Abstract
Background
The mechanisms by which behavioral therapies for substance use disorders (SUDs) exert their effects and the components of treatment that contribute most to substance use outcome remain unclear. Disruptions to aspects of impulse control and attention have been hypothesized to contribute to the development and maintenance of addiction; moreover, alterations in these processes may underlie responses to treatment.
Methods
Individuals participating in a randomized clinical trial evaluating computer-assisted cognitive behavioral therapy (CBT) for substance abuse participated in fMRI Stroop before and after treatment. A non-substance-using comparison group performed the same task under test-retest conditions.
Results
The patient group demonstrated decreased Stroop-related BOLD signal in regions including the anterior cingulate, inferior frontal gyrus and midbrain at post-treatment relative to pre-treatment, and displayed a greater decrease in the subthalamic nucleus and surrounding regions compared to healthy controls following test-retest.
Conclusions
Behavioral therapies may be associated with reduction in substance use and effects on neural systems involved in cognitive control, impulsivity, motivation and attention.
Keywords: fMRI, substance use disorders, addiction, impulsivity, cognitive control, cognitive behavioral therapy
1. Introduction
While substance use may initially be primarily motivated by the acute reinforcing experience of the substance, progression to addiction is characterized by diminished behavioral control and habitual use despite negative consequences (APA, 2000). Although multiple behavioral therapies have demonstrated efficacy in treating substance use disorders (SUDs: Dutra et al., 2008), the factors that determine whether individuals will achieve abstinence and avoid relapse and the mechanisms underlying effective behavioral treatments for SUDs remain incompletely understood. The mechanism of therapeutic efficacy is likely to depend upon the behavioral therapy employed. For instance, cognitive behavioral therapy (CBT) is based on the premise that learning processes contribute to addiction (Carroll, 1998). CBT encourages patients to recognize and avoid situations where they may be likely to use substances and to use coping strategies to resist drug use and temporize their behavior (Carroll, 1998; Kiluk et al., 2010).
Therapeutic efficacy across forms of treatment may relate to action on one or more mechanisms proposed to contribute to addiction (e.g., attenuated drug-induced craving, improved impulse control). Several theories emphasize potential roles for attentional biases to drug-related stimuli coupled with poor impulse control in the development and maintenance of addiction (e.g., Everitt and Robbins, 2005; Jentsch and Taylor, 1999; Robinson and Berridge, 1993, 2008; Volkow et al., 2002)). Acute administration of drugs of abuse, including cocaine, alcohol and marijuana, induces phasic dopamine release in the nucleus accumbens (NAc; Hungund et al., 2003; Tanda et al., 1997) which contributes to drugs’ reinforcing properties, including the subjective ‘high’ (Di Chiara and Imperato, 1988; Taylor and Robbins, 1984). Endogenous phasic dopamine release in the NAc is also critically involved in associative learning processes and imbuing stimuli with motivational salience (Everitt and Robbins, 2005; Robinson and Berridge, 2008). Repeated drug administration may potentiate associative learning of drug-relevant stimuli resulting in a stronger ‘impulse’ to take the drug (Jentsch and Taylor, 1999) or enhance incentive motivation of drug-related stimuli (i.e., drug “wanting”) through long-term changes in cell functioning (‘incentive sensitization’) within circuits responsible for regulating incentive salience (Robinson and Berridge, 2008). Several mechanisms could result in an attentional bias towards the drug and drug-related stimuli at the expense of other reward cues (Torregrossa et al., 2011), possibly contributing to clinically observed neglect of other ‘natural’ rewards in favor of drug-seeking, and vulnerability to relapse in the presence of drug-associated cues.
Converging lines of evidence support a role for impulsivity in addiction; consistently, impulsivity has been proposed as a potential target for treatments of addiction (Moeller et al., 2001). Poor response inhibition has been proposed to contribute to the diminished control over drug-taking characteristic of addiction (Bechara, 2005; Jentsch and Taylor, 1999; Volkow et al., 2004). Drug-dependent individuals perform ‘impulsively’ on laboratory tasks (Garavan et al., 2008) and display functional and structural abnormalities in brain regions essential to response inhibition (e.g., inferior frontal gyrus (IFG); Aron and Poldrack, 2006) and cognitive control (e.g., anterior cingulate cortex (ACC); dorsolateral prefrontal cortex (dlPFC); Bechara, 2005; Bolla et al., 2004; Carter and van Veen, 2007; Liu et al., 1998; Stapleton et al., 1995; Volkow et al., 2004). Higher impulsivity and riskier decision-making on laboratory tasks prior to treatment have been associated with greater severity of substance dependence and poorer treatment outcome (Carroll et al., 2011; Krishnan-Sarin et al., 2007; Moeller et al., 2001). In rodent models of addiction, impulsivity is associated with vulnerability to transition from controlled drug self-administration to the compulsive drug-taking characteristic of addiction (Belin et al., 2008) and with higher frequency of relapse (Economidou et al., 2009).
The Stroop color-word interference task, a well-validated measure of cognitive control (MacLeod, 1991), may be particularly relevant in the study of addiction as it incorporates response inhibition and selective attention processes (Carpenter et al., 2006). In cocaine-dependent individuals, Stroop task performance is sensitive to cognitive impairments during abstinence, improves with acute cocaine administration and relates to substance abuse treatment outcome (Bolla et al., 2000; Brewer et al., 2008; Carpenter et al., 2006; Streeter et al., 2008). For instance, in cocaine-dependent individuals prior to treatment, Stroop-related brain activity in regions implicated in response inhibition (right inferior frontal gyrus (rIFG); Aron and Poldrack, 2006), risky decision-making (ventromedial prefrontal cortex (vmPFC); Clark et al., 2004) and associative learning (striatum; McClure et al., 2003) has been associated with cocaine-use outcomes after behavioral treatments (Brewer et al., 2008).
To build upon these findings and investigate behavioral therapies’ potential influences on cognitive functioning, the current study assessed how Stroop-related regional brain activity changes following 8-weeks of behavioral treatment. Several previous studies have combined behavioral therapies with neuroimaging to investigate other psychiatric disorders (e.g., Frewen et al., 2008; Siegle et al., 2006) or to identify pre-treatment fMRI measures associated with SUD treatment outcome (Brewer et al., 2008), and studies have investigated changes following group counselling for tobacco dependence in terms of regional cerebral blood flow (Costello et al., 2009) and smoking-induced changes in intrasynaptic dopamine concentration (Brody et al., 2010). However, to our knowledge this is the first assessment of how functional brain activity changes before and after behavioral therapies in individuals with other SUDs.
First, we hypothesized that following a course of behavioral therapy for SUDs, patients would show improved functional efficiency in regions implicated in cognitive control (dlPFC, ACC), response inhibition (rIFG) and reward-related-learning (midbrain, striatum). Second, we hypothesized that these changes would differ from those in healthy control (HC) subjects’ test/retest performance.
2. Methods
2.1. Participants
2.1.1. Patients from a Randomized Clinical Trial (RCT)
Treatment-seeking, substance-dependent individuals involved in an RCT of computer-assisted CBT were invited to participate in this study prior to treatment (Carroll et al., 2008). In the RCT (see full description (Carroll et al., 2008)), participants were randomly assigned to eight weeks of treatment as usual (TAU) in a community-based outpatient drug treatment program consisting of weekly individual plus group sessions (SUDTAU) or TAU supplemented by twice-weekly access to a multimedia computer-assisted version of CBT (SUDCBT).
Participants included in the RCT were English-speaking adults who met current DSM-IV criteria for substance dependence as determined by the Structured Clinical Interview for DSM-IV (SCID; First et al., 1996), had used the substance in the previous 28 days, could commit to completing 8 weeks of treatment, and did not have an untreated psychotic disorder that precluded outpatient treatment. RCT participants were not invited to participate in the fMRI study component if pregnant, breastfeeding, left-handed or colorblind. Of 73 patients initiating the RCT, 12 were eligible for, opted to participate in, and completed the pre- and post-treatment fMRI protocol components (6 SUDCBT, 6 SUDTAU). Given the limited sample size, results should be viewed as preliminary.
2.1.2. Healthy Control Subjects
Twelve healthy control (HC) subjects were recruited from the local community using advertisements (e.g., newspaper, internet) and were 18–50 years old, right-handed and excluded for current medical illness (e.g., diabetes, medicated high blood pressure), prescription medications (except birth control pills), color-blindness or pregnancy. HCs had no current or history of Axis I psychiatric disorder, illicit drug use, alcohol abuse or dependence based on the Structured Clinical Interview for DSM-IV Axis I Disorders Non-patient Edition (First et al., 2007).
All participants provided written informed consent as approved by the Yale University School of Medicine Human Investigations Committee.
2.2. Study Protocol
All participants underwent two fMRI sessions, spaced at least two months apart. Patients completed fMRI sessions prior to starting and following completion of treatment. HCs performed test/retest scans without treatment. Shipley Institute of Living Scale (SILS) measured estimated IQ (Zachary, 1991). During treatment, patients were asked to submit urine samples for toxicology screens twice weekly.
2.2.1. fMRI Task
Participants performed six runs of the event-related fMRI Stroop color-word interference task (Brewer et al., 2008) and were asked to silently name the ink color of congruent or incongruent color-word pairs (e.g., ‘red’ written in red (congruent) or blue (incongruent) ink). Runs consisted of 105 stimuli, presented for 1300 milliseconds, with an inter-trial interval of 350 milliseconds, including seven incongruent events which were presented pseudo-randomly every 13 to 16 congruent stimuli.
2.2.2. Behavioral Stroop Measures
Stroop performance was measured out-of-scanner with five runs completed directly following scanning (Brewer et al., 2008). Verbal responses made into a microphone recorded reaction times to trials. Incongruent trial errors were manually recorded by research staff. Two practice runs prior to scanning familiarized participants with the task.
2.2.3. Image Acquisition
Images were obtained with a Siemens Trio 3T magnetic resonance imaging (MRI) system (Siemens AG, Erlangen, Germany). Localizer images were acquired for prescribing the functional image volumes, aligning the eighth slice parallel to the plane transecting the anterior and posterior commissure. Functional images were collected using an echo-planar image gradient-echo pulse sequence (repetition time/echo time [TR/TE] 1500/27 millisecond, flip angle 60°, field of view [FOV] 22cm × 22 cm, 64 × 64 matrix, 3.4 mm × 3.4 mm in-plane resolution, 5 mm effective slice thickness, 25 slices). Stimulus runs consisted of 124 volumes, including an initial 9-second rest period (to achieve signal stability) that was removed from analyses.
2.3. Data Analyses
2.3.1. Stroop Behavioral Data
Stroop reaction time data were analyzed using repeated-measures ANOVA including session (pre- and post-treatment) and trial-type (congruent, incongruent) as within-subject factors and group (SUD, HC) as a between-subject factor. Errors during incongruent trials were square-root transformed to meet parametric assumptions then included in a repeated-measure ANOVA with session as a within-subjects factor and group as a between-subject factor. Additional ANOVAs were conducted within group or trial-type when necessary to clarify significant main effects or interactions from the full ANOVA.
2.3.2. fMRI Analyses
Functional images were analyzed using SPM2 (Wellcome Functional Imaging Laboratory, London, United Kingdom). Each run, separately realigned using INRIAlign (Freire et al., 2002), was examined for head motion in excess of one acquisition voxel. Approximately 30 images were removed from the beginning and end of three SUD subjects’ fMRI acquisitions accounting for 0.5% of the images acquired for the study, or 4% of affected subjects’ total images. A mean functional image volume was constructed from realigned image volumes for each session and used for normalization to Montreal Neurological Institute (MNI) standardized space. Normalization parameters for each participant were applied to corresponding functional image volumes using an automated spatial transformation resulting in an isometric voxel size of 4 × 4 × 4 mm3. Normalized images were smoothed with a 9-mm full-width-at-half-maximum Gaussian filter.
Data were analyzed using the general linear model approach. Analysis was performed by modeling the times of congruent and incongruent stimulus presentation separately in an event-related design using the hemodynamic response function with time derivative provided by SPM2. A high-pass filter (cutoff period=128 sec) removed low-frequency signals. Resulting images representing the estimated hemodynamic response amplitude (positive and negative) for each condition were re-estimated with a latency-variation amplitude-correction method (Calhoun et al., 2004). Latency-corrected contrast images were used in random-effects and correlational group analyses.
At the single-subject level, the ‘Stroop-effect’ (incongruent vs. congruent trials) was modeled for each visit (pre-treatment, post-treatment) and for change across sessions (post-treatment vs. pre-treatment).
Changes in Stroop-related activation were assessed with paired t-tests separately within groups (i.e., SUDPost-Treatment vs. SUDPre-Treatment, HCSession2 vs. HCSession1; see Table 5.I). The primary planned analysis assessed between-group differences in change in Stroop-related activation across sessions using two sample t-tests (SUD (Post-Treatment vs. Pre-Treatment) vs. HC (Session2 vs. Session1); see Table 5.II). Inclusion of the HC group accounts for effects of repeated testing, so significant group differences across time may be attributable to factors specific to the patient group, like treatment. Within-group analyses applied a conjoint voxel-level threshold of p<0.005 with a cluster-level threshold of pcorrected<0.05. A mask generated from pre-treatment and post-treatment p<0.05 F-tests of Stroop-effect within the SUD group was applied to between-group analyses. Between-group analyses applied a conjoint voxel-level threshold of p<0.005 and cluster extent of k=19. AlphaSim was employed to estimate effective family-wise-error (FWE) thresholds reported for all conjoint voxel-level and cluster extent thresholds ((Ward, 2000); www.neuroelf.net). The effective FWE for the within-group analyses was pFWE<0.001 at the whole-brain level and effective FWE for between-group analyses was pFWE<0.05 for the masked area.1
Table 5. Change in fMRI Stroop Effect.
- Within group changes in the ‘Stroop effect’ (incongruent vs. congruent trials) across sessions were assessed with paired t-tests and a threshold of voxel-level p<0.005 with conjoint cluster-level pcorrected<0.05.
- Group differences in change scores were assessed with a two sample t-test in SPM2 comparing change in BOLD signal activity following treatment (or re-test) masked for regions engaged by the SUD group at pre or post-treatment to a threshold of voxel-level p<0.005 and conjoint cluster extent of k=19.
| Cluster Description | k | T | Peak coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| I. Within-Group Change in ‘Stroop Effect’ Related Regional BOLD Signal Following Treatment | |||||
| i. SUD Post-treatment < SUD Pre-treatment | |||||
| R/L midbrain/ lentiform nucleus/ thalamus/ cerebellum/ brainstem/ subthalamic nucleus | 176 | 8.35 | 8 | −12 | −4 |
| L middle and superior temporal gyrus/ BA 37 | 65 | 6.30 | −52 | −36 | −8 |
| R inferior frontal gyrus/ caudate/ middle temporal gyrus/ BA 10, 13, 46, 47 | 206 | 6.02 | 48 | 36 | 8 |
| R cuneus/ BA 18, 30, 31 | 70 | 5.96 | 20 | −80 | 16 |
| R anterior cingulate gyrus/ dorsolateral PFC/ BA 9, 32 | 54 | 5.87 | 8 | 36 | 24 |
| R middle frontal gyrus/ BA 8, 9 | 60 | 5.23 | 52 | 12 | 36 |
| R superior frontal gyrus/ BA 6, 8 | 62 | 4.74 | 0 | 8 | 68 |
| ii. HC Session 2 < HC Session 1 | |||||
| L lentiform nucleus/ putamen/ lateral globus pallidus | 70 | 5.21 | −24 | 12 | 8 |
| II. Group Difference in Change in ‘Stroop Effect’ Related Regional BOLD Signal Following Treatment | |||||
| i. SUD(Post-Treatment vs. Pre-Treatment) < HC(Session2 vs. Session 1) | |||||
| R lateral globus pallidus/ lentiform nucleus/ midbrain/ subthalamic nucleus | 21 | 4.05 | 8 | −12 | −4 |
3. Results
3.1. Demographics and Clinical Measures
SUD and HC groups did not significantly differ in age (t=1.67, p>0.1). Although SUD group had lower IQ (t=2.65, p=0.02) and fewer days between fMRI sessions (t=3.61, p=0.002), results did not appear driven by these variables.2 Seven SUD patients reported current (one substance-induced mood disorder, three anti-social personality disorder, two post-traumatic stress disorder) and lifetime (one major depression) diagnoses. One HC subject was identified as a cigarette smoker and nine patients reported daily cigarette smoking. Substance use characteristics of patients at treatment onset are presented in Table 2 and measures of SUD treatment engagement and abstinence in Table 3.
Table 2. Substance Use Characteristics Amongst SUD Patients.
SD: Standard Deviation; %: Percent of group; N: number of subjects; n/a: not applicable. “Days of use in 28 days prior to treatment” and “Years of lifetime substance use” measures are only presented for patients who reported any recent or lifetime use of the substance indicated, respectively. Heroin use was assessed separately from other opioids in all cases except for “Current substance use disorders” where the heroin category refers to a broader class of opioids.
| Substance Use Characteristics | Cocaine | Alcohol | Marijuana | Heroin | Primary Drug |
|---|---|---|---|---|---|
| Primary drug of use/abuse: %, N | 75.0, 9 | 16.7, 2 | 8.3, 1 | 0.0, 0 | n/a |
| Current Substance Use Disorders: %, N | 75.0, 9 | 25.0, 3 | 16.7, 2 | 16.7, 2 | 100.0, 12 |
| Days used in 28 days prior to treatment: Mean (SD), N | 10.8 (10.9), 9 | 8.9 (9,1), 7 | 19.3 (15.0), 3 | 1.7 (1.2), 3 | 11.7 (10.8), 12 |
| Years of Lifetime Substance Use: Mean (SD), N | 12.8 (7.7), 10 | 12.9 (9.8), 12 | 9.5 (6.5), 11 | 12.2 (11.7), 6 | 14.2 (7.2), 12 |
Table 3. Treatment Engagement and Outcome.
Urines were tested for cocaine, opioids, marijuana, amphetamine and methamphetamine. TAU: treatment as usual; CBT: computerized cognitive behavioral therapy; SD: standard deviation; N: number of subjects.
| Treatment Engagement and Outcome Measures | Mean (SD), N |
|---|---|
| Treatment Components Completed | |
| Days in Treatment | 41.3 (16.9), 12 |
| TAU Sessions | 8.3 (5.9), 12 |
| CBT Computerized Sessions | 5.8 (1.6), 6 |
| CBT Homework Assignments | 4.0 (1.2), 6 |
| Substance Use Measures During Treatment | |
| Longest Abstinence Duration (in Days) | 35.3 (17.8), 12 |
| Percent of Urines Negative for All Drugs | 70.8 (39.2), 12 |
3.2. Stroop Behavioral Data
There was a main effect of trial-type (F=87.13, p<0.001) on mean reaction time, demonstrating the Stroop-effect (i.e., slower reaction times during incongruent relative to congruent trials) in both groups. A main effect of group (F=10.36, p=0.006) reflected slower reaction times in the SUD relative to the HC group. There was a trend towards a group-by-session interaction (F=4.46, p=0.052), and significant trial-type-by-group (F=5.20, p=0.038) and group-by-session-by-trial-type (F=11.92, p=0.004) interactions. Both groups demonstrated a main effect of trial-type due to slower performance on the incongruent trials (SUD: F=75.89, p<0.001; HC: F=22.09, p=0.002), and neither group showed a significant effect of session on congruent trial reaction time (SUD: F=1.69, p=0.230; HC: F=2.94, p=0.130). Only the SUD group showed a significant decrease in incongruent reaction time (SUD: F=5.42, p=0.048; HC: F=3.96, p=0.087). There were no significant effects of group (F=0.35, p=0.563), session (F=0.29, p=0.599) or group-by-session interactions (F=0.08, p=0.792) on incongruent trial errors (see Table 4). Stroop behavioral data were not available for three SUD and four HC subjects due to microphone recording device malfunctioning.
Table 4. Stroop Behavioral Measures.
Data are displayed as mean (standard deviation). Reaction times are reported in milliseconds. RT: reaction time; SUD: patient group; HC: healthy control group; N: number of subjects. Stroop behavioral data were not available for three SUD and four HC subjects due to the microphone recording device malfunctioning during testing.
| Session | Stroop Behavioral Measure | SUD (N=9) | HC (N=8) |
|---|---|---|---|
| Pre-treatment | Congruent Mean RT | 6009 (494) | 4580 (1595) |
| Incongruent Mean RT | 8392 (760) | 5516 (2620) | |
| Incongruent Trial Errors | 4.63 (3.11) | 6.33 (7.63) | |
| Post-treatment | Congruent Mean RT | 6118 (432) | 5804 (862) |
| Incongruent Mean RT | 8091 (694) | 7514 (1237) | |
| Incongruent Trial Errors | 4.37 (3.78) | 5.58 (6.02) |
3.3. fMRI Results
3.3.1. fMRI Stroop-effect at Each Session
Stroop-effect-related increases in BOLD signal reached significance in the anterior cingulate, frontal and subcortical regions in SUD and HC groups in both sessions, consistent with previous reports of fMRI Stroop-effect (Carter and van Veen, 2007).3
3.3.2. Within-Group Change in fMRI Stroop across Sessions
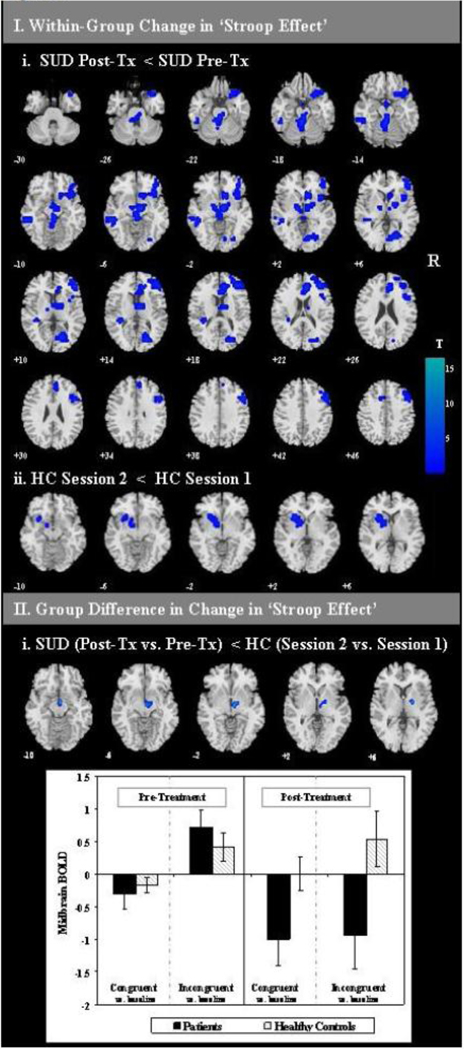
The SUD group demonstrated decreased Stroop-effect-related BOLD signal at post-treatment relative to pre-treatment in regions including the midbrain extending into the thalamus, lentiform nucleus and subthalamic nucleus, inferior frontal gyrus extending into the caudate, anterior cingulate gyrus, middle and superior frontal gyri, middle and superior temporal gyri and cuneus (see Table 5.Ii; Figure 1.Ii).
Figure 1. Change in fMRI ‘Stroop-effect’.
I. Change in fMRI BOLD signal across sessions on Stroop-effect (incongruent vs. congruent trials) contrast is displayed. Slice locations are indicated by MNI z levels. R: right side of brain images. Tx: treatment. Color bar indicates size of effect in t-values where blue tones indicate relative decreases in BOLD signal. i. Within-group changes across sessions were assessed with paired t-tests and a threshold of voxel-level p<0.005 with conjoint cluster-level pcorrected<0.05. ii. Group differences in change scores were assessed with a two sample t-test comparing change in BOLD signal activity following treatment (or re-test) masked for regions engaged by the SUD group at pre or post-treatment to a threshold of voxel-level p<0.005 and conjoint cluster extent of k=19. This cluster of significant interaction was saved as a mask in xjview and the mean signal intensity was extracted from the cluster mask region for incongruent vs. baseline and congruent vs. baseline contrasts from each participant at each session. The bar graph illustrates group means (±1 standard error of the mean) of the mean signal intensity from the cluster from each contrast at each time point.
The HC group showed decreased BOLD signal in the left putamen extending into the lentiform nucleus and lateral globus pallidus at session two relative to session one (see Table 5.Iii; Figure 1.Iii).
3.3.3. Group Differences (SUD vs. HC) in Changes in fMRI Stroop across Sessions
To clarify which components of the SUD group’s change in fMRI BOLD from pre- to post-treatment may have been attributable to treatment involvement or reductions in substance use, after accounting for test-retest effects, SUD and HC groups were compared on their changes in fMRI Stroop activation from pre- to post-treatment versus test-retest, respectively. The patient group (relative to HC test-retest) had significantly reduced Stroop-effect-related activation in the subthalamic nucleus (STN) extending into the ventral tegmental area (VTA), globus pallidus, hypothalamus and thalamus at post-treatment relative to pre-treatment (see Table 5.Iii; Figure 1.IIi). This cluster of significant interaction was saved as a mask in xjView (http://people.hnl.bcm.tmc.edu/cuixu/xjView/) and the mean signal intensity was extracted from the cluster mask region for incongruent vs. baseline and congruent vs. baseline contrasts from each participant at each session and entered into SPSS 16.0. Within this region of significant interaction, the patient group demonstrated a post-treatment reduction in the regional BOLD signal for incongruent trials (t=3.65, p=0.004) relative to pre-treatment, but no significant change in congruent trial activation (t=1.62, p=0.13). The HC group’s regional BOLD signal did not significantly change across the two visits for either trial-type (congruent t=−0.65, p=0.53; incongruent t=−0.36, p=0.72) (see Figure 1.IIi).
4. Discussion
This study assessed changes in functional brain activity across a course of behavioral treatment in SUD patients within an RCT context. Results generally supported our hypotheses. Firstly, the SUD group from pre- to post-treatment demonstrated improved task performance and reduced Stroop-effect-related BOLD signal change in several regions implicated in cognitive control, impulse control and motivational salience, including the ACC, right IFG, dlPFC and midbrain. Secondly, group difference in change in fMRI BOLD was seen in the subthalamic nucleus (STN), midbrain and surrounding regions, indicating greater decreases in BOLD signal in the SUD group from pre- to post-treatment than in the HC group from initial performance to re-test.
Cognitive control, or goal-directed guidance of behavior and information processing (Carter and van Veen, 2007), depends on a mechanism of determining how much control is required in a given situation (Botvinick et al., 2001) One proposed cognitive control system suggests the ACC detects degree of conflict while the dlPFC provides top-down control (Carter and van Veen, 2007; MacDonald et al., 2000). Decreased Stroop-effect-related activation in these regions, in the context of improved performance in the SUD group, may suggest more efficient ‘cognitive control’ mechanisms following treatment.
The relative speeding of responses during incongruent trials in the SUD group at post-versus pre-treatment without a concurrent increase in error rates was consistent with improved cognitive control in the SUD group. Simple motor or practice effects would be expected to affect trial-types or groups, respectively. If the incongruent response speeding indicated maladaptive impulsive responding in the SUD group post-treatment, it would be expected to be associated with increased incongruent trial error rates.
The effect of up to 8 weeks of outpatient substance abuse treatment in patients, after accounting for potential effects of repeated testing, was associated with decreased Stroop-effect-related activation in the STN and surrounding regions. The STN has been proposed to play an integral role in aspects of cognitive control, including response inhibition (Frank et al., 2007), and suggested as a viable target for treatments for addiction or compulsive disorders (Uslaner et al., 2008). The STN receives input from the ACC regarding the degree of conflict in a situation and in high-conflict situations (e.g., choice involving multiple reward options) can send a ‘no-go’ signal by increasing activation in the globus pallidus internal, which in turn exerts an inhibitory influence on thalamo-cortical loops, thus temporarily inhibiting responses and allowing more time to consider the decision (Frank et al., 2007). The STN may exert its role in response inhibition via hypothesized direct connections with the right IFG (Aron et al., 2007; Aron and Poldrack, 2006). Disruption of STN function impairs cognitive control (Hershey et al., 2004) and the ability to modulate responses in accordance with a situation’s degree of response conflict (Frank et al., 2007). STN dysfunction in rodents induces a profile of impulsive responding associated with vulnerability to addiction in animal models (Belin et al., 2008; Winstanley et al., 2005).
It was striking that the SUD group appeared to show diminished activation in the STN at post-treatment relative to pre-treatment, which at first may appear counter-intuitive as less STN activation could suggest decreased cognitive control and less response inhibition. However, behavioral data showing an improvement on these measures suggests alternate explanations. Firstly, due to the spatial resolution of fMRI, the regional activation (which shows peak significance in the STN) may be most accurately conceived of as reflecting the interplay of the functionally interconnected structures represented within the cluster (i.e., lateral globus pallidus, lentiform nucleus, midbrain, STN), rather than solely as a reflection of STN activation. Secondly, previous investigations suggest that it is the ability for the STN to respond effectively to endogenous signaling (not its absolute level of activity) which determines its effectiveness in exerting cognitive control. High frequency stimulation of the STN can produce behavioral effects akin to STN lesions, perhaps because exogenously induced STN firing interferes with responding to endogenous conflict signals (from the ACC) and prevents the STN from generating ‘no-go’ signals necessary for sufficient cognitive control in appropriate situations (Frank et al., 2007). Hypothetically, if drug administration provided an exogenous signal which interfered with the ability for the STN to respond to endogenous signals from the ACC, then diminished interference from such an exogenous signal could result in improved cognitive control, regardless of whether this manifested as an increase or decrease in overall STN activation. However, it should be noted that group differences in STN activation were not detected at the pre-treatment session in this sample (see Supplemental Table 34), so if the SUD group was experiencing STN dysfunction prior to treatment, it was not observable in this whole-brain fMRI analysis.
This study had numerous methodological strengths recommended by Frewen and colleagues (Frewen et al., 2008), which have not characterized much previous work on brain imaging evaluation of changes associated with behavioral therapies for non-nicotine-related SUDs. These strengths include randomization of patients to treatment conditions, inclusion of a healthy control group assessed at baseline and follow-up, use of a functional imaging task and correlation of clinical measures with neuroimaging data (Frewen et al., 2008).5 Several limitations should be noted. Patient and HC groups were not matched for time between test sessions, race, cigarette smoking, IQ or education, although time between scans and IQ do not appear to have accounted for fMRI results.6 Relatively small sample sizes within each treatment condition prevented direct comparison of treatment condition on change in the neural correlates of cognitive control. The fMRI Stroop paradigm employed prevents direct comparison of between-group in-scanner performance and does not allow for separate modelling of error trials. Although Stroop correct incongruent and error trials produce overlapping patterns of BOLD signal change (Kerns et al., 2004), differences in error rates could have contributed the observed group and treatment-associated effects on BOLD signal (Murphy and Garavan, 2004). The SUD group consisted of polydrug users with a range of primary substances of abuse, which may differentially influence the neural correlates of cognitive control or responsiveness to behavioral treatment. Variations in chronicity or recency of substance use may have contributed to individual variation in clinical presentation, cognition, brain structure or functional brain activity prior to treatment. A limitation in interpretation of the results is that decreases in regional brain activation in the patient group following treatment may have also been influenced by physiological changes related to decreased substance use or aspects of task performance. Abstinence in previously substance-dependent populations has been associated with decreased resting glucose metabolism in regions such as the ACC evident even four months following detoxification (Volkow et al., 1992). The ACC has also been implicated in craving such that greater ACC activity while viewing drug-related stimuli is associated with self-reported drug craving (Volkow et al., 2004). The degree of Stroop-related hypoactivity in the ACC and lateral PFC has been associated with severity of drug use prior to a period of abstinence, perhaps indicating that persistent functional brain abnormalities may arise from the drug use itself (Bolla et al., 2004). Therefore, such hypoactivations may not indicate task disengagement (Goldstein et al., 2009). Improvement on Stroop behavioral measures in this patient group from pre- to post-treatment argues against an explanation of decreased activation due to less task engagement.
Decreases in fMRI brain activity from pre- to post-outpatient treatment for SUDs were observed in regions with well-established roles in cognitive control, response impulsivity, motivation and attention, processes widely proposed to contribute to addiction. These preliminary findings may shed light on the mechanisms by which some behavioral therapies for substance use disorders achieve their effects.
Supplementary Material
Table 1. Sample Characteristics.
SD: Standard Deviation; %: Percent of group, N: number of subjects, SUD: substance use disorder patient group, HC: healthy control group. Asterisks indicate statistically significant (p<0.05) differences between the healthy control and patient groups.
| Demographics | SUD | HC |
|---|---|---|
| Total N (Female N) | 12 (5) | 12 (7) |
| Age, mean years (SD) | 37.2 (9.5) | 31.0 (8.6) |
| Race/ Ethnicity % (N) | ||
| White | 25.0 (3) | 75.0 (9) |
| Black | 50.0 (6) | 16.7 (2) |
| Asian | 25.0 (3) | 8.3 (1) |
| Hispanic ethnicity | 25.0 (3) | 8.3 (1) |
| Education Level % (N) | ||
| Partial High School | 25.0 (3) | 0.0 (0) |
| High School Completed | 41.7 (5) | 16.7 (2) |
| Partial College | 25.0 (3) | 33.3 (4) |
| College Completed | 8.3 (1) | 50.0 (6) |
| Shipley IQ mean (SD) * | 86.3 (14.3) | 101.1 (11.1) |
| Days between fMRI Sessions* | 84.4 (41.5) | 137.4 (29.4) |
Acknowledgements
The authors would like to thank Theresa Babuscio for her valuable assistance with data management and Jochen Weber for assistance with the implementation of AlphaSim.
Role of Funding Source This study was funded by the NIH grants P50-DA09241, RO1-DA020908, R37-DA15969, K05-DA00457, K05-DA00089, P20-DA027844 and the Veterans Integrated Service Network 1 Mental Illness Research, Education, and Clinical Center (MIRECC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of any of the funding agencies. EED was supported by T32-AA015496 and BIRCWH (K12DA031050), which was funded by the National Institute on Drug Abuse (NIDA), Office of Research on Women’s Health (ORWH) and National Institutes of Health (OD). HK is supported by K12 DA00167.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors MNP, KMC and BJR were responsible for the study concept and design. EED and PWD performed the imaging analysis. EED drafted the manuscript. HK advised on the implementation of AlphaSim. All authors critically reviewed the content. EED, PWD, MNP, HK and KMC approved the final version for publication.
Conflict of Interest Drs. DeVito, Carroll, Rounsaville, Kober, and Mr. Worhunsky declare no conflicts of interest. Dr. Potenza has received financial support or compensation for the following: consulted for and advised Boehringer Ingelheim; consulted for and has financial interests in Somaxon; received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, Forest Laboratories, Ortho-McNeil, Oy-Control/Biotie and Glaxo-SmithKline pharmaceuticals; participated in surveys, mailings, or telephone consultations related to drug addiction, impulse control disorders, or other health topics; consulted for law offices, governmental agencies and the federal public defender’s office in issues related to impulse control disorders; performed grant reviews for the National Institutes of Health and other agencies; given academic lectures in grand rounds, continuing medical education (CME) events, and other clinical or scientific venues; guest edited sections of journals; generated books or book chapters for publishers of mental health texts; and provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program.
Exploratory analyses assessed associations between regional changes in fMRI BOLD signal within the SUD group and measures of substance use history, treatment engagement and outcome. Methods and results are presented in Supplemental Table 2 Correlation analyses assessed whether relevant group differences (e.g., IQ) drove results (see Supplemental Table 3). Supplementary material found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary Table 3 can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary Table 1 can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material for this paper can be found by accessing the online version at http://dx.doi.org and by entering doi:…
See Supplemental Table 2 by accessing the online version at http://dx.doi.org and by entering doi:…
See Supplemental Table 3 by accessing the online version at http://dx.doi.org and by entering doi:….
References
- APA . Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat. Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J. Neuropsychiatry Clin. Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Funderburk FR, Cad et JL. Differential effects of cocaine and cocaine alcohol on neurocognitive performance. Neurology. 2000;54:2285–2292. doi: 10.1212/wnl.54.12.2285. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol. Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, London ED, Olmstead RE, Allen-Martinez Z, Shulenberger S, Costello MR, Abrams AL, Scheibal D, Farahi J, Shoptaw S, Mandelkern MA. Smoking-induced change in intrasynaptic dopamine concentration: effect of treatment for Tobacco Dependence. Psychiatry Res. 2010;183:218–224. doi: 10.1016/j.pscychresns.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Stevens MC, Pearlson GD, Kiehl KA. fMRI analysis with the general linear model: removal of latency-induced amplitude bias by incorporation of hemodynamic derivative terms. Neuroimage. 2004;22:252–257. doi: 10.1016/j.neuroimage.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Schreiber E, Church S, McDowell D. Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict. Behav. 2006;31:174–181. doi: 10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Carroll KM. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. Maryland: NIDA, Rockville; 1998. [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Nuro KF, Gordon MA, Portnoy GA, Rounsaville BJ. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. Am. J. Psychiatry. 2008;165:881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, Babuscio TA, Brewer JA, Potenza MN, Ball SA, Martino S, Rounsaville BJ, Lejuez CW. Cognitive function and treatment response in a randomized clinical trial of computer-based training in cognitive-behavioral therapy. Subst. Use Misuse. 2011;46:23–34. doi: 10.3109/10826084.2011.521069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn. Affect. Behav. Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Costello MR, Mandelkern MA, Shoptaw S, Shulenberger S, Baker SK, Abrams AL, Xia C, London ED, Brody AL. Effects of Treatment for Tobacco Dependence on Resting Cerebral Glucose Metabolism. Neuropsychopharmacology. 2009;35:605–612. doi: 10.1038/npp.2009.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am. J. Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol. Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Biometrics Research Department. New York: New York State Psychiatric Institution; 1996. Structured Clinical Interview for DSM-IV Axis I Disorders--Patient Edition. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York: New York State Psychiatric Institute; 2007. Structured Clinical Inter view for DSM-IV-TR Axis I Disorders -- Non-patient Edition (SC ID-I/P, 1/2007 revision) [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans. Med. Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJ, Lanius RA. Neuroimaging studies of psychological interventions for mood and anxiety disorders: empirical and methodological review. Clin. Psychol. Rev. 2008;28:228–246. doi: 10.1016/j.cpr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc. Natl. Acad. Sci. USA. 2009;106:9453–9458. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle A, Gibson PS, Dowling JL, Perlmutter JS. Stimulation of STN impairs aspects of cognitive control in PD. Neurology. 2004;62:1110–1114. doi: 10.1212/01.wnl.0000118202.19098.10. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J. Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl.) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kiluk BD, Nich C, Babuscio T, Carroll KM. Quality versus quantity: acquisition of coping skills following computerized cognitive-behavioral therapy for substance use disorders. Addiction. 2010;105:2120–2127. doi: 10.1111/j.1360-0443.2010.03076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Cavallo DA, Carroll KM, Potenza MN. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Matochik JA, Cadet JL, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacology. 1998;18:243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol. Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J. Subst. Abuse Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Murphy K, Garavan H. Artifactual fMRI group and condition differences driven by performance confounds. Neuroimage. 2004;21:219–228. doi: 10.1016/j.neuroimage.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain. Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am. J. Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Stapleton JM, Morgan MJ, Phillips RL, Wong DF, Yung BC, Shaya EK, Dannals RF, Liu X, Grayson RL, London ED. Cerebral glucose utilization in polysubstance abuse. Neuropsychopharmacology. 1995;13:21–31. doi: 10.1016/0893-133X(94)00132-J. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008;33:827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl.) 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol. Learn. Mem. 2011 doi: 10.1016/j.nlm.2011.02.014. Epub before print, PMID: 21376820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Dell’Orco JM, Pev zner A, Robinson TE. The influence of subthalamic nucleus lesions on sign-tracking to stimuli paired with food and drug rewards: facilitation of incentive salience attribution? Neuropsychopharmacology. 2008;33:2352–2361. doi: 10.1038/sj.npp.1301653. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol. Learn. Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Ward BD. AFNI 3dDeconvolve Documentation. Medical College of Wisconsin; 2000. Simultaneous intereference for FMRI data. [Google Scholar]
- Winstanley CA, Baunez C, Theobald DE, Robbins TW. Lesions to the subthalamic nucleus decrease impulsive choice but impair autoshaping in rats: the importance of the basal ganglia in Pavlovian conditioning and impulse control. Eur. J. Neurosci. 2005;21:3107–3116. doi: 10.1111/j.1460-9568.2005.04143.x. [DOI] [PubMed] [Google Scholar]
- Zachary RA. The Manual of the Shipley Institute of Living Scale-Revised. Los Angeles: Western Psychological Services; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.