Abstract
A simplified method for the preparation of Fmoc-serine and Fmoc-threonine glycosides for use in O-linked glycopeptide synthesis is described. Lewis acids promote glycoside formation, but also promote undesired reactions of the glycoside products. Use of “minimally competent” Lewis acids such as InBr3 promotes the desired activation catalytically, and with greatly reduced side products from sugar peracetates.
Glycoside construction remains as a tedious process that has not yielded to general methods. Intense efforts have been expended to develop synthetic methods for glycosides,i largely due to increased understanding of glycobiology, and subsequent demand for glycopeptides. The ideal methodology should produce high yields in a stereoselective manner and tolerate diverse functionality; it should be economical, environmentally friendly, easily reproduced, and scalable; it should use inexpensive, stable, and readily accessible glycosyl donors. Many existing methods produce high yields of the desired anomers, but often require the production of labile glycosyl donors, or very reactive (e.g. unstable) promoters.ii All of these methods require scrupulously dry conditions.
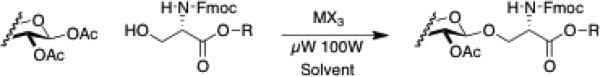
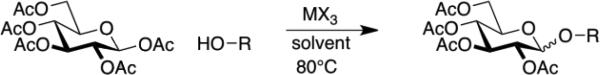
Efficient production of O-linked glycopeptides requires the availability of acetate-protected glycosides of Fmoc serine and threonine, but direct approaches to these precursors have been problematic, principally due difficulties in purification.1a,10 The first example of this approach was provided by Kihlberg, et al.1a This study describes the glycosylation of Fmoc-L-Ser-OBn, Fmoc-L-Ser-OH, (Table 1) and simple alcohols (Table 2) with sugar peracetates in the presence of Sc(OTfl)3 or In(III) salts (Figures 1 & 2). Both Sc(OTfl)3 and InBr3 proved to be excellent glycosylation promoters. The trans- or β-glycosides were produced in moderate to excellent yield.11,12 For simple alcohol acceptors anomeric ratios depended on reaction times and solvent polarity.
Table 1.
Glycosylation of Fmoc-Protected L-Serine and L-Serine Benzyl Ester with β-Peracetate Donors and Sc(III) or In(III) Salts
| Reaction | Donor | R | Promoter | Solvent | Time (min) | Temp (°C) | Isolated Yield |
|---|---|---|---|---|---|---|---|
| 1 | β-Glc | Bn | 1.0 eq Sc(OTfl)3 | PhCH3 CH2Cl2 |
3 | 80° | 85% |
| 2 | β-Xyl | Bn | 1.0 eq Sc(OTfl)3 | PhCH3 CH2Cl2 |
3 | 80° | 90% |
| 3 | β-Lact | Bn | 1.0 eq Sc(OTfl)3 | PhCH3 CH2Cl2 |
3 | 80° | 81% |
| 4 | β-Glc | H | 1.0 eq Sc(OTfl)3 | ClCH2CH2Cl | 5 | 80° | 71% |
| 5 | β-Xyl | H | 1.0 eq Sc(OTfl)3 | ClCH2CH2Cl | 5 | 80° | 68% |
| 6 | β-Lact | H | 1.0 eq. Sc(OTfl)3 | ClCH2CH2Cl | 5 | 80° | 74% |
| 7 | β-Glc | Bn | 1.0 eq InBr3 | PhCH3 CH2Cl2 |
2 | 80° | 90% |
| 8 | β-Glc | Bn | 0.5 eq InBr3 | PhCH3 CH2Cl2 |
5 | 80° | 93% |
| 9 | β-Glc | Bn | 0.2 eq InBr3 | PhCH3 CH2Cl2 |
12 | 80° | 92% |
| 10 | β-Glc | Bn | 0.1eq InBr3 | PhCH3CH2Cl2 | 20 | 80° | 93% |
| 11 | β-Glc | Bn | 0.05 eq InBr3 | PhCH3 CH2Cl2 |
45 | 80° | 93% |
| 12 | β-Glc | Bn | 0.01 eq InBr3 | PhCH3 CH2Cl2 |
90 | 80° | 92% |
| 13 | β-Glc | Bn | 0.5 eq InCl3\HBr | PhCH3 CH2Cl2 |
5 | 80° | 91% |
| 14 | β-Glc | H | 0.5 eq InCl3 | CH2Br2 | 5 | 100° | 84% |
| 15 | β-Glc | H | 0.1 eq InBr3 | PhCH3 CH2Br2 |
5 | 80°-100° | 42% (no chrom.) |
| 16 | β-Lact | H | 0.15 eq InBr3 | PhCH3 CH2Br2 |
300 | 80° | 61% (no chrom.) |
Table 2.
Glycosylation of simple a Icohols with β-Glucose Peracetate and Sc(OTfl)3 or InBr3
| R | Promoter | Solvent | Time (min) | Isolated Yield | α:β (HPLC) | |
|---|---|---|---|---|---|---|
| 1 | Me | 1.0 eq. Sc(OTfl)3 | PhCH3 | 1.00 | 75% | 47 : 53 |
| 2 | Me | 1.0 eq. Sc(OTfl)3 | PhCH3 | 5.00 | 32% | 92 : 8 |
| 3 | Me | 1.0 eq. Sc(OTfl)3 | PhCH3 | 15.00 | - | - |
| 4 | Me | 1.0 eq. Sc(OTfl)3 | DCE | 1.00 | 42% | 95 : 5 |
| 5 | Me | 1.0 eq. Sc(OTfl)3 | DCE | 5.00 | 27% | 90 : 10 |
| 6 | Me | 1.0 eq. Sc(OTfl)3 | DCE | 15.00 | - | |
| 7 | Me | 0.5 eq InBr3 | PhCH3 | 1.00 | 96% | α |
| 8 | Me | 0.5 eq InBr3 | PhCH3 | 5.00 | 95% | α |
| 9 | Me | 0.5 eq InBr3 | PhCH3 | 15.00 | 95% | α |
| 10 | Me | 0.5 eq InBr3 | DCE | 1.00 | 96% | α |
| 11 | Me | 0.5 eq InBr3 | DCE | 5.00 | 97% | α |
| 12 | Me | 0.5 eq InBr3 | DCE | 15.00 | 97% | α |
| 13 | Et | 1.0 eq. Sc(OTfl)3 | PhCH3 | 1.00 | 71% | 9 : 91 |
| 14 | Et | 1.0 eq. Sc(OTfl)3 | PhCH3 | 5.00 | 69% | 19 : 81 |
| 15 | Et | 1.0 eq. Sc(OTfl)3 | PhCH3 | 15.00 | 66% | 51 : 49 |
| 16 | Et | 1.0 eq. Sc(OTfl)3 | DCE | 1.00 | 62% | 6 : 94 |
| 17 | Et | 1.0 eq. Sc(OTfl)3 | DCE | 5.00 | 60% | 25 : 75 |
| 18 | Et | 1.0 eq. Sc(OTfl)3 | DCE | 15.00 | 58% | 62 : 38 |
| 19 | Et | 0.5 eq InBr3 | PhCH3 | 1.00 | 83% | 2 : 98 |
| 20 | Et | 0.5 eq InBr3 | PhCH3 | 5.00 | 84% | 4 : 96 |
| 21 | Et | 0.5 eq InBr3 | PhCH3 | 15.00 | 81% | 10 : 90 |
| 22 | Et | 0.5 eq InBr3 | DCE | 1.00 | 82% | 5 : 95 |
| 23 | Et | 0.5 eq InBr3 | DCE | 5.00 | 83% | 25 : 75 |
| 24 | Et | 0.5 eq InBr3 | DCE | 15.00 | 85% | 57 : 43 |
| 25 | i-Pr | 1.0 eq. Sc(OTfl)3 | PhCH3 | 1.00 | 72% | 20 : 80 |
| 26 | i-Pr | 1.0 eq. Sc(OTfl)3 | PhCH3 | 5.00 | 70% | 52 : 48 |
| 27 | i-Pr | 1.0 eq. Sc(OTfl)3 | PhCH3 | 15.00 | 65% | 80 : 20 |
| 28 | i-Pr | 1.0 eq. Sc(OTfl)3 | DCE | 1.00 | 65% | 13 : 87 |
| 29 | i-Pr | 1.0 eq. Sc(OTfl)3 | DCE | 5.00 | 64% | 19 : 81 |
| 30 | i-Pr | 1.0 eq. Sc(OTfl)3 | DCE | 15.00 | 61% | 57 : 43 |
| 31 | i-Pr | 0.5 eq InBr3 | PhCH3 | 1.00 | 90% | 2 : 98 |
| 32 | i-Pr | 0.5 eq InBr3 | PhCH3 | 5.00 | 88% | 6 : 94 |
| 33 | i-Pr | 0.5 eq InBr3 | PhCH3 | 15.00 | 89% | 15 : 85 |
| 34 | i-Pr | 0.5 eq InBr3 | DCE | 1.00 | 88% | 21 : 79 |
| 35 | i-Pr | 0.5 eq InBr3 | DCE | 5.00 | 90% | 64 : 36 |
| 36 | i-Pr | 0.5 eq InBr3 | DCE | 15.00 | 89% | 84 : 16 |
| 37 | C6H11 | 1.0 eq. Sc(OTfl)3 | PhCH3 | 1.00 | 67% | 28 : 72 |
| 38 | C6H11 | 1.0 eq. Sc(OTfl)3 | PhCH3 | 5.00 | 66% | 53 : 47 |
| 39 | C6H11 | 1.0 eq. Sc(OTfl)3 | PhCH3 | 15.00 | 63% | 80 : 20 |
| 40 | C6H11 | 1.0 eq. Sc(OTfl)3 | DCE | 1.00 | 55% | 14 : 86 |
| 41 | C6H11 | 1.0 eq. Sc(OTfl)3 | DCE | 5.00 | 54% | 37 : 63 |
| 42 | C6H11 | 1.0 eq. Sc(OTfl)3 | DCE | 15.00 | 51% | 70 : 30 |
| 43 | C6H11 | 0.5 eq InBr3 | PhCH3 | 1.00 | 79% | 5 : 95 |
| 44 | C6H11 | 0.5 eq InBr3 | PhCH3 | 5.00 | 76% | 10 : 90 |
| 45 | C6H11 | 0.5 eq InBr3 | PhCH3 | 15.00 | 77% | 23 : 77 |
| 46 | C6H11 | 0.5 eq InBr3 | DCE | 1.00 | 77% | 46 : 54 |
| 47 | C6H11 | 0.5 eq InBr3 | DCE | 5.00 | 75% | 86 : 14 |
| 48 | C6H11 | 0.5 eq InBr3 | DCE | 15.00 | 77% | 87 : 13 |
Figure 1.
Glycosylation with β-Peracetate Donors.
Figure 2.
Glycosylation of simple alcohols with β-Glucose Peracetate.
Various InIII salts displayed striking differences in reactivity. Under reaction conditions utilizing halogenated solvents CH2Cl2 or ClCH2CH2Cl, In(OAc)3, InCl3, InF3 and InI3 salts proved to be ineffective as glycosylation promoters, whereas InBr3 effected nearly quantitative conversion of Fmoc-L-Ser-OBn to th eβ-glycoside within minutes. Upon addition of catalytic amounts of HBr, or use of CH2Br2 as a solvent, InCl3 became effective. Athorough investigation of InBr3 mediated reactions showed this Lewis acid to be a superior promoter of O-glycosylation. All glycosyl donors tested a fforded the desired Fmoc amino acid O-glycosides in moderate to excellent yield. InBr3 promoted reactions of the Fmoc serine benzylesters provided greater than 90% isolated yield of the desired β–glycoside. The complementary InBr3- or Sc(OTfl)3-promoted reactions with Fmoc-Ser-OH gave somewhat lower yields which mirrored each other. Both microwave heated and traditional oil bath reflux glycosylations promoted by InBr3 allowed for the use of catalytic quantities of the Lewis acid.
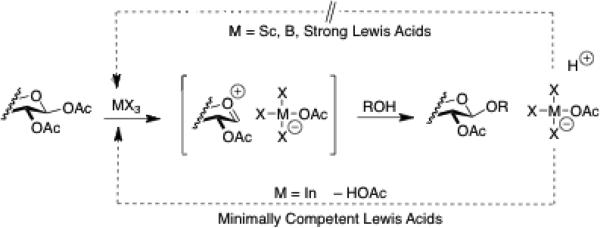
Of many Lewis acids tested, Sc(OTfl)3 and I nBr3 proved to be the most effective at promoting glycosylation with sugar peracetates. In the case of the more active promoter Sc(OTfl)3 optimal yields required strict control of reaction conditions. The InBr3 promoted glycosylations on the other hand, were much milder, allowing reactions to be run for longer times at higher temperatures without incurring significant side product formation. This suggests that In Br3 possesses sufficient Lewis acid competency to activate the anomeric acetate with neighboring group participation from the 2-position, but lacks the degree of reactivity associated with side product formation via Brønsted acid catalysis (Scheme 1). This minimal competency is also reflected in the promoter's ability function catalytically. Similar patterns of Lewis acid reactivity have been demonstrated in Denmark's studies.iii,iv
Scheme 1.
Minimally competent Lewis acids such as InBr3 can dissociate (lower pathway) from the displaced acetate to form acetic acid and regenerate the Lewis acid catalyst. Stronger Lewis acids remain a ssociated with the acetate (upper pathway) to produce a Brønsted acid, and generally require a full equivalent of the Lewis acid.
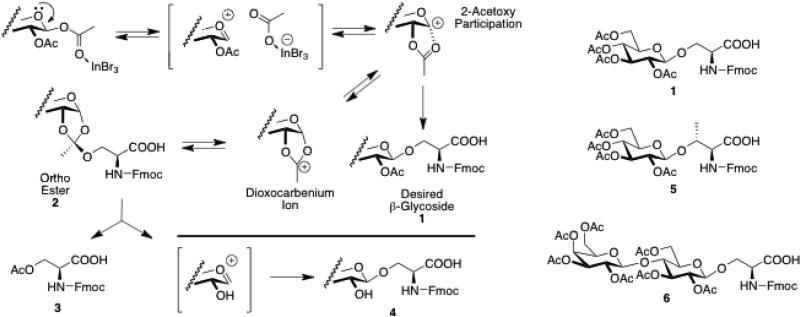
The results are consistent with the mechanism in Scheme 2. The product profiles of reactions involving Fmoc-serine glycosyl acceptors provide compelling evidence for an orthoester intermediate.v Although in none of the examples were the orthoesters isolated, evidence for the existence of this intermediate was provided by the isolation of acylated serine 3, and the deacylated glycosides 4, all presumably arising from the orthoester 2. The lower yields observed with the free acid derivatives can be rationalized by the work of Szabo and Polt showing that the amino acid carboxylic acid can react with both the anomeric center and the carbonyl carbon of the participating group in the dioxocarbenium ion.1b
Scheme 2.
Participation by the 2-acetoxy group produces anint ermediate th at largely undergoes desired trans β-glycoside formation. Formation of an ortho ester can decompose in 3 ways, leading to the desired glycoside 1, or acyl transfer to the serine acceptor (c.f. 4), which produces a 2-hydroxyl sugar oxoniumion that rapidly glycosylates another acceptor in situ . Lactose β-peracetate and Fmoc-L-threonine have been used as well to provide 5 and 6 in good yields and without the use of molecular sieves or chromatography.
The anomeric α-acetates are of limited use in this reaction.2c Earlier studies of Lewis acid promoted glycosylations by Moraru also showed that the α-anomers were unreactive.13 This suggests that the orthoester 2 (Scheme 2) may not be an important intermediate in the glycosylation pathway under the described conditions.vi This conclusion is consistent with the observation of complete selectivity for the β-product in the case of Fmoc-serine glycosyl acceptors. Lewis acid activation of anomeric acetates has been used quite effectively for 2-deoxy-2-iodo-sugarsvii due to the relatively electropositive iodine that is participating, but not as deactivating as the more electron-withdrawing trans-1,2-diacetate.viii Other donors, such as lactose peracetate and acceptors, such as Fmoc-L-threonine have been used to provide good yields of compounds 5 and 6 as well. Other halogenated solvent systems, such as neat CHCl3 and CCl4, have also been used to good effect.
The InBr3-catalyzed reaction has been accomplished with several 2° alcohols (Table 2), including the production of the Fmoc-L-threonine-β-D-glucoside, (Table 1, entry 15).ix It is important to emphasize that the InBr3 catalytic system is extremely moisture tolerant, in addition to demonstrating reduced sensitivity to overheating or extended reaction times. The free acids of Fmoc-Ser-OH and Fmoc-Thr-OH have been converted to their corresponding glucoside peracetates in good yield, high purity, and in one step without chromatography.7,9,x
The very reactive and non-bulky acceptor CH3OH lead to α-glycosides (Table 2, entries 1—12), and prolonged heating leads to higher proportions of the α-glycoside, especially in the presence of the stronger Lewis acid Sc(OTfl)3 that is not “minimally competent,” requiring a full equivalent of this Lewis acid promotor such as BF3•Et2O, SnCl4 or AgOTfl.1a,10,11,12 These observations are quite consistent with what is known about the anomeric effect, and it is not surprising that the axial glycosides ultimately predominate as reaction times are extended. Further studies of minimally competent Lewis acid promotors with more complex donors and other acceptors is probably warranted.
Supplementary Material
Acknowledgment
We thank the Office of Naval Research (N00014-05-1-0807 & N00014-02-1-0471), the National Science Foundation (CHE-607917) and the National Institutes of Health (NINDS-NS-052727) for Support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- i.a Elofsson M, Walse B, Kihlberg J. Tetrahedron Lett. 1991;32:7613–7616. [Google Scholar]; b Szabo L, Polt R. Carbohydr. Res. 1994;258:293–297. doi: 10.1016/0008-6215(94)84096-2. [DOI] [PubMed] [Google Scholar]; c Paulsen H, Peters S, Bielfeldt T, Meldal M, Bock K. Carbohydr. Res. 1995;268:17–34. doi: 10.1016/0008-6215(94)00292-n. [DOI] [PubMed] [Google Scholar]
- ii.a Seibel J, Hillringhaus L, Moraru R. Carbohydr. Res. 2005;340:507–11. doi: 10.1016/j.carres.2004.12.014. [DOI] [PubMed] [Google Scholar]; b Wei G, Lv X, Du Y. Carbohydr. Res. 2008;343:3096–9. doi: 10.1016/j.carres.2008.09.003. [DOI] [PubMed] [Google Scholar]; c Keyari CM, Polt R. J. Carbohydr. Chem. 2010;29:181–206. and references therein. [Google Scholar]; d Yao N, Fung G, Malekan H, Ye L, Kurth MJ, Lam KS. Carbohydr. Res. 2010;345:2277–2281. doi: 10.1016/j.carres.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- iii.Denmark SE, Beutner GL, Wynn T, Eastgate MD. J. Am. Chem. Soc. 2005;127:3774–3789. doi: 10.1021/ja047339w. [DOI] [PubMed] [Google Scholar]
- iv.Denmark SE, Wynn T. J. Am. Chem. Soc. 2001;123:6199–6200. doi: 10.1021/ja016017e. [DOI] [PubMed] [Google Scholar]
- v.a Garegg PJ, Kvarnstrom I. Acta Chem. Scand. B. 1976;30:655–658. [Google Scholar]; b Wallace JE, Schroeder LR. J. Chem. Soc., Perkin Trans. 2. 1977:795–802. [Google Scholar]; c Banoub J, Bundle DR. Can. J. Chem. 1979;57:2091. [Google Scholar]; d Garegg PJ, Konradsson P, Kvarnstrom I, Norberg T, Svensson SCT, Wigilius B. Acta Chem. Scand. B. 1985;39:569–577. [Google Scholar]; e Polt R, Szabò L, Treiberg J, Li Y, Hruby VJ. J. Am. Chem. Soc. 1992;114:10249–10258. [Google Scholar]
- vi.A 250 mL round bottom flask equipped with a magnetic stirrer and a reflux condenser was charged with 17.8 g (45.6 mMol) β-glucose peracetate, 4.98 g (15.2 mMol) Fmoc-L-serine and 808 mg (1.52 mMol) InBr3. Next, 80 mL dry PhCH3 and 8 mL dry (filtered through a plug of SiO2) CH2Br2 were added, and the reaction was placed in an oil bath at 65°–70°C for 20 min until the starting materials had dissolved forming a pink solution. The oil bath was heated to 110°C and the reaction run for 3–4 hrs until the Fmoc-serine had disappeared by TLC. The reaction was cooled, and the solvent evaporated to form a glassy solid, which was redissolved in 250 mL dry EtOAc, washed 3X with 75 mL saturated NaHCO3, then extracted 3X with 75 mL deionized H2O, alternating between washing and extracting each time. The neutral extractions were combined, and the organic phase and basic washes were discarded after verifying no product was present by TLC. The combined neutral extractions were acidified to pH 2–3 with HCl, and the resulting precipitate recrystallized from EtOAc/hexanes or iPrOAc/hexanes to provide 5.0 g (67 %) of Fmoc-L-serine-β-D-glucoside-peracetate 1, m.p. 160–162°C. See Supporting Information for HPLC traces and NMR spectra.
- vii.a Handa M, Smith WJ, Roush WR. J. Org. Chem. 2008;73:1036–9. doi: 10.1021/jo7022526. [DOI] [PubMed] [Google Scholar]; b Chong PY, Roush WR. Org. Lett. 2002;4:4523–4526. doi: 10.1021/ol027066e. [DOI] [PubMed] [Google Scholar]
- viii.Ogawa T, Beppu K, Nakabayashi S. Carbohydr. Res. 1981;93:C6–9. [Google Scholar]
- ix.A 50 mL triple-walled Pyrex® tube with a threaded Teflon® plug was charged with 3.90 g β-glucoseperacetate, 1.14 g Fmoc-L-threonine and 118 mg InBr3, 1 mL CH2Br2 and 9 mL PhCH3. The tube was tightly sealed with the Teflon plug, and the reaction mixture was heated in a Emerson commercial 1000 watt microwave oven (purchased at home depot) for 30 second increments at 50% power setting. After each increment of heating the reaction was shaken, temperature was monitored, and an aliquot removed for TLC or HPLC analysis. The temperature never exceeded 100°C. After several 30 second heating increments 4—5% of the original Fmoc-L-threonine was evident by HPLC analysis. The mixture was evaporated to a viscous oil and redissolved in 50 mL dry EtOAc. This solution was gently washed (to avoid formation of an emulsion) with 15 mL saturated NaHCO3– very little product was in this basic wash layer. The EtOAc solution was extracted with 15 mL deionized H2O– this neutral extract contained the glycoside product. This washing and extraction process was repeated 4X, and the neutral washings were combined. The combined neutral washings were stirred vigorously and acidified to pH = 3 with 1 N HCl while stirring continued. The resulting white precipitate was collected and dried in vacuo. Recrystallization from 10 mL EtOAc and 5 mL hexanes provided 0.95 g of Fmoc-L-threonine-β-D-glucoside-peracetate 5, m.p. 180—182°C. See Supporting Information for HPLC traces and NMR spectra.
- x.Salvador LA, Elofsson M, Kihlberg J. Tetrahedron. 1995;51:5643–5656. [Google Scholar]
- 11.Garg HG, Hasenkamp T, Paulsen H. Carbohydr. Res. 1986;151:225–232. doi: 10.1016/s0008-6215(00)90343-4. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell SA, Pratt MR, Hruby VJ, Polt R. J. Org. Chem. 2001;66:2327–2342. doi: 10.1021/jo005712m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.