Abstract
Microglia are the resident immune cells within the brain and their production of immune molecules such as cytokines and chemokines is critical for the processes of normal brain development including neurogenesis, axonal migration, synapse formation, and programmed cell death. Notably, sex differences exist in many of these processes throughout brain development; however, it is unknown whether a sex difference concurrently exists in the colonization, number, or morphology of microglia within the developing brain. We demonstrate for the first time that the number and morphology of microglia throughout development is dependent upon the sex and age of the individual, as well as the brain region of interest. Males have overall more microglia early in postnatal development (postnatal day (P) 4), whereas females have more microglia with an activated/amoeboid morphology later in development, as juveniles and adults (P30-60). Finally, gene expression of a large number of cytokines, chemokines and their receptors shifts dramatically over development, and is highly dependent upon sex. Taken together, these data warrant further research into the role that sex-dependent mechanisms may play in microglial colonization, number, and function, and their potential contribution to neural development, function, or potential dysfunction.
Keywords: male, female, glia, cytokines, chemokines
INTRODUCTION
Microglia are the resident immune cells within the brain and their releasable factors, including cytokines and chemokines, have profound effects on neural function and behavior. In the adult brain, microglia maintain a predominantly ramified state and express low levels of inflammatory cytokines and chemokines. Microglia respond rapidly to disturbances in homeostasis, such as infection or injury, with a shift in morphology and increased production of pro- and anti-inflammatory cytokines and chemokines (see (Harry and Kraft, 2008; Ransohoff and Perry, 2009) for review). During development, however, microglia show features of being reactive or amoeboid as they migrate and proliferate throughout the developing nervous system. These cells gradually differentiate into mature/ramified microglia as the brain matures [see (Cuadros and Navascués, 1998) for review].
Among their many proposed functions throughout brain development, microglia support the survival of new neurons and synaptic connections, prune spurious synaptic connections, and phagocytose cells programmed for death (Boulanger, 2009; Deverman and Patterson, 2009; Garay and McAllister, 2010; Stevens, Allen, Vazquez, Howell, Christopherson, Nouri, Micheva, Mehalow, Huberman, Stafford, Sher, Litke, Lambris, Smith, John, and Barres, 2007). Many of these processes are sexually dimorphic within the developing and adult rodent brain (Arias, Zepeda, Hernandez-Ortega, Leal-Galicia, Lojero, and Camacho-Arroyo, 2009; Forger, Rosen, Waters, Jacob, Simerly, and de Vries, 2004; Forger, 2006; Johnson, Breedlove, and Jordan, 2008; Krebs-Kraft, Hill, Hillard, and McCarthy, 2010; Mong, Glaser, and McCarthy, 1999; Reyna-Neyra, Arias, Ferrera, Morimoto, and Camacho-Arroyo, 2004; Schwarz and McCarthy, 2008). Taken together, because microglia are critical for normal neural development but also maintain their role as primary CNS immune cells, we have hypothesized that early brain development represents a particularly sensitive window during which diverse immune-activating challenges can induce long-term changes in brain function. For instance, bacterial infection in male rat pups (on P4) causes significant long-term changes in microglial function, which underlie long-term alterations in brain cytokine expression and behavioral changes in adulthood (Bilbo, Levkoff, Mahoney, Watkins, Rudy, and Maier, 2005; Bilbo, Barrientos, Eads, Northcutt, Watkins, Rudy, and Maier, 2008). Importantly, infection on P30 does not induce the same long-term changes (Bilbo, Rudy, Watkins, and Maier, 2006). Notable as well is that females do not exhibit the same vulnerability to infection at P4, suggesting that females may be less sensitive to early-life challenges of the immune system (Bilbo, Smith, and Schwarz, 2011). These findings are consistent with other reports in the literature that use either early-life immune challenges or models of neonatal brain damage and find that males often fair far worse than females (Hilton, Nunez, and McCarthy, 2003; Hodgson and Knott, 2002; Llorente, Gallardo, Berzal, Prada, Garcia-Segura, and Viveros, 2009; Nunez, Alt, and McCarthy, 2003; Santos-Galindo, Acaz-Fonseca, Bellini, and Garcia-Segura, 2011; Wynne, Horvat, Osei-Kumah, Smith, Hansbro, Clifton, and Hodgson, 2011).
Despite this evidence, very little is known about the effect of sex on the colonization, morphology, or function of microglia either during brain development or in adulthood. Thus the purpose of these experiments was two-fold: 1) To characterize the colonization and morphological phenotype of microglia within the rodent brain in males and females in brain regions important for cognition, and 2) To examine gene expression of immune molecules important for glial migration and function in males and females throughout brain development. We report that microglial morphology and the expression of cytokines and chemokines shifts dramatically throughout development and is highly dependent upon sex. We specifically show that males have significantly more microglia than females at P4, within the parietal cortex (PCX), the CA1, CA3 and dentate gyrus (DG) of the hippocampus, and the amygdala (AMY). In contrast, females have significantly more microglia than males within these same brain regions at P30 and again at P60. No sex differences in glial colonization were found within the paraventricular nucleus (PVN) of the hypothalamus, a brain region important for stress responsivity. These collective data suggest that males and females may have profound differences in glial function that are dependent upon age and brain region. Moreover, these data may lend valuable insight into potentially distinct windows of vulnerability to immune challenges between the sexes.
MATERIALS AND METHODS
Animals
Adult male and female Sprague–Dawley rats (55–65 days) were obtained from Harlan (Indianapolis, IN) and housed in same sex pairs in polypropylene cages with ad libitum access to food and water. The colony was maintained at 22 °C on a 12:12-hour light-dark cycle (lights on at 0700 EST). Males and females were paired into breeders. Female breeders were visually examined daily for confirmation of pregnancy (E1 = first day of sperm presence), and male breeders were removed from cages prior to the day of birth (P0). Sentinel animals were housed in the colony room and screened periodically for the presence of common rodent diseases; all screens were negative. All experiments were conducted with protocols approved by the Duke University Institutional Animal Care and Use Committee.
Tissue Collection
Male and female brains were collected at the following ages: embryonic day (E) 17, P0, 4, 30, and 60 (n = 6 pups/sex at E17 and P0 and n = 4 pups/sex at P4, P30, and P60). Rats were sexed at P0, 4, 30, and 60 based on anogenital distance (P0 and P4) or the appearance of the gonads (P30 and P60). E17 rats were sexed by subsequent PCR analysis of the sry gene from tail (see below). E17 was selected as a time point for analysis as it is prior to the onset of testosterone secretion in males, and thus would allow us to examine glial colonization prior to hormone exposure. P0, or the day of birth, was selected for analysis, as this time point represents the peak of testosterone secretion in neonatal males. P4 was selected for analysis based on our work with neonatal bacterial infection in male and female pups as described above (Bilbo, Levkoff, Mahoney, Watkins, Rudy, and Maier, 2005; Bilbo, Barrientos, Eads, Northcutt, Watkins, Rudy, and Maier, 2008). Moreover, this time point closely follows the peak of testosterone secretion in neonatal males. P30 was selected for analysis as it is after the critical period of sexual differentiation of the brain, yet prior to the onset of circulating hormones of adulthood. P60 was selected for analysis as it reflects adult levels of circulating hormones. Estrous cycle was monitored in all females at P60, but did not significantly affect glial morphology or gene expression.
At the time of tissue collection, rats were perfused with cold 0.9% saline followed by 4% paraformaldehyde. Brains from P30 and P60 rats were removed and placed into cold 4% paraformaldehyde for 24 hours before being transferred into 30% sucrose for 48 hours. Brains from E17, P0, and P4 rats were removed and placed into cold 4% paraformaldehyde for 1 hour before being submerged in 10% gelatin solution (prepared in distilled water). After the gelatin solidified, the gelatin submerged brains were transferred back to 4% paraformaldehyde for 24 hours before being transferred into 30% sucrose for 48 hours.
Immunohistochemical detection of Iba1 protein
Brains were sectioned (14 microns) throughout the brain on a Leica cryostat at -20°C and thaw-mounted directly onto Superfrost++ Micro Slides (VWR) where they were allowed to dry before being stored at 4°C. The ionized calcium-binding adaptor molecule (Iba)-1 protein was used because it is specific to microglia, and its expression is constitutive. Slides were washed with PBS and incubated for 1 h in PBS with 1% H2O2, 10% normal goat serum (NGS), and 0.9% Triton X in order to quench endogenous peroxidase, block, and permeabilize, respectively. Slides were washed and incubated with 200 μl of primary antibody (rabbit anti-Iba1, 1:500 Wako Chemicals, Richmond, VA, USA) overnight at RT. On day 2, slides were washed and incubated with a biotinylated secondary antibody (goat anti-rabbit IgG, 1:200; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 2 hours at room temperature. Slides were washed, and immunostaining was identified by the streptavidin/HRP technique (Vectastain ABC kit; Vector Laboratories, Burlingame, CA, USA) with diaminobenzidine (DAB) as the chromagen. Slides were dehydrated and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA, USA).
Unbiased Stereology
Iba1 labeled cells were counted using the optical fractionator method within StereoInvestigator software (Microbrightfield Inc., Williston, VT) (Bilbo, Wieseler, Barrientos, Tsang, Watkins, and Maier, 2010; Glaser, Green, and Hendricks, 2007; Mouton, 2002). For analysis, we set an optical dissector height of 4 μm with a 1-μm guard zone on top and bottom, and counted stained cells within each frame using a 100X oil objective lens. Cells were only counted as positive if the entire, rounded cell body was visible (average diameter = between 15 and 25 microns) and the stain appeared uniformly dark and apparent throughout the cell, in order to avoid counting a cell fragment. Only cells that were within the tissue were counted (i.e. cells were not counted if they were located at the outermost edges of the tissue). While we could not completely exclude the inclusion of perivascular macrophages, which also stain positive with Iba1, we have previous data from our lab indicating that they only represent approximately 4% of the Iba1+ cell population within the brain when sorted with FACS, based on high CD45 expression (Williamson, Sholar, Mistry, Smith, and Bilbo, 2011); and would have little if any influence on the data described below.
Because the sections were relatively thin, we used an exhaustive 50 μm by 50 μm counting frame to count cells throughout each section for each section counted. For each animal, we analyzed every tenth section throughout the following brain regions and analyzed the following number of representative sections in each brain region: the parietal cortex (PCX) (10 sections/animal), the amygdala (AMY) (8 sections/animal), the CA1 of the hippocampus (10 sections/animal), the CA3 of the hippocampus (10 sections/animal), the dentate gyrus (DG) of the hippocampus (10 sections/animal), and the paraventricular nucleus (PVN) (4 sections/animal). The definitions according to the atlas, subregions delineated within the PCX and AMY, and the number of exhaustive counting frames for each brain region analyzed is listed in Supplementary Table 1. We also obtained estimates of the volume from the same sections using Cavalieri’s principle. For each section examined, the area was calculated by the StereoInvestigator software and was based on the boundaries of the contour tracings. Volume estimates were obtained by summing the areas given by the Cavalieri estimator, then multiplying the sum of these areas with the pre-histology thickness of each sample by the number of sections examined. Volumes and the corresponding statistical analyses are listed in Table 1.
Table 1. Average volumes for each brain region compared across sex.
The average volume ± SEM for each brain region at each age is listed along with the statistical analysis for this data. We found a significant effect of sex at P0 within the CA3 of the hippocampus, as the volume of the female CA3 was significantly larger than the male CA3; and we found a significant effect of sex at P4 within the PCX, as the volume of the PCX was significantly larger in males than in females.
| Age | Region | Avg. Volume (μm3) ± SEM | Avg. Volume (μm3) ± SEM | Statistics |
|---|---|---|---|---|
| MALES | FEMALES | |||
| E17 | PCX | 17,185,000 ± 142,821 | 19,926,666 ± 1,895,364 | t8= 1.16; p = 0.27 |
| E17 | AMY | 30,286,666 ± 4,146,839 | 34,603,333 ± 1,489,884 | t8= 0.98; p = 0.35 |
| E17 | CA1 | 4,608,333 ± 311,174 | 4,882,500 ± 335,556 | t8= 0.60; p = 0.56 |
| E17 | CA3 | 3,125,692 ± 311,160 | 3,727,500 ± 455,112 | t6= 1.09; p = 0.32 |
| E17 | DG | 3,596,250 ± 473,120 | 4,889,304 ± 789,592 | t6= 1.41; p = 0.21 |
| E17 | PVN | 1,417,500 ± 355,354 | 778,750 ± 57,820 | t6= -1.77; p = 0.13 |
| P0 | PCX | 199,727,500 ± 15,796,814 | 180,955,834 ± 16,788,394 | t6= -0.81; p = 0.45 |
| P0 | AMY | 88,305,000 ± 9,252,067 | 91,700,000 ± 3,013,220 | t6= 0.35; p = 0.74 |
| P0 | CA1 | 13,912,500 ± 581,200 | 19,232,500 ± 1,700,204 | t6= 2.96; p = 0.02 |
| P0 | CA3 | 13,226,108 ± 1,164,681 | 17,762,500 ± 733,261 | t6= 3.29; p = 0.02* |
| P0 | DG | 10,640,000 ± 1,806,205 | 13,396,250 ± 722,586 | t6= 1.42; p = 0.21 |
| P0 | PVN | 4,436,250 ± 551,250 | 5,783,750 ± 979,101 | t6= 1.20; p = 0.28 |
| P4 | PCX | 470,096,667 ± 20,828,640 | 283,360,000 ± 46,750,048 | t6= -3.6; p = 0.02* |
| P4 | AMY | 156,248,750 ± 23,043,942 | 162,146,250 ± 24,951,106 | t6= 0.17; p = 0.87 |
| P4 | CA1 | 54,845,000 ± 10,190,260 | 55,720,000 ± 11,098,762 | t6= 0.06; p = 0.96 |
| P4 | CA3 | 49,201,250 ± 5,038,123 | 45,718,750 ± 7,196,683 | t6= -0.40; p = 0.71 |
| P4 | DG | 58,100,000 ± 9,494,681 | 57,540,000 ± 2,587,239 | t6= -0.05; p = 0.95 |
| P4 | PVN | 4,410,000 ± 533,678 | 5,013,750 ± 784,236 | t6= 0.64; p = 0.55 |
| P30 | PCX | 338,152,500 ± 22,458,741 | 330,455,412 ± 40,735,467 | t6= -0.17; p = 0.87 |
| P30 | AMY | 310,590,000 ± 52,838,994 | 301,096,250 ± 76,112,164 | t6= -0.10; p = 0.92 |
| P30 | CA1 | 41,956,250 ± 4,099,992 | 35,673,750 ± 2,273,625 | t6= -1.34; p = 0.23 |
| P30 | CA3 | 35,612,500 ± 3,570,757 | 47,710,831 ± 7,168,026 | t6= 1.51; p = 0.18 |
| P30 | DG | 59,351,250 ± 13,275,118 | 68,985,000 ± 10,304,464 | t6= 0.57; p = 0.59 |
| P30 | PVN | 3,202,500 ± 294,395 | 3,255,000 ± 705,375 | t6= 0.07; p = 0.95 |
| P60 | PCX | 315,953,750 ± 16,844,101 | 343,568,750 ± 12,659,927 | t6= 1.31; p = 0.24 |
| P60 | AMY | 217,017,500 ± 26,143,248 | 171,605,000 ± 13,181,245 | t6= -1.55; p = 0.17 |
| P60 | CA1 | 62,868,750 ± 5,759,518 | 72,527,850 ± 5,590,957 | t6= 1.20; p = 0.27 |
| P60 | CA3 | 63,560,000 ± 5,907,937 | 69,685,000 ± 5,869,730 | t6= 0.74; p = 0.49 |
| P60 | DG | 91,446,250 ± 11,536,003 | 75,993,750 ± 7,698,249 | t6= -1.14; p = 0.31 |
| P60 | PVN | 4,121,250 ± 135,836 | 3,788,750 ± 523,905 | t6= -0.61; p = 0.56 |
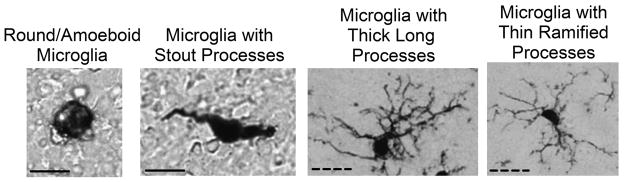
Iba1-positive cells were classified into four morphological types based on their cell shape and configuration of their processes (Gomez-Gonzalez and Escobar, 2010; Kreutzberg, 1996; Wu, Wen, Shieh, and Ling, 1992; Wu, Wen, Shieh, and Ling, 1993), see Figure 1. These 4 cell types consisted of round/amoeboid microglia, microglia with stout processes, microglia with thicker longer processes, and microglia with thinner, more ramified processes.
Figure 1. Classification of microglial morphology.
Representative photos depicting the four classifications of microglial morphology that correspond to differences in microglial function, round/amoeboid microglia (40X), microglia with stout processes (40X), microglia with thick long processes (20X), and microglia with thin ramified processes (20X). Scale bars: solid line = 10 μm, dashed line = 50 μm.
Analysis of Cell Counts and Brain Region Volumes
The total number of each classification of Iba1-positive cells was obtained by an experimenter blind to sex, across all representative sections for each brain region. The total cell counts for all sections were analyzed for microglial morphology and sex using within-subjects ANOVA for each brain region at each age and are presented within the figures. Significant interactions (p < 0.05) of microglial morphology and sex at each age point were followed up with a Holm-Sidak post-hoc test to determine group differences within each category of microglial morphology. The volumes of each brain region were analyzed using a Student t-test with α-level at 0.05. The graphs represent the average number of Iba1+ cells in each morphological category across all sections analyzed ± the SEM (number of sections analyzed for each brain region are list in Supplementary Table 1).
Quantitative Real-time PCR
RNA was isolated from the hippocampus and PCX or the AMY using the TRIzol method and DNASE-treated. The hippocampus and PCX were used for most of the gene expression analysis based on the data obtained from Iba-1 analysis which indicated that all subregions of the hippocampus and the cortex displayed significant sex differences across development, and previous reports indicating that infiltration of primitive microglia occurs primarily along the subcortical areas of the brain, including the hippocampus and corpus callosum (Wang, Wu, Shieh, and Wen, 2002; Xu, Kaur, and Ling, 1993). Complimentary DNA (cDNA) was synthesized from 500 ng of isolated RNA using the RT2 First Strand Kit (Cat No C - 03) SABiosciences/Qiagen (Frederick, MD). Gene expression was measured using quantitative real-time PCR with primers designed to measure 85 rat inflammatory cytokines, chemokines, and receptors (SABiosciences/Qiagen; Cat. No PARN-011) using the RT2 SYBR® Green qPCR Master Mix (Cat. No PA-010) from SABiosciences/Qiagen (Frederick, MD) following the manufacturer’s protocol. CXCL9 primers Forward: ATGCTCCTGCGAATCAGCG Reverse: TCTCCCATTCTTTCATCATCAG.
qRT-PCR Analysis
Threshold amplification cycle number (Ct) was determined for each reaction within the linear phase of the amplification plot, and relative gene expression was determined using the 2−ΔΔCT method. Relative gene expression across all groups was compared using a 2-Way ANOVA with sex and age as factors (α-level = 0.001 for main effects or interactions). Significant interactions using a 2-Way ANOVA were followed up with the Holm-Sidak post-hoc test, with p < 0.05, to determine potential group differences.
IL-1β and IL-10 Enzyme-linked immunosorbent assay (ELISA)
Hippocampal/PAR samples were sonicated in 300 μl cold homogenization buffer (50 mM Tris buffer, 1 mM EDTA, 100 mM Amino-n-caproic acid, 5 mM Benzamidine, 0.2 mM PMSF) and IL-1β and IL-10 were measured from the supernatant using a commercially available ELISA kit (R&D Systems), using 50 μl of supernatant in duplicate for each assay. The detection limit for IL-1β is typically 5 pg/ml, and less than 10 pg/ml for IL-10. To adjust for variability in tissue sample sizes, total protein concentrations were determined by Coomassie Brilliant Blue AG-250 protein assay. Cytokine data are expressed as picograms per 100 μg of total protein.
DNA isolation and Sex Determination at E17
Tails from E17 rats were homogenized in lysis buffer (50 mmol/L Tris HCl, pH 7.5, 100 mmol/L EDTA, 100 mmol/L NaCl, 1% SDS), and DNA was precipitated using phenol:chloroform:isoamyl alcohol extraction. Following rehydration in nuclease-free water, 1 μl of DNA from each sample was run in a quantitative real-time PCR reaction with QuantiTect SYBR Green Master Mix following manufacturer’s protocol (Qiagen, California) and the following primers for the sry gene (Sex-determining Region of the Y-Chromosome), Forward primer: TACAGCCTGAGGACAT, Reverse primer: CTGCTTGCTGATCTCTGAAT at a concentration of 0.4 μmol/L. The sex of each individual was determined by the presence (in males) or absence (in females) of the sry gene using the threshold amplification cycle number (Ct) of each sample in comparison to P60 samples of known sex [average Ct in males = 18 and average Ct in females = 30], then further confirmed by electrophoresis on 1% agarose gel as a product of 148 bp.
Microglial Isolations and Flow Cytometry
Microglia were isolated and enriched from hippocampus as previously reported (Frank, Wieseler-Frank, Watkins, and Maier, 2006). Briefly, dissociated tissue was re-suspended in 70% isotonic Percoll (GE Healthcare) layered with a 50% isotonic Percoll and a final layer of 1X Phosphate-buffered saline (PBS). Following centrifugation, two distinct layers were visible. The lower layer between the 70% and 50% Percoll phases contained a highly enriched and quiescent population of microglia (average = 91% CD11b+ cells in male samples and average = 88% CD+ cells in female samples), which were removed and washed with 1X PBS, and stained with CD11b as previously reported by our lab (Williamson, Sholar, Mistry, Smith, and Bilbo, 2011). Cells were incubated with 5 μl of rat Fc receptor block (CD32; BD Biosciences Pharmingen) for 5 min at 4°C. Next, 100 μl of PE-conjugated mouseanti-rat CD11b/c (BD Biosciences Pharmingen), diluted 1:500 in buffer was added and cells were incubated for 15 min at 4°C in the dark. Cells were washed in buffer, spun down at 350 × g for 5 min, and then fixed in 200 μl of 1.5% paraformaldehyde before analysis using a FACSCanto II flow cytometer (BD Biosciences) and FlowJo software (Tree Star). For each sample, 3,000 events were collected and doublets were excluded from the analysis based on properties of size (forward scatter height and area). Mean fluorescence intensity (MFI) was measured from microglia isolated from 4 males and 4 females and analyzed using a t-test (α level = 0.05).
RESULTS
Microglial morphology and number are significantly affected by sex, age, and the brain region of analysis
Embryonic day 17
At E17, we found no significant effect of sex on the volume of any brain region analyzed PCX (p = 0.27), AMY (p = 0.35), CA1 (p = 0.56), CA3 (p = 0.32), DG (p = 0.21), and the PVN (p = 0.13, Table 1).
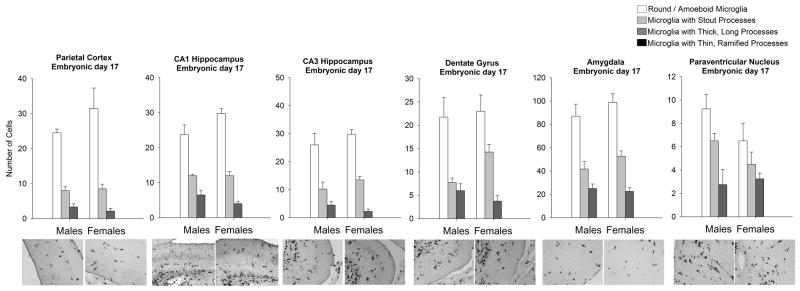
Within-subjects ANOVA of microglial morphology and sex revealed a significant effect of morphology in every brain region analyzed, including the PCX (F1,8=73.82; p < 0.0001), CA1 (F1,6=192.28; p < 0.001), CA3 (F1,6=82.02; p < 0.001), and DG (F1,6= 53.63; p < 0.0001) of the hippocampus, AMY (F1,6= 138.6; p < 0.001) and the PVN (F1,6= 28.7; p < 0.001; Figure 2). Specifically, at this age, more amoeboid microglia and microglia with stout processes were detected than ramified microglia (microglia with longer or thinner processes).
Figure 2. Microglial morphology and number are not affected by sex at embryonic day (E) 17.
Analysis of microglial morphology within the parietal cortex, CA1 hippocampus, CA3 hippocampus, dentate gyrus of the hippocampus, amygdala, and paraventricular nucleus indicated that all brain regions had significantly more amoeboid microglia and microglia with stout processes than microglia with thicker or thinner processes. No brain region analyzed displayed a significant effect of sex on microglia number or morphology at this age (within-subjects ANOVA, n = 4 rats/group). Data represent the mean ± SEM of all Iba1+ cells in each morphological category across all sections analyzed.
This analysis revealed no overall effects of sex on microglial counts or morphology within the same brain regions (PCX: F1,8=0.75; p = 0.41, CA1: F1,6 =0.614; p = 0.462, CA3: F1,6 =0.841; p = 0.394, DG: F1,6 = 0.45; p = 0.52, AMY: F1,6 = 0.88; p = 0.38, PVN: F1,6 = 1.87; p = 0.22), and no interaction of sex and microglial morphology within these brain regions (PCX: F1,8=1.56; p = 0.22, CA1: F1,6= 1.12; p = 0.342, CA3: F1,6= 1.06; p = 0.389, DG: F1,6= 1.98; p = 0.153, AMY: F1,6= 1.19; p = 0.33, PVN: F1,6= 1.52; p = 0.24). These data indicate that within the prenatal brain, microglia exhibit a predominantly amoeboid morphology that is not affected by sex.
Postnatal day 0 (Day of Birth)
At P0, we found no significant effect of sex on the volume for the following brain regions: PCX (p = 0.45), AMY (p = 0.74), DG (p = 0.21), and the PVN (p = 0.28). There was a significant effect of sex within the CA1 (t6= 2.96; p= .02) and CA3 (t6= 3.29; p= .02) of the hippocampus, as the CA1 and the CA3 were significantly larger in females (Table 1).
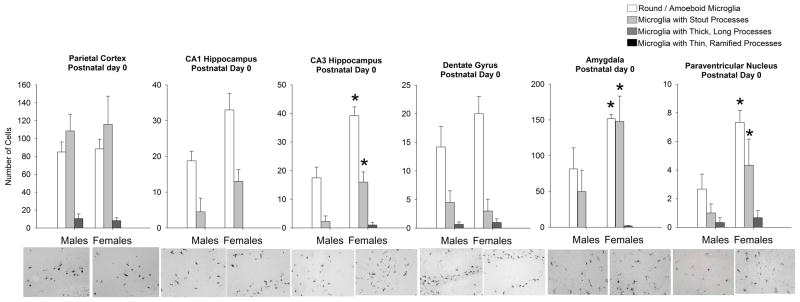
Within-subjects ANOVA revealed a significant effect of microglial morphology within the PCX (F1,6=35.01; p < 0.001), CA1 of the hippocampus (F1,6= 65.01; p < 0.001), DG (F1,8= 38.326; p < 0.0001), and PVN (F1,8 = 14.62; p = <.0001; Figure 3). Within these same brain regions, analysis revealed no effect of sex (PCX: F1,6=0.35; p = 0.85, CA1: F1,6 =4.87; p = 0.114, DG: F1,8 = 1.67; p = 0.23), and no significant interactions of sex and microglial morphology (PCX: F1,6=0.51; p = 0.98, CA1: F1,6= 2.67; p = 0.111, DG: F1,8= 2.2; p = 0.11).
Figure 3. Microglial morphology and number are significantly affected by sex at postnatal day (P) 0, the day of birth, in select brain regions.
At P0, analysis of microglial morphology revealed a significant effect of morphology within all brain regions analyzed. Specifically, all regions analyzed had significantly more amoeboid microglia and microglia with stout processes than microglia with thicker or thinner processes. Within the CA3 of the hippocampus and the amygdala, analysis revealed a significant interaction of sex and microglial morphology (within-subjects ANOVA, p < 0.05; n = 4 rats/group). Specifically, females had significantly more amoeboid microglia and microglia with stout processes than males at this time within these two brain regions (post hoc, *p < 0.05 in females v. the same cell type in males). Data represent the mean ± SEM of all Iba1+ cells in each morphological category across all sections analyzed.
Within the CA3 of the hippocampus, the AMY, and the PVN, however, analysis revealed a significant interaction of sex and microglial morphology (CA3: F1,6= 9.32; p = 0.0006, AMY: F1,6= 3.82; p = 0.027; PVN: F1,8= 10.452; p=.009), and post-hoc tests revealed that females had significantly more round microglia and microglia with stout processes than males (p < 0.05 for both cell types) within these three brain regions. Though the effect size is large, the sex difference seen within the CA3 may be attributed to the significant sex difference seen in volume at this age.
Postnatal day 4
At P4, we found no significant effect of sex on the volume of the following brain regions: AMY (p = 0.87), CA1 (p = 0.96), CA3 (p = 0.71), DG (p = 0.95), and the PVN (p = 0.55). There was a significant effect of sex within the PCX at this age (t6= -3.6; p = 0.02), as the PCX was significantly larger in males than in females (Table 1).
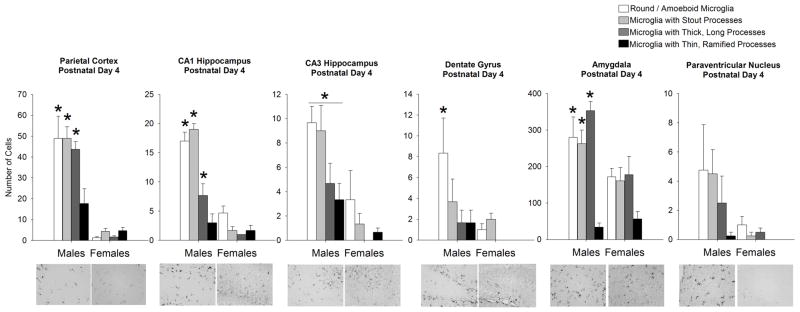
Within-subjects ANOVA for microglial morphology and sex revealed a significant interaction of sex and microglial morphology within the PCX (F1,6 = 11.56; p = 0.0007), CA1 (F1,6 = 33.25; p < 0.001), DG (F1,6 = 9.96; p = 0.01), and AMY (F1,6 = 5.76; p = 0.006; Figure 4). Post hoc tests of data from the PCX revealed that males have significantly more round microglia, stout microglia, and microglia with thick processes than females (p < 0.05). There was, however, no significant effect of sex on the few microglia with thin processes at this age. The same results were found following post hoc analysis of the CA1. Within the DG, post hoc tests revealed that males had significantly more round microglia than females at this age (p < 0.05). Within the AMY post-hoc tests indicated that males have significantly more round microglia, microglia with stout processes, and microglia with thicker, long processes than females at this age (p < 0.05 for all cell types). There was no significant difference in the few microglia with thin, ramified processes at this age within the AMY.
Figure 4. Microglia morphology and number are significantly affected by sex at postnatal day 4 in many brain regions analyzed.
At P4, analysis of microglial morphology revealed a significant effect of morphology within all brain regions analyzed. Specifically, all brain regions displayed more amoeboid microglia and microglia with stout processes than microglia with thicker or thinner processes. Within the parietal cortex, CA1, CA3, dentate gyrus, and amygdala analysis revealed a significant interaction of sex and microglial morphology (within-subjects ANOVA, p < 0.05; n = 4 rats/group), as males had significantly more amoeboid microglia, microglia with stout processes and microglia within thick, long processes than females within the parietal cortex, CA1 hippocampus, and amygdala (p < 0.05 for each cell type within males when compared to females). Within the CA3 of the hippocampus, males had significantly more microglia in all morphological categories than females at this age (p < 0.05 for each cell type within males when compared to females). Within the dentate gyrus of the hippocampus, males had significantly more amoeboid microglia than females (*p < 0.05 compared to females for this cell type). Within the paraventricular nucleus, no effects of sex on glial morphology or number were detected. Data represent the mean ± SEM of all Iba1+ cells in each morphological category across all sections analyzed.
Within the CA3 of the hippocampus, the sex difference seen at P0 reversed, as analysis revealed a significant overall effect of microglial morphology (F1,6= 8.09; p = 0.003) and a significant overall effect of sex (F1,6= 11.13; p = 0.028). Post hoc tests indicated that males had significantly more microglia within all categories of morphology than females (p < 0.05).
Within the PVN, analysis revealed no significant effect of microglial morphology (F1,6 = 2.88; p = 0.06), no significant effect of sex (F1,6= 2.52; p = 0.16), and no significant interaction of the two factors (F1,6= 1.64; p = 0.21).
Thus, within many of the brain regions analyzed, a significant effect of sex was determined as males had significantly more microglia with an activated morphology. In addition, microglia within many of these brain regions demonstrated a significant shift in morphology, and we observed the first appearance of more ramified microglia with small cell bodies and thin long processes. While it appears that the overall cell counts decreased within certain brain regions, including the CA1 and PCX, from P0 to P4 we hypothesize that this significant decrease in cell counts may have been caused by migration, as other brain regions (such as the AMY) show a robust increase in microglia from P0 to P4.
Postnatal day 30
At P30, we found no significant effect of sex on the volume of any brain region analyzed PCX (p = 0.87), AMY (p = 0.92), CA1 (p = 0.23), CA3 (p = 0.18), DG (p = 0.59), and the PVN (p = 0.95; Table 1).
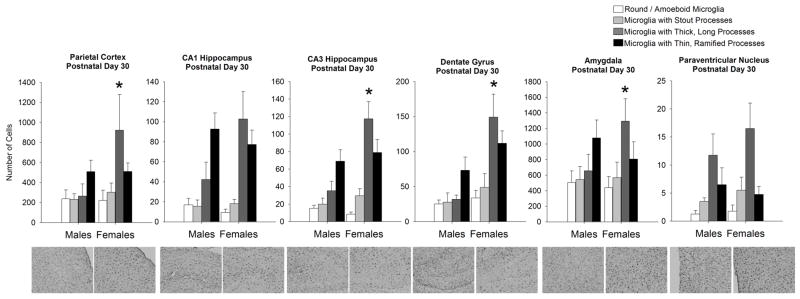
By P30, microglial morphology had shifted dramatically towards a more ramified/quiescent phenotype than that seen previously, and more interestingly, the sex difference in glial number/morphology at P4 was reversed at this age within many brain regions. Within-subjects ANOVA revealed a significant interaction of microglial morphology and sex for the PCX (F1,6=3.17; p = 0.046), CA3 (F1,6= 5.24; p = 0.009), DG, (F1,6= 3.74; p = 0.029, and AMY (F1,6= 7.72; p = 0.001; Figure 5), and post-hoc tests indicated that females had significantly more microglia with thick, long processes than males (p < 0.05) in each of these brain regions. No sex differences were observed for any other microglial morphology at this age.
Figure 5. Microglial morphology and number are significantly affected by sex at postnatal day 30 within many brain regions analyzed.
At P30, analysis of microglial morphology revealed a significant effect of morphology within all brain regions, as all brain regions had significantly more microglia with thicker, longer or thinner/ramified processes than microglia with stout processes or amoeboid morphology. Within the parietal cortex, CA3 and dentate gyrus of the hippocampus, and within the amygdala females had significantly more microglia with a more activated morphology (thick, long processes) than males (within-subjects ANOVA, *p < 0.05 compared to males within the same morphology; n = 4 rats/group). The CA1 of the hippocampus and the paraventricular nucleus do not exhibit a sex difference in microglial morphology at this age. Data represent the mean ± SEM of all Iba1+ cells in each morphological category across all sections analyzed.
Analysis of the CA1 and the PVN revealed a significant effect of morphology as the cells mature into a more ramified phenotype (CA1: F1,6= 12.09; p = 0.001, PVN: F1,6= 9.21; p = 0.007), however, no sex differences (CA1: F1,6 =1.56; p = 0.257, PVN: F1,6= 0.48; p = 0.51) or interactions of sex and morphology (CA1: F1,6= 2.6; p = 0.084, PVN: F1,6= 0.58; p = 0.63), were detected within these brain regions at this age.
Postnatal day 60
At P60, we found no significant effect of sex on the volume of any brain region analyzed PCX (p = 0.24), AMY (p = 0.17), CA1 (p = 0.27), CA3 (p = 0.49), DG (p = 0.31), and the PVN (p = 0.56; Table 1).
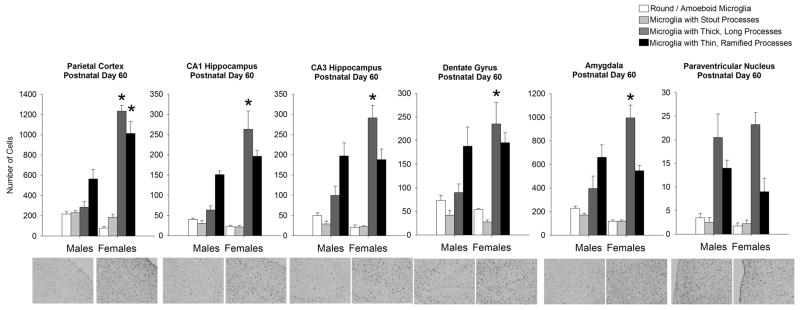
The sex difference in morphological phenotype seen at P30 within many brain regions analyzed was maintained at P60. Analysis revealed a significant interaction of microglial morphology and sex for the PCX (F1,6=45.15; p < 0.001), CA1 (F1,6= 14.5; p < 0.001), CA3 (F1,6=15.10; p < 0.001), DG (F1,6 = 6.4; p = 0.003), and AMY (F1,6 = 16.6; p < 0.001; Figure 6). Post hoc tests of data collected from the PCX revealed that females had significantly more microglia with thick, long processes and thin, long processes than males (p < 0.05 for both cell types), while post hoc tests of data collected from the CA1, CA3, DG, and AMY indicated that females had significantly more microglia with thick, long processes than males at this age (p < 0.05, females v. males for all brain regions).
Figure 6. Microglial morphology and number are significantly affected by sex at postnatal day 60 within many brain regions analyzed.
At P60, analysis of microglial morphology revealed a significant effect of morphology as all brain regions analyzed had significantly more microglia with thicker, longer and thinner/ramified processes than microglia within stout processes or amoeboid microglia. A significant interaction of microglial morphology and sex was detected within the parietal cortex, CA1, CA3, dentate gyrus, and amygdala (within-subjects ANOVA, p < 0.05; n = 4 rats/group). Within the parietal cortex, females had significantly more microglia with both thicker longer processes and microglia with thin ramified processes than males (*p < 0.05 for both cell types in females compared to males). Within the CA1, CA3, dentate gyrus, and amygdala, females had significantly more microglia with thick long processes than males (*p < 0.05 compared to the same cell morphology in males). Data represent the mean ± SEM of all Iba1+ cells in each morphological category across all sections analyzed.
As seen at most age points, analysis of the PVN revealed a significant effect of microglial morphology (F1,6= 34.08; p < 0.001), yet no significant effect of sex (F1,6= 0.31; p = 0.57), and no significant interaction of the two factors (F1,6= 1.04; p = 0.39).
Gene expression of immune factors is dependent upon sex and age
Taken together, the above data indicate that many brain regions important for cognition, including the PCX, hippocampus, and AMY, exhibit a sex difference in the number and morphology of microglia that shifts as the brain develops. Early in development, around P4, males have significantly more round microglia and microglia with stout processes than females within the PCX, CA1, CA3, DG, and the AMY. Later in development, after P30 and again at P60, females have significantly more cells that display an active phenotype (thicker, longer processes) than males within these same brain regions. Interestingly, the PVN within the hypothalamus only exhibits a sex difference in microglial morphology at birth.
Based on these data we sought to identify candidate immune factors that could either drive the sex difference in glial colonization (such as chemokines), or that might suggest a difference in glial function between the sexes throughout development (such as cytokines and glial proteins). We analyzed gene expression within the hippocampus and PCX at P0, P4, and at P60, as these brain regions showed a robust sex difference in our morphological analysis. Each gene was analyzed using a two-way ANOVA for age and sex.
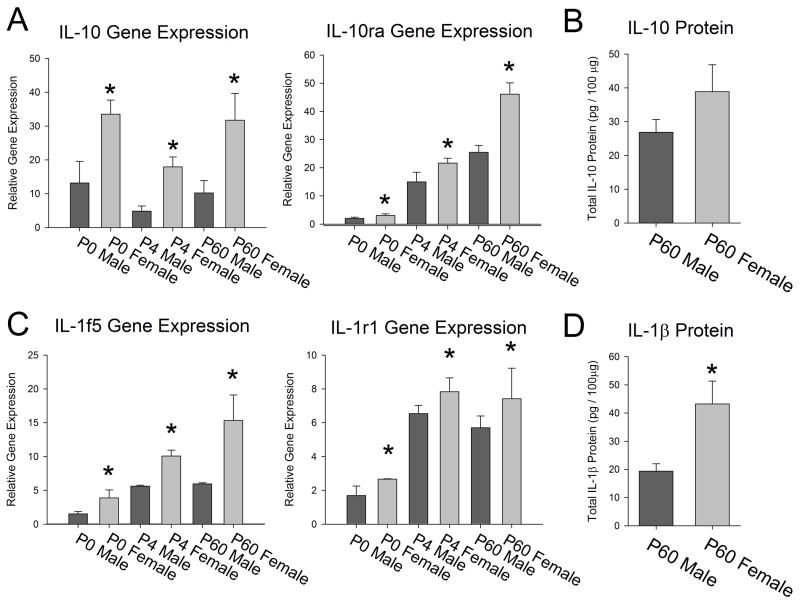
Many genes were significantly affected by age (Table 2). A few genes showed a significant effect of sex, independent of age (Figure 7). These included the anti-inflammatory cytokine Interleukin (IL) -10 and its receptor, IL-10ra, which were both significantly increased in females when compared to males at all ages analyzed (IL-10: overall effect of sex F1,17 = 20.738; p < 0.001, and IL-10ra: overall effect of sex F1,17 = 21.029; p < 0.001, Figure 7A). We analyzed the protein levels of IL-10 from the hippocampus and cortex of males and females at P60 and found no significant difference in the protein levels (p = 0.14; Figure 7B), suggesting that the mRNA is either not transcribed or the protein is degraded in such a way that the sex difference is lost at the protein level, at least basally. IL1f5 and IL1r1 were also both significantly increased in females when compared to males at all ages (IL1f5: overall effect of sex F1,17 = 15.75; p < 0.001, and IL1r1: overall effect of sex F1,17 = 20.31; p < 0.001, Figure 7C). We analyzed the protein levels of IL-1β from the hippocampus and cortex of males and females at P60 and determined that IL-1β protein is significantly increased in females when compared to males at this age (t8 = -3.57; p = 0.007, Figure 7D), suggesting that much of the IL-1 pathway is significantly up-regulated in females when compared to males in adulthood.
Table 2. Gene expression of immune factors affected by age within the hippocampus and cortex (all genes listed had no significant effect of Sex or significant interaction of Age × Sex).
Gene expression of immune factors affected by age within the hippocampus and cortex. Gene expression analysis of many immune factors revealed a significant main effect of age (two-way ANOVA, p < 0.001 for all genes depicted) and no significant effect of sex or interaction of age × sex. Genes highlighted in bold (Ccl12, Ccl2, Ccl3, Ccl6, Ccl7, and Cxcl6) showed significantly increased expression within the developing brain when compared to the adult brain, and have a demonstrated role in the migration of microglia during brain development. Genes highlighted in italics (C3, Cx3cl1, IL4, and IL8ra) showed significantly increased expression within the adult brain when compared to the developing brain.
| Gene | Reference ID | P0 Male | P0 Female | P4 Male | P4 Female | P60Male | P60 Female | Main Effect of Age |
|---|---|---|---|---|---|---|---|---|
| Abcf1 | XM_001056151 | 15.4±0.2 | 15.76±2.1 | 6.0±0.3 | 6.2±0.2 | 4.0±0.2 | 3.9±0.2 | F1,17=96.2, p < 0.0001 |
| Bcl6 | XM_221333 | 6.8±2.4 | 8.2±1.0 | 1.22±0.1 | 2.6±0.1 | 4.6±0.2 | 13.26±0.2 | F1,17=23.6, p < 0.0001 |
| Casp 1 | NM_012762 | 5.5±0.6 | 6.6±0.3 | 1.4±0.2 | 1.8±0.1 | 1.8±0.09 | 2.0±0.2 | F1,17=119.0, p < 0.0001 |
| Ccl12 | XM_213425 | 16.5±2.9 | 13.7±1.5 | 6.7±2.0 | 8.3±1.5 | 1.6±0.07 | 1.05±0.02 | F1,17=33.1, p < 0.0001 |
| Ccl2 | NM_031530 | 77.7±22.7 | 59.7±10.5 | 2.4±0.9 | 3.1±0.6 | 2.0±0.3 | 1.1±0.05 | F1,17=28.2, p < 0.0001 |
| Ccl3 | NM_013025 | 15.2±3.2 | 22.8±1.4 | 7.0±1.2 | 7.8±1.3 | 1.4±0.1 | 1.0±0.08 | F1,17=61.0, p < 0.0001 |
| Ccl6 | NM_001004202 | 9.2±1.1 | 11.2±1.6 | 2.5±0.2 | 3.2±0.1 | 1.1±0.09 | 1.0±0.01 | F1,17=71.6, p < 0.0001 |
| Ccl7 | NM_001007612 | 59.2±13.7 | 50.2±1.0 | 7.3±2.3 | 13.4±1.0 | 2.6±1.3 | 3.4±0.8 | F1,17=46.9, p < 0.0001 |
| Ccr3 | NM_053958 | 2.5±1.2 | 3.1±0.4 | 6.4±0.7 | 11.5±1.5 | 15.8±4.4 | 16.6±1.2 | F1,17=20.6, p < 0.0001 |
| Ccr4 | NM_133532 | 2.3±0.4 | 2.0±0.8 | 11.2±0.2 | 20.6±2.8 | 13.5±1.5 | 7.2±0.1 | F1,17=51.4, p < 0.0001 |
| CD200 | NM_031518 | 3.5±0.2 | 5.4±0.4 | 4.5±0.3 | 4.5±0.1 | 1.0±0.07 | 1.3±0.09 | F1,17=70.1, p < 0.0001 |
| CD68 | NM_001031638 | 7.6±1.7 | 9.9±1.1 | 8.2±0.6 | 5.2±0.5 | 1.8±0.09 | 1.1±0.1 | F1,17=24.3, p < 0.0001 |
| C3 | NM_016994 | 1.2±0.1 | 1.1±0.1 | 4.6±0.5 | 6.6±0.3 | 98.3±2.4 | 163.5±5.6 | F1,17=1680.5, p < 0.0001 |
| Cx3cl1 | NM_134455 | 2.3±1.0 | 2.5±0.3 | 35.1±8.4 | 38.7±4.9 | 162.8±6.0 | 194.4±30.6 | F1,17=97.2, p < 0.0001 |
| Cxcl6 | NM_022214 | 16.6±1.3 | 12.4±0.9 | 20.4±2.4 | 16.7±2.5 | 1.9±0.3 | 1.3±0.2 | F1,17=62.2, p < 0.0001 |
| Ccr10 | XM_343968 | 2.2±0.3 | 1.7±0.3 | 4.1±0.8 | 6.5±1.3 | 6.9±1.6 | 10.1±1.1 | F1,17=19.1, p < 0.0001 |
| IL11 | NM_133519 | 7.6±1.7 | 10.9±0.5 | 5.9±0.1 | 6.5±1.0 | 1.1±0.06 | 2.1±0.4 | F1,17=38.8, p < 0.0001 |
| IL13 | NM_053828 | 1.2±0.1 | 2.7±0.4 | 7.4±0.6 | 7.3±0.1 | 6.1±0.06 | 8.4±1.01 | F1,17=68.1, p < 0.0001 |
| IL16 | XM_218851 | 1.5±0.2 | 1.6±0.1 | 6.4±0.1 | 7.2±0.4 | 8.7±0.4 | 21.1±0.4 | F1,17=867.2, p < 0.0001 |
| IL1β | NM_031512 | 5.9±1.5 | 8.2±1.2 | 1.3±0.08 | 2.7±0.6 | 1.3±0.2 | 3.13±0.24 | F1,17=21.4, p < 0.0001 |
| IL1r2 | NM_053953 | 9.4±1.6 | 11.4±1.5 | 4.2±0.7 | 5.5±0.7 | 1.33±0.1 | 2.9±1.0 | F1,17=28.7, p < 0.0001 |
| IL2rb | NM_013195 | 3.7±0.9 | 2.8±0.2 | 6.9±0.8 | 6.3±0.3 | 1.2±0.1 | 1.1±0.05 | F1,17=51.5, p < 0.0001 |
| IL2rg | NM_080889 | 5.9±0.9 | 6.6±0.2 | 12.0±0.9 | 16.0±0.7 | 1.1±0.1 | 2.2±0.1 | F1,17=193.8, p < 0.0001 |
| IL4 | NM_201270 | 1.7±0.3 | 2.1±0.5 | 6.6±3.1 | 7.9±0.9 | 19.57±1.24 | 27.4±4.3 | F1,17=74.5, p < 0.0001 |
| IL6ra | NM_017020 | 2.5±0.5 | 3.17±0.2 | 1.17±0.08 | 1.52±0.08 | 7.5±0.1 | 9.02±0.9 | F1,17=128.4, p < 0.0001 |
| IL8ra | NM_019310 | 1.8±0.7 | 2.9±0.8 | 5.4±0.1 | 25.6±10.2 | 127.5±10.5 | 155.5±44.7 | F1,17=31.9, p < 0.0001 |
| Ltb | NM_212507 | 6.3±0.6 | 7.7±0.5 | 4.2±0.1 | 4.5±0.05 | 1.4±0.07 | 1.5±0.4 | F1,17=104.0, p < 0.0001 |
| Tnfrsf1a | NM_013091 | 1.6±0.3 | 1.92±0.2 | 7.3±0.04 | 7.8±0.6 | 2.7±0.4 | 4.7±0.5 | F1,17=100.1, p < 0.0001 |
| CD40lg | NM_053353 | 1.9±0.6 | 1.1±0.07 | 6.9±0.1 | 5.6±0.6 | 7.38±0.2 | 4.5±0.1 | F1,17=95.9, p < 0.0001 |
Figure 7. Gene expression of immune factors within the hippocampus and cortex is significantly affected by sex, independent of age.
A) Analysis of IL-10 and its receptor, IL10ra, revealed a significant effect of sex (two-way ANOVA, p < 0.001 for both genes). Specifically, females had significantly more IL-10 and IL0ra than males at all age points (IL10ra main effect of age, p = 0.006; did not meet criterion for statistical significance). B) Analysis of IL-10 protein within the male and female hippocampus/cortex at P60 revealed no significant differences (t-test, p = 0.14). C) Analysis of IL1f5 and IL1 receptor 1 (IL1r1) of the IL1 family of genes revealed a significant effect of sex (two-way ANOVA, p < 0.001 for both genes), as females had significantly higher levels of both genes at all age points (IL1f5 main effect of age, p = 0.002 and IL1r1 p = 0.001; do not meet criterion for statistical significance). D) Analysis of IL-1β protein within the male and female hippocampus/cortex at P60 revealed a significant effect of sex, as females had significantly higher levels of the IL-1β protein than males (t-test, *p < 0.01). Data represent the mean ± SEM.
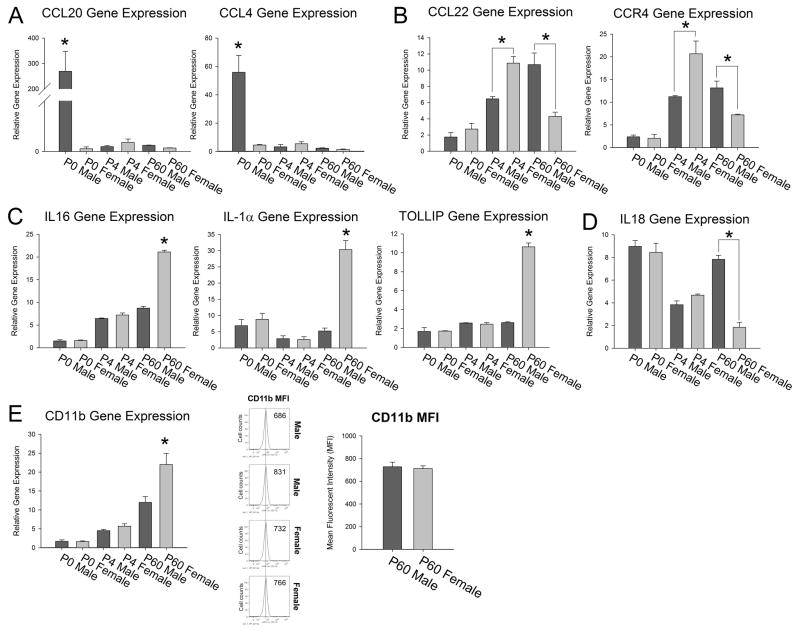
Many genes showed a significant interaction of sex and age. At P0, Chemokine (C-C motif) ligand 20 [(CCL) 20] was nearly 200-fold higher in males than in females and CCL4 was nearly 50-fold higher in males than in females (CCL20 sex × age: F1,17 = 11.5; p < 0.00, CCL4 sex × age: F1,17 = 18.36; p < 0.001, Figure 8A). At P4, females had significantly higher levels of CCL22 and its receptor CCR4 within the hippocampus/cortex, however this sex difference reversed completely at P60 at which point males had significantly higher levels of CCL22 and CCR4 than females (CCL22 sex × age: F1,17 = 23.2; p < 0.001, CCR4 sex × age: F1,17 = 16.4; p < 0.001, Figure 8B). These data suggest that a sex difference in chemokines may explain the sex difference in the colonization of microglia in the brain at P4. At P60, females had significantly higher levels of IL-16, IL1α, and TOLLIP than males (IL-16 sex × age: F1,17= 226.9; p < 0.001; IL1α sex × age: F1,17 = 34.11; p < 0.001, TOLLIP sex × age: F1,17 = 175.9; p < 0.001, Figure 8C) and males had significantly higher levels of IL18 than females (sex × age: F1,17 = 28.3; p < 0.001, Figure 8D), suggesting these molecules may play an important role in the function of glia in response to immune challenges in adulthood. We also found that females had significantly higher levels of CD11b, a general marker of glial activation, at P60 when compared to males (CD11b sex × age: F1,17 = 7.72; p < 0.001, Figure 8E); however, flow cytometry of CD11b staining of isolated/enriched microglia from the hippocampus of males and females revealed no significant difference in the mean fluorescence intensity (t6= 0.33; p=0.75; Figure 8E) suggesting that the significant difference in CD11b gene expression is due to the significant increase in the number of cells within the female hippocampus/cortex when compared to the male.
Figure 8. Gene expression of immune factors within the hippocampus and cortex is significantly affected by sex and dependent upon age.
Within the hippocampus and cortex, gene expression analysis of immune factors at P0, P4 and P60 revealed a significant interaction of sex and age (two-way ANOVA interaction: p < 0.001 for all genes depicted). A) Ccl20 and Ccl4 were significantly up-regulated in males at P0 when compared to females at the same age point. This effect was not present at P4 and at P60. B) Ccl22 and its receptor Ccr4 were not significantly different in males and females at P0, but at P4 showed a significant increase in females when compared to males (post hoc, * p < 0.05). This sex difference reversed at P60, at which point males had significantly higher levels of these two genes when compared to females (post hoc, * p < 0.05). C) IL16, IL-1α, and Tollip were all significantly up-regulated in females when compared to males only at P60 (post-hoc, * p < 0.05 in P60 females compared to all other groups). D) IL18 expression within the hippocampus showed a steady decrease in expression across development within females, but in males showed a significant increase at P60 again when compared to females (post hoc, * p < 0.05 in P60 males compared to P60 females). E) CD11b was significantly up-regulated within the hippocampus/cortex of females when compared to males only at P60 (post hoc, * p < 0.05 in P60 females compared to all other groups); however, analysis of surface staining of CD11b on isolated microglia from the hippocampus revealed no significant difference in the mean fluorescence intensity (MFI) between males and females (p = 0.75). Data represent the mean ± SEM.
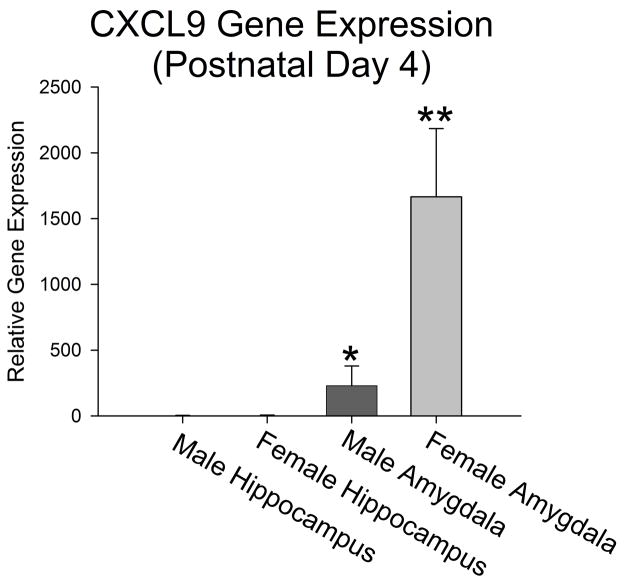
The analysis of microglial cell counts across age also indicates that the overall number of microglia significantly decreases within many of the brain regions analyzed in females from P0 to P4, in particular the PCX (~ 212 cells total to ~12 cells total), CA1 (~ 46 cells total to ~26 cells total), and CA3 (~56 cells total to ~19 cells total), which may contribute to the significant sex difference in microglial number detected at P4 (males > females). In contrast, the amygdala continues to show a significant increase in microglial number from P0 to P4 (~300 cells total to ~567 cells total) suggesting microglia may migrate to the amygdala from the hippocampus and cortex more so in females than in males. To investigate this further, we ran a PCR array of cytokines and chemokines on tissue from the female hippocampus and the amygdala at P4. Only one gene out of 84 was significantly (p < 0.005) increased in the female amygdala when compared to the hippocampus, and this was CXCL9. Individual PCR analysis of CXCL9 on tissue from the hippocampus/cortex and amygdala from both males and females revealed that both males and females had undetectable levels of CXCL9 in the hippocampus at P4; but females had nearly 30-fold more CXCL9 in the amygdala than males at this age (t6= -2.17, p = 0.03; Figure 9). This suggests that CXCL9 may be a particular factor that is important for attracting microglia from the female hippocampus/cortex to the amygdala at this time.
Figure 9. Gene expression CXCL9 within the hippocampus and amygdala of males and females on postnatal day 4.
Real-time PCR analysis of CXCL9 within the hippocampus and amygdala of males and females at P4 indicates that expression is relatively undetectable within the hippocampus. In contrast, females show significantly elevated levels of CXCL9 within the amygdala when compared to males at this same age (* and ** p < 0.005 compared to all other groups). Data represent the mean ± SEM.
DISCUSSION
These data are the first to identify a sex difference in the colonization and morphology of microglia across development, within brain regions important for cognition. Moreover, we have identified several candidate immune genes that change dramatically over development, are differentially affected by sex, and may have a role in the observed changes in microglial morphology or number, though this remains to be explored. We have determined that males have significantly increased numbers of microglia at P4 within the PCX, the CA1, CA3 and DG of the hippocampus, and the AMY. This sex difference reverses later in development around P30 and is maintained past adolescence into adulthood, at which time females have significantly more microglia with an activated phenotype within these same brain regions. Interestingly, a sex difference in glial morphology or number was only present within the PVN of the hypothalamus, a brain region important for stress responsivity, at birth. These data indicate that the sex difference in glial number and morphology is dependent upon brain region, and thus may be driven by different factors within the PVN at other age points.
The sex differences in glial colonization observed early in development (P4) may be induced by the difference in sex hormones that occurs at this time. At E18, the male testes begin to secrete testosterone, while the female ovaries remain quiescent. The testes produce a spike in testosterone at birth that is necessary for the masculinization of the rodent brain. We determined that at E17, prior to the onset of testosterone secretion, no sex differences exist in the number or morphology of microglia in any brain region analyzed, consistent with this hypothesis. While a sex difference was observed at the time of birth in two brain regions (the CA3 and AMY), the sex difference in the CA3, at least, may be attributed to the sex difference in CA3 volume which emerged exclusively at this age. Moreover, the predominant sex differences identified within the neonatal brain occurred shortly after the testosterone surge, at P4, at which point males had significantly more microglia with an activated morphology than females in most brain regions analyzed. While we did find a significant effect of sex on the volume of the PCX at P4, the magnitude of this effect (35% increase in volume in males vs. females) could not adequately account for the more robust effect of sex on cell counts (10-fold increase in males vs. females) observed at this time in the PCX.
A second notable observation from these data indicate that the overall number of microglia significantly decreases within many brain regions, in particular the PCX, CA1, and CA3 of females from P0 to P4. All sections were stained at the same time using the same preparation of Iba1 antibody and all animals from a particular age group were collected on the same day, using the same fixative; suggesting that the observed effect was not due to a difference in staining. More importantly, while there is a significant decrease in overall cell counts from P0 to P4 within females in the PCX, CA1 and CA3, this is not the case in the AMY which shows a robust increase in cell counts from P0 to P4 within the same tissue. Thus, the microglia which initially colonize around the hippocampus and subcortical regions may migrate to other brain regions, such as the amygdala, within these few days. This effect appears to be specific to females, as microglia either remain or continue to colonize the hippocampus of the P4 male. Consistent with this observation, we also determined that the chemokine, CXCL9, is significantly elevated within the amygdala at this time when compared to the hippocampus and significantly more so in females than in males (nearly 30-fold). Our hypothesis that microglia migrate to the amygdala more so in females may relate to a recent report which indicates that females have significantly increased cell proliferation within the amygdala during this stage of development, P4 (Krebs-Kraft, Hill, Hillard, and McCarthy, 2010). Thus, it is possible that microglia may migrate to the amygdala in females to support the on-going increase in cell proliferation at this time.
We also determined that at P0, at the time of the testosterone surge, the male brain had strikingly higher levels of two chemokines, CCL20 and CCL4 (200- and 50-fold respectively) when compared to the female brain. We predict these two factors may be increased by testosterone exposure beginning at P0, and might drive an increase in colonization or a decrease in migration of microglia within the brain of males measured at P4. Many chemokines have an important role in microglial migration and neural development within the healthy brain (Cowell and Silverstein, 2003; Cowell, Xu, Parent, and Silverstein, 2006; Cross and Woodroofe, 1999; Rezaie, Trillo-Pazos, Everall, and Male, 2002), and it is known that microglia colonize the brain via infiltration of primitive macrophage precursors from the yolk sac (Ginhoux, Greter, Leboeuf, Nandi, See, Gokhan, Mehler, Conway, Ng, Stanley, Samokhvalov, and Merad, 2010), initially entering the embryonic brain around subcortical regions such as the hippocampus and around the corpus callosum (Wang, Wu, Shieh, and Wen, 2002; Xu, Kaur, and Ling, 1993). We found that at P0 the following chemokines were up-regulated within the hippocampus and cortex, regardless of sex, when compared to later time points, CCL2, CCL3, CCL6, CCL7, CCL12, and Chemokine (C-X-C motif) ligand 6 [CXCL6] (highlighted in bold in Table 1). We predict that these cytokines may be important for attracting primitive macrophages/premature microglia into the brain from the periphery during early brain development or maintaining these immature populations within the hippocampus and subcortical regions. Many of these same cytokines (CCL2, CCL3, and CCL7) are expressed by astrocytes and neural progenitor cells (Hahn, Vo, Fitting, Block, Hauser, and Knapp, 2010; McKimmie and Graham, 2010; Rezaie, Trillo-Pazos, Everall, and Male, 2002), and have a demonstrated role in regulating the development of microglial cells within the human brain (Rezaie, Trillo-Pazos, Everall, and Male, 2002). Thus, further investigation into the role of these chemokines and the effect of early sex hormone exposure on glial colonization within the developing brain is needed.
Early in development, at E17 and P0, analysis revealed a profound difference in microglial morphology within every brain region analyzed, and post hoc tests indicated that most of the microglia within the brain at this time of development display a round, amoeboid morphology. Consistent with this amoeboid morphology, we also found a significant increase in CD68 expression, a marker of macrophages, within the hippocampus/cortex at birth that rapidly decreases with age. As these cells mature, finish migrating, and reach their final destination within the brain, they begin to express a fundamentally different morphology and biochemistry, more similar to that seen in the healthy adult brain [see (Cuadros and Navascués, 1998) for review]. Even four days later, by P4, every brain region analyzed began to display microglia with smaller cell bodies and thin processes, and decreased CD68, indicating that by this point the cells are rapidly maturing and shifting into a more ramified morphology, with representation from each morphological category. By P30 and P60, the predominant morphological phenotype observed was the more quiescent morphology. Consistent with this observation, we also detected a nearly 200-fold increase in the chemokine CX3CL1 within the P60 hippocampus/cortex when compared to the levels expressed at P0 in the hippocampus/cortex, though we found no significant difference in the expression of its receptor CX3CR1 across development. CX3CL1 is produced and released by mature neurons and is thought to maintain microglia in a ramified/quiescent state (Adler, Geller, Chen, and Rogers, 2006; Nishiyori, Minami, Ohtani, Takami, Yamamoto, Kawaguchi, Kume, Akaike, and Satoh, 1998). At P60, we also detected a nearly 100-fold increase in complement component 3, C3, when compared to the developing brain. C3 is an immune protein which has a well-established role for tagging spurious synapses for elimination, a process important for neural function (Stevens, Allen, Vazquez, Howell, Christopherson, Nouri, Micheva, Mehalow, Huberman, Stafford, Sher, Litke, Lambris, Smith, John, and Barres, 2007).
Along with the shift in microglial phenotype that occurs with age, another sex difference emerged. Specifically, a larger subset of the microglia appear to be maintained in a more activated state, characterized by longer and thicker processes, within the adult female brain when compared to the adult male brain. While we did measure increased gene expression levels of CD11b, a microglial activation marker, within the hippocampus/cortex of adult females when compared to males, we did not detect a significant difference in the surface expression of this particular protein on isolated microglia. Taken together, these data suggest that the increase in CD11b gene expression is most likely related to the increase in the total number of microglia in females than in males. We did, however, find that females had significantly higher levels of several cytokines at this age, specifically within the IL-1 family (IL-1α, IL1f5, and IL1r1 mRNA expression and IL-1β protein) and the IL-10 family (IL-10 and IL10ra), as well as the Toll-like receptor signaling protein, TOLLIP, suggesting that microglia within the female juvenile and adult brain may have be functionally different or have a fundamentally different biochemistry than microglia within the male brain.
The shift in the sex difference in microglial number was initially observed in most brain regions at P30 and was still observed in these same brain regions at P60. We specifically chose the P30 time point for analysis as it is prior to the onset of adult circulating hormones that occurs at adolescence (P35-45). Thus, we initially expected to see few or no sex differences in glial number or morphology at this time. Despite this, however, we see that the sex difference in glial morphology/number is established at P30 and is maintained until P60, even after the onset of circulating adult sex hormones. Thus, while at P60 the sex difference in glial morphology may (or may not) be affected by adult circulating hormones, the same may not be said for the sex difference observed at P30. Instead, the sex difference observed in the juveniles and adults may be related to differences in the genes expressed by the sex chromosomes (Arnold, Xu, Grisham, Chen, Kim, and Itoh, 2004) or other factors not yet determined.
While we report these striking and novel findings that sex affects the number and morphology of microglia within the developing brain and in the adult brain, it is unclear at this time how this sex difference may affect the function of these cells during brain development or later in adulthood. As mentioned previously, microglia have an important role in brain development, as ongoing cell genesis, synaptogenesis, and cell death occurs (Ashwell, 1990; Ashwell, 1991; Boulanger, 2009; Deverman and Patterson, 2009; Garay and McAllister, 2010; Stevens, Allen, Vazquez, Howell, Christopherson, Nouri, Micheva, Mehalow, Huberman, Stafford, Sher, Litke, Lambris, Smith, John, and Barres, 2007). Many of these processes are well-known to be sexually dimorphic within the neonatal brain (Forger, Rosen, Waters, Jacob, Simerly, and de Vries, 2004; Forger, 2006; Krebs-Kraft, Hill, Hillard, and McCarthy, 2010; Mong, Glaser, and McCarthy, 1999; Schwarz and McCarthy, 2008), the adolescent brain (Sisk and Zehr, 2005; Zehr, Todd, Schulz, McCarthy, and Sisk, 2006), and even in the adult brain (Arias, Zepeda, Hernandez-Ortega, Leal-Galicia, Lojero, and Camacho-Arroyo, 2009; Johnson, Breedlove, and Jordan, 2008; Reyna-Neyra, Arias, Ferrera, Morimoto, and Camacho-Arroyo, 2004). Thus it is not clear whether the sex differences in glial colonization occur in response to sex differences in these processes, which are caused by hormone exposure; or whether glial colonization may be differentially affected by sex or hormones and subsequently affect these ongoing processes in a sex-dependent manner.
In addition to the important role of microglia in normal brain development and function into adulthood, microglia also have a critical role in the response to changes in homeostasis caused by infection, injury, ischemia, stress, drugs of abuse, and exposure to environmental toxicants. Despite this, very few studies have investigated how sex differences in microglial number or function may affect outcomes following immune challenges such as behavior and cognition, the development of pain or neurodegeneration, or recovery from infection or injury during development or in adulthood. Many studies have investigated the role of sex hormones on glial function (Baker, Brautigam, and Watters, 2004; Drew and Chavis, 2000; Drew, Chavis, and Bhatt, 2003; Kipp, Karakaya, Johann, Kampmann, Mey, and Beyer, 2007; Soucy, Boivin, Labrie, and Rivest, 2005), but these are often performed ex vivo and are not compared across sex. A few studies have determined that either an early life immune challenge or an early-life traumatic brain injury (neonatal model of ischemia) can cause significantly greater damage to the male brain than to the female brain; with significant long-term consequences for behavior and hippocampal function into adulthood (Hilton, Nunez, and McCarthy, 2003; Hodgson and Knott, 2002; Llorente, Gallardo, Berzal, Prada, Garcia-Segura, and Viveros, 2009; Nunez, Alt, and McCarthy, 2003; Santos-Galindo, Acaz-Fonseca, Bellini, and Garcia-Segura, 2011; Wynne, Horvat, Osei-Kumah, Smith, Hansbro, Clifton, and Hodgson, 2011). Based on the data presented here, further investigation into the role of the immune system and microglia function may lend even greater insight into the mechanisms underlying this differential sensitivity to perinatal immune activation and injury.
Finally, within the human literature it is well-known a strong sex bias exists in the prevalence of many neuropsychiatric disorders that display not only a difference in the latency to onset but also a strong dysregulation of the immune system. Specifically, males are more likely to be diagnosed with early-onset or developmental neurological disorders such as autism, learning disabilities (dyslexia), and schizophrenia. In contrast, females are more likely to be diagnosed with conditions that emerge later in life, following the onset of adolescence, such as depression, anxiety, and panic disorders [see (Bao and Swaab, 2010) for a recent review]. Thus, we hypothesize that the sex differences observed here in microglial number and morphology, first an increase in males early in development and then an increase in females later in life, may have relevance for disparities in the onset, prevalence, or severity of symptoms associated with many of these neuropsychiatric disorders. Future research aimed at investigating the effects of sex, microglial function, and behavior either during postnatal brain development or adulthood is thus warranted.
Supplementary Material
Acknowledgments
Supported by NIH grants R01 MH083698, R01DA025978 and F32DA030136. The authors would like to thank Sonia M. Sabater and Dr. Susan H. Smith for technical assistance.
Footnotes
The authors declare no conflict of interest.
References
- Adler MW, Geller EB, Chen X, Rogers TJ. Viewing chemokines as a third major system of communication in the brain. AAPS J. 2006;7(4):E865–70. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Zepeda A, Hernandez-Ortega K, Leal-Galicia P, Lojero C, Camacho-Arroyo I. Sex and estrous cycle-dependent differences in glial fibrillary acidic protein immunoreactivity in the adult rat hippocampus. Horm Behav. 2009;55(1):257–263. doi: 10.1016/j.yhbeh.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Xu J, Grisham W, Chen X, Kim YH, Itoh Y. Minireview: Sex chromosomes and brain sexual differentiation. Endocrinology. 2004;145(3):1057–62. doi: 10.1210/en.2003-1491. [DOI] [PubMed] [Google Scholar]
- Ashwell K. The distribution of microglia and cell death in the fetal rat forebrain. Brain Res Dev Brain Res. 1991;58(1):1–12. doi: 10.1016/0165-3806(91)90231-7. [DOI] [PubMed] [Google Scholar]
- Ashwell K. Microglia and cell death in the developing mouse cerebellum. Brain Res Dev Brain Res. 1990;55(2):219–230. doi: 10.1016/0165-3806(90)90203-b. [DOI] [PubMed] [Google Scholar]
- Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145(11):5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- Bao AM, Swaab DF. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist. 2010;16(5):550–565. doi: 10.1177/1073858410377005. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Barrientos RM, Eads AS, Northcutt A, Watkins LR, Rudy JW, Maier SF. Early-life infection leads to altered BDNF and IL-1beta mRNA expression in rat hippocampus following learning in adulthood. Brain Behav Immun. 2008;22(4):451–455. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005;119(1):293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav Brain Res. 2006;169(1):39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Smith SH, Schwarz JM. A Lifespan Approach to Neuroinflammatory and Cognitive Disorders: A Critical Role for Glia. J Neuroimmune Pharmacol. 2011 doi: 10.1007/s11481-011-9299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Wieseler JL, Barrientos RM, Tsang V, Watkins LR, Maier SF. Neonatal bacterial infection alters fever to live and simulated infections in adulthood. Psychoneuroendocrinology. 2010;35(3):369–381. doi: 10.1016/j.psyneuen.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Boulanger LM. Immune Proteins in Brain Development and Synaptic Plasticity. Neuron. 2009;64(1):93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Silverstein FS. Developmental changes in the expression of chemokine receptor CCR1 in the rat cerebellum. J Comp Neurol. 2003;457(1):7–23. doi: 10.1002/cne.10554. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Xu H, Parent JM, Silverstein FS. Microglial expression of chemokine receptor CCR5 during rat forebrain development and after perinatal hypoxia-ischemia. J Neuroimmunol. 2006;173(1–2):155–165. doi: 10.1016/j.jneuroim.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Cross AK, Woodroofe MN. Chemokines induce migration and changes in actin polymerization in adult rat brain microglia and a human fetal microglial cell line in vitro. J Neurosci Res. 1999;55(1):17–23. doi: 10.1002/(SICI)1097-4547(19990101)55:1<17::AID-JNR3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, Navascués J. The origin and differentiation of microglial cells during development. Prog Neurobiol. 1998;56(2):173–189. doi: 10.1016/s0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64(1):61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111(1–2):77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA, Bhatt R. Sex steroid regulation of microglial cell activation: relevance to multiple sclerosis. Ann N Y Acad Sci. 2003;1007:329–334. doi: 10.1196/annals.1286.031. [DOI] [PubMed] [Google Scholar]
- Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138(3):929–38. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci U S A. 2004;101(37):13666–71. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: Immunophenotypic and functional characteristics. J Neurosci Methods. 2006;151(2):121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Garay PA, McAllister AK. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Front Synaptic Neurosci. 2010;2:136. doi: 10.3389/fnsyn.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser J, Green G, Hendricks S. Stereology for Biological Research 2007 [Google Scholar]
- Gomez-Gonzalez B, Escobar A. Prenatal stress alters microglial development and distribution in postnatal rat brain. Acta Neuropathol. 2010;119(3):303–315. doi: 10.1007/s00401-009-0590-4. [DOI] [PubMed] [Google Scholar]
- Hahn YK, Vo P, Fitting S, Block ML, Hauser KF, Knapp PE. beta-Chemokine production by neural and glial progenitor cells is enhanced by HIV-1 Tat: effects on microglial migration. J Neurochem. 2010;114(1):97–109. doi: 10.1111/j.1471-4159.2010.06744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry GJ, Kraft AD. Neuroinflammation and microglia: considerations and approaches for neurotoxicity assessment. Expert Opin Drug Metab Toxicol. 2008;4(10):1265–1277. doi: 10.1517/17425255.4.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton GD, Nunez JL, McCarthy MM. Sex differences in response to kainic acid and estradiol in the hippocampus of newborn rats. Neuroscience. 2003;116(2):383–91. doi: 10.1016/s0306-4522(02)00716-9. [DOI] [PubMed] [Google Scholar]
- Hodgson DM, Knott B. Potentiation of tumor metastasis in adulthood by neonatal endotoxin exposure: sex differences. Psychoneuroendocrinology. 2002;27(7):791–804. doi: 10.1016/s0306-4530(01)00080-4. [DOI] [PubMed] [Google Scholar]
- Johnson RT, Breedlove SM, Jordan CL. Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala. J Comp Neurol. 2008;511(5):599–609. doi: 10.1002/cne.21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipp M, Karakaya S, Johann S, Kampmann E, Mey J, Beyer C. Oestrogen and progesterone reduce lipopolysaccharide-induced expression of tumour necrosis factor-alpha and interleukin-18 in midbrain astrocytes. J Neuroendocrinol. 2007;19(10):819–822. doi: 10.1111/j.1365-2826.2007.01588.x. [DOI] [PubMed] [Google Scholar]
- Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc Natl Acad Sci U S A. 2010;107(47):20535–20540. doi: 10.1073/pnas.1005003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Llorente R, Gallardo ML, Berzal AL, Prada C, Garcia-Segura LM, Viveros MP. Early maternal deprivation in rats induces gender-dependent effects on developing hippocampal and cerebellar cells. Int J Dev Neurosci. 2009;27(3):233–241. doi: 10.1016/j.ijdevneu.2009.01.002. [DOI] [PubMed] [Google Scholar]
- McKimmie CS, Graham GJ. Astrocytes modulate the chemokine network in a pathogen-specific manner. Biochem Biophys Res Commun. 2010;394(4):1006–1011. doi: 10.1016/j.bbrc.2010.03.111. [DOI] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19(4):1464–72. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists 2002 [Google Scholar]
- Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429(2):167–172. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Alt JJ, McCarthy MM. A novel model for prenatal brain damage. II Long-term deficits in hippocampal cell number and hippocampal-dependent behavior following neonatal GABAA receptor activation. Exp Neurol. 2003;181(2):270–80. doi: 10.3201/eid0906.020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Reyna-Neyra A, Arias C, Ferrera P, Morimoto S, Camacho-Arroyo I. Changes in the content and distribution of microtubule associated protein 2 in the hippocampus of the rat during the estrous cycle. J Neurobiol. 2004;60(4):473–480. doi: 10.1002/neu.20042. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Trillo-Pazos G, Everall IP, Male DK. Expression of beta-chemokines and chemokine receptors in human fetal astrocyte and microglial co-cultures: potential role of chemokines in the developing CNS. Glia. 2002;37(1):64–75. doi: 10.1002/glia.1128. [DOI] [PubMed] [Google Scholar]
- Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, Garcia-Segura LM. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol Sex Differ. 2011;2(1):7. doi: 10.1186/2042-6410-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, McCarthy MM. Steroid-induced sexual differentiation of the developing brain: multiple pathways, one goal. J Neurochem. 2008;105(5):1561–1572. doi: 10.1111/j.1471-4159.2008.05384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Soucy G, Boivin G, Labrie F, Rivest S. Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. J Immunol. 2005;174(10):6391–6398. doi: 10.4049/jimmunol.174.10.6391. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Wang C, Wu C, Shieh J, Wen C. Microglial distribution and apoptosis in fetal rat brain. Dev Brain Res. 2002;139(2):337–342. doi: 10.1016/s0165-3806(02)00584-9. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Sholar PW, Mistry RM, Smith SH, Bilbo SD. Microglia and Memory: Modulation by Early-Life Infection. Journal of Neuroscience. 2011 doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Wen CY, Shieh JY, Ling EA. A quantitative study of the differentiation of microglial cells in the developing cerebral cortex in rats. J Anat. 1993;182(Pt 3):403–413. [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Wen CY, Shieh JY, Ling EA. A quantitative and morphometric study of the transformation of amoeboid microglia into ramified microglia in the developing corpus callosum in rats. J Anat. 1992;181(Pt 3):423–430. [PMC free article] [PubMed] [Google Scholar]
- Wynne O, Horvat JC, Osei-Kumah A, Smith R, Hansbro PM, Clifton VL, Hodgson DM. Early life infection alters adult BALB/c hippocampal gene expression in a sex specific manner. Stress. 2011;14(3):247–261. doi: 10.3109/10253890.2010.532576. [DOI] [PubMed] [Google Scholar]
- Xu J, Kaur C, Ling EA. Variation with age in the labelling of amoeboid microglial cells in rats following intraperitoneal or intravenous injection of a fluorescent dye. J Anat. 1993;182(Pt 1):55–63. [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 2006;66(6):578–90. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.