Abstract
Netrin-4, a member of the netrin family, is a potent regulator of embryonic development. It promotes neurite extension and regulates pulmonary airway branching, vasculogenesis patterning, and endothelial proliferation in pathological angiogenesis. The initial characterization of netrin-4 expression was focused on epithelial-derived organs (kidney, lung and salivary gland) and the central nervous system. Ocular development is an ideal system to study netrin-4 expression and function, as it involves both ectodermal (cornea, lens and retina) and mesodermal (sclera and choroid) derivatives and has an extensive and well-characterized angiogenic process. Netrin-4 is expressed in all ocular tissues. It is a prominent component of the basement membranes of the lens and cornea, as well as all three basement membranes of the retina: the inner limiting membrane, vascular basement membranes, and Bruch’s membrane. Netrin-4 is differentially deposited in vascular basement membranes, with more intense anti-netrin-4 reactivity on the arterial side. The retinal microcirculation also expresses netrin-4. In order to test the function of netrin-4 in vivo, we generated a conventional mouse lacking Ntn4 expression. Basement membrane formation in the cornea, lens and retina is undisrupted by netrin-4 deletion, demonstrating that netrin-4 is not a major structural component of these basement membranes. In the Ntn4 homozygous null (Ntn4−/−) cornea, the overall morphology of the cornea, as well as the epithelial, stromal and endothelial stratification are normal; however, epithelial cell proliferation is increased. In the Ntn4−/− retina, neurogenesis appears to proceed normally, as does retinal lamination. In the Ntn4−/− retina, retinal ganglion cell targeting is intact, although there are minor defects in axon fasciculation. In the retinal vasculature of the Ntn4−/− retina, the distribution patterns of astrocytes and the vasculature are largely normal, with the possible exception of increased branching in the deep capillary plexus, suggesting that netrin-4 may act as a negative regulator of angiogenesis. These data, taken together, suggest that netrin-4 is a negative regulator of corneal epithelial cell proliferation and retinal vascular branching in vivo, whereas netrin-4 may be redundant with other members of the netrin family in other ocular tissue development. Ntn4−/− mice may serve as a good model in which to study the role of netrins in vivo of the pathobiologic vascular remodeling in the retina and cornea.
Keywords: Extracellular matrix, netrin, angiogenesis, cornea, retina, axonal pathfinding
1. Introduction
Ocular development involves neural tube-derived components (retina) and ectoderm-derived components (lens and cornea) (Cvekl and Tamm, 2004). In mouse, retinal ganglion cells (RGCs) are generated prenatally (E11 to E19) in an approximately centrifugal order (Drager, 1985; Young, 1985). Their axons exit the eye starting at E11.5 also in a centrifugal order; thus, axons of the later-born RGCs arise from more peripheral regions and travel greater distances before they turn to exit into the optic nerve (Mann et al., 2004). Along their elongation path, RGC axons are in close contact with Müller cell endfeet and with the inner limiting membrane (ILM) (Mann et al., 2004; Stuermer and Bastmeyer, 2000).
The anterior segment follows parallel pathways of development. At E12.5-13.5, cells derived from the neural crest begin to migrate into the space between the anterior epithelium of the lens vesicle and the surface ectoderm. By E14.5-E15.5, the cells closest to the lens form an endothelial monolayer; the surface ecotoderm becomes the corneal epithelium; cells between the endothelium and epithelium differentiate into keratocytes of the corneal stroma (Cvekl and Tamm, 2004).
During the development of these ocular structures, many extracellular matrix (ECM) molecules play important roles. For example, netrin-1, laminin α1β1γ1, and ephrin-B are all implicated in RGC axon pathfinding (Hopker et al., 1999; Mann et al., 2004), and ECM molecules are thought to have a role in corneal development and maturation (Kabosova et al., 2007).
Netrins are a family of ECM molecules that have homology with the short arm of laminin molecules. Five members of the netrin family have been identified in mammals: netrin-1 (Kennedy et al., 1994), netrin-3 (Van Raay et al., 1997; Wang et al., 1999), netrin-4 (Koch et al., 2000; Yin et al., 2000), netrin-G1 and netrin-G2 (Nakashiba et al., 2000; Nakashiba et al., 2002). All netrin genes encode secreted proteins, but in contrast to netrin-1, −3 and −4, netrin-G1 and -G2 are bound to the membrane via a glycosylphosphatidylinositol (GPI) linkage.
Netrins were first recognized as long range guidance cues regulating the migration of neurons and the behavior of axon growth cones (Barallobre et al., 2005; Kennedy et al., 1994). However, they have wide-spread expression outside the nervous system, and netrins are involved in a wide variety of developmental events, including angiogenesis (Lu et al., 2004; Wilson et al., 2006), cell-cell adhesion (Srinivasan et al., 2003; Yebra et al., 2003), and branching morphogenesis of lung, mammary gland and salivary glands (Liu et al., 2004; Schneiders et al., 2007; Srinivasan et al., 2003).
In the embryo, netrin-1 is deposited at the exit of the optic nerve, and RGC axons express the netrin-1 receptor, DCC (de la Torre et al., 1997; Deiner et al., 1997; Keino-Masu et al., 1996; Livesey and Hunt, 1997) suggesting a role in guidance. In both netrin-1-hypomorphic and DCC-null mouse retinas, RGC axons navigate to the optic disc. Some axons fail to exit into the optic nerve, indicating that netrin-1 is not required for guiding retinal axons to the optic disc, but may be required for proper exit of RGC axons (Deiner et al., 1997). Importantly, DCC-null mice have a more severe phenotype than netrin-1 hypomorphic mice, suggesting there may be additional ligands for the DCC receptor (Fazeli et al., 1997).
We first identified netrin-4 (β-netrin), and demonstrated its expression in multiple tissues (Koch et al., 2000). We showed that, in vitro, netrin-4 promoted the neurite growth from E14 rat olfactory bulb explants, suggesting netrin-4 might be important as a haptotactic factor in promoting axonal elongation or as a substrate for neuronal migration. Netrin-4 has also been shown to regulate epithelial branching and endothelial proliferation (Lejmi et al., 2008; Liu et al., 2004; Park et al., 2004; Schneiders et al., 2007). This study details netrin-4 expression in the mouse eye, provides the first characterization of a Ntn4−/− mouse, and uses that netrin-4-null mouse to define the role of netrin-4 in the development of ocular components in vivo.
2. Materials and Methods
All procedures involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Tufts University and State University of New York - Downstate Medical Center and were in accordance with the National Institute of Health Guide for the Care and Use of Animals.
2.1 Recombinant expression and purification of murine netrin-4 fragment
A truncated netrin molecule, Δnetrin-4 (AF281278, nucleotides 311-1,672), was amplified by PCR and subcloned into a modified pCEP-Pu expression vector (an 8 histidine tag and a thrombin cleavage site were introduced either at the N-terminal or the C-terminal end of the protein sequence). The expression vector was transfected into 293-EBNA cells with FuGENE 6 Transfection Reagent (Roche Diagnostics, Indianapolis, IN). Clones with the highest protein expression were expanded for large-scale production. The purification of the secreted proteins was performed as previously described (Koch et al., 2000).
2.2 Antibody production
KR1, a rabbit anti-netrin-4 antibody, was produced as described previously (Koch et al., 2000). Recombinant mouse Δnetrin-4 (AF281278, nucleotides 311-1,672), from which the His tag was removed, was injected intradermally into a rabbit following standard procedures (Harlow and Lane, 1988). The KR1 antiserum was purified over a protein G column (Amersham Biosciences, Piscataway, NJ) and eluted with triethylamine (Sigma, St. Louis, MO). The neutralized elute was affinity-purified on a netrin-4 protein column. To prepare this column, Δnetrin-4, from which the His tag was removed, was coupled to activated CNBr-Sepharose (Amersham Biosciences). Bound antibodies were eluted with triethylamine and immediately neutralized. Recombinant DCC (NM_007831, amino acids 26-1,097) was expressed and purified similarly. Guinea pig polyclonal antibody against purified recombinant DCC protein was produced similarly.
2.3 Immunohistochemistry
For fresh-frozen samples, adult or embryonic tissues were dissected, embedded immediately in OCT compound (Sakura, Torrance, CA), and frozen by immersion in dry ice-cooled ethanol. For fixed tissues, mice were anesthetized by intramuscular injection of ketamine and xylamine, then transcardially perfused with phosphate buffered saline (PBS) followed by 4% paraformaldhyde (PFA). Tissues were dissected and fixed in 4% PFA by immersion at 4°C overnight, then processed through 10%, 20% and 30% sucrose before being embedded in OCT. For both fresh-frozen and 4% PFA fixed tissues, 12μm sections were cut with a Reichert-Jung 2800 Frigocut cryostat and placed onto Superfrost Plus slides (Brain Research Laboratory, Newton, MA). Slides were stored at −80°C until use.
Upon use, slides were returned to room temperature and air dried. Fresh-frozen tissue slides were immersed in absolute methanol at −20°C for 2min; 4% PFA fixed tissue slides directly went to the next step. Slides were then washed in PBS and blocked with 1% BSA and 5% goat or donkey serum in PBS for 1hr. The sections were incubated in primary antibody at room temperature for 2 hr or at 4°C overnight. The primary antibodies used were against: netrin-4 (KR1, rabbit polyclonal, 1:1,000; R33, rabbit polyclonal, 1:2,000) (Koch et al., 2000), DCC (guinea pig polyclonal, 1:1,000), perlecan (rat monoclonal, 1:50,000, Chemicon, Temecula, CA), vimentin (rabbit polyclonal, 1:5,000, Chemicon), calretinin (mouse monoclonal, 1: 1,000, Chemicon), glutamine synthetase (mouse monoclonal, 1:1,000, Chemicon), PKCα (MC5, mouse monoclonal, 1:1,000, Chemicon), and rhodopsin (RetP1, mouse monoclonal, 1:10,000, Sigma), PECAM-1 (CD31, rat monoclonal, BD Biosciences, San Jose, CA), Phospho-Histone H3 (Rabbit polyclonal, Upstate Biotechnology, Lake Placid, NY). The primary antibodies were diluted in PBS containing 1% BSA and 1% goat or donkey serum. After a PBS wash, slides were incubated in species-appropriate, affinity-purified, fluorescently labeled secondary antibodies (Invitrogen, Carlsbad, CA) at room temperature for 1hr. Some sections were counter-stained with DAPI (Sigma). After a PBS wash, slides were mounted in ProLong (Invitrogen).
Slides were examined and images were captured using OpenLab (4.0.2) or Volocity (5.0) software (Improvision Inc., Lexington, MA) with a Hamamatsu Orca camera on a Nikon E800 microscope. The figures shown here were created using Adobe Photoshop 7.0 and no adjustment other than contrast and brightness was made to the images. To make a montage image, series of images were taken using OpenLab 4.0.2 with a motorized X-Y stage and were put into a montage using GraphicConverter software (Lemke Software GmbH, Peine, Germany) after the background of these images was subtracted using OpenLab 4.0.2.
2.4 Mice
Ntn4−/− mice were generated using standard recombination methods. The targeting vector was designed to delete a 3.9kb fragment containing part of the first exon, the first intron and part of the second exon, inserting in its place a neomycin resistance (Neo) cassette flanked by LoxP sites and a LacZ reporter gene. The mutation was introduced into 129/SvJ embryonic stem (ES) cells (Li et al., 1992). Following neomycin selection and cloning, Southern analysis was used to identity ES cells for chimera production. Two probes were used one at the 5′ end and one at the 3′ end. Genomic DNA was digested with PvuIII for the 5′ probe or HindIII for the 3′ probe. The recombination event was confirmed in two lines (119 and 146) by the presence of mutant bands migrating above the wild-type bands. ES injection into blastocysts was performed in the transgenic mouse facility at the Cutaneous Biology Research Center, Massachusetts General Hospital.
Following identification of founder mice, Ntn4+/− mice were back-crossed to C57BL/6 wild-type mice for 9 generations. The genotypes of the offspring were determined by PCR analysis of genomic DNA prepared from tail or toe samples. Primers used: wild type and null allele forward AGCAGCCTTTAAACATCCTGAG, wild type allele reverse GAAAGCTCCGGGCAGACACTATGTG, and null allele reverse CAAATGTGTCAGTTTCATAGCC. B6.Cg-Tg(Thy1-YFPH)2Jrs/J mice (Feng et al., 2000) were crossed with Ntn4+/− mice to generate a line of EYFP+/Ntn4+/− mice. The primers used to detect the EYFP transgene were as described (Feng et al., 2000). All animals used in this study were littermates; both Ntn4 lines (Ntn4+/− and EYFP+/Ntn4+/) were maintained as heterozygotes.
2.5 Cornea whole-mounts
Mice were euthanized by carbon dioxide and the eyes were enucleated. The cornea was dissected and fixed in 4% PFA for 2hrs. Four radial cuts were made on the cornea to ease the flattening. The cornea was then flattened onto a glass slides. Processing followed the procedures for retinal whole-mounts below. Whole-mounts were imaged using Volocity 5.5.
2.6 Retinal whole-mounts
Mice were anesthetized by intramuscular injection of ketamine and xylamine and then then transcardially perfused by PBS followed by 4% PFA. Eyes were enucleated from the mice and the anterior segment was discarded.
The eyecups were further fixed in 4% PFA by immersion at room temperature for 30 min. The sclera was peeled away and the pigment epithelium was removed. The residual vitreous was carefully removed under a dissecting microscope to help flatten the retina. Four radial cuts were made to allow for further flattening. EYFP+/Ntn4+/+ and EYFP+/Ntn4−/− retina whole-mounts were mounted in glycerol and the coverslips were sealed to the slides with nail polish.
The whole-mount immunohistology protocol was modified from Cellerino et al. (Cellerino et al., 1998). Retinas were dehydrated in ethanol with ascending concentrations (50%, 70%, 80%, 90%, 95%, and 100%) for 10min each, and then incubated in xylene at room temperature for 10min. They were rehydrated in ethanol with descending concentrations (100%, 95%, 90%, 80%, 70%, and 50%) for 10min each before being washed in PBS for 30min. The retinas were blocked in 1% BSA with 20% goat or donkey serum and 0.3% Triton X-100 overnight, and then incubated in primary antibodies in 1% BSA with 5% goat or donkey serum at 4°C for 3 days with continuous agitation. The primary antibodies used were against: netrin-4 (KR1, 1:5,000), neurotubulin (TuJ-1, mouse monoclonal, 1:500, Covance Innovative, Berkeley, CA) and integrin β4 (346-11A, rat monoclonal, 1:100, Chemicon). After a PBS wash, the retinas were incubated in species-appropriate, affinity-purified, fluorescently labeled secondary antibodies (Invitrogen) at 4°C for 1 day with continuous agitation. After a PBS wash, the retinas were flattened onto slides with the ganglion cell layer side facing up and mounted in ProLong (Invitrogen). Whole-mount retina slides were stored at 4°C between examinations.
2.7 DiI labeling
Retinal organotypic cultures of P7 wild type and mutant mice were prepared as previously described (Pinzon-Duarte et al., 2000). DiI crystals were applied onto the retinas and cultured for 2 weeks. The retinas were then fixed in 4% PFA and mounted for photographing.
3. Results
3.1 Netrin-4 expression in the mouse eye
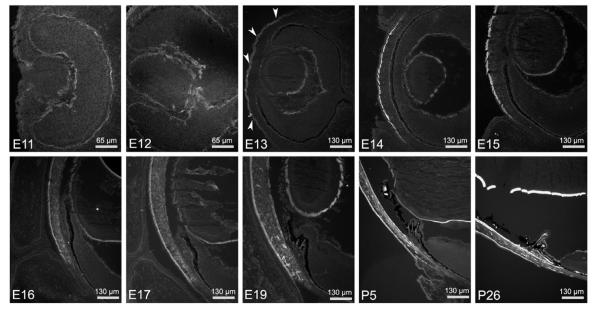
Netrin-4 is first detected in the BM of the presumptive corneal surface epithelium at E13 (Fig. 1; arrowheads). As development proceeds, netrin-4 immunoreactivity (IR) becomes more intense in the corneal epithelial BM, i.e., Bowman’s membrane. Concomitant with the migration of mesenchymeal cells, netrin-4 IR is found within the developing corneal stroma beginning at approximately E16. An abrupt boundary in netrin-4 expression at the corneal limbus is evident at this early age. This striking boundary is maintained throughout development and into adulthood (Fig. 1). Starting at E17, netrin-4 IR becomes detectable in the corneal endothelial BM, i.e., Descemet’s membrane. At first the expression in Descemet’s membrane is discontinuous; however, it becomes a continuous sheet near birth (Fig. 1).
Figure 1.
Developmental expression of netrin-4 in cornea and lens capsule. Netrin-4 is detected in cornea epithelium from E13 (arrowheads) and persists into adulthood. Starting at E16, netrin-4 is deposited in the corneal stroma, especially at the peripheral cornea, resulting in a clear boundary between the cornea and the sclera. Netrin-4 IR in Decemet’s membrane, the discontinuous corneal endothelium basement membrane, was first detected at E17. The netrin-4 IR in Decemet’s membrane persists to adulthood. Netrin-4 is also detected in lens capsule as early as E11; the netrin-4 IR is stronger in the posterior lens capsule than that in the anterior capsule.
The lens capsule is a specialized BM surrounding the lens (Seland, 1992). It serves as a basal attachment site for lens epithelial cells and provides stable anchoring sites for zonular fibers, filamentous structures that couple the ciliary muscle to the lens during accommodation reflexes (Kelley et al., 2002). Many extracellular matrix proteins have been found in the lens capsule, including entactin/nidogen (Cammarata and Spiro, 1985), laminin and fibronectin (Parmigiani and McAvoy, 1984), heparin sulfate proteoglycan (Mohan and Spiro, 1986), collagen XVIII (Halfter et al., 1998), and collagen IV (Schmut, 1978); indeed, laminin β2 was isolated from the lens capsule (Hunter et al., 1989). Netrin-4 is detectable in the BM of the developing lens placode as early as E11, the earliest time point that we examined (Fig. 1). Netrin-4 continues to be expressed in the lens BM as the lens placode detaches from the surface ectoderm, and is expressed through adulthood (Fig. 1). Thus, netrin-4 is deposited in the BM compartment separating the invaginating lens and the neural retina. Throughout development (E14 to P5) there appears to be a differential expression of netrin-4 in the lens capsule - netrin-4 deposition in the posterior capsule appears higher than in the anterior capsule (Fig. 1; e.g., compare anterior with posterior at E19). Although it may be that netrin-4 is important in this developmental gradient, it may also be that the apparent difference in the intensity of netrin-4 IR in the anterior versus the posterior lens capsule reflects the relative thickness of the anterior versus the posterior lens capsule during development (Kelley et al., 2002) rather than a change in concentration of the molecule.
3.2 Netrin-4 in retina and optic nerve head
Netrin-1 and its receptor, DCC, are expressed in the optic nerve and are critical components of the molecular mechanism guiding RGC axons out of the eye. Thus, we were interested in discovering if netrin-4 overlapped with these molecules in expression and deposition. From the earliest point in development that we assayed (E11), netrin-4 is deposited in the BM of the invaginating eye cup (i.e., the ILM), the BM of the RPE (Bruch’s membrane) and the BM of the ciliary and iris epithelium (Figs. 1, 2). Its expression persists into adulthood (Fig. 1, P26; Fig. 2C). There is also continuous deposition of netrin-4 in the pars plana of the retina (Fig. 1, P26).
Figure 2.
Developmental expression of netrin-4 in mouse retina and optic nerve. (A) Cross sections of E13 mouse eye through optic nerve head. Netrin-4 IR is present at the ILM, Bruch’s membrane and the BM along the optic fissure associated with the hyaloid vessels. In cross sections of E14 to E19 (B) and P5 to P26 (C) retinas, in addition to netrin-4 IR in the ILM and Bruch’s membrane, netrin-4 IR is present in hyaloid vessels (arrows) and, at later times, on retinal vasculature BMs. Netrin-4 IR is also present postnatally in the epineurium of the optic nerve.
At E11-12, netrin-4 is present along the ILM and along the optic fissure, the exit point for RGC axons and the entry point for the hyaloid and retinal arteries (Fig. 1). Netrin-4 expression is not found surrounding RGC axons, i.e, in the endo- or perineurium, rather netrin-4 is associated with the hyaloid artery from E13 through E19 (Fig. 2A, B, arrows).
Retinal vascularization proceeds first by growth along the ILM/vitreal border (from P0 to P10), then arterial branches penetrate deep into the retina (from P4 to P14), forming a capillary bed at the synaptic bases of photoreceptors in the outer retina (Connolly et al., 1988; Stone et al., 1995). Netrin-4 IR remains as a component of the vascular BM throughout retinal vasculogenesis and persists in mature retina (Fig 2C).
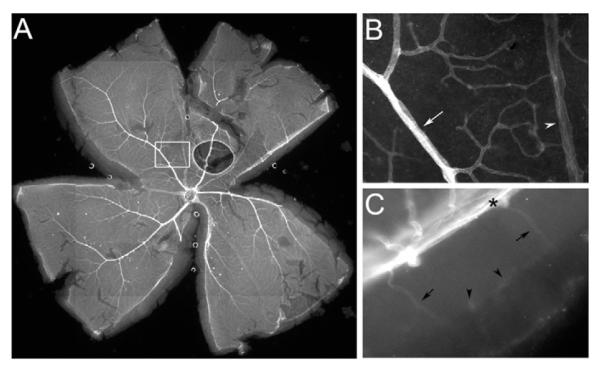
We ascertained whether netrin-4 is evenly distributed over the retinal vascular tree using adult retina whole-mounts. Netrin-4 deposition appears more intense on the arterial side of the vascular tree and extends deeply into the capillary bed, whereas netrin-4 in the veins appears considerably less intense (Fig. 3). Netrin-4 IR is deposited in a circumferential pattern on the artery (Fig. 3B, arrow) with punctate regions of increased staining in the micro-vessels particularly at branch points, whereas netrin-4 is deposited longitudinally on the vein (Fig. 3B, arrowhead). This pattern matches the smooth muscle distribution or pericyte distribution on the respective vessels.
Figure 3.
Netrin-4 IR in the retinal vasculature. (A) Netrin-4 IR is stronger in arteries than that in veins. (B) Higher magnification of the boxed area in (A), demonstrating stronger netrin-4 IR in an artery (arrow) than a vein (arrowhead). (C) From the cut edge of a retina whole-mount, netrin-4 IR can be detected at the ILM (*) and in vascular branches (arrows) and capillaries (arrowheads).
In addition to the vascular BM deposition, netrin-4 is a prominent component of the ILM. This distribution is apparent in whole-mounts (Fig. 3A) as well as radial sections (Fig. 4); moreover, netrin-4 is expressed in the epineurial sheath of the nerve (Fig. 4).
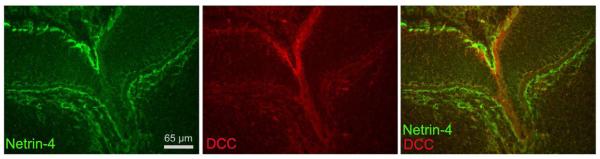
Figure 4.
Spatial relationship of netrin-4 IR and DCC IR at the E13 optic nerve head. Netrin-4 IR (left) is concentrated in the ILM and DCC IR (center) is concentrated in RGC axons. When superimposed (right), DCC-expressing RGC axons can be discerned running under the netrin-4 rich ILM at the embryonic optic nerve head.
To determine whether the netrin receptor, DCC, is expressed near netrin-4 in the retina, we labeled sections with antibodies against DCC and netrin-4; co-localization of DCC and netrin-4 signals is present only at the very margins of the optic nerve and at the junction of the ILM and nerve fiber layer of the mouse retina (Fig. 4). This arrangement is also present in rat retina (data not shown). Thus, embryonic RGC axons (expressing DCC) run along the netrin-4-rich ILM until the RGC axons turn and exit into the optic nerve. This co-localization of netrin-4 with DCC is consistent with immunoprecipitation data demonstrating that netrin-4 directly binds to DCC in retina (data not shown).
3.3 Generation and verification of Ntn4−/− mice
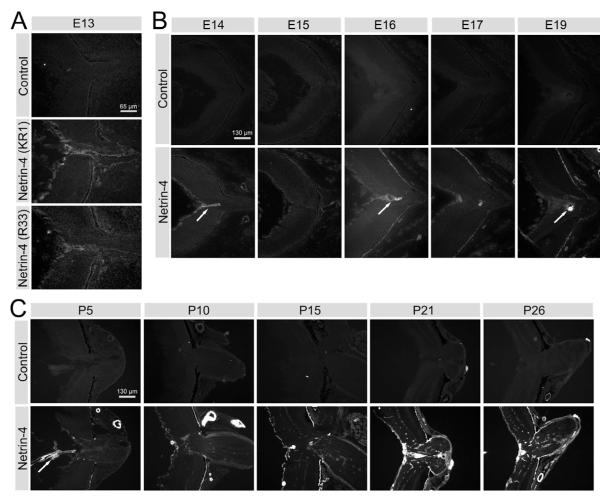
To understand the function of netrin-4 in the mouse CNS, we generated Ntn4−/− mice. We confirmed that Ntn4−/− mice did not express netrin-4 protein using immunohistological methods (Fig. S1). Although netrin-4 is widely expressed in wild-type retina, no netrin-4 IR was detectable in Ntn4−/− retina at any stage in development. Therefore we conclude that there is no netrin-4 protein in Ntn4−/− mice. These antibodies against netrin-4 have been additionally characterized elsewhere (Koch et al., 2000; Schneiders et al., 2007).
3.4 Proliferation in cornea of Ntn4−/− mice
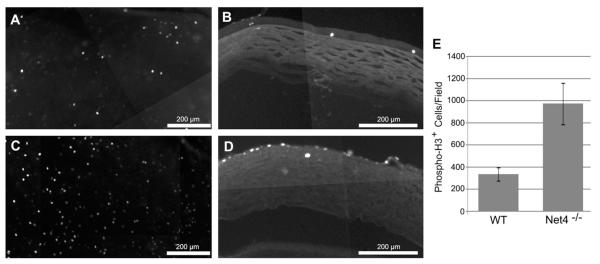
Netrin-4 is present in the cornea (Figs. 1, 2). Because netrins may play roles in cellular proliferation, we assayed whether proliferation was altered in the cornea of the netrin-4-null mouse. Cellular proliferation was assayed by Phospho-Histone H3 expression. In both whole-mount and radially sectioned cornea, low levels of proliferation are detectable (Fig, 5 A,B). In the cornea of Ntn4−/− mice, Phospho-Histone H3-expressing cells are considerably more numerous than in wild-type mice (compare Fig. 5C to A). The proliferation appears to occur in the apical levels of the epithelium (compare Fig. 5D to B). These data are quantified in Fig 5E.; the increase is statistically significant. This increase in proliferation in cornea in the absence of netrin-4 likely reflects a negative regulation of proliferation in cornea by netrin-4 during normal corneal development and maintenance. However, although there is increased proliferation in the cornea in the absence of netrin-4, there is no change in the thickness of the cornea, suggesting that an increase in cell death leads to an overall homeostasis in the thickness of the cornea.
Figure 5.
Phospho-Histone H3-expressing cells in the cornea of Ntn4+/+ and Ntn4−/− mice. (A, B) Ntn4+/+ cornea; (C, D) Ntn4−/− cornea. (A, C) cornea whole-mounts; (B, D) cross-sections of the cornea. (E) Cells expressing Phospho-Histone H3 were captured using Volocity and Phospho-Histone H3-expressing cell numbers were averaged. The number of cells expressing Phospho-Histone H3 is increased in the absence of netrin-4. Mean ± SEM for P33-34 animals (n = 4).
3.5 RGC axon fasciculation and pathfinding in Ntn4−/− mice
Because RGC axons are in proximity to the netrin-4-rich ILM while running in fascicles toward the optic nerve head, we asked whether RGC axon pathfinding was affected by netrin-4 ablation. We used three parallel sets of experiments; we first labeled all RGCs non-selectively and in subsequent experiments we selectively labeled RGCs with either a transgenic marker or DiI.
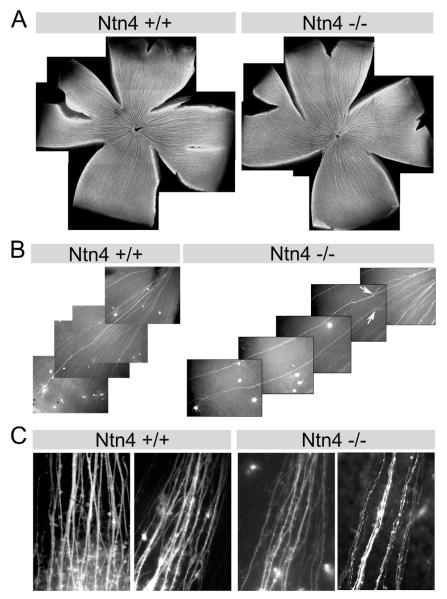
We used an anti-neurotubulin antibody (TuJ1) to label all RGC axons (Fig. 6A). In the Ntn4+/+ retina, RGC axon fascicles were formed in the periphery and ran centripetally to reach the optic nerve head following the nearest path. A similar pattern was seen in the Ntn4−/− retina; i.e., there was no gross misrouting of RGC axon fascicles in the Ntn4−/− retina. In addition, the diameter of the Ntn4−/− optic nerve is similar to that of the Ntn4+/+ optic nerve (Fig. S2).
Figure 6.
Axon pathfinding and fasciculation in the Ntn4−/− retina. (A) RGC axon fascicles, revealed with anti-neurotubulin, reach the optic nerve head. No ectopic axon fascicles were observed in the Ntn4−/− retina. (B) In retinae expressing EYFP in a subset of RGCs, some of the EYFP (+) RGCs fasciculate with each other in EYFP+/Ntn4+/+ as well as in EYFP+/Ntn4−/− retinas. However, the fascicles appear to be less stable in the EYFP+/Ntn4−/− retina, occasionally defasciculating to form ‘fork-like’ structures (Ntn4−/−, arrows). (C) DiI labeling of Ntn4+/+ and Ntn4−/− retinas. The fascicles in the Ntn4−/− retina appear narrower, and more individual axons are present.
In the wild-type retina, some RGC axons switch from one fascicle to another, a process called selective fasciculation (Raper et al., 1983). Thus, in order to look more closely at the events of fasciculation, we turned to a transgenic approach to allow us to selectively visualize individual axons. We crossed the Ntn4+/− mouse with a Thy1.1-EYFP transgenic mouse, in which ~10-30% RGCs express the EYFP transgene and, thereby, have endogenous fluorescence (Feng et al., 2000). The resultant EYFP+/Ntn4+/+ and EYFP+/Ntn4−/− mouse retinas are compared in Figure 5B. In both genotypes, some of the EYFP (+) RGC axons fasciculate with one another. In EYFP+/Ntn4+/+ retinas, the EYFP (+) fascicles are largely stable: no or only minor defasciculation was present before the axons reach the optic nerve head. However, in EYFP+/Ntn4−/− retina, EYFP (+) fascicles sometimes defasciculated to form ‘fork-like’ structures before reaching the optic nerve (Fig. 6B, arrows), suggesting the RGC axon fasciculation in the EYFP+/Ntn4−/− retina may be less stable.
We also labeled adjacent quadrants of the retina using DiI. Consistent with results using other methods (Figs. 6A,B), RGC axon targeting to the head of the optic nerve assayed with DiI was not affected by Ntn4 ablation. However, dense fascicles of axons were present in the wild-type retina, most containing multiple RGC axons; rarely did we observe a single axon in isolation (Fig. 6C); in contrast, the fascicles were smaller in the Ntn4−/− retina and appeared to be composed of fewer axons, with frequent single axons present (Fig. 6C).
3.6 Retinal structure in the Ntn4−/− mouse
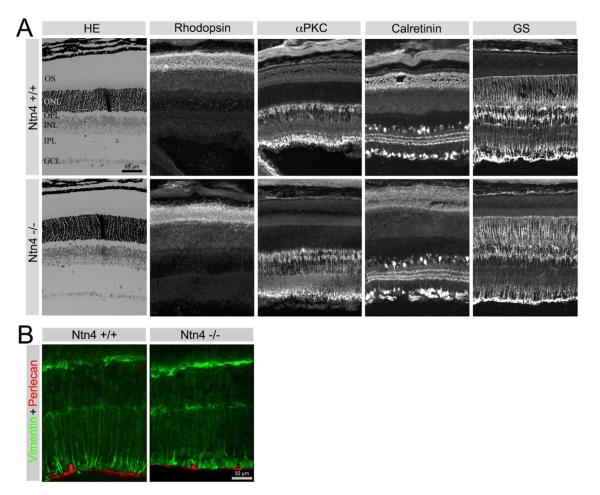
We also investigated whether retinal cell types other than RGCs were affected in the Ntn4−/− retina (Fig. 7). We observed no gross changes in retinal structure or lamination in the Ntn4−/− retina. The Ntn4−/− retina was well laminated and photoreceptors were normal in morphology and expressed and localized rhodopsin normally. Bipolar cells, visualized with antibodies to PKCα, were unchanged either in density or stratification of the axonal terminals in the deepest layers of the inner plexiform layer (IPL). Subsets of amacrine and ganglion cells were revealed with calretinin immunoreactivity. In the wild type retina, calretinin-expressing cell bodies were present in both the inner nuclear layer (INL) and the ganglion cell layer (GCL); there was a tri-laminate expression of calretinin in the IPL. Calretinin immunoreactivity was unchanged in the Ntn4−/− retina.
Figure 7.
Deletion of netrin 4 leads to no obvious change of cell organization in the retina. (A) In the Ntn4−/− retina, there are no lamination defects or ectoptic ganglion cells (HE); there are no obvious morphological changes in photoreceptors (Rhodopsin), bipolar cells (αPKC), amacrine cells (Calretinin) and Müller cells (GS). (B) The relationship between Müller cell endfeet (visualized with anti-vimentin, green) and the ILM (visualized with anti-perlecan, red) is not disturbed in the Ntn4−/− retina.
We also examined the glial cells of the retina - Müller cells and astrocytes. Müller cells are radial glial elements that span the entire thickness of the neural retina; their basal endfeet lie in close contact with the ILM, where netrin-4 is richly deposited. Müller cells were visualized by examining the presence of two different Müller cell proteins, glutamine synthetase (GS, Fig. 7A) and vimentin (Fig. 7B).
The orientation, density and organization of Müller cells were not altered in the netrin-4 null retina. The apical ends of the Müller cell (photoreceptor side) form tight junctions with each other and with photoreceptors, forming the external limiting membrane (ELM). The ELM was not disrupted in the netrin-4 null retina. The basal ends of the Müller cells sit on the perlecan-expressing ILM (Fig. 7B). In the netrin-4 null retina, perlecan expression was not affected and the Müller cell endfeet were well organized and properly oriented (Fig. 7B).
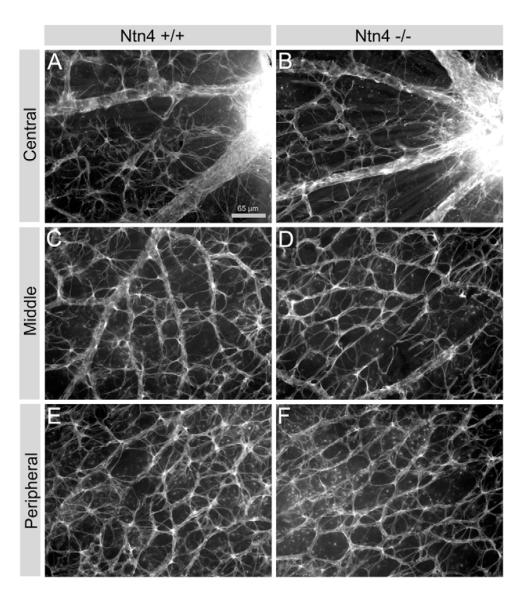
Astrocytes invade the neural retina via the optic nerve and their entry precedes and parallels vasculogenesis in the retina (Gariano, 2003). Astrocytes are the only cells that express GFAP in the normal murine retina; therefore, we used this marker for them to determine whether the number of astrocytes was altered in the netrin-4 null retina. In radial sections, GFAP-expressing cells formed a sparse layer of cells over the nerve fiber layer of the retina, and there was no difference between wild type and netrin-4 null retinae (data not shown). In retina whole-mount preparations, astrocytes invested the vascular supply of the retina and we observed no apparent difference in the number and the branching structure of astrocytes in all regions of the netrin-4 null retina (Fig. 8).
Figure 8.
Deletion of netrin 4 leads to no change in astrocyte morphology and density in the retina. Astroctyes were visualized with GFAP immunoreactivity. (A, C, and E), Ntn4+/+; (B, D, and F), Ntn4−/−. (A, B) near the optic nerve; (C, D) near the fundus; (E, F) at the peripheral retina.
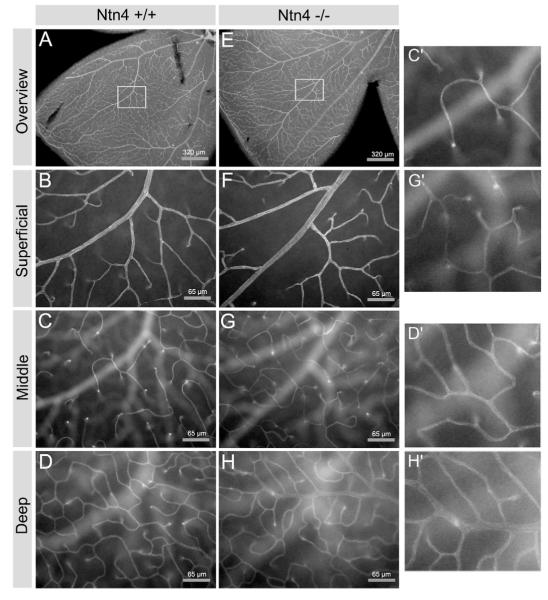
Because astrocytes guide retinal vascularization (Stone et al., 1995) and netrins including netrin-4 regulate endothelial migration and proliferation in vitro (Larrivee et al., 2007; Lejmi et al., 2008), we analyzed potential changes in retinal vascularization in the netrin-4 null retina. We labeled the vascular tree with anti-PECAM-1 (CD31), and examined detailed branching structure of microvessels at the superficial (vitreal) surface, in the middle of the IPL, and deep in the retina at the level of the OPL in the adult retina (approximately 1 month old) (Fig. 9). While branching patterns appear largely normal at the surface of the retina, there appears to be some increased branching, particularly in the middle levels of the microvessels (compare Fig. 9C, G) and somewhat less in the deep levels of the microvessels (compare Fig. 9D, H). A more extensive examination of the potentially increased branching awaits further experiments.
Figure 9.
Comparison of vascular branching in the Ntn4+/+ and Ntn4−/− retina. Retinal vasculature was visualized using an antibody to an endothelial marker, PECAM-1. (A, B, C, and D), Ntn4+/+; (E, F, G, and H), Ntn4−/−. (A, E) low power view of a retina quadrant; (B, F) higher magnification of the boxed area from the fundus with the focus at the vitreal surface; (C, G) the same area and magnification as (A, E), with the focus at INL-IPL border; (D, H) the same area and magnification as (A, E), with the focus at the OPL. In the absence of netrin-4, there is some increased branching in the middle and deep levels of the microvessels. Portions of Figs. 9 C, D, G and H are shown at higher magnification in C’, D’, G’ and H’.
4. Discussion
4.1 Conclusions and Summary of Principle Findings
We, and others, have suggested roles for netrin-4 by localization in vivo (Liu et al., 2004), by analyzing effects of netrin-4 on cells in vitro (Lejmi et al., 2008; Park et al., 2004), and by exogenous netrin-4 in vivo (Larrieu-Lahargue et al., 2010). However, this study is the first direct demonstration of a role for netrin-4 in vivo. Although data reported by others have suggested roles for netrin-4 by localization and exogenous application of netrin-4, our results are the first to demonstrate a role for netrin-4 in cellular proliferation and morphogenesis in vivo. Additional data on the role for netrin-4 in regulation of proliferation in the cerebellum are being prepared for publication (Li and Brunken, unpublished data).
Netrin-4 is expressed in all BMs of ocular tissue. This is particularly clear in early development, where netrin-4 is present as a continuous band around the eyecup and surrounding the lens placode. As development proceeds, netrin-4 remains as a prominent component of the BM underlying both ectoderm-derived neural tissues (the iris epithelium, the ciliary epithelium and the retinal pigmented epithelium; i.e., Bruch’s membrane) and the neural retina (i.e., the ILM). Netrin-4 is also expressed in the vascular BM where it appears to be tightly associated with smooth muscle or pericytes.
4.2 Netrin-4 Is Not Required for BM Integrity
Structural integrity of the ILM is important for retinal histogenesis (Halfter et al., 2001). Components of the ILM include laminins, nidogens, agrin and collagens (Halfter et al., 2000). With this report, we add netrin-4 to these components.
The ILM may be a stable attachment site for both ganglion cells and Müller cell endfeet (Halfter et al., 2001). Simultaneous deletion of the laminin β2 and γ3 genes disrupts Müller cell adhesion to the ILM and causes ganglion cell ectopia and other more profound defects in the retina (Pinzón-Duarte et al., 2010). Other laminin mutations or deletions also produce profound disruptions in the organization of the BM, similar to those produced by collagen IV mutations (Poschl et al., 2004). However, deletion of netrin-4 in the Ntn4−/− mouse did not disrupt the BMs of the retina, cornea or lens, suggesting that netrin-4 is not a structural element of ocular BMs, but netrin-4 might function in other ways, regulating proliferation or migration.
4.3 Netrin-4 and retinal vasculogenesis
Netrin-4 is richly deposited in vascular BMs. Vascular endothelial cells are likely the cell source of netrin-4, as vascular endothelial cells have been reported as a source of netrin-4 (by in situ hybridization, Koch et al., 2000; and microarray, Ho et al., 2003). However, the spatial distribution of netrin-4 protein is similar to the distribution of smooth muscle cells in arteries and veins, and pericytes in microvessels. Netrin-4 has been reported to be anti-adhesive to endothelial cells (Wilson et al., 2006). Given the uneven distribution of netrin-4 in vascular BMs, netrin-4 may contribute to the arterial-veinous axis; however, we detected no disruption in this axis in the null mouse.
Two other observations concerning netrin-4 and retinal angiogenesis are worthy of further comment. First, netrin-4 expression is temporally linked to the maturation of the vascular system at the vitreal border. Angiogenesis is directed by astrocyte migration over the retinal surface. Netrin-4 is expressed over the ILM, and therefore is placed to contribute to astrocyte guidance. However, astrocyte numbers and distribution are not altered in the Ntn4−/− mouse, suggesting that netrin-4 is not involved in astrocyte migration or proliferation, and any modulation of vascular development by netrin-4 is not via a direct action on astrocyte migration or proliferation. In addition, superficial angiogenesis proceeds normally in netrin-4 null mice, suggesting that endothelial-astrocyte interactions are not altered in the absence of netrin-4.
Second, the branching of deeper retinal microvessels may be increased in the absence of netrin-4, suggesting that the patterning of superficial and deeper capillary plexus may be genetically and mechanistically separable. The sprouting and plexus formation of capillary beds in the inner and outer retina are driven by Müller cell interactions, certainly by growth factors and, possibly, by cell-cell interactions. Our in vivo data demonstrate that both middle and deep plexuses may be altered by the Ntn4 deletion, though subtly. Because netrin-4 inhibits endothelial cell proliferation in vitro (Lejmi et al., 2008), the possible increase in branching in the Ntn4−/− mouse may be due to a direct effect of the loss of netrin-4.
Mutations of several ECM genes, including collagens XV and XVIII (from which endostatin is derived), produce persistent hyaloid vessels (Hurskainen et al., 2005). We did not observe persistent hyaloid vessels in netrin-4 null mice. Local production of netrin-4 has been reported to promote proangiogenic remodeling (Wilson et al., 2006). In this regard, it will be of interest to determine whether remodeling events in retinal vasculature, such as hypoxia-driven remodeling, are altered in the Ntn4 null mouse. In addition, neovascularization at the vitreal border leads to the blinding disorder, proliferative vitreoretinopathy (PVR), many ECM molecules are up-regulated in PVR (Hiscott, et al., 1999; Hiscott et al., 2002), and matrix metalloproteinases are implicated in complications of diabetic retinopathy (Yang et al., 2007). In this regard, the netrin-4 null mouse will be an important tool for studying the role of netrins in neovascularization.
4.4 Netrin-4 in the Cornea and Epithelial Proliferation
One striking spatial and temporal pattern of netrin-4 expression is its corneal expression. Netrin-4 is expressed first in the corneal epithelial BM (Bowman’s) and later in the stroma and endothelial basement membrane (Descemet’s membrane). There is an abrupt transition from high level of expression and deposition in these regions to completely negative expression in the adjacent sclera, with the limbus acting as the boundary. The cornea is avascular, transparent and richly innervated. However, we discerned no primary defect in corneal organization in the netrin-4 null mice, suggesting that netrin-4 is not required for epithelial differentiation or guidance of the neural crest cells into the corneal endothelium or stroma. However, the finding that corneal epithelium proliferation was considerably increased in these mice suggests that netrin-4 may be involved in the regulation of epithelial turnover and renewal. Thus, it will be of interest to investigate whether corneal wound healing is altered in netrin-4-null animals.
4.5 Netrin-4 and RGC axon guidance and fasciculation
In the developing mouse retina, RGCs differentiate between E11 and P3 (Young, 1985). RGC axons follow complex paths to several targets in the brain, each of which serves a distinct visual function including perception and reflex coordination. Along the path, RGC axons traverse many extracellular cues and guidance molecules (Stuermer and Bastmeyer et al., 2000). The first, and perhaps most critical, guidance event occurs within the eye, where axons are directed down the optic fissure into the developing optic nerve. Within the eye, RGC axons emerge from the cell soma, then grow toward the vitreal border and the ILM, where they make a sharp perpendicular turn to run parallel to the retinal surface in a narrow zone beneath the ILM and Müller cell endfeet. They are guided along the retinal surface towards the optic fissure and the later optic nerve head, where they turn again to enter the optic nerve.
Many molecular components have been suggested to be involved in RGC axon guidance to the optic nerve head, such as chondroitin sulfate (CS) (Brittis et al., 1992), slit 1 (Jin et al., 2003), Eph/Ephrins (Birgbauer et al., 2000), and netrin-1/DCC (Deiner et al., 1997). In the Ntn4−/− mouse retina, we detected no ectopic axons, suggesting either that netrin-4 is not a critical molecule for RGC axon guidance or there is redundancy in the netrin family such that another netrin, such as netrin-1, might have substituted for netrin-4. The critical test for redundancy would be to substitute Ntn4 in the Ntn1 locus and the reverse thereby dissecting the individual functions of these family members.
Supplementary Material
Highlights.
Netrin-4 is expressed in corneal stroma and basement membranes
Netrin-4 knockout has increased corneal epithelial proliferation
Netrin-4 is expressed in retinal vascular basement membrane
Netrin-4 knockout has increased deep vascular branching
Netrin-4 knockout retina is normal
Acknowledgements
Portions of this study were completed as part of the doctoral dissertation of Y.N. Li. The authors thank Dale D Hunter, PhD, JD for his careful and insightful editing of the manuscript. Supported by Grants: NS 39502 and EY 12767 (WJB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barallobre MJ, Pascual M, Del Rio JA, Soriano E. The netrin family of guidance factors: emphasis on netrin-1 signaling. Brain Res. Rev. 2005;49:22–47. doi: 10.1016/j.brainresrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Birgbauer E, Cowan CA, Sretavan DW, Henkemeyer M. Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development. 2000;127:1231–1241. doi: 10.1242/dev.127.6.1231. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Canning DR, Silver J. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science. 1992;255:733–736. doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- Cammarata PR, Spiro RG. Identification of noncollagenous components of calf lens capsule: evaluation of their adhesion-promoting activity. J. Cell Physiol. 1985;125:393–402. doi: 10.1002/jcp.1041250306. [DOI] [PubMed] [Google Scholar]
- Cellerino A, Pinzon-Duarte G, Carroll P, Kohler K. Brain-derived neurotrophic factor modulates the development of the dopaminergic network in the rodent retina. J. Neurosci. 1998;18:3351–3362. doi: 10.1523/JNEUROSCI.18-09-03351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SE, Hores TA, Smith LE, D’Amore PA. Characterization of vascular development in the mouse retina. Microvasc. Res. 1988;36:275–290. doi: 10.1016/0026-2862(88)90028-3. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre JR, Hopker VH, Ming GL, Poo MM, Tessier-Lavigne M, Hemmati-Brivanlou A, Holt CE. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19:1211–1224. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Drager UC. Birth dates of retinal ganglion cells giving rise to the crossed and uncrossed optic projections in the mouse. Proc. Roy. Soc. Lond. B Biol. Sci. 1985;224:57–77. doi: 10.1098/rspb.1985.0021. [DOI] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Gariano RF. Cellular mechanisms in retinal vascular development. Prog. Ret. Eye. Res. 2003;22:295–306. doi: 10.1016/s1350-9462(02)00062-9. [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Balasubramani M, Bier ME. Temporary disruption of the retinal basal lamina and its effect on retinal histogenesis. Dev. Biol. 2001;238:79–96. doi: 10.1006/dbio.2001.0396. [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Schurer B, Cole GJ. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J. Biol. Chem. 1998;273:25404–25412. doi: 10.1074/jbc.273.39.25404. [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Schurer B, Osanger A, Schneider W, Ruegg M, Cole GJ. Composition, synthesis, and assembly of the embryonic chick retinal basal lamina. Dev. Biol. 2000;220:111–128. doi: 10.1006/dbio.2000.9649. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1988. [Google Scholar]
- Hiscott P, Sheridan C, Magee RM, Grierson I. Matrix and the retinal pigment epithelium in proliferative retinal disease. Prog. Ret. Eye Res. 1999;18:167–190. doi: 10.1016/s1350-9462(98)00024-x. [DOI] [PubMed] [Google Scholar]
- Hiscott P, Hagan S, Heathcote L, Sheridan CM, Groenewald CP, Grierson I, Wong D, Paraoan L. Pathobiology of epiretinal and subretinal membranes: possible roles for the matricellular proteins thrombospondin 1 and osteonectin (SPARC) Eye. 2002;16:393–403. doi: 10.1038/sj.eye.6700196. [DOI] [PubMed] [Google Scholar]
- Ho M, Yang E, Matcuk G, Deng D, Sampas N, Tsalenko A, Tabibiazar R, Zhang Y, Chen M, Talbi S, Ho YD, Wang J, Tsao PS, Ben-Dor A, Yakhini Z, Bruhn L, Quertermous T. Identification of endothelial cell genes by combined database mining and microarray analysis. Physiol. Genomics. 2003;13:249–262. doi: 10.1152/physiolgenomics.00186.2002. [DOI] [PubMed] [Google Scholar]
- Hopker VH, Shewan D, Tessier-Lavigne M, Poo MM, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Shah V, Merlie JP, Sanes JR. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989;338:229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Hurskainen M, Eklund L, Hagg PO, Fruttiger M, Sormunen R, Ilves M, Pihlajaniemi T. Abnormal maturation of the retinal vasculature in type XVIII collagen/endostatin deficient mice and changes in retinal glial cells due to lack of collagen types XV and XVIII. FASEB J. 2005;19:1564–1566. doi: 10.1096/fj.04-3101fje. [DOI] [PubMed] [Google Scholar]
- Jin Z, Zhang J, Klar A, Chedotal A, Rao Y, Cepko CL, Bao ZZ. Irx4-mediated regulation of Slit1 expression contributes to the definition of early axonal paths inside the retina. Development. 2003;130:1037–1048. doi: 10.1242/dev.00326. [DOI] [PubMed] [Google Scholar]
- Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones C, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, Sundarraj N, Timpl R, Virtanen I, Ljubimov AV. Compositional differences between infant and adult human corneal basement membranes. Invest. Ophthalmol. Vis. Sci. 2007;48:4989–99. doi: 10.1167/iovs.07-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kelley PB, Sado Y, Duncan MK. Collagen IV in the developing lens capsule. Matrix Biol. 2002;21:415–423. doi: 10.1016/s0945-053x(02)00014-8. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Koch M, Murrell JR, Hunter DD, Olson PF, Jin W, Keene DR, Brunken WJ, Burgeson RE. A novel member of the netrin family, beta-netrin, shares homology with the beta chain of laminin: identification, expression, and functional characterization. J. Cell Biol. 2000;151:221–234. doi: 10.1083/jcb.151.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu-Lahargue F, Welm AL, Thomas KR, Li DY. Netrin-4 induces lymphangiogenesis in vivo. Blood. 2010;115:5418–5426. doi: 10.1182/blood-2009-11-252338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrivee B, Freitas C, Trombe M, Lv X, Delafarge B, Yuan L, Bouvree K, Breant C, Del Toro R, Brechot N, Germain S, Bono F, Dol F, Claes F, Fischer C, Autiero M, Thomas JL, Carmeliet P, Tessier-Lavigne M, Eichmann A. Activation of the UNC5B receptor by netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21:2433–2447. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejmi E, Leconte L, Pedron-Mazoyer S, Ropert S, Raoul W, Lavalette S, Bouras I, Feron JG, Maitre-Boube M, Assayag F, Feumi C, Alemany M, Jie TX, Merkulova T, Poupon MF, Ruchoux MM, Tobelem G, Sennlaub F, Plouet J. Netrin-4 inhibits angiogenesis via binding to neogenin and recruitment of Unc5B. Proc. Nat’l. Acad. Sci. U S A. 2008;105:12491–12496. doi: 10.1073/pnas.0804008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Liu Y, Stein E, Oliver T, Li Y, Brunken WJ, Koch M, Tessier-Lavigne M, Hogan BL. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr. Biol. 2004;14:897–905. doi: 10.1016/j.cub.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey FJ, Hunt SP. Netrin and netrin receptor expression in the embryonic mammalian nervous system suggests roles in retinal, striatal, nigral, and cerebellar development. Mol. Cell. Neurosci. 1997;8:417–429. doi: 10.1006/mcne.1997.0598. [DOI] [PubMed] [Google Scholar]
- Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- Mann F, Harris WA, Holt CE. New views on retinal axon development: a navigation guide. Int’l. J. Dev. Biol. 2004;48:957–964. doi: 10.1387/ijdb.041899fm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan PS, Spiro RG. Macromolecular organization of basement membranes. Characterization and comparison of glomerular basement membrane and lens capsule components by immunochemical and lectin affinity procedures. J. Biol. Chem. 1986;261:4328–4336. [PubMed] [Google Scholar]
- Nakashiba T, Ikeda T, Nishimura S, Tashiro K, Honjo T, Culotti JG, Itohara S. Netrin-G1: a novel glycosyl phosphatidylinositol-linked mammalian netrin that is functionally divergent from classical netrins. J. Neurosci. 2000;20:6540–6550. doi: 10.1523/JNEUROSCI.20-17-06540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Nishimura S, Ikeda T, Itohara S. Complementary expression and neurite outgrowth activity of netrin-G subfamily members. Mech. Dev. 2002;111:47–60. doi: 10.1016/s0925-4773(01)00600-1. [DOI] [PubMed] [Google Scholar]
- Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, Kuo CJ, Li DY. The axonal attractant netrin-1 is an angiogenic factor. Proc. Nat’l. Acad. Sci. U S A. 2004;101:16210–16215. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmigiani C, McAvoy J. Localisation of laminin and fibronectin during rat lens morphogenesis. Differentiation. 1984;28:53–61. doi: 10.1111/j.1432-0436.1984.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Pinzón-Duarte G, Kohler K, Arango-Gonzalez B, Guenther E. Cell differentiation, synaptogenesis, and influence of the retinal pigment epithelium in a rat neonatal organotypic retina culture. Vision Res. 2000;40:3455–3465. doi: 10.1016/s0042-6989(00)00185-1. [DOI] [PubMed] [Google Scholar]
- Pinzón-Duarte G, Daly G, Li YN, Koch M, Brunken WJ. Defective formation of the inner limiting membrane in laminin beta2- and gamma3-null mice produces retinal dysplasia. Invest. Ophthalmol. Vis. Sci. 2010;51:1773–1782. doi: 10.1167/iovs.09-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- Raper JA, Bastiani M, Goodman CS. Pathfinding by neuronal growth cones in grasshopper embryos. II. Selective fasciculation onto specific axonal pathways. J. Neurosci. 1983;3:31–41. doi: 10.1523/JNEUROSCI.03-01-00031.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmut O. The organization of tissues of the eye by different collagen types. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1978;207:189–199. doi: 10.1007/BF00411053. [DOI] [PubMed] [Google Scholar]
- Schneiders FI, Maertens B, Bose K, Li Y, Brunken WJ, Paulsson M, Smyth N, Koch M. Binding of netrin-4 to laminin short arms regulates basement membrane assembly. J. Biol. Chem. 2007;282:23750–23758. doi: 10.1074/jbc.M703137200. [DOI] [PubMed] [Google Scholar]
- Seland JH. The lens capsule and zonulae. Acta Ophthalmol. Suppl. 1992;205:7–12. doi: 10.1111/j.1755-3768.1992.tb02175.x. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev. Cell. 2003;4:371–382. doi: 10.1016/s1534-5807(03)00054-6. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuermer CA, Bastmeyer M. The retinal axon’s pathfinding to the optic disk. Prog. Neurobiol. 2000;62:197–214. doi: 10.1016/s0301-0082(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Raay TJ, Fosket,t SM, Connor,s TD, Klinger KW, Landes GM, Burn TC. The NTN2L gene encoding a novel human netrin maps to the autosomal dominant polycystic kidney disease region on chromosome 16p13.3. Genomics. 1997;41:279–282. doi: 10.1006/geno.1997.4659. [DOI] [PubMed] [Google Scholar]
- Wang H, Copeland NG, Gilbert DJ, Jenkins NA, Tessier-Lavigne M. Netrin-3, a mouse homolog of human NTN2L, is highly expressed in sensory ganglia and shows differential binding to netrin receptors. J. Neurosci. 1999;19:4938–4947. doi: 10.1523/JNEUROSCI.19-12-04938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GA, Thorne T, Silver M, Thomas KR, Chien CB, Losordo DW, Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Liu H, Williams I, Chaqour B. Matrix metalloproteinase-2 expression and apoptogenic activity in retinal pericytes. Ann. N.Y. Acad. Sci. 2007;1103:_196–201. doi: 10.1196/annals.1394.000. [DOI] [PubMed] [Google Scholar]
- Yebra M, Montgomery AM, Diaferia GR, Kaido T, Silletti S, Perez B, Just ML, Hildbrand S, Hurford R, Florkiewicz E, Tessier-Lavigne M, Cirulli V. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev. Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- Yin Y, Sanes JR, Miner JH. Identification and expression of mouse netrin-4. Mech. Dev. 2000;96:115–119. doi: 10.1016/s0925-4773(00)00369-5. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat. Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.