Abstract
Dietary n-6 polyunsaturated fatty acid (PUFA) deprivation in rodents reduces brain arachidonic acid (20:4n-6) concentration and 20:4n-6-preferring cytosolic phospholipase A2 (cPLA2-IVA) and cyclooxygenase (COX)-2 expression, while increasing brain docosahexaenoic acid (DHA, 22:6n-3) concentration and DHA-selective Ca2+-independent iPLA2-VIA expression. We hypothesized that these changes are accompanied by upregulated brain DHA metabolic rates. Using a fatty acid model, brain DHA concentrations and kinetics were measured in unanesthetized male rats fed, for 15 weeks post-weaning, an n-6 PUFA “adequate” (31.4 wt% linoleic acid) or “deficient” (2.7 wt% linoleic acid) diet, each lacking 20:4n-6 and DHA. [1-14C]DHA was infused intravenously, arterial blood was sampled, and the brain was microwaved at 5 min and analyzed. Rats fed the n-6 PUFA deficient compared with adequate diet had significantly reduced n-6 PUFA concentrations in brain phospholipids but increased eicosapentaenoic acid (EPA, 20:5n-3), docosapentaenoic acidn-3 (DPAn-3, 22:5n-3) and DHA (by 9.4%) concentrations, particularly in ethanolamine glycerophospholipid. Incorporation rates of unesterified DHA from plasma, which represent DHA metabolic loss from brain, were increased 45% in brain phospholipids, as was DHA turnover. Increased DHA metabolism following dietary n-6 PUFA deprivation may increase brain concentrations of antiinflammatory DHA metabolites, which with a reduced brain n-6 PUFA content, likely promote neuroprotection. (199 words)
Keywords: linoleic acid, arachidonic PUFA, diet, turnover, metabolism, docosahexaenoic, kinetics, brain, alpha-linolenic, rat
INTRODUCTION
The brain has high concentrations of arachidonic acid (20:4n-6) and docosahexaenoic acid (DHA, 22:6n-3). These long chain polyunsaturated fatty acids (PUFAs) serve as constituents of cell membranes and as signaling molecules, and can be metabolized to bioactive eicosanoids and docosanoids, respectively (Uauy & Dangour 2006, DeGeorge et al. 1991, Jones et al. 1997, Rapoport 2008, Bazan 2007, Serhan et al. 2008). They cannot be synthesized in vertebrates de novo, but must be obtained through the diet or be elongated and desaturated (primarily in the liver) from their respective nutritionally essential precursors, linoleic acid (18:2n-6) and α-linolenic acid (18:3n-3) (Holman 1986, DeMar et al. 2005, Jump et al. 2005, Nakamura & Nara 2003, Sprecher 2000, Igarashi et al. 2005).
We reported (Igarashi et al. 2009) effects in rats fed an n-6 PUFA deficient diet lacking 20:4n-6 or DHA and containing 18:2n-6 at 9.9 wt% of the daily estimated requirement, 1200 mg/100 g (Bourre et al. 1990), which was n-3 PUFA adequate with regard to organ composition and function. In rats fed the n-6 PUFA deficient compared with an n-6 PUFA adequate diet for 15 weeks, 20:4n-6 concentrations were decreased in serum (-86%), while concentrations of eicosapentaenoic acid (EPA, 20:5n-3) and DHA were elevated. The serum concentration of eicosatrienoic acid (20:3n-9), a marker of 18:2n-6 deficiency (Lundberg 1980, Bazinet et al. 2003), also was increased. The total n-3 PUFA concentration in brain was increased by 15%, reflecting largely an 11% increased DHA concentration (Igarashi et al. 2009). Expression of enzymes of the 20:4n-6 cascade, cytosolic phospholipase A2 (cPLA2) IVA and COX-2, was downregulated, while expression of DHA-preferring calcium-independent iPLA2 VIA (Strokin et al. 2004, Basselin et al. 2011, Garcia & Kim 1997, Ramadan et al. 2010) and of 15-lipoxygenase (LOX) was upregulated (Kim et al. 2011).
In view of the reported elevation in brain DHA concentration and expression of DHA-metabolizing iPLA2-VIA in the n-6 PUFA deprived animals, we predicted that rates of brain DHA metabolism also would be upregulated. To test this prediction, in the present paper we used our in vivo fatty acid infusion model to determine incorporation rates, turnovers and half-lives of DHA in brain lipids of rats that had been fed, for 15 weeks post-weaning, a diet deficient or adequate in n-6 PUFA content (Robinson et al. 1992, Rapoport 2001, Bourre et al. 1990, Igarashi et al. 2009). Unanesthetized rats were infused intravenously with [1-14C]DHA, arterial plasma was sampled, the brain was subjected to high energy microwaving after 5 min of infusion, then chemically analyzed (Rapoport 2001, Robinson et al. 1992, Chang et al. 1999). We found that dietary n-6 PUFA deprivation increased the rate of incorporation of unesterified DHA from plasma into and DHA turnover within brain phospholipids as well as brain DHA concentration.
MATERIALS AND METHODS
Materials
[1-14C]DHA in ethanol was purchased from Moravek Biochemicals (Brea, CA, USA). Its specific activity was 54 mCi/mmol and its purity was > 95%, as determined by high performance liquid chromatography (HPLC) and scintillation counting. Diheptadecanoate phosphatidylcholine (di-17:0 PtdCho), free heptadecanoic acid (17:0), and thin-layer chromatography (TLC) standards for cholesterol, triacylglycerol, and cholesteryl esters were purchased from Sigma-Aldrich (St. Louis, MO, USA). Standards for fatty acid methyl esters (FAMEs) for gas chromatography (GC) and for HPLC were obtained from NuChek Prep (Elysian, MN, USA). 6-p-Toluidine-2-naphthalene sulfonic acid was from Acros Organics (Fair Lawn, NJ, USA). Liquid scintillation cocktail (Ready Safe™) was purchased from Beckman Coulter™ (Fullerton, CA, USA). Solvents were HPLC grade and were purchased from Fisher Scientific (Fair Lawn, NJ, USA) or EMD Chemicals (Gibbstown, NJ, USA). Other chemicals and reagents were purchased from Sigma-Aldrich or Fisher Scientific.
Animals
The protocol was approved by the Animal Care and Use Committee of the Eunice Kennedy Schriver National Institute of Child Health and Human Development and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23). Fischer-344 (CDF) male rat pups (18 days old) and their surrogate mothers, purchased from Charles River Laboratories (Portage, MI, USA), were housed in an animal facility having regulated temperature, humidity, and a 12 h light/12 h dark cycle. The pups were allowed to nurse until weaning at 21 days of age. Lactating rats had free access to water and rodent chow formulation NIH-31 18-4, which contained 4% (wt/wt) crude fat (Zeigler Bros., Gardners, PA, USA) and whose fatty acid composition has been reported (Igarashi et al. 2007b, Igarashi et al. 2007d). 18:3n-3, EPA, and DHA contributed 4.7%, 2.1% and 2.8% of total fatty acid (wt%), respectively, whereas 18:2n-6 and 20:4n-6 contributed 48.3 wt% and 0.3 wt%, respectively. After weaning, the pups were divided randomly into two groups, fed an n-6 PUFA adequate (n = 8) or deficient (n = 8) diet for 15 weeks (see below). They had free access to food and water, and their food was replaced every 2 or 3 days.
n-6 PUFA adequate and deficient diets
The n-6 PUFA adequate and deficient diets were prepared by Dyets Inc. (Bethlehem, PA, USA) based on the AIN-93G formulation (Reeves et al. 1993, Igarashi et al. 2009), and each contained 10% fat. The n-6 PUFA adequate diet contained hydrogenated coconut oil (6 g/100 g diet), safflower oil (3.23 g/100 g) and flaxseed oil (0.77 g/100 g) (Supplementary Table 1). The n-6 PUFA deficient diet contained hydrogenated coconut oil (8.73 g/100 g), flaxseed oil (0.77 g/100 g), and olive oil (0.5 g/100 g), but not safflower oil.
The n-6 PUFA adequate diet contained 18:2n-6 at 14.8 mg/g diet (31.4 wt% of total fatty acid; 3.3% energy), whereas the deficient diet contained 18:2n-6 at 1.2 mg/g (2.7 wt%; 0.27% energy) (Igarashi et al. 2009), which is 10% of the minimum requirement for rodents (12 mg/g) (Bourre et al. 1990) (Supplementary Table 2). Both diets contained α-LNA at 2.4-2.5 mg/g (5.0-5.7 wt%), which is close to the minimum requirement for dietary n-3 PUFA adequacy in rodents (Bourre et al. 1989a, Bourre et al. 1989b), and oleic acid (18:1n-9) at 3.8-4.1 mg/g (8.7-8.9 wt%). Other n-3 and n-6 PUFAs were not found in either diet (Igarashi et al. 2009).
Surgery
A rat was anesthetized with 1-3% halothane (Shirley Aldred & Co., South Yorkshire, Great Britain). Polyethylene catheters (PE 50, Intramedic™, Clay Adams™, Becton Dickinson, Sparks, MD, USA) filled with heparinized saline (100 IU/ml) were surgically implanted into the right femoral artery and vein, then the skin was closed with staples and treated with 1% lidocaine (Hospira, Lake Forest, IL, USA) for pain control. The rat was wrapped loosely in a fast-setting plaster cast taped to a wooden block, and allowed to recover from anesthesia for 3-4 h. Body temperature was maintained at 36-38 °C using a feedback-heating element with a rectal probe (Indicating Temperature Controller, Yellow Springs Instruments, Yellow Springs, OH, USA). The animal was provided food the night prior to surgery but not on the morning of surgery.
Radiotracer infusion
The unanesthetized rat was infused via the femoral vein catheter with 150 μCi/kg [1-14C]DHA (DeMar et al. 2005, Chang et al. 1999). An aliquot of [1-14C]DHA in ethanol was dried under nitrogen, and the residue was dissolved in HEPES buffer (pH 7.4) containing 50 mg/ml fatty acid-free bovine serum albumin. The mixture was sonicated at 40 °C for 20 min and mixed by vortexing. A computer-controlled variable speed pump (No. 22; Harvard Apparatus, South Natick, MA, USA) was used to infuse 1.3 ml tracer solution at a rate of 0.223 (1+e-1.92t) ml/min (t in min) to rapidly establish steady-state plasma radioactivity (Washizaki et al. 1994, Igarashi et al. 2007c, DeMar et al. 2005). Arterial blood (180 μl) was collected in centrifuge tubes (polyethylene-heparin lithium fluoride-coated, Beckman) at 0, 0.25, 0.5, 0.75, 1.5, 3, 4, and 5 min after starting the infusion. The samples were centrifuged at 10,000 g for 1 min, and plasma was collected and stored at -80 °C. After 5 min of infusion, the rat was euthanized with an overdose of sodium pentobarbital (Ovation Pharmaceuticals, Deerfield, IL, USA) (100 mg/kg i.v.). The head was subjected immediately to high-energy focused beam microwave irradiation (5.5 kW, 4.8 sec) (Model S6F, Cober Electronics; Stamford, CT, USA) to interrupt brain metabolism (Deutsch et al. 1997), and the brain was removed, weighed and stored at -80 °C until it was used chemical and radioactive measurements (see below).
Separation and analysis of lipids in plasma and brain
Approximately 0.8 g brain (one half-brain) or 150 μl plasma was used for lipid extraction by the Folch procedure (Folch et al. 1957). The aqueous extraction phases were washed once with an equal volume of chloroform to remove residual lipid, and radioactivity in the aqueous and total lipid fractions was counted (see below). Total lipid extracts from plasma and brain were separated into neutral lipid subclasses by TLC on silica gel 60 plates (EM Separation Technologies, Gibbstown, NJ, USA) using heptane /diethyl ether /glacial acetic acid (60:40:3, v/v/v) (Skipski et al. 1968). Total lipid extract from brain was separated into phospholipid classes by TLC on silica gel 60 plates using chloroform/methanol/glacial acetic acid/water (60:40:1:4, v/v/v/v) to separate ethanolamine glycerophospholipids (EtnGpl), choline glycerophospholipids (ChoGpl), phosphatidylserine (PtdSer), and phosphatidylinositol (PtdIns) (Skipski et al. 1967). Authentic standards of triacylglycerol, cholesterol, cholesteryl ester and unesterified fatty acids for neutral lipid separation, and individual phospholipids for phospholipid separation, were run on the plates to identify lipids. Plates were dried, sprayed with 0.03% 6-p-toluidine-2-naphthalene sulfonic acid in 50 mM Tris-HCl buffer (pH 7.4) (w/v), and lipid bands were visualized under ultraviolet light. The bands were scraped, and the silica gel was used to directly quantify radioactivity by scintillation counting, to prepare FAMEs and to analyze individual phospholipid concentrations (described below).
Lipid concentrations
An aliquot of total brain lipid extract, prepared as described above, was dried to prepare for digestion to measure the total phospholipid concentration in brain. To measure an individual phospholipid concentration, total lipid extracts were separated by TLC as described above. Dried total lipid extract or scraped silica gels were added to 0.5 ml of water and 0.65 ml of perchloric acid (70%) for digestion, and were treated at 180 °C for 1 h (Rouser et al. 1970). After the sample was cooled to room temperature, 0.5 ml of ascorbic acid solution (10%, w/v), 0.5 ml of ammonium molybdate solution (2.5%, w/v), and 3.0 ml of water were added. The mixture was boiled for 5 min to develop color, and absorbance was read at 797 nm after sample cooling. Standards for this assay were purchased from Sigma, and phosphorus concentrations were determined using standard curves. To quantify concentrations of total cholesterol and triacylglycerol, the lipid extract was dried, and the residue was dissolved in 0.1% Triton X-100. Total cholesterol was determined with a cholesterol/cholesteryl ester quantitation kit (BioVision Research Products, Mountain View, CA, USA). Triacylglycerol was determined with a free glycerol determination kit (Sigma-Aldrich).
Radioactivity
Samples for measuring radioactivity were placed in scintillation vials and dissolved in liquid scintillation cocktail (Ready Safe™ plus 1% glacial acetic acid). Radioactivity was determined using a liquid scintillation analyzer (2200CA, TRI-CARB®, Packard Instruments, Meriden, CT, USA).
Fatty acid methyl ester preparation
Unesterified and esterified fatty acids (total phospholipids, individual phospholipids, triacylglycerol, and cholesterol esters) were transmethylated to FAMEs with 1% H2SO4-methanol for 3 h at 70 °C (Makrides et al. 1994, DeMar et al. 2004). Before transmethylation, an appropriate quantity of di-17:0 PtdCho (for phospholipids, triacylglycerol and cholesteryl esters) or of 17:0 fatty acid (for unesterified fatty acids) was added as an internal standard. The prepared FAMEs were analyzed by GC and HPLC, as described below.
GC analysis
Fatty acid concentrations (nmol/g brain wet wt) in brain and plasma lipids were determined using a GC (6890N, Agilent Technologies, Palo Alto, CA, USA) equipped with an SP™-2330 fused silica capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness) (Supelco, Bellefonte, PA, USA) and a flame ionization detector (DeMar et al. 2004). Peaks were identified from retention times of the standards for the FAMEs. Concentrations were calculated by proportional comparison of peak areas to the area of the added 17:0 internal standard.
HPLC analysis
To determine fatty acid radioactivity in brain and plasma lipid samples, FAMEs were quantified by HPLC by the method of Aveldano et al. (Aveldano et al. 1983) with modifications. Total lipids were alkalinized with KOH solution, and extracted twice with n-hexane. The hexane phase was dried and transmethylated as described above. FAMEs were dissolved in acetonitrile, and the solution was fractionated by reversed phase column-HPLC using a pump (System GOLD 126, Beckman Coulter, Fullerton, CA, USA) outfitted with a UV detector (UV/VIS-151, Gilson, Middleton, WI, USA) and an on-line continuous scintillation counter detector (β-RAM Model 2, IN/US Systems, FL, USA) mixing in liquid scintillation cocktail (IN-FLOW™ 2:1, IN/US Systems). The reversed-phase column, Luna 5 μC18 (2) (5 μm particle size, 4.6 × 250 mm), was obtained from Phenomenex (Torrance, CA, USA). For brain FAME samples, HPLC eluate was collected every 30 sec and subjected to liquid scintillation counting to obtain a radioactivity profile. Chromatography was performed using a linear gradient system of water and acetonitrile. The acetonitrile was held at 85% for 30 min, increased to 100% over 10 min, and held at 100% for 20 min. The flow rate was 1.0 ml/min. The UV detector was set at 205 nm.
Eight samples were pooled equally to analyze HPLC profiles of FAMEs. Analyses were carried out in duplicate. Peaks were identified from retention times of the standards for the FAMEs. Percentages of radioactivity in DHA and other fatty acids in the brain and plasma total lipid fractions were determined from these HPLC profiles.
Long chain acyl-CoAs
Long chain acyl-CoAs were extracted from brain using an affinity chromatography method with slight modification (Deutsch et al. 1994). After an appropriate amount of heptadecanoyl-CoA (17:0-CoA) was added as an internal standard to ~0.5 g of brain, the sample was sonicated in 25 mM KH2PO4 for 30 sec on ice with a probe sonicator (Model W-225, Misonix, Farmingdale, NY, USA). To the homogenate was added 2 ml of 2-isopropanol and the sample was sonicated for 30 sec. 0.25 ml of saturated (NH4)2SO4 was added to the homogenate to precipitate protein, and 4 ml of acetonitrile was added before the sample was mixed vigorously for 5 min at 3,000 rpm. The supernatant was diluted with 1.25 vol of 25 mM KH2PO4. The solution was passed 3 times through an oligonucleotide purification cartridge (ABI Masterpiece™, OPC®, Applied Biosystems, Foster City, CA, USA), and the cartridge was washed with 10 ml of 25 mM KH2PO4. Acyl-CoA species were eluted with 0.4 ml of isopropanol /1 mM glacial acetic acid (75:25 v/v)
Extracted acyl-CoAs were separated on a reversed phase HPLC column (Symmetry, 5 μm particle size, 4.6 mm × 250 mm, Waters Corporation, Milford, MA, USA), using a pump coupled with a UV/VIS detector (System Gold, Model 168, Beckman). Chromatography was performed using a linear gradient system of 75 mM KH2PO4 and acetonitrile. At the start, acetonitrile was 44% and held for 1 min, then was increased to 49% over 25 min, increased to 68% over 10 min, held at 68% for 4 min, returned to 44% over 6 min, and held for 6 min (52 min total run time). The flow rate was 1.0 ml/min. UV detection was set at 260 nm for integration of concentrations and at 280 nm for identifying acyl-CoAs (260/280 = 4:1) (Deutsch et al. 1994). Peaks were identified from retention times of acyl-CoA standards. The acyl-CoA standards for α-LNA, EPA and DHA were prepared from the free fatty acids and the free CoA by an enzymatic method (Taylor et al. 1990). Endogenous acyl-CoA concentrations (nmol/g brain) were calculated by direct proportional comparison with the peak area of the 17:0-CoA internal standard. The DHA-CoA peak was collected in each sample, and its radioactivity was counted with a liquid scintillation counter.
Calculations
Pulse-labeling equations for determining in vivo kinetics of brain fatty acid metabolism following intravenous infusion with a radiolabeled fatty acid (Robinson et al. 1992, Rapoport et al. 2001, DeMar et al. 2005), were applied in the rats fed an n-6 PUFA adequate or deficient diet and infused with [1-14C]DHA. Incorporation coefficients (ml/sec/g brain), representing transfer of unesterified [1-14C]DHA from plasma into brain lipid i (phospholipid, triacylglycerol or cholesteryl ester), were calculated as:
| (Eq. 1) |
where (nCi/g brain) is DHA radioactivity in i at time T (5 min) after starting tracer infusion, t is time after starting infusion, and (nCi/ml plasma) is radioactivity of unesterified plasma DHA (DeMar et al. 2005).
The incorporation rate of unlabeled unesterified DHA from plasma into brain lipid i, Jin, i(DHA), was calculated in units of nmol/s/g brain,
| (Eq. 2) |
where cplasma(DHA) is the concentration (nmol/ml) of unlabeled unesterified DHA in plasma.
A “dilution factor” λDHA-CoA equals the steady-state ratio of brain DHA-CoA specific activity to specific activity of unesterified DHA in plasma,
| (Eq. 3) |
The rate of incorporation JFA, i(DHA) of unlabeled DHA from the brain DHA-CoA pool into brain lipid i equals, in units of nmol/g/sec,
| (Eq. 4) |
Turnover FFA, i(DHA) of DHA within brain lipid i equals,
| (Eq. 5) |
where cbrain, i(DHA) is the concentration of total DHA in i. The corresponding half-life of DHA in i equals,
| (Eq. 6) |
Statistical analysis
Data are expressed as mean ± SD (n = 8). An unpaired Student’s t-test was used to compare means in 2 groups having possible equal variance with the Levene-test. The Welch test was used to compare the means of 2 groups having unequal variances. p ≤ 0.05 was used as a cutoff for statistical significance.
RESULTS
Growth and tissue weight
Body and brain weights did not differ significantly between animals on the n-6 PUFA adequate and deficient diets. Initial body weight at 21 days of age was 29 ± 1 g and 31 ± 2 g in the two groups, respectively (p = 0.13), whereas body weight after 15 weeks on a diet equaled 385 ± 14 g and 383 ± 14 g, respectively (p = 0.92). Brain weights after 15 weeks were 1.5 ± 0.1 g and 1.4 ± 0.3 g in the adequate and deficient groups, respectively (p = 0.40).
Lipid concentrations in plasma
Fifteen weeks of n-6 PUFA deprivation compared with control decreased the mean unesterified plasma concentration of n-6 PUFAs by 84% (Table 1). The change in the total unesterified n-3 PUFA concentration was statistically insignificant, although unesterified DHA and EPA concentrations with the deficient diet were increased by 53% and 79%, respectively. The unesterified n-6/n-3 PUFA concentration ratio in plasma was reduced from 5.2 to 1.2.
Table 1.
Fatty acid concentrations in plasma in rats after 15 weeks on n-6 PUFA adequate or deficient diet
| Fatty acid | Unesterified fatty acids | Phospholipids | Triacylglycerol | Cholesteryl esters | ||||
|---|---|---|---|---|---|---|---|---|
| Adequate | Deficient | Adequate | Deficient | Adequate | Deficient | Adequate | Deficient | |
| nmol/ml plasma | ||||||||
| n-6 PUFA | ||||||||
| 18:2 | 231 ± 74 | 26 ± 5 *** | 242 ± 36 | 135 ± 19*** | 161 ± 71 | 14 ± 8*** | 183 ± 26 | 94 ± 19*** |
| 18:3 | 6.5 ± 5.9 | 1.5 ± 1.1 * | 2.4 ± 1.7 | 1.4 ± 0.4 | 5.5 ± 2.9 | 1.0 ± 0.3 ** | 5.7 ± 4.0 | 3.8 ± 1.6 |
| 20:3 | 17 ± 10 | 35 ± 5 ** | 15 ± 6 | 34 ± 13** | 13 ± 7 | 19 ± 7 | 19 ± 13 | 37 ± 7 ** |
| 20:4 | 28 ± 13 | 4.7 ± 2.2 ** | 591 ± 93 | 135 ± 17*** | 55 ± 21 | 5 ± 3 *** | 957 ± 179 | 201 ± 30 *** |
| 22:4 | 5.4 ± 2.8 | 0.8 ± 0.3 ** | 5.7 ± 0.9 | 2.4 ± 1.7*** | 7.2 ± 3.3 | 0.54 ± 0.39 *** | ND | ND |
| 22:5 | 1.5 ± 0.9 | ND*** | 5.4 ± 2.6 | 2.1 ± 0.8 *** | 2.2 ± 0.7 | ND | ND | ND |
| Total n-6 PUFA | 289 ± 91 | 68 ± 10 *** | 861 ± 128 | 309 ± 41*** | 241 ± 97 | 40 ± 17 *** | 1165 ± 211 | 342 ± 52 *** |
| n-3 PUFA | ||||||||
| 18:3 | 24 ± 8 | 18 ± 4 * | 2.2 ± 0.9 | 4.7 ± 1.1*** | 13 ± 4 | 7 ± 5* | 3.6 ± 3.3 | 11 ± 2 *** |
| 20:5 | 5.6 ± 2.9 | 10 ± 3 * | 5.0 ± 1.5 | 109 ± 28 *** | 12 ± 4 | 12 ± 4 | 14 ± 3 | 232 ± 63*** |
| 22:5 | 11 ± 4 | 9.4 ± 2.7 | 18 ± 3 | 25 ± 3 *** | 11 ± 6 | 9 ± 6 | ND | ND |
| 22:6 | 15 ± 5 | 23 ± 6 * | 121 ± 19 | 187 ± 25 *** | 19 ± 10 | 13 ± 6 | 30 ± 11 | 39± 5 |
| Total n-3 PUFA | 55 ± 18 | 60 ± 14 | 146 ± 23 | 324 ± 43 *** | 54 ± 20 | 40 ± 16 | 48 ± 16 | 290 ± 71 *** |
| Others | ||||||||
| 16:0 | 376 ± 112 | 378 ± 91 | 409 ± 56 | 371 ± 36 | 298 ± 160 | 203 ± 132 | 141 ± 76 | 77 ± 12 |
| 16:1n-7 | 48 ± 25 | 72 ± 43 | 13 ± 5 | 28 ± 7 *** | 25 ± 15 | 33 ± 23 | 31 ± 12 | 73 ± 15 *** |
| 18:0 | 126 ± 79 | 71 ± 12 | 646 ± 96 | 509 ± 46 ** | 252 ± 196 | 171 ± 153 | 33 ± 11 | 20 ± 4 * |
| 18:1n-9 | 209 ± 62 | 273 ± 67 | 72 ± 22 | 159 ± 30*** | 141 ± 80 | 173 ± 118 | 42 ± 6 | 85 ± 14*** |
| 18:1n-7 | 41 ± 15 | 49 ± 11 | 47 ± 6 | 61 ± 11** | 38 ± 21 | 30 ± 19 | 13 ± 4 | 18± 2 ** |
| 20:3n-9 | 1.6 ± 0.8 | 1.6 ± 0.8 | 1.1 ± 0.3 | 21 ± 5 *** | 2.5 ± 1.4 | 3.6 ± 1.2 | 2.7 ± 1.7 | 14 ± 5 *** |
| Total saturated | 502 ± 141 | 449 ± 101 | 1056 ± 150 | 880 ± 75* | 551 ± 176 | 374 ± 134 * | 174 ± 71 | 97 ± 12* |
| Total mono | 298 ± 99 | 394 ± 110 | 132 ± 32 | 248 ± 38 | 205 ± 112 | 236 ± 159 | 86 ± 22 | 177 ± 32 *** |
| Total n-9 | 211 ± 62 | 275 ± 66 | 74 ± 22 | 181 ± 33 | 144 ± 81 | 176 ± 119 | 45 ± 5 | 100 ± 20 *** |
| Total | 1146 ± 270 | 972 ± 229 | 2196 ± 328 | 1783 ± 182 ** | 1053 ± 287 | 694 ± 266 * | 1475 ± 255 | 919 ± 159 *** |
| n-6/n-3 | 5.2 ± 0.3 | 1.2 ± 0.2*** | 5.9 ± 0.2 | 1.0 ± 0.2 *** | 4.3 ± 0.8 | 1.0 ± 0.2 *** | 26 ± 5 | 1.2 ± 0.2 *** |
Values are means ± SD (n = 8 for both groups).
p < 0.05,
p < 0.01,
p < 0.001, differs significantly from mean in adequate group.
ND = not detected (< 0.1 nmol/ml plasma, taken as 0 ± 0)
n-6 PUFA deprivation also significantly reduced esterified n-6 PUFA concentrations in plasma phospholipid, triacylglycerol and cholesteryl ester, and significantly increased n-3 PUFA concentrations in phospholipid (Table 1), giving a final esterified n-6/n-3 PUFA concentration ratio of 1.0 compared with 5.9 with the adequate diet. Other changes, summarized in Table 1, are comparable to prior results (Igarashi et al. 2009).
Structural lipids in brain
Brain concentrations of phospholipids, cholesterol and triacylglycerol did not differ significantly between rats on the n-6 PUFA deficient compared with adequate diet (Table 2). Concentrations of total n-6 PUFAs, and of 18:2n-6, 20:4n-6, and docosatetraenoic acid (22:4n-6), were decreased significantly by 11-49% in EtnGpl, ChoGpl, PtdSer, and PtdIns (Table 3). Docosapentaenoic acid n-6 (DPAn-6, 22:5n-6) was not detected in these lipids in rats fed the deficient diet.
Table 2.
Brain lipid concentrations in rats after 15 weeks on n-6 PUFA adequate or deficient diet
| Lipids | Dietary groups
|
|
|---|---|---|
| Adequate | Deficient | |
| μmol/g brain | ||
| Total phospholipids | 57.4 ± 10.4 | 65.2 ± 5.8 |
| Ethanolamine Glycerophospholipids | 22.5 ± 2.0 | 21.6 ± 2.3 |
| Phosphatidylinositol | 3.7 ± 0.7 | 3.3 ± 0.4 |
| Phosphatidylserine | 9.6 ± 0.5 | 9.7 ± 1.2 |
| Choline glycerophospholipids | 26.0 ± 1.4 | 25.6 ± 2.6 |
| Sphingomyelin | 2.3 ± 0.8 | 3.0 ± 0.9 |
| Total cholesterol | 42.6 ± 9.0 | 48.9 ± 11.9 |
| Triacylglycerol | 0.27 ± 0.08 | 0.27 ± 0.08 |
Values are mean ± SD (n = 8 for both groups)
Table 3.
Fatty acid concentrations in brain phospholipids
| Fatty acid | EtnGpl | ChoGpl | PtdSer | PtdIns | ||||
|---|---|---|---|---|---|---|---|---|
| Ade | Def | Ade | Def | Ade | Def | Ade | Def | |
| nmol/g brain | ||||||||
| n-6 PUFA | ||||||||
| 18:2 | 292 ± 31 | 207 ± 42 *** | 350 ± 41 | 241 ± 73 ** | 48 ± 6 | 37 ± 8* | 74 ± 13 | 49 ± 18** |
| 20:3 | 341 ± 55 | 420 ± 87* | 87 ± 11 | 137 ± 11*** | 90 ± 15 | 111 ± 32* | 23 ± 3 | 34 ± 7** |
| 20:4 | 5482 ± 527 | 4395 ± 594 ** | 2731 ± 249 | 2171 ± 406 ** | 760 ± 82 | 569 ± 102** | 2240 ± 179 | 1989 ± 270* |
| 22:4 | 2663 ± 218 | 1778 ± 387 *** | 366 ± 48 | 227 ± 71 *** | 781 ± 67 | 514 ± 119 *** | 139 ± 32 | 71 ± 21*** |
| 22:5 | 162 ± 20 | ND*** | 57 ± 12 | ND*** | 118 ± 14 | ND*** | 7.7 ± 0.9 | ND*** |
| Total | 8940 ± 786 | 6766± 846 *** | 3591 ± 321 | 2776 ± 542 ** | 1797 ± 168 | 1231 ± 223 *** | 2483 ± 210 | 2144 ± 308 * |
| n-3 PUFA | ||||||||
| 18:3 | ND | ND | ND | ND | ND | ND | ND | ND |
| 20:5 | ND | 83 ± 23*** | ND | 42 ± 13*** | ND | 10 ± 4*** | ND | 25 ± 4*** |
| 22:5 | 114 ± 11 | 313 ± 75 ** | 38 ± 6 | 70 ± 11*** | 40 ± 4 | 91 ± 19 *** | 7.7 ± 4.0 | 16 ± 4*** |
| 22:6 | 8093 ± 586 | 8852 ± 701 * | 2053 ± 210 | 2259 ± 279 | 4115 ± 349 | 4142 ± 373 | 377 ± 57 | 379 ± 67 |
| Total | 8207 ± 597 | 9248 ± 733 ** | 2091 ± 214 | 2371 ± 273* | 4155 ± 351 | 4243 ± 381 | 385 ± 54 | 420 ± 71 |
| Others | ||||||||
| 16:0 | 2636 ± 181 | 2675 ± 251 | 19082 ± 1500 | 19318 ± 1749 | 336 ± 50 | 322 ± 55 | 723 ± 86 | 727 ± 108 |
| 16:1n-7 | 246 ± 67 | 275 ± 112* | 252 ± 31 | 300 ± 35* | 17 ± 3 | 22 ± 4** | 37 ± 5 | 44 ± 8 |
| 18:0 | 7008 ± 521 | 6900 ± 537 | 7061 ± 607 | 7260 ± 852 | 7874 ± 611 | 7640 ± 714 | 2422 ± 191 | 2331 ± 319 |
| 18:1n-9 | 6767 ± 910 | 7519 ± 1059 | 10891 ± 1114 | 11828 ± 996 | 3523 ± 551 | 3648 ± 529 | 1151 ± 157 | 1218 ± 137 |
| 18:1n-7 | 1827 ± 231 | 2164 ± 272 * | 3006 ± 239 | 3163 ± 275 | 616 ± 96 | 662 ± 72 | 458 ± 58 | 495 ± 103 |
| 20:3n-9 | 73 ± 16 | 179 ± 41 *** | 26 ± 8 | 96 ± 54** | 28 ± 6 | 63 ± 18*** | 9.2 ± 0.9 | 39 ± 11*** |
| Total saturated | 9644 ± 679 | 9575 ± 768 | 26143 ± 2076 | 26578 ± 2567 | 8210 ± 646 | 7963 ± 766 | 3145 ± 268 | 3059 ± 424 |
| Total mono | 8840 ± 1160 | 10002 ± 1181 | 14149 ± 1240 | 15292 ± 1259 | 4156 ± 646 | 4332 ± 594 | 1645 ± 214 | 1758 ± 235 |
| Total n-9 FA | 7108 ± 964 | 7938 ± 1096 | 10917 ± 1117 | 11924 ± 996 | 3551 ± 556 | 3711 ± 537 | 1160 ± 158 | 1258 ± 139 |
| Total | 35704 ± 2889 | 35803 ± 3067 | 46001 ± 3718 | 47112 ± 4390 | 18345 ± 1503 | 17830 ± 1751 | 7667 ± 718 | 7420 ± 1010 |
| n-6/n-3 | 1.1±0.0 | 0.7±0.1*** | 1.7±0.2 | 1.2±0.2*** | 0.4±0.0 | 0.3±0.0*** | 6.5±0.6 | 5.1±0.4*** |
Values are mean ± SD (n = 8 for both groups).
p < 0.05,
p < 0.01,
p < 0.001, differs significantly from mean in adequate group,
ND = not detected, < 1.0 nmol/g brain (taken as 0 ± 0)
The total esterified n-3 PUFA concentration was increased significantly in brain EtnGpl by 13% and in ChoGpl by 13%, respectively, in rats fed the deficient compared with adequate diet, whereas DHA was increased in EtnGpl by 9.4% (Table 3). Increments in EPA, DPAn-3, and 20:3n-9, were significant in each of the four phospholipids examined. EPA was detected in brain only in rats on the deficient diet.
Plasma radioactivity during infusion
Mean radioactivity in the total lipid and aqueous phases of plasma during the 5-min [1-14C]DHA infusion in rats on each of the two diets is shown in Figure 1. Steady-state radioactivity was achieved within 1 min in both groups, and at 5 min > 95% of radioactivity was in the total lipid phase while < 5% was in the aqueous phase. Integrated plasma radioactivity during infusion equaled 207748 ± 16227 nCi.sec/ml and 201149 ± 56491 nCi.sec/ml in the n-6 PUFA adequate and deficient groups, respectively, and did not differ significantly between groups.
Figure 1.
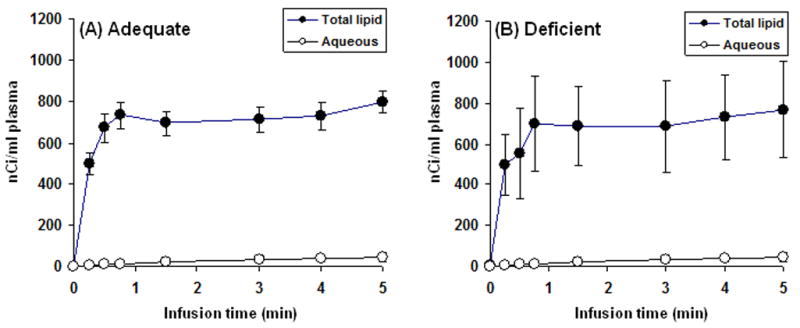
Radioactivity in total lipids (●) and aqueous (○) phase from plasma of n-6 PUFA adequate and deficient rats during i.v. infusion of [1-14C]DHA. Values are mean ± SD (n = 8 for both groups).
In each diet group after 5 min of [1-14C]DHA infusion (Fig. 2), > 92% of radioactivity was in the unesterified fatty acid pool of total plasma lipid, and the remaining 8% was in phospholipids, triacylglycerol, cholesterol and cholesteryl esters. At 5 min, > 97% of plasma total lipid radioactivity was [1-14C]DHA in both diet groups (HPLC chromatogram not shown), indicating minimal metabolism.
Figure 2.
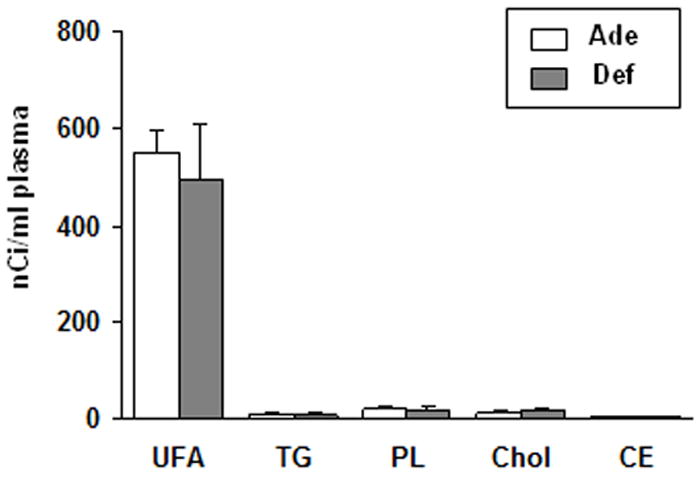
Radioactivity of plasma lipids in n-6 PUFA adequate and deficient rats after a 5-min i.v. infusion of [1-14C]DHA. Abbreviations: UFA, unesterified fatty acid; TG, triacylglycerol; PL, phospholipid; CE, cholesteryl ester; Chol, cholesterol. Values are mean ± SD (n = 8 for both groups).
Brain radioactivity
Radioactivity in different brain lipids after the 5-min [1-14C]DHA infusion is given in the first two data columns of Table 4. More than 86% of brain radioactivity was in the total lipid fraction in both dietary groups, less than 14% in the aqueous fraction. In the total lipid fraction, > 96% of radioactivity was [1-14C]DHA. Less than 2% was labeled palmitic acid (16:0), which likely was produced by recycling of radiolabeled carbon following β-oxidation of [1-14C]DHA (chromatograms not shown). Of net brain lipid radioactivity, > 78% was in total phospholipid, 2% in cholesteryl ester, 10% in triacylglycerol, and 8% in cholesterol. Of total phospholipid radioactivity due to [1-14C]DHA, 40% was in EtnGpl, 30% in ChoGpl, 23% in PtdIns, and 7% in PtdSer. There was no statistically significant group difference in these values.
Table 4.
Incorporation coefficients and incorporation rates (Jin, i(DHA)) of plasma DHA into brain lipids in unanesthetized rats on n-6 PUFA adequate or deficient diets
| Radioactivity (nCi/g brain) | Incorporation coefficient, ki* (ml/sec/g ×10-4) | Incorporation rate, Jin, i (nmol/sec/g × 10-4) | ||||
|---|---|---|---|---|---|---|
| Adequate | Deficient | Adequate | Deficient | Adequate | Deficient | |
| Total lipids | 45.1 ± 5.5 | 44.5 ± 12.6 | 2.2 ± 0.3 | 2.2 ± 0.3 | ||
| Aqueous phase | 6.6 ± 1.2 | 6.9 ± 1.6 | ||||
| Total phospholipids | 36.3 ± 5.0 | 34.6 ± 9.7 | 1.8 ± 0.3 | 1.7 ± 0.2 | 25.1 ± 6.8 | 36.4 ± 10.5 * |
| EtnGpl | 21.7 ± 3.2 | 19.8 ± 5.4 | 1.0 ± 0.2 | 0.99 ± 0.12 | 15.0 ± 4.0 | 20.9 ± 6.1 * |
| PtdIns | 10.7 ± 1.4 | 12.1 ± 2.9 | 0.52 ± 0.05 | 0.61 ± 0.07** | 7.5 ± 2.5 | 12.7 ± 3.4 ** |
| PtdSer | 3.2 ± 0.3 | 4.0 ± 1.1 | 0.15 ± 0.02 | 0.20 ± 0.03** | 2.3 ± 0.8 | 4.2 ± 1.2 ** |
| ChoGpl | 15.3 ± 3.3 | 14.7 ± 4.5 | 0.74 ± 0.19 | 0.73 ± 0.11 | 10.4 ± 2.8 | 15.5 ± 5.0 * |
| Neutral lipids | ||||||
| Triacylglycerol | 4.5 ± 0.5 | 5.1 ± 1.8 | 0.22 ± 0.03 | 0.25 ± 0.05 | 3.1 ± 0.8 | 5.4 ± 2.1 * |
| Cholesterol | 3.6 ± 0.3 | 4.0 ± 1.0 | 0.18 ± 0.02 | 0.20 ± 0.03 | ||
| Cholesteryl ester | 0.69 ± 0.10 | 0.81 ± 0.24 | 0.034 ± 0.005 | 0.041 ± 0.008* | ||
Values are mean ± SD (n = 8 for both groups).
p < 0.05,
p < 0.01, differs significantly from mean in adequate group
DHA incorporation coefficients and rates in stable brain lipids
Incorporation coefficients of plasma unesterified DHA into brain lipids i, were calculated by dividing radioactivity in lipid i by integrated plasma radioactivity in the same experiment (Eq. 1) (the second two data columns in Table 4). For PtdIns and PtdSer, was increased significantly by 1.2- and 1.3-fold, respectively, in rats fed the deficient compared with adequate diet, but it was not changed significantly for EtnGpl or ChoGpl. also was increased significantly into cholesteryl ester.
Incorporation rates Jin, i(DHA) of unesterified plasma DHA into brain lipids i were calculated by multiplying by the unesterified unlabeled plasma DHA concentration, determined prior to [1-14C]DHA infusion (Table 1) (Eq. 2). Jin, i(DHA) was increased significantly into total phospholipid (by 45%), triacylglycerol (by 74%), and individual phospholipids EtnGpl (39%), PtdIns (69%), PtdSer (83%) and ChoGpl (49%) in rats fed the n-6 PUFA deficient compared with adequate diet (third pair of data columns in Table 4)
Brain acyl-CoA concentrations and dilution coefficients λDHA-CoA
HPLC separation of the aqueous brain extract yielded unlabeled and radiolabeled acyl-CoA species (Table 5). Dietary n-6 PUFA deprivation significantly decreased unlabeled 18:2n-6-CoA and 20:4n-6-CoA concentrations by 70% and 64%, respectively (Table 5), but did not change concentrations of the other measured acyl-CoAs. DHA-CoA radioactivity was not affected by diet (Table 5). Mean values for the dilution coefficient, λDHA–CoA, the steady-state ratio of brain DHA-CoA specific activity to plasma unesterified DHA specific activity at the end of the 5-min infusion (Eq. 3) (Robinson et al. 1992), equaled 0.024 ± 0.007 and 0.025 ± 0.004 in the n-6 PUFA adequate and deprived rats, respectively, and did not differ significantly (Table 5).
Table 5.
Acyl-CoA concentrations and radioactivity and λDHA–CoA from dietary n-6 PUFA adequate and deficient rats following 5 min of i.v. [1-14C]DHA infusion
| Diet
|
||
|---|---|---|
| Adequate | Deficient | |
| Concentration (nmol/g brain) | ||
| 14:0-CoA, 18:3n-3-CoA, EPA-CoA | 1.1 ± 0.4 | 1.5 ± 0.4 |
| 16:0-CoA | 12.0 ± 2.3 | 11.8 ± 3.9 |
| 18:0-CoA | 3.5 ± 0.5 | 3.6 ± 1.4 |
| 18:1-CoA | 13.2 ± 2.2 | 12.9 ± 4.5 |
| 18:2n-6-CoA | 0.77 ± 0.31 | 0.23 ± 0.18*** |
| 20:4n-6-CoA | 1.7 ± 0.8 | 0.61 ± 0.22** |
| DHA-CoA | 1.3 ± 0.5 | 1.5 ± 0.4 |
| Radioactivity (nCi/g brain) | ||
| DHA-CoA | 1.69 ± 0.62 | 1.31 ± 0.37 |
| λDHA–CoA | 0.024 ± 0.007 | 0.025 ± 0.004 |
Values are mean ± SD (n = 8 for both groups).
p < 0.01,
<0.001, differs significantly from mean in adequate group.
DHA turnover and half-life in brain phospholipids
Jin, i(DHA) for each experiment was divided by λ DHA–CoA to calculate the incorporation rate JFA, i(DHA) of DHA from the precursor brain DHA-CoA pool into brain lipid i, using Eq. 4 (Table 6). n-6 PUFA deprivation significantly increased JFA, i(DHA) into total phospholipid by 1.4-fold, into PtdIns by 1.7-fold, into PtdSer by 1.9-fold, and into ChoGpl by 1.5-fold, but did not change JFA, i(DHA) into EtnGpl.
Table 6.
Dilution factors (λDHA-CoA), incorporation rates from brain DHA-CoA (JFA, i(DHA)), turnovers (FFA, i(DHA)) and half-lives of DHA in brains of rats fed an n -3 PUFA adequate or deprived diet
| JFA (nmol/sec/g × 10-4) | Turnover, FFA (%/h) | Half-life, t1/2 (h) | ||||
|---|---|---|---|---|---|---|
| Adequate | Deficient | Adequate | Deficient | Adequate | Deficient | |
| Total phospholipid | 1053 ± 1810 | 1500 ± 464 * | 2.28 ± 0.39 | 2.94 ± 0.83 | 31 ± 5 | 26 ± 8 |
| EtnGpl | 628 ± 104 | 864 ± 282 | 2.79 ± 0.42 | 3.52 ± 1.10 | 25 ± 4 | 22 ± 7 |
| PtdIns | 317 ± 82 | 526 ± 159 ** | 30.6 ± 7.9 | 50.4 ± 13.0 ** | 2.4 ± 0.7 | 1.5 ± 0.5 ** |
| PtdSer | 94 ± 10 | 175 ± 60 ** | 0.83 ± 0.23 | 1.53 ± 0.51 ** | 89 ± 26 | 50 ± 17 ** |
| ChoGpl | 439 ± 75 | 640 ± 226 * | 7.70 ± 1.18 | 10.0 ± 2.6* | 9.2 ± 1.4 | 7.4 ± 2.2 |
λDHA–CoA
Diet-adequate: 0.024 ± 0.007; Diet-Deficient: 0.025 ± 0.004
Values are mean ± SD (n = 8 for both groups).
p < 0.05,
p < 0.01, differs significantly from mean in adequate group
DHA turnover in phospholipid i, FFA, i(DHA), was calculated by Eq. 5 using individual values of JFA, i(DHA) and of the unlabeled DHA concentration in phospholipid i (Table 6). Dietary n-6 PUFA deprivation significantly increased DHA turnover in PtdIns by 1.6-fold, in PtdSer by 1.8-fold, and in ChoGpl by 1.3-fold. Corresponding DHA half-lives were shortened by 21-46% in the deprived compared with adequate group (Table 6).
DISCUSSION
Fifteen weeks of dietary n-6 PUFA deprivation following weaning in rats produced multiple changes in plasma and brain PUFA concentrations, in agreement with a previous study (Igarashi et al. 2009). In plasma, these changes represented an 84% reduction in the net unesterified n-6 PUFA concentration, including an 83% decline in unesterified 20:4n-6, compared with 50-80% increments in unesterified DHA and EPA concentrations. The result is decline in the plasma unesterified n-6/n-3 PUFA concentration ratio from 5.2 to 1.2. In the n-6 PUFA deficient brain, esterified n-6 PUFA concentrations were decreased by 11-49% in individual phospholipids, while esterified n-3 PUFAs were increased by 13% in ChoGpl and EtnGpl, the change in EtnGpl including a 9.4% increase in DHA (Igarashi et al. 2009). Concentrations of less abundant EPA, DPAn-3 and 20:3n-9 also were increased in individual brain phospholipids.
Brain PUFA content largely depends on incorporation of unesterified PUFAs from plasma, because of the inability of the vertebrate brain to synthesize PUFAs de novo, and its very limited ability to elongate or desaturate 18:2n-6 or 18:3n-3 (Holman 1986, Igarashi et al. 2007a). The increases in n-3 PUFA kinetics and concentrations in brain phospholipids with deprivation were related to increased n-3 PUFA availability in plasma, due to their increased hepatic synthesis, and to the reduced availability of n-6 PUFAs in plasma for brain incorporation (Robinson et al. 1992, DeMar et al. 2004, Smith & Nagura 2001, Gao et al. 2011).
Our hypothesis that brain DHA metabolism would be increased in the n-6 PUFA deprived rats was based on evidence of an increased brain DHA concentration and increased expression of DHA-selective iPLA2-VIA (Igarashi et al. 2009, Kim et al., Strokin et al. 2004, Garcia & Kim 1997) in such deprived rats. Consistent with the hypothesis incorporation rates Jin, i(DHA) of unesterified plasma DHA were increased into total brain phospholipid (45%), triacylglycerol (74%), EtnGpl (39%), PtdIns (69%), PtdSer (83%) and ChoGpl (49%) in rats fed the n-6 PUFA deficient compared with adequate diet, and DHA turnovers (deacylation-reacylation) (Robinson et al. 1992, Lands & Crawford 1976) were increased as well. Jin, i(DHA) represents net replacement by plasma DHA of brain DHA that has been lost by metabolism, since DHA cannot be resynthesized de novo in vertebrates and less than 1% of plasma α-LNA taken up by brain is elongated to DHA (Holman 1986, DeMar et al. 2004, DeMar et al. 2005, Rapoport et al. 2001). Thus, the 45% increments in Jin, i(DHA) demonstrate that the deficient diet markedly increased brain DHA metabolism and loss.
In rats fed the n-6 PUFA adequate diet, the DHA incorporation rate was highest in EtnGpl followed by ChoGpl followed by PtdIns followed by PtdSer (Table 4), and the DHA concentration was highest in EtnGpl (Table 3), in agreement with studies in rats fed other PUFA-adequate diets (DeGeorge et al. 1989, Nariai et al. 1994). Taking into account DHA concentrations and the incorporation coefficient λDHA-CoA, which corrects for the difference in steady-state specific activity between the precursor brain DHA-CoA pool and plasma DHA (Robinson et al. 1992), DHA turnover was in the following order: PtdIns > ChoGpl > EtnGpl> PtdSer (Table 6). These results identify PtdIns, ChoGpl and EtnGpl as having the most active DHA metabolism, probably associated with phospholipase A2-mediated deacylation and reacylation (Lands & Crawford 1976, Robinson et al. 1992, Purdon & Rapoport 1998), and PtdSer as having the least. The infusion method assumes rapid (within 5 min) pulse-labeling of phospholipid by plasma-derived radioactive fatty acid, and equilibration of specific activity between the plasma and brain precursor pool. The data suggest that pulse labeling is least applicable to PtdSer, which is synthesized from EtnGpl by a head group exchange reaction (Vance 2008). Delayed labeling of PtdSer following labeling of EtnGpl in rat brain has been demonstrated directly during intravenous [1-14 C]20:4n-6 infusion (Washizaki et al. 1994).
DHA can be hydrolyzed from the sn-2 position of EtnGpl (which includes ethanolamine plasmalogen) by iPLA2 VIA, whose brain expression is increased in rats fed the n-6 PUFA deficient diet (Strokin et al. 2004, Basselin et al. 2011, Garcia & Kim 1997, Kim et al. 2011), or by a plasmalogen-specific PLA2 selective for ethanolamine plasmalogen (Farooqui et al. 2006, Farooqui & Horrocks 2001). In agreement, of [3H]DHA incorporated into neural membranes in vitro, most was in the sn-2 position of EtnGpl (Farooqui & Horrocks 2001). Studies using intravenous infusion of [1,1-3H]hexadecanol in unanesthetized rats have shown that plasmalogens turn over rapidly in brain (Rosenberger et al. 2002).
DHA incorporation rates from plasma Jin, i(DHA) were increased significantly in all phospholipids in rats on the n-6 PUFA deficient compared with adequate diet (by 39 to 82%, Table 4), and DHA turnovers were increased in all phospholipids (by 29 to 84%, Table 6) but EtnGpl. These large statistically significant elevations in incorporation rates and turnovers in the different phospholipids, compared with the slight although statistically significant 9.4% increment in DHA concentration only in EtnGpl, show that the kinetic analysis using radiolabeled DHA infusion provided more compelling evidence of changes in active brain DHA metabolic processes with deprivation than did measuring unlabeled brain DHA concentrations.
DHA can be lost from brain by β-oxidation, by conversion to bioactive docosanoids such as resolvins, docosatrienes, and neuroprotectins by enzymes such as 15-LOX, and by other metabolic pathways (Hong et al. 2003, Gleissman et al. 2009, Robinson et al. 1992, Bazan et al. 2010, Groeger et al. 2010, Arnold et al. 2010, Gavino & Gavino 1991). The n-6 PUFA deficient diet did not affect β-oxidation, however, since the diet did not significantly change radioactivity in the brain aqueous fraction after the 5-min [1-14C]DHA infusion (Figure 1, Table 4) (Igarashi et al. 2007a, Igarashi et al. 2007c). Thus the measured increments in incorporation and turnover likely corresponded to increased production of bioactive DHA metabolites, many of which have anti-inflammatory and anti-apoptotic properties (Bazan 2007, Basselin et al., Serhan et al. 2004). In contrast, 20:4n-6 incorporation and turnover, and production of proinflammatory eicosanoids, likely were reduced in the n-6 PUFA deficient rats, in view of the reduced plasma and brain 20:4n-6 concentrations and brain expression of 20:4n-6-selective cPLA2-IVA and of COX-2 (Igarashi et al. 2009, Kim et al., Farooqui et al. 2007, Six & Dennis 2000), but this remains to be confirmed experimentally (Rapoport 2003).
The n-6 PUFA deficient diet also increased brain concentrations of EPA and DPAn-3. DPAn-3 has physiological effects, and is metabolized to 11- and 14-hydroxy DPA in human platelets (Sprecher 1986, Kanayasu-Toyoda et al. 1996). EPA like DHA can be converted by lipoxygenases (e.g. LOX-15) to 17-hydroxy metabolites, including resolvins and neuroprotectins that have antiinflammatory properties (Hong et al. 2003, Serhan et al. 2008). In view of the increases in brain DHA turnover and incorporation in the n-6 PUFA deprived rats and in brain concentrations of n-3 PUFAs, and the functional relevance of n-3 PUFA metabolites, we predict that brain n-3 PUFA metabolite concentrations are elevated following n-6 PUFA deprivation, and that the brain would have increased resistance to neuroinflammatory and other insults.
The diet of our ancestors contained approximately equal amounts of n-6 and n-3 PUFAs, while the n-6/n-3 PUFA concentration ratio approximates 10 to 1 in the current “Western” diet (Simopoulos 2000, Kris-Etherton et al. 2000). Some argue that this higher ratio increased the incidence of a number of human brain diseases, thus that current dietary n-6 PUFA content should be reduced (Hibbeln et al. 2006, Bazan et al. 1995, Simopoulos 2000). Our data suggest that such reductions also would be beneficial by reducing the brain 20:4n-6 content and expression of 20:4n-6-metabolizing enzymes (e.g. cPLA2-IVA and COX-2), that are upregulated in inflammatory and excitotoxic brain disease, and by increasing brain concentrations of DHA and its bioactive metabolites (McGeer & McGeer 2006, Chang et al. 2008, Basselin et al., Kim et al. 2011). A balanced diet containing n-6 PUFAs is desired, however, because 20:4n-6 is necessary for optimal brain function. For example, dietary 20:4n-6 supplementation in aged rats improved membrane fluidity, synaptic plasticity and spatial cognition (Okaichi et al. 2005, Kotani et al. 2003, Fukaya et al. 2007).
Because integrated plasma radioactivity during the 5-min [1-14C]DHA infusion did not differ significantly between the two dietary groups, the plasma half-life and thus the rate of disappearance of unesterified DHA from plasma also did not differ (Rapoport et al. 1982). Furthermore, since the n-6 PUFA deficient diet was DHA-free, the elevated plasma DHA concentration in the deficient group likely reflected increased hepatic DHA synthesis from circulating shorter-chain n-3 PUFA precursors, particularly α-LNA (Gao et al. 2009a, Gao et al. 2009b, Igarashi et al. 2007c, Gao et al. 2011). Of these, the plasma concentration of unesterified α-LNA was reduced while concentrations of EPA and DPAn-3 were increased with dietary deficiency (Table 1).
DHA and 20:4n-6 can be elongated and desaturated from 18:2n-6 and 18:3n-3 respectively through a series of enzymatic steps in the liver, whereas rates of brain and heart conversion are much less and unresponsive to diet (Nakamura & Nara 2003, Jump 2004, Igarashi et al. 2007a, Igarashi et al. 2005, Igarashi et al. 2008, Gao et al. 2009a, Gao et al. 2009b, Hagve & Sprecher 1989). Competition between the precursors for the hepatic enzymes helps to determine the rates of formation of 20:4n-6 and DHA, in addition to changes in enzyme expression (Yoshikawa et al. 2002, Tu et al. 2010). This makes it likely that the 95% reduction in plasma unesterified 18:2n-6 and in concentrations of other shorter-chain unesterified n-6 PUFAs in the deprived animals caused the increases in hepatic synthesis and plasma DHA concentration (Gao et al. 2011). In the addition, β-oxidation of n-3 PUFAs likely is downregulated in n-6 PUFA deprived rats (Igarashi et al. 2009). Consistent with enzyme competition, n-6 PUFA deprivation increased plasma 20:3n-9, which is synthesized from oleic acid (18:1n-9) through the same pathway and is a marker of 18:2n-6 deficiency (Lundberg 1980, Bazinet et al. 2003).
The data on the rats fed the n-6 PUFA adequate diet are comparable to data in rats fed other PUFA “adequate” diets (Contreras et al. 2000, DeMar et al. 2004). In one such study, Jin, DHA for total phospholipids equaled 22 nmol.g-1.s-1 × 10-4, λDHA–CoA equaled 0.03, and DHA turnover equaled 0.9%/h (Contreras et al. 2000), close to the respective values in the n-6 PUFA adequate animals in this study (Tables 4-6). In another, intracerebral [4,5-3H]DHA injection gave Jin, DHA as 28.9 × 10-4 g-1.s-1 in rats fed a PUFA adequate diet for 15 weeks (DeMar et al. 2004), close to rates obtained following intravenous infusion. It also is possible to measure Jin, DHA in humans when infusing [1-11C]DHA intravenously and using positron emission tomography for brain imaging (Umhau et al. 2009).
In summary, fifteen weeks of dietary n-6 PUFA deprivation post-weaning increased brain EPA, DPAn-3 and DHA concentrations, DHA incorporation rates from plasma and the brain DHA-CoA pool into brain phospholipids, and DHA turnover within phospholipids of adult male rats, while decreasing brain n-6 PUFA concentrations. The increments in DHA kinetic parameters were proportionately greater than the slight increment in DHA concentration, demonstrating the utility of the kinetic approach to better characterize brain metabolic changes during dietary PUFA manipulation.
Supplementary Material
Acknowledgments
This research was supported entirely by the Intramural Research Program of the National Institute on Aging. The authors thank the NIH Fellows Editorial Board for editorial assistance.
Abbreviations
- COX
cyclooxygenase
- cPLA2
cytosolic phospholipase A2
- iPLA2
calcium-independent phospholipase A2
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EPA
eicosapentaenoic acid
- FAME
fatty acid methyl ester
- GC
gas chromatography
- HPLC
high performance liquid chromatography
- PUFA
polyunsaturated fatty acid
- EtnGpl
ethanolamine glycerophospholipids
- ChoGpl
choline glycerophospholipids
- PtdSer
phosphatidylserine
- PtdIns
phosphatidylinositol
- sn
stereospecifically numbered
- TLC
thin layer chromatography
Footnotes
No author has a conflict of interest with regard to this manuscript.
References
- Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacological reports : PR. 2010;62:536–547. doi: 10.1016/s1734-1140(10)70311-x. [DOI] [PubMed] [Google Scholar]
- Aveldano MI, VanRollins M, Horrocks LA. Separation and quantitation of free fatty acids and fatty acid methyl esters by reverse phase high pressure liquid chromatography. J Lipid Res. 1983;24:83–93. [PubMed] [Google Scholar]
- Basselin M, Ramadan E, Igarashi M, Chang L, Chen M, Kraft AD, Harry GJ, Rapoport SI. Imaging upregulated brain arachidonic acid metabolism in HIV-1 transgenic rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:486–493. doi: 10.1038/jcbfm.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. Journal of lipid research. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Rodriguez de Turco EB, Allan G. Mediators of injury in neurotrauma: intracellular signal transduction and gene expression. J Neurotrauma. 1995;12:791–814. doi: 10.1089/neu.1995.12.791. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Douglas H, Cunnane SC. Whole-body utilization of n-3 PUFA in n-6 PUFA-deficient rats. Lipids. 2003;38:187–189. doi: 10.1007/s11745-003-1050-8. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Durand G, Pascal G, Youyou A. Brain cell and tissue recovery in rats made deficient in n-3 fatty acids by alteration of dietary fat. J Nutr. 1989a;119:15–22. doi: 10.1093/jn/119.1.15. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr. 1989b;119:1880–1892. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Piciotti M, Dumont O, Pascal G, Durand G. Dietary linoleic acid and polyunsaturated fatty acids in rat brain and other organs. Minimal requirements of linoleic acid. Lipids. 1990;25:465–472. doi: 10.1007/BF02538090. [DOI] [PubMed] [Google Scholar]
- Chang MC, Bell JM, Purdon AD, Chikhale EG, Grange E. Dynamics of docosahexaenoic acid metabolism in the central nervous system: lack of effect of chronic lithium treatment. Neurochem Res. 1999;24:399–406. doi: 10.1023/a:1020989701330. [DOI] [PubMed] [Google Scholar]
- Chang YC, Kim HW, Rapoport SI, Rao JS. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: cross-talk between excitotoxicity and neuroinflammation. Neurochem Res. 2008;33:2318–2323. doi: 10.1007/s11064-008-9731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras MA, Greiner RS, Chang MC, Myers CS, Salem N, Jr, Rapoport SI. Nutritional deprivation of alpha-linolenic acid decreases but does not abolish turnover and availability of unacylated docosahexaenoic acid and docosahexaenoyl-CoA in rat brain. J Neurochem. 2000;75:2392–2400. doi: 10.1046/j.1471-4159.2000.0752392.x. [DOI] [PubMed] [Google Scholar]
- DeGeorge JJ, Nariai T, Yamazaki S, Williams WM, Rapoport SI. Arecoline-stimulated brain incorporation of intravenously administered fatty acids in unanesthetized rats. J Neurochem. 1991;56:352–355. doi: 10.1111/j.1471-4159.1991.tb02603.x. [DOI] [PubMed] [Google Scholar]
- DeGeorge JJ, Noronha JG, Bell JM, Robinson P, Rapoport SI. Intravenous injection of [1-14C]arachidonate to examine regional brain lipid metabolism in unanesthetized rats. J Neurosci Res. 1989;24:413–423. doi: 10.1002/jnr.490240311. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. alpha-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J Neurochem. 2005;94:1063–1076. doi: 10.1111/j.1471-4159.2005.03258.x. [DOI] [PubMed] [Google Scholar]
- Deutsch J, Grange E, Rapoport SI, Purdon AD. Isolation and quantitation of long-chain acyl-coenzyme A esters in brain tissue by solid-phase extraction. Anal Biochem. 1994;220:321–323. doi: 10.1006/abio.1994.1344. [DOI] [PubMed] [Google Scholar]
- Deutsch J, Rapoport SI, Purdon AD. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochem Res. 1997;22:759–765. doi: 10.1023/a:1022030306359. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA. Plasmalogens, phospholipase A2, and docosahexaenoic acid turnover in brain tissue. Journal of molecular neuroscience : MN. 2001;16:263–272. doi: 10.1385/jmn:16:2-3:263. discussion 279-284. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Ong WY, Horrocks LA. Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev. 2006;58:591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fukaya T, Gondaira T, Kashiyae Y, Kotani S, Ishikura Y, Fujikawa S, Kiso Y, Sakakibara M. Arachidonic acid preserves hippocampal neuron membrane fluidity in senescent rats. Neurobiol Aging. 2007;28:1179–1186. doi: 10.1016/j.neurobiolaging.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Gao F, Kiesewetter D, Chang L, Ma K, Bell JM, Rapoport SI, Igarashi M. Whole-body synthesis-secretion rates of long-chain n-3 PUFAs from circulating unesterified {alpha}-linolenic acid in unanesthetized rats. J Lipid Res. 2009a;50:749–758. doi: 10.1194/jlr.D800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gao F, Kiesewetter D, Chang L, Ma K, Rapoport SI, Igarashi M. Whole-body synthesis-secretion of docosahexaenoic acid from circulating Eicosapentaenoic acid in anesthetized rats. J Lipid Res. 2009b;50:2463–2470. doi: 10.1194/jlr.M900223-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gao F, Kim HW, Igarashi M, Kiesewetter D, Chang L, Ma K, Rapoport SI. Liver conversion of docosahexaenoic and arachidonic acids from their 18-carbon precursors in rats on a DHA-free but alpha-LNA-containing n-3 PUFA adequate diet. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbalip.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Garcia MC, Kim HY. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Res. 1997;768:43–48. doi: 10.1016/s0006-8993(97)00583-0. [DOI] [PubMed] [Google Scholar]
- Gavino GR, Gavino VC. Rat liver outer mitochondrial carnitine palmitoyltransferase activity towards long-chain polyunsaturated fatty acids and their CoA esters. Lipids. 1991;26:266–270. doi: 10.1007/BF02537135. [DOI] [PubMed] [Google Scholar]
- Gleissman H, Yang R, Martinod K, Lindskog M, Serhan CN, Johnsen JI, Kogner P. Docosahexaenoic acid metabolome in neural tumors: identification of cytotoxic intermediates. FASEB J. 2009 doi: 10.1096/fj.09-137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nature chemical biology. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagve TA, Sprecher H. Metabolism of long-chain polyunsaturated fatty acids in isolated cardiac myocytes. Biochim Biophys Acta. 1989;1001:338–344. doi: 10.1016/0005-2760(89)90118-5. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- Holman RT. Control of polyunsaturated acids in tissue lipids. J Am Coll Nutr. 1986;5:183–211. doi: 10.1080/07315724.1986.10720125. [DOI] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from α-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007a;48:1150–1158. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. J Lipid Res. 2007b;48:1150–1158. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Upregulated liver conversion of α-linolenic acid to docosahexaenoic acid in rats on a 15 week n-3 PUFA-deficient diet. J Lipid Res. 2007c;48:152–164. doi: 10.1194/jlr.M600396-JLR200. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Gao F, Kim HW, Ma K, Bell JM, Rapoport SI. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochim Biophys Acta. 2009;1791:132–139. doi: 10.1016/j.bbalip.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, DeMar JC, Jr, Rapoport SI. α-linolenic acid is minimally converted to docosahexaenoic acid in brain and liver of adult rats fed a DHA-containing diet. Soc Neurosci Abstr. 2005;35:188–114. [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007d;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Rat heart cannot synthesize docosahexaenoic acid from circulating α-linolenic acid because it lacks elongase-2. J Lipid Res. 2008;49:1735–1745. doi: 10.1194/jlr.M800093-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CR, Arai T, Rapoport SI. Evidence for the involvement of docosahexaenoic acid in cholinergic stimulated signal transduction at the synapse. Neurochem Res. 1997;22:663–670. doi: 10.1023/a:1027341707837. [DOI] [PubMed] [Google Scholar]
- Jump DB. Fatty acid regulation of gene transcription. Crit Rev Clin Lab Sci. 2004;41:41–78. doi: 10.1080/10408360490278341. [DOI] [PubMed] [Google Scholar]
- Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- Kanayasu-Toyoda T, Morita I, Murota S. Docosapentaenoic acid (22:5, n-3), an elongation metabolite of eicosapentaenoic acid (20:5, n-3), is a potent stimulator of endothelial cell migration on pretreatment in vitro. Prostaglandins Leukot Essent Fatty Acids. 1996;54:319–325. doi: 10.1016/s0952-3278(96)90045-9. [DOI] [PubMed] [Google Scholar]
- Kim HW, Rao JS, Rapoport SI, Igarashi M. Dietary n-6 PUFA deprivation downregulates arachidonate but upregulates docosahexaenoate metabolizing enzymes in rat brain. Biochim Biophys Acta. 2011;1811:111–117. doi: 10.1016/j.bbalip.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S, Nakazawa H, Tokimasa T, Akimoto K, Kawashima H, Toyoda-Ono Y, Kiso Y, Okaichi H, Sakakibara M. Synaptic plasticity preserved with arachidonic acid diet in aged rats. Neurosci Res. 2003;46:453–461. doi: 10.1016/s0168-0102(03)00123-8. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–188S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- Lands WEM, Crawford CG. Enzymes of membrane phospholipid metabolism. In: Martonosi A, editor. The Enzymes of Biological Membranes. Vol. 2. Plenum; New York: 1976. pp. 3–85. [Google Scholar]
- Lundberg WO. The significance of cis, cis, cis 5,8,11 eicosatrienoic acid in essential fatty acid deficiency. Nutr Rev. 1980;38:233–235. doi: 10.1111/j.1753-4887.1980.tb05910.x. [DOI] [PubMed] [Google Scholar]
- Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr. 1994;60:189–194. doi: 10.1093/ajcn/60.2.189. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: Epidemiological, animal model and clinical studies. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Nakamura MT, Nara TY. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot Essent Fatty Acids. 2003;68:145–150. doi: 10.1016/s0952-3278(02)00264-8. [DOI] [PubMed] [Google Scholar]
- Nariai T, DeGeorge JJ, Greig NH, Genka S, Rapoport SI, Purdon AD. Differences in rates of incorporation of intravenously injected radiolabeled fatty acids into phospholipids of intracerebrally implanted tumor and brain in awake rats. Clinical & experimental metastasis. 1994;12:213–225. doi: 10.1007/BF01753889. [DOI] [PubMed] [Google Scholar]
- Okaichi Y, Ishikura Y, Akimoto K, Kawashima H, Toyoda-Ono Y, Kiso Y, Okaichi H. Arachidonic acid improves aged rats’ spatial cognition. Physiol Behav. 2005;84:617–623. doi: 10.1016/j.physbeh.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Purdon AD, Rapoport SI. Energy requirements for two aspects of phospholipid metabolism in mammalian brain. Biochem J. 1998;335(Pt 2):313–318. doi: 10.1042/bj3350313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan E, Rosa AO, Chang L, Chen M, Rapoport SI, Basselin M. Extracellular-derived calcium does not initiate in vivo neurotransmission involving docosahexaenoic acid. Journal of lipid research. 2010;51:2334–2340. doi: 10.1194/jlr.M006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI. In vivo fatty acid incorporation into brain phospholipids in relation to plasma availability, signal transduction and membrane remodeling. J Mol Neurosci. 2001;16:243–261. doi: 10.1385/JMN:16:2-3:243. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. In vivo approaches to quantifying and imaging brain arachidonic and docosahexaenoic acid metabolism. J Pediatr. 2003;143:S26–34. doi: 10.1067/s0022-3476(03)00399-8. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79:153–156. doi: 10.1016/j.plefa.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI, Chang MC, Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res. 2001;42:678–685. [PubMed] [Google Scholar]
- Rapoport SI, Fitzhugh R, Pettigrew KD, Sundaram U, Ohno K. Drug entry into and distribution within brain and cerebrospinal fluid: [14C]urea pharmacokinetics. Am J Physiol. 1982;242:R339–348. doi: 10.1152/ajpregu.1982.242.3.R339. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr. 1993;123:1923–1931. doi: 10.1093/jn/123.11.1923. [DOI] [PubMed] [Google Scholar]
- Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: Review and critical analysis. Brain Res Brain Res Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- Rosenberger TA, Oki J, Purdon AD, Rapoport SI, Murphy EJ. Rapid synthesis and turnover of brain microsomal ether phospholipids in the adult rat. J Lipid Res. 2002;43:59–68. [PubMed] [Google Scholar]
- Rouser G, Fkeischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Human requirement for N-3 polyunsaturated fatty acids. Poult Sci. 2000;79:961–970. doi: 10.1093/ps/79.7.961. [DOI] [PubMed] [Google Scholar]
- Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Skipski VP, Barclay M, Reichman ES, Good JJ. Separation of acidic phospholipids by one-dimensional thin-layer chromatography. Biochim Biophys Acta. 1967;137:80–89. doi: 10.1016/0005-2760(67)90010-0. [DOI] [PubMed] [Google Scholar]
- Skipski VP, Good JJ, Barclay M, Reggio RB. Quantitative analysis of simple lipid classes by thin-layer chromatography. Biochim Biophys Acta. 1968;152:10–19. doi: 10.1016/0005-2760(68)90003-9. [DOI] [PubMed] [Google Scholar]
- Smith QR, Nagura H. Fatty acid uptake and incorporation in brain: studies with the perfusion model. J Mol Neurosci. 2001;16:167–172. doi: 10.1385/JMN:16:2-3:167. [DOI] [PubMed] [Google Scholar]
- Sprecher H. The metabolism of (n-3) and (n-6) fatty acids and their oxygenation by platelet cyclooxygenase and lipoxygenase. Prog Lipid Res. 1986;25:19–28. doi: 10.1016/0163-7827(86)90007-x. [DOI] [PubMed] [Google Scholar]
- Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 2000;1486:219–231. doi: 10.1016/s1388-1981(00)00077-9. [DOI] [PubMed] [Google Scholar]
- Strokin M, Sergeeva M, Reiser G. Role of Ca2+-independent phospholipase A2 and n-3 polyunsaturated fatty acid docosahexaenoic acid in prostanoid production in brain: perspectives for protection in neuroinflammation. Int J Dev Neurosci. 2004;22:551–557. doi: 10.1016/j.ijdevneu.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Taylor DC, Weber N, Hogge LR, Underhill EW. A simple enzymatic method for the preparation of radiolabeled erucoyl-CoA and other long-chain fatty acyl-CoAs and their characterization by mass spectrometry. Anal Biochem. 1990;184:311–316. doi: 10.1016/0003-2697(90)90686-4. [DOI] [PubMed] [Google Scholar]
- Tu WC, Cook-Johnson RJ, James MJ, Muhlhausler BS, Gibson RA. Omega-3 long chain fatty acid synthesis is regulated more by substrate levels than gene expression. Prostaglandins, leukotrienes, and essential fatty acids. 2010;83:61–68. doi: 10.1016/j.plefa.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev. 2006;64:S24–33. doi: 10.1301/nr.2006.may.s24-s33. discussion S72-91. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Zhou W, Carson RE, et al. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J Lipid Res. 2009;50:1259–1268. doi: 10.1194/jlr.M800530-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. Journal of lipid research. 2008;49:1377–1387. doi: 10.1194/jlr.R700020-JLR200. [DOI] [PubMed] [Google Scholar]
- Washizaki K, Smith QR, Rapoport SI, Purdon AD. Brain arachidonic acid incorporation and precursor pool specific activity during intravenous infusion of unesterified [3H]arachidonate in the anesthetized rat. J Neurochem. 1994;63:727–736. doi: 10.1046/j.1471-4159.1994.63020727.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Shimano H, Yahagi N, et al. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. The Journal of biological chemistry. 2002;277:1705–1711. doi: 10.1074/jbc.M105711200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


