. Colloids Surf B Biointerfaces. 2009 Nov 1;74(1):37–44. doi: 10.1016/j.colsurfb.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 118.Gregoriadis G, Davis D, Davies A. Liposomes as immunological adjuvants: antigen incorporation studies. Vaccine. 1987 Jun;5(2):145–51. doi: 10.1016/0264-410x(87)90063-6. [DOI] [PubMed] [Google Scholar]
- 119.Dietz V, Galazka A, van Loon F, Cochi S. Factors affecting the immunogenicity and potency of tetanus toxoid: implications for the elimination of neonatal and non-neonatal tetanus as public health problems. Bull World Health Organ. 1997;75(1):81–93. [PMC free article] [PubMed] [Google Scholar]
- 120.Nakano Y, Mori M, Yamamura H, Naito S, Kato H, Taneichi M, et al. Cholesterol inclusion in liposomes affects induction of antigen-specific IgG and IgE antibody production in mice by a surface-linked liposomal antigen. Bioconjug Chem. 2002 Jul-Aug;13(4):744–9. doi: 10.1021/bc0155667. [DOI] [PubMed] [Google Scholar]
- 121.Nakano Y, Mori M, Nishinohara S, Takita Y, Naito S, Kato H, et al. Surface-linked liposomal antigen induces ige-selective unresponsiveness regardless of the lipid components of liposomes. Bioconjug Chem. 2001 May-Jun;12(3):391–5. doi: 10.1021/bc0001185. [DOI] [PubMed] [Google Scholar]
- 122.Ellens H, Bentz J, Szoka FC. Fusion of phosphatidylethanolamine-containing liposomes and mechanism of the L alpha-HII phase transition. Biochemistry (Mosc) 1986 Jul 15;25(14):4141–7. doi: 10.1021/bi00362a023. [DOI] [PubMed] [Google Scholar]
- 123.Legendre JY, Szoka FC., Jr Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: comparison with cationic liposomes. Pharm Res. 1992 Oct;9(10):1235–42. doi: 10.1023/a:1015836829670. [DOI] [PubMed] [Google Scholar]
- 124.Nair S, Zhou F, Reddy R, Huang L, Rouse BT. Soluble proteins delivered to dendritic cells via pH-sensitive liposomes induce primary cytotoxic T lymphocyte responses in vitro. J Exp Med. 1992 Feb 1;175(2):609–12. doi: 10.1084/jem.175.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li W, Szoka FC., Jr Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007 Mar;24(3):438–49. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 126.Conwell CC, Huang L. Recent advances in non-viral gene delivery. Adv Genet. 2005;53:3–18. doi: 10.1007/4-431-27879-6_1. [DOI] [PubMed] [Google Scholar]
- 127.Ahmad N, Deeba F, Faisal SM, Khan A, Agrewala JN, Dwivedi V, et al. Role of fusogenic non-PC liposomes in elicitation of protective immune response against experimental murine salmonellosis. Biochimie. 2006 Oct;88(10):1391–400. doi: 10.1016/j.biochi.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 128.Hayashi A, Nakanishi T, Kunisawa J, Kondoh M, Imazu S, Tsutsumi Y, et al. A novel vaccine delivery system using immunopotentiating fusogenic liposomes. Biochem Biophys Res Commun. 1999 Aug 11;261(3):824–8. doi: 10.1006/bbrc.1999.1044. [DOI] [PubMed] [Google Scholar]
- 129.Owais M, Gupta CM. Liposome-mediated cytosolic delivery of macromolecules and its possible use in vaccine development. Eur J Biochem. 2000 Jul;267(13):3946–56. doi: 10.1046/j.1432-1327.2000.01447.x. [DOI] [PubMed] [Google Scholar]
- 130.Perrie Y, Frederik PM, Gregoriadis G. Liposome-mediated DNA vaccination: the effect of vesicle composition. Vaccine. 2001 Apr 30;19(23–24):3301–10. doi: 10.1016/s0264-410x(00)00432-1. [DOI] [PubMed] [Google Scholar]
- 131.Holten-Andersen L, Doherty TM, Korsholm KS, Andersen P. Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect Immun. 2004 Mar;72(3):1608–17. doi: 10.1128/IAI.72.3.1608-1617.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bekierkunst A, Levij IS, Yarkoni E. Suppression of urethan-induced lung adenomas in mice treated with trehalose-6,6-dimycolate (cord factor) and living bacillus Calmette Guerin. Science. 1971 Dec 17;174(15):1240–2. doi: 10.1126/science.174.4015.1240. [DOI] [PubMed] [Google Scholar]
- 133.Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010 Mar 15;184(6):2756–60. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997 Nov 28;278(5343):1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 135.Karasavvas N, Beck Z, Tong J, Matyas GR, Rao M, McCutchan FE, et al. Antibodies induced by liposomal protein exhibit dual binding to protein and lipid epitopes. Biochem Biophys Res Commun. 2008;366(4):982–7. doi: 10.1016/j.bbrc.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 136.Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, et al. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem. 1995 Jun 9;38(12):2176–87. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 137.Li S, Wu SP, Whitmore M, Loeffert EJ, Wang L, Watkins SC, et al. Effect of immune response on gene transfer to the lung via systemic administration of cationic lipidic vectors. Am J Physiol. 1999 May;276(5 Pt 1):L796–804. doi: 10.1152/ajplung.1999.276.5.L796. [DOI] [PubMed] [Google Scholar]
- 138.Whitmore M, Li S, Huang L. LPD lipopolyplex initiates a potent cytokine response and inhibits tumor growth. Gene Ther. 1999 Nov;6(11):1867–75. doi: 10.1038/sj.gt.3301026. [DOI] [PubMed] [Google Scholar]
- 139.Stamatatos L, Leventis R, Zuckermann MJ, Silvius JR. Interactions of cationic lipid vesicles with negatively charged phospholipid vesicles and biological membranes. Biochemistry (Mosc) 1988 May 31;27(11):3917–25. doi: 10.1021/bi00411a005. [DOI] [PubMed] [Google Scholar]
- 140.Yan W, Chen W, Huang L. Reactive oxygen species play a central role in the activity of cationic liposome based cancer vaccine. J Control Release. 2008;130(1):22–8. doi: 10.1016/j.jconrel.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 141.Vasievich EA, Chen W, Huang L. Enantiospecific adjuvant activity of cationic lipid DOTAP in cancer vaccine. Cancer Immunol Immunother. 2011 May;60(5):629–38. doi: 10.1007/s00262-011-0970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ruysschaert JM, el Ouahabi A, Willeaume V, Huez G, Fuks R, Vandenbranden M, et al. A novel cationic amphiphile for transfection of mammalian cells. Biochem Biophys Res Commun. 1994 Sep 30;203(3):1622–8. doi: 10.1006/bbrc.1994.2372. [DOI] [PubMed] [Google Scholar]
- 143.Tanaka T, Legat A, Adam E, Steuve J, Gatot JS, Vandenbranden M, et al. DiC14-amidine cationic liposomes stimulate myeloid dendritic cells through Toll-like receptor 4. Eur J Immunol. 2008 May;38(5):1351–7. doi: 10.1002/eji.200737998. [DOI] [PubMed] [Google Scholar]
- 144.Lonez C, Vandenbranden M, Ouali M, Legat A, Ruysschaert JM, Elouahabi A. Free diC14-amidine liposomes inhibit the TNF-alpha secretion induced by CpG sequences and lipopolysaccharides: role of lipoproteins. Mol Membr Biol. 2006 May-Jun;23(3):227–34. doi: 10.1080/09687860600574436. [DOI] [PubMed] [Google Scholar]
- 145.Lonez C, Legat A, Vandenbranden M, Ruysschaert JM. DiC14-amidine confers new anti-inflammatory properties to phospholipids. Cell Mol Life Sci. 2008 Feb;65(4):620–30. doi: 10.1007/s00018-007-7520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007 Jul 13;317(5835):256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 147.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 148.DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011 Mar 10;471(7337):220–4. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ozpolat B, Lopez-Berestein G. Liposomal-all-trans-retinoic acid in treatment of acute promyelocytic leukemia. Leuk Lymphoma. 2002 May;43(5):933–41. doi: 10.1080/10428190290021678. [DOI] [PubMed] [Google Scholar]
- 150.Watson DS, Huang Z, Szoka FC., Jr All-trans retinoic acid potentiates the antibody response in mice to a lipopeptide antigen adjuvanted with liposomal lipid A. Immunol Cell Biol. 2009 Nov-Dec;87(8):630–3. doi: 10.1038/icb.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005 May 1;174(9):5390–7. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 152.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009 Nov;29(11):1944–9. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 153.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011 May;12(5):408–15. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kong W, Yen JH, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav Immun. 2011 Jul;25(5):872–82. doi: 10.1016/j.bbi.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Draper E, Reynolds CM, Canavan M, Mills KH, Loscher CE, Roche HM. Omega-3 fatty acids attenuate dendritic cell function via NF-kappaB independent of PPARgamma. J Nutr Biochem. 2010 Nov 25; doi: 10.1016/j.jnutbio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 156.Bonilla DL, Ly LH, Fan YY, Chapkin RS, McMurray DN. Incorporation of a dietary omega 3 fatty acid impairs murine macrophage responses to Mycobacterium tuberculosis. PLoS ONE. 2010;5(5):e10878. doi: 10.1371/journal.pone.0010878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Camuesco D, Galvez J, Nieto A, Comalada M, Rodriguez-Cabezas ME, Concha A, et al. Dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with DSS-induced colitis. J Nutr. 2005 Apr;135(4):687–94. doi: 10.1093/jn/135.4.687. [DOI] [PubMed] [Google Scholar]
- 158.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000 May 4;405(6782):85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 159.Otsuka M, Negishi Y, Aramaki Y. Involvement of phosphatidylinositol-3-kinase and ERK pathways in the production of TGF-beta1 by macrophages treated with liposomes composed of phosphatidylserine. FEBS Lett. 2007 Jan 23;581(2):325–30. doi: 10.1016/j.febslet.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 160.Mori M, Nishida M, Maekawa N, Yamamura H, Tanaka Y, Kasai M, et al. An increased adjuvanticity of liposomes by the inclusion of phosphatidylserine in immunization with surface-coupled liposomal antigen. Int Arch Allergy Immunol. 2005 Jan;136(1):83–9. doi: 10.1159/000082588. [DOI] [PubMed] [Google Scholar]
- 161.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–86. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 162.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008 Oct;8(10):753–63. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004 Jan 22;427(6972):355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 164.Idzko M, Panther E, Corinti S, Morelli A, Ferrari D, Herouy Y, et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J. 2002 Apr;16(6):625–7. doi: 10.1096/fj.01-0625fje. [DOI] [PubMed] [Google Scholar]
- 165.Fueller M, Wang DA, Tigyi G, Siess W. Activation of human monocytic cells by lysophosphatidic acid and sphingosine-1-phosphate. Cell Signal. 2003 Apr;15(4):367–75. doi: 10.1016/s0898-6568(02)00117-1. [DOI] [PubMed] [Google Scholar]
- 166.Zheng Y, Kong Y, Goetzl EJ. Lysophosphatidic acid receptor-selective effects on Jurkat T cell migration through a Matrigel model basement membrane. J Immunol. 2001 Feb 15;166(4):2317–22. doi: 10.4049/jimmunol.166.4.2317. [DOI] [PubMed] [Google Scholar]
- 167.Chan LC, Peters W, Xu Y, Chun J, Farese RV, Jr, Cases S. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA) J Leukoc Biol. 2007 Nov;82(5):1193–200. doi: 10.1189/jlb.0407221. [DOI] [PubMed] [Google Scholar]
- 168.Panther E, Idzko M, Corinti S, Ferrari D, Herouy Y, Mockenhaupt M, et al. The influence of lysophosphatidic acid on the functions of human dendritic cells. J Immunol. 2002 Oct 15;169(8):4129–35. doi: 10.4049/jimmunol.169.8.4129. [DOI] [PubMed] [Google Scholar]
- 169.Perrin-Cocon L, Agaugue S, Coutant F, Saint-Mezard P, Guironnet-Paquet A, Nicolas JF, et al. Lysophosphatidylcholine is a natural adjuvant that initiates cellular immune responses. Vaccine. 2006 Feb 27;24(9):1254–63. doi: 10.1016/j.vaccine.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 170.Oussoren C, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection: III. Influence of surface modification with poly(ethyleneglycol) Pharm Res. 1997 Oct;14(10):1479–84. doi: 10.1023/a:1012145410859. [DOI] [PubMed] [Google Scholar]
- 171.Oussoren C, Zuidema J, Crommelin DJ, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid compostion and lipid dose. Biochim Biophys Acta. 1997 Sep 4;1328(2):261–72. doi: 10.1016/s0005-2736(97)00122-3. [DOI] [PubMed] [Google Scholar]
- 172.Harrington KJ, Rowlinson-Busza G, Syrigos KN, Uster PS, Vile RG, Stewart JS. Pegylated liposomes have potential as vehicles for intratumoral and subcutaneous drug delivery. Clin Cancer Res. 2000 Jun;6(6):2528–37. [PubMed] [Google Scholar]
- 173.Oussoren C, Velinova M, Scherphof G, van der Want JJ, van Rooijen N, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. IV. Fate of liposomes in regional lymph nodes. Biochim Biophys Acta. 1998 Mar 13;1370(2):259–72. doi: 10.1016/s0005-2736(97)00275-7. [DOI] [PubMed] [Google Scholar]
- 174.Even-Or O, Joseph A, Itskovitz-Cooper N, Samira S, Rochlin E, Eliyahu H, et al. A new intranasal influenza vaccine based on a novel polycationic lipid-ceramide carbamoyl-spermine (CCS). II. Studies in mice and ferrets and mechanism of adjuvanticity. Vaccine. 2011 Mar 16;29(13):2474–86. doi: 10.1016/j.vaccine.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 175.Richards RL, Habbersett RC, Scher I, Janoff AS, Schieren HP, Mayer LD, et al. Influence of vesicle size on complement-dependent immune damage to liposomes. Biochim Biophys Acta. 1986 Feb 27;855(2):223–30. doi: 10.1016/0005-2736(86)90168-9. [DOI] [PubMed] [Google Scholar]
- 176.Bradley AJ, Brooks DE, Norris-Jones R, Devine DV. C1q binding to liposomes is surface charge dependent and is inhibited by peptides consisting of residues 14–26 of the human C1qA chain in a sequence independent manner. Biochim Biophys Acta. 1999 Apr 14;1418(1):19–30. doi: 10.1016/s0005-2736(99)00013-9. [DOI] [PubMed] [Google Scholar]
- 177.Phillips MC, Johnson WJ, Rothblat GH. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim Biophys Acta. 1987 Jun 24;906(2):223–76. doi: 10.1016/0304-4157(87)90013-x. [DOI] [PubMed] [Google Scholar]
- 178.Kirby C, Clarke J, Gregoriadis G. Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem J. 1980 Feb 15;186(2):591–8. doi: 10.1042/bj1860591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kaur R, Bramwell VW, Kirby DJ, Perrie Y. Pegylation of DDA:TDB liposomal adjuvants reduces the vaccine depot effect and alters the Th1/Th2 immune responses. J Control Release. 2011 Oct 18; doi: 10.1016/j.jconrel.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 180.Serre K, Machy P, Grivel JC, Jolly G, Brun N, Barbet J, et al. Efficient presentation of multivalent antigens targeted to various cell surface molecules of dendritic cells and surface Ig of antigen-specific B cells. J Immunol. 1998 Dec 1;161(11):6059–67. [PubMed] [Google Scholar]
- 181.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009 Jan;9(1):15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 182.Schwendener RA, Lagocki PA, Rahman YE. The effects of charge and size on the interaction of unilamellar liposomes with macrophages. Biochim Biophys Acta. 1984 Apr 25;772(1):93–101. doi: 10.1016/0005-2736(84)90521-2. [DOI] [PubMed] [Google Scholar]
- 183.Miller CR, Bondurant B, McLean SD, McGovern KA, O’Brien DF. Liposome-cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry (Mosc) 1998 Sep 15;37(37):12875–83. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]
- 184.Lee KD, Hong K, Papahadjopoulos D. Recognition of liposomes by cells: in vitro binding and endocytosis mediated by specific lipid headgroups and surface charge density. Biochim Biophys Acta. 1992 Jan 31;1103(2):185–97. doi: 10.1016/0005-2736(92)90086-2. [DOI] [PubMed] [Google Scholar]
- 185.Xu Y, Szoka FC., Jr Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry (Mosc) 1996 May 7;35(18):5616–23. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 186.Sonawane ND, Szoka FC, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003 Nov 7;278(45):44826–31. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 187.Siegel DP, Epand RM. The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: implications for membrane fusion mechanisms. Biophys J. 1997 Dec;73(6):3089–111. doi: 10.1016/S0006-3495(97)78336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhou F, Huang L. Liposome-mediated cytoplasmic delivery of proteins: an effective means of accessing the MHC class I-restricted antigen presentation pathway. Immunomethods. 1994 Jun;4(3):229–35. doi: 10.1006/immu.1994.1025. [DOI] [PubMed] [Google Scholar]
- 189.Zhou F, Watkins SC, Huang L. Characterization and kinetics of MHC class I-restricted presentation of a soluble antigen delivered by liposomes. Immunobiology. 1994 Feb;190(1–2):35–52. doi: 10.1016/S0171-2985(11)80282-2. [DOI] [PubMed] [Google Scholar]
- 190.Rao M, Rothwell SW, Wassef NM, Pagano RE, Alving CR. Visualization of peptides derived from liposome-encapsulated proteins in the trans-Golgi area of macrophages. Immunol Lett. 1997 Nov;59(2):99–105. doi: 10.1016/s0165-2478(97)00107-7. [DOI] [PubMed] [Google Scholar]
- 191.Rothwell SW, Wassef NM, Alving CR, Rao M. Proteasome inhibitors block the entry of liposome-encapsulated antigens into the classical MHC class I pathway. Immunol Lett. 2000 Oct 3;74(2):141–52. doi: 10.1016/s0165-2478(00)00206-6. [DOI] [PubMed] [Google Scholar]
- 192.Peachman KK, Rao M, Palmer DR, Zidanic M, Sun W, Alving CR, et al. Functional microtubules are required for antigen processing by macrophages and dendritic cells. Immunol Lett. 2004 Aug 15;95(1):13–24. doi: 10.1016/j.imlet.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 193.Peachman KK, Rao M, Alving CR, Palmer DR, Sun W, Rothwell SW. Human dendritic cells and macrophages exhibit different intracellular processing pathways for soluble and liposome-encapsulated antigens. Immunobiology. 2005;210(5):321–33. doi: 10.1016/j.imbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 194.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 195.Andrieu M, Loing E, Desoutter JF, Connan F, Choppin J, Gras-Masse H, et al. Endocytosis of an HIV-derived lipopeptide into human dendritic cells followed by class I-restricted CD8(+) T lymphocyte activation. Eur J Immunol. 2000 Nov;30(11):3256–65. doi: 10.1002/1521-4141(200011)30:11<3256::AID-IMMU3256>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 196.Andrieu M, Desoutter JF, Loing E, Gaston J, Hanau D, Guillet JG, et al. Two human immunodeficiency virus vaccinal lipopeptides follow different cross-presentation pathways in human dendritic cells. J Virol. 2003 Jan;77(2):1564–70. doi: 10.1128/JVI.77.2.1564-1570.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stittelaar KJ, Hoogerhout P, Ovaa W, van Binnendijk RR, Poelen MC, Roholl P, et al. In vitro processing and presentation of a lipidated cytotoxic T-cell epitope derived from measles virus fusion protein. Vaccine. 2002 Oct 12;20(1–2):249–61. doi: 10.1016/s0264-410x(01)00265-1. [DOI] [PubMed] [Google Scholar]
- 198.Kamps JA, Scherphof GL. Receptor versus non-receptor mediated clearance of liposomes. Adv Drug Deliv Rev. 1998 Jun 8;32(1–2):81–97. doi: 10.1016/s0169-409x(97)00133-6. [DOI] [PubMed] [Google Scholar]
- 199.Yotsumoto S, Aramaki Y, Kakiuchi T, Tsuchiya S. Induction of antigen-dependent interleukin-12 production by negatively charged liposomes encapsulating antigens. Vaccine. 2004 Sep 3;22(25–26):3503–9. doi: 10.1016/j.vaccine.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 200.Szoka F, Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–8. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Holmgren J, Lycke N, Czerkinsky C. Cholera toxin and cholera B subunit as oral-mucosal adjuvant and antigen vector systems. Vaccine. 1993 Sep;11(12):1179–84. doi: 10.1016/0264-410x(93)90039-z. [DOI] [PubMed] [Google Scholar]
- 202.Watanabe J, Miyazaki Y, Zimmerman GA, Albertine KH, McIntyre TM. Endotoxin contamination of ovalbumin suppresses murine immunologic responses and development of airway hyper-reactivity. J Biol Chem. 2003 Oct 24;278(43):42361–8. doi: 10.1074/jbc.M307752200. [DOI] [PubMed] [Google Scholar]
- 203.Bito LZ. Inflammatory effects of endotoxin-like contaminants in commonly used protein preparations. Science. 1977 Apr 1;196(4285):83–5. doi: 10.1126/science.557238. [DOI] [PubMed] [Google Scholar]
- 204.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000 Jun 15;164(12):6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 205.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004 Nov;22(5):460–6. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- 206.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002 Nov;2(11):845–58. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 207.Albert S, Johnson RM. The effect of foster-nursing on mouse lymph nodes. Cancer Res. 1960 Feb;20:242–5. [PubMed] [Google Scholar]
- 208.Torchilin V. Nanoparticulates as drug carriers. World Scientific Publishing Company; 2006. [Google Scholar]
- 209.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004 Mar 1;172(5):2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 210.Brunel F, Darbouret A, Ronco J. Cationic lipid DC-Chol induces an improved and balanced immunity able to overcome the unresponsiveness to the hepatitis B vaccine. Vaccine. 1999;17:2192–203. doi: 10.1016/s0264-410x(98)00492-7. [DOI] [PubMed] [Google Scholar]
- 211.Pialoux G, Hocini H, Perusat S, Silberman B, Salmon-Ceron D, Slama L, et al. Phase I study of a candidate vaccine based on recombinant HIV-1 gp160 (MN/LAI) administered by the mucosal route to HIV-seronegative volunteers: the ANRS VAC14 study. Vaccine. 2008 May 19;26(21):2657–66. doi: 10.1016/j.vaccine.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 212.Thyagarajan R, Arunkumar N, Song W. Polyvalent antigens stabilize B cell antigen receptor surface signaling microdomains. J Immunol. 2003 Jun 15;170(12):6099–106. doi: 10.4049/jimmunol.170.12.6099. [DOI] [PubMed] [Google Scholar]
- 213.Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000 Aug;176:154–70. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 214.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006 Apr 17;203(4):1081–91. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jegerlehner A, Storni T, Lipowsky G, Schmid M, Pumpens P, Bachmann MF. Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation. Eur J Immunol. 2002 Nov;32(11):3305–14. doi: 10.1002/1521-4141(200211)32:11<3305::AID-IMMU3305>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 216.Rao M, Alving CR. Delivery of lipids and liposomal proteins to the cytoplasm and Golgi of antigen presenting cells. Advanced Drug Delivery Reviews. 2000;41:171–88. doi: 10.1016/s0169-409x(99)00064-2. [DOI] [PubMed] [Google Scholar]
- 217.Mabrey S, Mateo PL, Sturtevant JM. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry (Mosc) 1978 Jun 13;17(12):2464–8. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- 218.Huang Z, Szoka F. Sterol-modified phospholipids: cholesterol and phospholipid chimeras with improved biomembrane properties. J Am Chem Soc. 2008;130(46):15702–12. doi: 10.1021/ja8065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008 Aug;9(8):847–56. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci U S A. 2009 Jan 20;106(3):870–5. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003 Mar 28;278(13):11303–11. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 222.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005 Jan;11(1):90–4. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 223.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009 Oct 2;284(40):27384–92. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006 May 11;441(7090):235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 225.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008 Dec;9(12):1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 226.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011 Feb 24;470(7335):543–7. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhu Q, Egelston C, Gagnon S, Sui Y, Belyakov IM, Klinman DM, et al. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J Clin Invest. 2010 Feb 1;120(2):607–16. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Even-Or O, Samira S, Rochlin E, Balasingam S, Mann AJ, Lambkin-Williams R, et al. Immunogenicity, protective efficacy and mechanism of novel CCS adjuvanted influenza vaccine. Vaccine. 2010 Sep 7;28(39):6527–41. doi: 10.1016/j.vaccine.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 229.Lay M, Callejo B, Chang S, Hong DK, Lewis DB, Carroll TD, et al. Cationic lipid/DNA complexes (JVRS-100) combined with influenza vaccine (Fluzone) increases antibody response, cellular immunity, and antigenically drifted protection. Vaccine. 2009 Jun 12;27(29):3811–20. doi: 10.1016/j.vaccine.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hartikka J, Bozoukova V, Ferrari M, Sukhu L, Enas J, Sawdey M, et al. Vaxfectin enhances the humoral immune response to plasmid DNA-encoded antigens. Vaccine. 2001 Feb 28;19(15–16):1911–23. doi: 10.1016/s0264-410x(00)00445-x. [DOI] [PubMed] [Google Scholar]
- 231.Sullivan SM, Doukas J, Hartikka J, Smith L, Rolland A. Vaxfectin: a versatile adjuvant for plasmid DNA- and protein-based vaccines. Expert Opin Drug Deliv. 2010 Dec;7(12):1433–46. doi: 10.1517/17425247.2010.538047. [DOI] [PubMed] [Google Scholar]
- 232.Leroux-Roels I, Koutsoukos M, Clement F, Steyaert S, Janssens M, Bourguignon P, et al. Strong and persistent CD4+ T-cell response in healthy adults immunized with a candidate HIV-1 vaccine containing gp120, Nef and Tat antigens formulated in three Adjuvant Systems. Vaccine. 2010 Oct 8;28(43):7016–24. doi: 10.1016/j.vaccine.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 233.Garcon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines. 2011 Apr;10(4):471–86. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 234.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007 Jul 26;25(30):5467–84. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 235.Poland GA, Ovsyannikova IG, Jacobson RM, Smith DI. Heterogeneity in vaccine immune response: the role of immunogenetics and the emerging field of vaccinomics. Clin Pharmacol Ther. 2007 Dec;82(6):653–64. doi: 10.1038/sj.clpt.6100415. [DOI] [PubMed] [Google Scholar]
- 236.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009 Jan;10(1):116–25. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002 Aug;8(8):878–84. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- 238.Ahsan F, Rivas IP, Khan MA, Torres Suarez AI. Targeting to macrophages: role of physicochemical properties of particulate carriers--liposomes and microspheres--on the phagocytosis by macrophages. J Control Release. 2002 Feb 19;79(1–3):29–40. doi: 10.1016/s0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]
- 239.Manicassamy S, Pulendran B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin Immunol. 2009;21:22–7. doi: 10.1016/j.smim.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]