Abstract
The two cell types that populate the human conventional outflow pathway, Schlemm's canal (SC) and trabecular meshwork (TM) regulate intraocular pressure. In culture, SC and TM cells have been useful tools toward understanding their respective roles in conventional outflow homeostasis. Unfortunately, currently available protein markers that distinguish SC from TM cells are limited, motivating the present study. Antibodies that specifically recognize different vascular endothelial markers were used to probe lysates from mature cell monolayers subjected to SDS-PAGE followed by western blot analyses. Results show that SC and TM cells both expressed many of the endothelial candidate proteins investigated, such as Robo1/4, Tie2/TEK, VEGF-R1/R2, VCAM-1, eNOS and neuropilin-1. In contrast, all SC cell strains tested (n=11) expressed two proteins, fibulin-2 and vascular endothelial (VE) cadherin, not expressed by TM cells. To examine changes in VE-cadherin expression and cell-cell junction formation, indicated by transendothelial electrical resistance (TEER), SC cells were seeded onto filters at confluence and growth factors were withdrawn. Culturing cells in media containing adult bovine serum rather than fetal bovine serum resulted in a 75% mean increase in TEER and 67% corresponding average increase in VE-cadherin expression (p<0.05). While both TM and SC cells form monolayers, are contact inhibited, share some endothelial responsibilities and several endothelial protein markers, SC cells uniquely express at least two proteins which likely reflect a distinction in cellular responsibilities in vivo. One of these responsibilities, maintenance of the blood-aqueous barrier, can be modeled in culture upon withdrawal of growth factors from SC cell monolayers.
Keywords: Aqueous humor, Glaucoma, Intraocular Pressure, Trabecular meshwork
Introduction
Pathology that underlies ocular hypertension associated with primary open-angle glaucoma resides in the conventional outflow pathway (Grant, 1951). Dysfunction with one or both of the two cell types, trabecular meshwork (TM) and Schlemm's canal (SC), that populate the conventional outflow pathway likely are responsible for the increased resistance to outflow responsible for ocular hypertension. Outflow cells have many unique features and individual responsibilities that contribute to the generation and regulation of outflow resistance in both health and disease; maintaining intraocular pressure (IOP) in most people within a couple millimeters of mercury over a lifetime (David et al., 1987; Klein et al., 1992). Unfortunately, much is unknown about the cell biology responsible for the maintenance of health and the development of disease.
Primary cultures of cells isolated from the conventional outflow pathway of human donor eyes have been useful to better understand the cellular mechanisms that contribute to the regulation of conventional outflow and thus IOP. Methods have been developed, and are commonly used to specifically culture TM cells (Polansky et al., 1979; Stamer et al., 1995; Steely et al., 1992). Cultured cells can be differentiated in the laboratory and used to study a variety of cell activities, including contraction, phagocytosis, receptor activation and second messenger signaling. For example, studies using cultured TM cells have identified a number of cell surface receptors that participate in conventional outflow regulation, either increasing or decreasing outflow (Millard et al., 2011; Shearer and Crosson, 2002; Stamer et al., 2010; Sumida and Stamer, 2011; Tripathi et al., 1993; Wax et al., 1989). Similarly, cultures of TM cells have been used to characterize regulation of extracellular matrix turnover [reviewed by (Yue and Elvart, 1987)]. The study of such signaling pathways in cell culture has been critical for understanding the molecular mechanisms that underlie the clinical efficacy of laser trabeculoplasty (Hosseini et al., 2006) and the discovery of the glaucoma-causing protein, myocilin (Nguyen et al., 1998; Resch and Fautsch, 2009).
Due to research focus and relative ease of culturing primary TM cells, the majority of studies to date have targeted TM cell biology. Thus morphologic features, positive protein markers and cell behaviors have been used to describe and identify cultured TM cells. For example, a distinguishing response of TM cells from potential neighboring cell contaminants is the dramatic induction of myocilin protein upon treatment with corticosteroids (Polansky et al., 2000; Shepard et al., 2001; Stamer et al., 1998; Tamm et al., 1999). In contrast, other ocular cells have lower basal myocilin expression levels and fail to respond to corticosteroid treatment (Polansky et al., 2000). Additional protein markers identified for TM cells in culture include elevated secretion of tissue plasminogen activator (Seftor et al., 1994), plus distinctive expression of matrix GLA, αB-crystalin and smooth muscle actin by specific populations of cells in the TM (i.e.: juxtacanalicular versus uveal TM cells, (Pang et al., 1994; Tamm et al., 1999; Tamm et al., 1996; Xue et al., 2006)).
Work with the second major cell type in the conventional outflow pathway, SC endothelia, has lagged behind research with TM cells despite its noteworthy role in the conventional outflow pathway. The inner wall of SC is strategically located where the majority of resistance to outflow is generated, the juxtacanalicular region (Bahler et al., 2004; Grant, 1963; Johnson et al., 1992; Maepa and Bill, 1992; Rosenquist et al., 1989). Hence, disruption of SC-TM interactions dramatically decreases outflow resistance (Lu et al., 2008; Overby et al., 2002) while perfusion of eyes with tracer that occludes junctions between SC cells of the inner wall increases outflow resistance (Ethier and Chan, 2001). Moreover, the inner wall of SC is the only continuous monolayer in the outflow tract, serving an important function in maintaining part of the blood-aqueous barrier (Overby et al., 2009). Finally, the inner wall of SC may function as a mechanical capacitor, accommodating IOP transients via the formation of transcellular/paracellular pores and giant vacuoles (Allingham et al., 1992; Johnstone and Grant, 1973).
Not surprisingly, several methods to isolate and characterize SC cells have been developed over the last few years, each with its specific advantage. For example, a cannulation method captures a greater number of cells than an immunopanning method, but takes months to expand and characterize cells for use in experiments (Alvarado et al., 2004; Stamer et al., 1998). Immunopanning isolates much fewer primary cells, but experiments can be performed within days on primary isolates (Karl et al., 2005). Recently, a novel method using puromycin treatment of conventional outflow tissue digests has been developed to isolate SC cells, taking advantage of endothelial upregulation of the efflux transporter, p-glycoprotein by SC cells but not TM cells (Lei et al., 2010). This method isolates many aqueous plexus endothelial cells from porcine eyes (human SC equivalent), but has not been reported using human eyes.
In a short amount of time working with cultured SC cells, remarkable progress has been made to better understand their unique biology. For instance, SC cell monolayers in culture respond to pressure transients by activating signaling pathways (Stamer et al., 1999) plus forming giant vacuoles (Alvarado et al., 2004; Pedrigi et al., 2011), similar to cells in vivo. Moreover, activation of receptors on SC cell monolayers modifies second messenger pathways and cytoskeletal arrangements that are coincident with monolayer/barrier function alterations in eyes treated in situ (Alvarado et al., 1998; Rao et al., 2001; Sumida and Stamer, 2010; Underwood et al., 1999).
Due to the close proximity and common “endothelial nature” of TM and SC cells, the discovery of distinguishing protein markers for SC cells in culture is important for future studies involving cultured SC cells. Currently exclusion criteria, not positive markers are used to discriminate between SC and TM cells. Thus, the purpose of the present study was to examine a series of endothelial proteins in a large number of SC and TM cell strains with the goal of establishing novel characteristic markers for SC cells. In addition to finding two novel markers, this study reports an innovative way to differentiate SC cells in culture, monitoring expression of positive cell markers during the process.
Methods
Cell Isolation and Culture
Human Schlemm's canal (SC) and trabecular meshwork (TM) cells were isolated using previously described methods. SC cells were isolated using the cannulation method (Stamer et al., 1998), while TM cells were isolated using a method involving a blunt dissection followed by extracellular matrix digestion (Stamer et al., 1995). Both cell types were grown to confluence for one week in Dulbecco's Modified Essential Medium (DMEM) (Invitrogen) supplemented with a penicillin (100 units/ml) streptomycin (100ug/ml) glutamine (0.29 mg/ml) solution (Invitrogen) and 10% fetal bovine serum (FBS) (Hyclone). At one week, TM cells were switched to DMEM supplemented with 1% FBS and maintained for at least one additional week prior to lysis, while SC cells remained in 10% FBS. The specific cell strains tested for a certain marker protein was dependent upon availability at the time of the experiment.
Western Blot
Confluent monolayers of cells were rinsed with ice-cold 1× phosphate buffered saline (PBS) and then lysed in radioimmunoprecipitation assay buffer (RIPA, Sigma) with protease inhibitors (Roche) by scrapping with a disposable cell scraper and then subjected to centrifugation (14,000g for 8 minutes) to remove cell debris. Protein concentration from cleared cell lysates was determined using a BCA protein assay (Pierce Biotechnology). Cell lysates were added to equal volumes of 2× Laemmli buffer, boiled prior to loading (10μg) onto a gel slab and subjected to SDS-PAGE (7.5% or 12%). After fractionation, proteins were transferred electrophoretically to nitrocellulose membranes. After transfer, membranes were first incubated in blocking buffer [5% nonfat dry milk in Tris buffered saline containing 0.2% Tween-20 (TBS-T)]. Antibodies (supplemental table 1) that bind specifically to candidate marker proteins were diluted in blocking buffer and were incubated with membranes overnight at 4°C. Membranes were next washed with TBS-T (4 times: 10 minutes each) and were then transferred to blocking buffer containing horseradish peroxidase-conjugated secondary antibodies (Jackson Immunoresearch). After 2 hours, membranes were again washed with TBS-T (4 times: 10 minutes each) and proteins were visualized by briefly (~ 1 min) incubating membranes with enhanced chemiluminescence solution (ECL, Amersham Biosciences) and exposing to X-ray film (Genesee). Protein bands of interest on film were visualized and quantified by densitometry using the Syngene gel documentation system. Only bands within the linear range of the X-ray film were included in analyses.
Immunoprecipitation
Cell lysates from mature SC and TM cell monolayers were prepared in RIPA buffer as described above. Lysates (20 or 200 μg total protein) were first precleared with protein G beads (washed in RIPA buffer) for 1 hour at 4°C and were then incubated with 1μg of integrin α6 antibody (J1B5) for 1 hr with rotation at 4°C. Antibody complexes were isolated from lysates by adding protein G beads (50 μl of 50% slurry) and incubation with rotation for 12 hr at 4°C. Beads were pelleted by centrifugation at 6000×g for 1 minute and then were rinsed three times with 1 ml of RIPA buffer. Integrin α6 was liberated from beads by boiling in 40 μl of 1× Laemmli sample buffer for ten minutes. Beads were briefly centrifuged and supernatant was analyzed by SDS-PAGE/western blotting using integrin α3/α6 IgGs (AA6A).
Differentiation of SC Cell Monolayers
SC cells were plated at confluence onto polycarbonate Snapwell permeable supports (Costar) and maintained in 10% FBS supplemented DMEM until reaching a net transendothelial electrical resistance (TEER) of at least 10 Ohm*cm2 (2–3 weeks). Cells were then maintained in 10% FBS DMEM or switched to DMEM containing 10% adult bovine serum (ABS, Invitrogen). TEER measurements were taken using an Endohm device (World Precision Instruments) every two days for 16 days. Experiments were terminated by rinsing cells with 0.5 ml ice cold PBS and then scraping cells into ice cold RIPA buffer (40 μl). Cell lysates from Snapwells or from 12 well culture plates were diluted with equal volume of 2× Laemmli sample buffer, boiled and subjected to SDS-PAGE followed by western blot analysis for VE-cadherin, PECAM-1 and β-actin content.
Statistical Analyses
Comparisons were executed between data obtained from paired groups of cells that were treated with adult bovine serum or remained in fetal bovine serum. For western blots, only digitized bands captured onto x-ray film that corresponded to proteins of interest and fell within the linear range of the x-ray film were used for analyses. Data in both cases were subjected to a student's two-tailed t-test assuming unequal variance and differences were determined significant at p<0.05.
Results
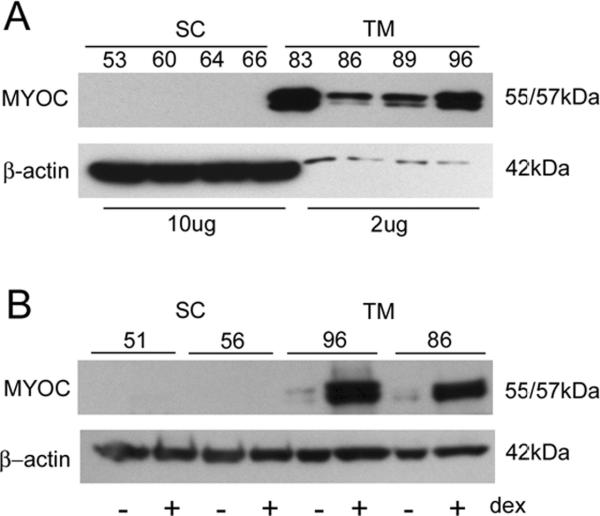
Due to current absence of positive protein markers, SC cells are identified based upon their spindle-like morphology, contact inhibition, generation of significant transendothelial electrical resistance and absence of myocilin induction upon treatment with corticosteroids, a positive marker for TM cells (Stamer et al., 1998). Thus, all SC cell strains isolated in our laboratory as a routine are treated for 5 days with 100 nM dexamethasone and examined for myocilin expression by western blot analyses and compared to TM cells. Extending results from our initial report that tested only two SC cell strains, we found here that all TM cell strains tested (n=10), but none of the SC cell strains (n=23) express myocilin at high levels (figure 1, panel A) nor responded to dexamethasone treatment. Shown in (figure 1, panel B) are results from representative cell strains of each cell type analyzed by western blot showing that myocilin expression in TM cells, but not SC cells is dramatically upregulated upon dexamethasone treatment. Moreover, SC cells have low basal levels of myocilin (data not shown) (Stamer et al., 1998).
Figure 1. Myocilin expression by conventional outflow cells in the presence and absence of corticosteroid treatment.
Panel A shows relative expression of myocilin in Schlemm's canal versus trabecular meshwork cell monolayers by western blot. Panel B shows western blot analyses of cell lysates taken from mature Schlemm's canal (SC) and trabecular meshwork (TM) cell monolayers that were untreated (−) or treated (+) with dexamethasone (100 nM, dex) for 5 days. Representative cell strains from each cell types are displayed.
Due to its vascular origin, we screened SC cells for a series of proteins expressed by endothelial cells to find unique markers (table 1). After cells were confluent for at least one week, we compared expression patterns of SC and TM cells to human umbilical vein endothelial cells (HUVEC), our positive control. Similar to previous studies, we observed that typical endothelial proteins such as PECAM-1 (CD31) and VCAM-1 (CD106) were not observed in SC cell strains, likely down regulated in culture (Stamer et al., 1998). In contrast, other characteristic endothelial cell proteins like VEGF receptor -1,2 and eNOS were present in both vascular endothelia as well as TM cells. Interestingly, we also observed that a variety of other proteins expressed by vascular endothelia (Robo-1, Robo-4, Tie2/TEK, Annexin-A1, Erg and Neuropilin-1) were expressed by all three cell types tested.
Table 1.
Endothelial Proteins Examined in Human Conventional Outflow Cells
| Marker Cell | HUVEC (control) | SC (n ≥ 3) | TM (n ≥ 3) |
|---|---|---|---|
| Robo1 | + | + | + |
| Robo4 | + | + | + |
| Tie2/TEK | + | + | +/− |
| Annexm A1 | + | + | + |
| Erg-1/2/3 | + | + | + |
| Neuropilm 1 | + | + | + |
| VEGF R1 | + | + | + |
| VEGF R2 | + | + | + |
| VCAM-1 | − | − | − |
| eNOS | + | + | + |
| PECAM-1 | + | − | − |
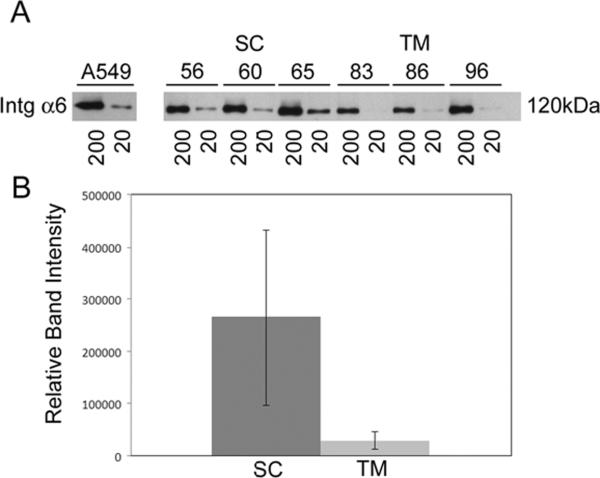
Using western blot analyses, we next tested the protein expression of endothelial candidates shown to be preferentially expressed by SC cells but not TM cells either at the mRNA level (fibulin-2) (Liton et al., 2005) or protein level in tissue sections of human eyes (VE-cadherin)(Heimark et al., 2002). We observed that both fibulin-2 and VE-cadherin were expressed by 11 SC cell strains tested (figure 2), each isolated from eyes of different individual donors. In addition, we expanded findings from a recent study (VanderWyst et al., 2011) and found expression of integrin-α6 in 11 additional SC cell strains (14 total). Interestingly, in the present study we tested additional TM cell strains and detected expression of integrin-α6 by some cell strains but not others. When examined more closely, we found that there was considerably less integrin-α6 protein in TM strains compared to SC strains (figure 3). A summary of these data from all SC and TM cells strains tested is shown in table 2.
Figure 2. Protein markers that distinguish Schlemm's canal (SC) endothelial from trabecular meshwork (TM) cells.
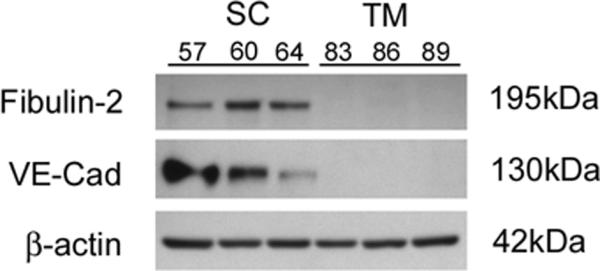
Shown are western blot analyses of lysates from mature cell monolayers using antibodies that specifically recognize Fibulin-2 or Vascular Endothelial (VE) cadherin. Results from three representative cell strains from each cell type are shown. Summary of data from all cell strains tested are shown in table 2.
Figure 3. Relative abundance of integrin-α6 expression in cultured monolayers of human conventional outflow cells.
Integrin-α6 (Intg α6)was immunoprecipitated from cell lysates (either 20 or 200 μg of total protein) as described in Methods. Panel A: Protein expression was evaluated by western blot analysis in three Schlemm's canal (SC) and three trabecular meshwork (TM) cell strains and compared to a positive control, A549 cells (adenocarcinomic human alveolar basal epithelial cells). Panel B: Relative abundance of integrin-α6 that was immunoprecipitated from SC and TM cell lysates (20 μg) shown in panel A was evaluated using densitometric analysis of western blots and compared statistically (p=0.07).
Table 2.
Positive protein markers for Schlemm's canal endothelia in culture
| Marker Cell | + Control | SC | TM |
|---|---|---|---|
| VE-Cadhenn | + | + (n=14) | − (n=10) |
| Fibulm-2 | + | + (n=11) | − (n=4) |
| Integcm-α6 | + | + (n=14) | + (n=5),− (n=3) |
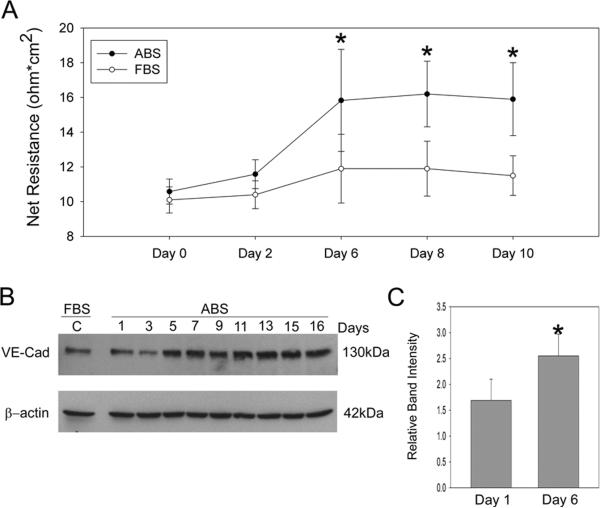
To investigate the effect of growth factor withdrawal on SC cell differentiation in culture, we switched SC cells from media containing 10% fetal bovine serum to 10% adult bovine serum. We hypothesized that the lower content of growth factors in adult serum may promote assembly of cell-cell junctions as evidenced by increase in TEER. Figure 4 shows that growth factor withdrawal did not affect TEER immediately, however beginning a day 6 TEER was significantly different in cells exposed to ABS compared to FBS; having a 75% mean change in TEER (p=0.02). Coincident with TEER elevation were changes in the adherens protein, VE-cadherin, at about the same time (~day 5). When examined closely at day 6 (first significant difference in TEER) in 4 different cell strains, we observed an average increase in VE-cadherin expression of 67% (p=0.03) in the presence of adult bovine serum (panel C). In contrast, another adherens protein, PECAM-1 expression was not detected in neither fetal nor adult bovine serum-treated SC cells (table 1).
Figure 4. Effect of growth factor withdrawal on transendothelial electrical resistance (TEER) and vascular endothelial (VE) cadherin expression by Schlemm's canal endothelia over time.
SC cell monolayers on filters were switched from fetal bovine serum (FBS) to adult bovine serum (ABS) after one week at confluence. Mature monolayers of SC cells were tested for TEER (panel A) or expression of the adherens protein, VE-cadherin (panels B) following change to media containing ABS every two days. Panel C shows data examining change in VE-cadherin protein in 4 different SC cell strains 6 days after switch to ABS, the time point showing first significant difference in TEER. Significant differences (p<0.05) in both VE-cadherin expression and TEER were detected following treatment with ABS (n=3–5 experiments using 4 different cell strains).
Discussion
Screening 15 potential endothelial markers in at least 11 different SC cell and 10 TM strains isolated from different donor eyes, the present study documents for the first time two proteins, fibulin-2 and VE-cadherin, with unique expression by human SC endothelia in culture. Moreover, experiments here confirm the expression of a laminin-binding protein, integrin-α6, recently identified as expressed by SC endothelia. We also observed that switching cultured SC cell monolayers from fetal to adult bovine serum stimulates differentiation in culture, significantly increasing both transendothelial electrical resistance and VE-cadherin expression. Taken together, these data show that the two cell types that populate the conventional outflow pathway, SC and TM express many of the same endothelial proteins, however two proteins were identified to be uniquely expressed that may be critical for SC cell function in the unique environment of the inner wall.
Despite their different embryological origin, TM (mesenchym/neural crest) and SC (mesoderm) cells both express a number of endothelial markers. Thus, expression of proteins by SC and TM cells are likely a function of their common “endothelial” role in maintaining a patent passageway for aqueous humor egress from the eye. For example, in the present study we found that both cell types express the endothelial proteins Robo1/4, Tie2/TEK, VEGF-R1/R2, eNOS and neuropilin-1. Likewise, we previously showed that both cell types expressed CD34, tPA, LDL/acetylated LDL receptor and CD54 (ICAM-1)(Stamer et al., 1998).
Fibulin-2, a component of the subendothelial matrix, was expressed by all SC but none of TM cell strains. As part of the internal elastic lamina, fibulin-2 functions to protect vessels from injury by directing the formation of elastic fibrils (Chapman et al., 2010). In the context of the conventional outflow pathway, the elastic nature of fibulin-2 may be important in helping accommodate dramatic pressure transients experienced by the inner wall of SC (Johnstone and Grant, 1973). Previously, fibulin-2 was identified as a preferentially expressed mRNA by SC cells by microarray analysis (Liton et al., 2005), results that we recently confirmed by microarray comparing two of our SC cell strains with two TM cell strains (data not shown). Here we extend these findings at the protein level, detecting expression in 11 different SC cell strains.
Similar to a recent report (VanderWyst et al., 2011), integrin-α6, an endothelial laminin binding protein, was found in 10 SC strains tested in the present study. Interestingly, integrin-α6 was also observed in 5 of 8 TM strains tested. This unexpected finding may be due to two factors: the nature of our blunt TM dissection technique, which sometimes isolates contaminant cell colonies from the inner wall (Stamer et al., 1995) and the high sensitivity of the immunoprecipitation method. To specifically detect integrin-α6, an immunoprecipitation procedure is needed (followed by western blot using anti-integrin α3/α6 IgGs) because available antibodies selective for integrin-α6 IgGs do not recognize denatured integrin-α6 on western blots. The net result of this procedure is that integrin-α6 protein is dramatically concentrated from samples, allowing for detection of very small quantities of protein. To examine whether the presence of integrin-α6 in TM cell cultures was due to SC contaminants or expression by small subpopulation of TM cells, we performed immunoprecipitations using 10 fold less total cell protein and found consistently more integrin-α6 in SC versus TM cells (figure 3). Regardless of these results, integrin-α6 does not appear to represent a good marker for distinguishing TM and SC cells in culture given current detection methods.
In a previous study looking at the expression of adherens proteins in the conventional outflow pathway, PECAM-1 and VE-cadherin were found to be uniquely expressed by SC endothelia in tissue sections of human donor eyes (Heimark et al., 2002). Here we report the expression of VE-cadherin in all 14 strains of SC cells but none of the TM strains tested. Interestingly, we found that once confluent, VE-cadherin expression was relatively stable over time (data not shown). However, upon exposure to adult bovine serum expression of VE-cadherin increased over time, coincident with increased TEER. Taken together, these results indicate that cell-cell junction complexes were maturing and cells were differentiating in culture. Thus, withdrawal of cells from fetal bovine serum containing concentrated amounts of growth factors appears to be sufficient to initiate differentiation. Interestingly, the expression of another adherens protein, PECAM-1, did not increase (become detectable) by adult bovine serum treatment.
While nine endothelial proteins were expressed by both TM and SC cells, we found two endothelial proteins that were uniquely expressed and one that was preferentially expressed by SC cells in culture. Expression of fibulin-2 and the laminin binding integrin-α6 subtype by SC cells suggest the importance of SC cell adherence with and the flexibility of the basement membrane of the inner wall. The expression of VE-cadherin by SC, but not TM suggests the importance of cell-cell adhesion and barrier function by the SC. Taken together, these results provide indicators of the unique role of SC cells in conventional outflow function plus accomplish the goal of the study, the identification of positive protein markers for SC cells in culture.
Supplementary Material
Highlights
Schlemm's canal, but not trabecular meshwork cells uniquely express VE cadherin and fibulin 2
Schlemm's canal and trabecular meshwork cells express many endothelial proteins
Upon withdrawal of growth factors, Schlemm's canal cells show signs of differentiation in culture
Acknowledgements
The authors thank Emely Hoffman for primary cultures of trabecular meshwork cells, Drs. Mark Johnson and Darryl Overby for helpful discussion during the course of the project and Dr. Anne Cress for the gift of antibodies that recognize α6-integrin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allingham RR, de Kater AW, Ethier CR, Anderson PJ, Hertzmark E, Epstein DL. The relationship between pore density and outflow facility in human eyes. Invest Ophthalmol Vis Sci. 1992;33:1661–1669. [PubMed] [Google Scholar]
- Alvarado J, Betanzos A, Franse-Carman L, Chen J, Gonzalez-Mariscal L. Endothelia of Schlemm's canal and trabecular meshwork: distinct molecular, functional, and anatomic features. Am. J. Physiol. 2004;286:C621–C634. doi: 10.1152/ajpcell.00108.2003. [DOI] [PubMed] [Google Scholar]
- Alvarado JA, Murphy CG, Franse-Carman L, Chen J, Underwood JL. Effect of beta-adrenergic agonists on paracellular width and fluid flow across outflow pathway cells. Invest Ophthalmol Vis Sci. 1998;39:1813–1822. [PubMed] [Google Scholar]
- Bahler C, Hann C, Fautsch M, Johnson D. Pharmacological disruption of Schlemm's canal cells and outflow facility in anterior segments of human eyes. Invest. Ophthalmol. Vis. Sci. 2004;45:2246–2254. doi: 10.1167/iovs.03-0746. [DOI] [PubMed] [Google Scholar]
- Chapman SL, Sicot FX, Davis EC, Huang J, Sasaki T, Chu ML, Yanagisawa H. Fibulin-2 and fibulin-5 cooperatively function to form the internal elastic lamina and protect from vascular injury. Arterioscler Thromb Vasc Biol. 2010;30:68–74. doi: 10.1161/ATVBAHA.109.196725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Zangwill L, Stone D, Yassur Y. Epidemiology of intraocular pressure in a population screened for glaucoma. Br J Ophthalmol. 1987;71:766–71. doi: 10.1136/bjo.71.10.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier C, Chan D. Cationic ferritin changes outflow facility whereas anionic ferritin does not. Invest. Ophthalmol. Vis. Sci. 2001;42:1795–1802. [PubMed] [Google Scholar]
- Grant WM. Clinical tonography. Trans Am Acad Ophthalmol Otolaryngol. 1951;55:774–81. [PubMed] [Google Scholar]
- Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- Heimark R, Kaochar S, Stamer W. Human Schlemm's canal cells express the endothelial adherens proteins, VE-cadherin and PECAM-1. Curr Eye Res. 2002;25:299–308. doi: 10.1076/ceyr.25.5.299.13495. [DOI] [PubMed] [Google Scholar]
- Hosseini M, Rose AY, Song K, Bohan C, Alexander JP, Kelley MJ, Acott TS. IL-1 and TNF induction of matrix metalloproteinase-3 by c-Jun N-terminal kinase in trabecular meshwork. Invest Ophthalmol Vis Sci. 2006;47:1469–76. doi: 10.1167/iovs.05-0451. [DOI] [PubMed] [Google Scholar]
- Johnson M, Shapiro A, Ethier CR, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci. 1992;33:1670–1675. [PubMed] [Google Scholar]
- Johnstone M, Grant M. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75:365–383. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- Karl MO, Fleischhauer JC, Stamer WD, Peterson-Yantorno K, Mitchell CH, Stone RA, Civan MM. Differential P1-purinergic modulation of human Schlemm's canal inner-wall cells. Am J Physiol Cell Physiol. 2005;288:C784–94. doi: 10.1152/ajpcell.00333.2004. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Linton KL. Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992;33:2224–8. [PubMed] [Google Scholar]
- Lei Y, Overby DR, Read AT, Stamer WD, Ethier CR. A new method for selection of angular aqueous plexus cells from porcine eyes: a model for Schlemm's canal endothelium. Invest Ophthalmol Vis Sci. 2010;51:5744–50. doi: 10.1167/iovs.10-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Liu X, Stamer WD, Challa P, Epstein DL, Gonzalez P. Specific targeting of gene expression to a subset of human trabecular meshwork cells using the chitinase 3-like 1 promoter. Investigative Ophthalmology & Visual Science. 2005;46:183–90. doi: 10.1167/iovs.04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Overby DR, Scott PA, Freddo TF, Gong H. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp Eye Res. 2008;86:271–81. doi: 10.1016/j.exer.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maepa O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res. 1992;54:879–883. doi: 10.1016/0014-4835(92)90151-h. [DOI] [PubMed] [Google Scholar]
- Millard LH, Woodward DF, Stamer WD. The Role of the Prostaglandin EP4 Receptor in the Regulation of Human Outflow Facility. Invest Ophthalmol Vis Sci. 2011;52:3506–13. doi: 10.1167/iovs.10-6510. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Chen P, Huang W, Chen H, Johnson D, Polansky J. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- Overby D, Gong H, Qiu G, Freddo TF, Johnson M. The mechanism of increasing outflow facility during washout in the bovine eye. Invest Ophthalmol Vis Sci. 2002;43:3455–64. [PubMed] [Google Scholar]
- Overby DR, Stamer WD, Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp Eye Res. 2009;88:656–70. doi: 10.1016/j.exer.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang I, Shade D, Clark A, Steely HT, Destantis L. Preliminary characterization of a transformed cell strain derived form human trabecular meshwork. Curr. Eye Res. 1994;13:51–63. doi: 10.3109/02713689409042398. [DOI] [PubMed] [Google Scholar]
- Pedrigi RM, Simon D, Reed A, Stamer WD, Overby DR. A model of giant vacuole dynamics in human Schlemm's canal endothelial cells. Exp Eye Res. 2011;92:57–66. doi: 10.1016/j.exer.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky J, Fauss D, Zimmerman C. Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye. 2000;14:503–514. doi: 10.1038/eye.2000.137. [DOI] [PubMed] [Google Scholar]
- Polansky J, Weinreb R, Baxter J, Alvarado J. Human trabecular cells: Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979;18:1043–1049. [PubMed] [Google Scholar]
- Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–37. [PubMed] [Google Scholar]
- Resch ZT, Fautsch MP. Glaucoma-associated myocilin: a better understanding but much more to learn. Exp Eye Res. 2009;88:704–12. doi: 10.1016/j.exer.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist R, Epstein DL, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculectomy. Curr Eye Res. 1989;8:1233–1240. doi: 10.3109/02713688909013902. [DOI] [PubMed] [Google Scholar]
- Seftor R, Stamer W, Seftor E, Snyder R. Dexamethasone decreases tissue plasminogen activator activity in trabecular meshwork organ and cell culture. J Glaucoma. 1994;3:323–328. [PubMed] [Google Scholar]
- Shearer TW, Crosson CE. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002;43:3016–20. [PubMed] [Google Scholar]
- Shepard A, Jacobson N, Fingert J, Stone E, Sheffield V, Clark A. Delayed secondary glucocorticoid responsiveness of MYOC in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 2001;42:33173–3181. [PubMed] [Google Scholar]
- Stamer W, Roberts B, Epstein D. Hydraulic pressure stimulates adenosine 3', 5'-cyclic monophosphate accumulation in endothelial cells from Schlemm's canal. Invest. Ophthalmol. Vis. Sci. 1999;40:1983–1988. [PubMed] [Google Scholar]
- Stamer W, Seftor R, Williams S, Samaha H, Snyder R. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995;14:611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Piwnica D, Jolas T, Carling RW, Cornell CL, Fliri H, Martos J, Pettit SN, Wang JW, Woodward DF. Cellular basis for bimatoprost effects on human conventional outflow. Invest Ophthalmol Vis Sci. 2010;51:5176–81. doi: 10.1167/iovs.09-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Roberts BC, Howell DN, Epstein DL. Isolation, culture, and characterization of endothelial cells from Schlemm's canal. Invest Ophthalmol Vis Sci. 1998;39:1804–12. [PubMed] [Google Scholar]
- Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1992;33:2242–50. [PubMed] [Google Scholar]
- Sumida GM, Stamer WD. Sphingosine-1-phosphate enhancement of cortical actomyosin organization in cultured human Schlemm's canal endothelial cell monolayers. Invest Ophthalmol Vis Sci. 2010;51:6633–8. doi: 10.1167/iovs.10-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida GM, Stamer WD. S1P2 receptor regulation of sphingosine-1-phosphate effects on conventional outflow physiology. Am J Physiol Cell Physiol. 2011;300:C1164–71. doi: 10.1152/ajpcell.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm E, Russell P, Epstein D, Johnson D, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:2577–2582. [PubMed] [Google Scholar]
- Tamm ER, Russell P, Piatigorsky J. Development of characterization of a immortal and differentiated murine trabecular meshwork cell line. Invest Ophthalmol Vis Sci. 1999;40:1392–403. [PubMed] [Google Scholar]
- Tamm ER, Siegner A, Baur A, Lutjen-Drecoll E. Transforming growth factor-beta 1 induces alpha-smooth muscle-actin expression in cultured human and monkey trabecular meshwork. Exp Eye Res. 1996;62:389–97. doi: 10.1006/exer.1996.0044. [DOI] [PubMed] [Google Scholar]
- Tripathi RC, Borisuth NS, Kolli SP, Tripathi BJ. Trabecular cells express receptors that bind TGF-beta 1 and TGF-beta 2: a qualitative and quantitative characterization. Invest Ophthalmol Vis Sci. 1993;34:260–3. [PubMed] [Google Scholar]
- Underwood J, Murphy C, Chen J, Franse-Carman I, Wood I, Epstein D, Alvarado J. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intercellular junctions. Am J Physiol. 1999;277:C330–C342. doi: 10.1152/ajpcell.1999.277.2.C330. [DOI] [PubMed] [Google Scholar]
- VanderWyst SS, Perkumas KM, Read AT, Overby DR, Stamer WD. Structural basement membrane components and corresponding integrins in Schlemm's canal endothelia. Mol Vis. 2011;17:199–209. [PMC free article] [PubMed] [Google Scholar]
- Wax MB, Molinoff PB, Alvarado J, Polansky J. Characterization of beta-adrenergic receptors in cultured human trabecular cells and in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1989;30:51–7. [PubMed] [Google Scholar]
- Xue W, Wallin R, Olmsted-Davis EA, Borras T. Matrix GLA protein function in human trabecular meshwork cells: inhibition of BMP2-induced calcification process. Invest Ophthalmol Vis Sci. 2006;47:997–1007. doi: 10.1167/iovs.05-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue B, Elvart J. Biosynthesis of glycosaminoglycans by trabecular meshwork cells in vitro. Curr Eye Res. 1987;6:959–967. doi: 10.3109/02713688709034867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.