Abstract
Purpose
To probe susceptibility of retinal ganglion cells (RGC) to physiological stressors associated with moderate head-down body tilt in patients with suspicion of glaucoma or early manifest glaucoma.
Methods
One hundred nine subjects with best corrected visual acuity ≥20/20 and no disease other than glaucoma (glaucoma suspects, GS= 79, early manifest glaucoma, EMG=14, normal controls, NC= 16) and comparable age range were tested. Non-contact IOP, pattern electroretinogram (PERG), and brachial blood pressure/heart rate measurements were performed in three consecutive (~8 minutes apart) conditions: seated (baseline), −10 deg whole body head-down (HDT), and seated again (recovery). PERG amplitude and latency, IOP, and systolic/diastolic blood pressures, heart rate, calculated mean central retinal artery pressure, ocular perfusion pressure, and systolic/diastolic perfusion pressures were evaluated.
Results
During HDT, IOP significantly (P<0.001) increased in all groups approximately to the same extent (~20%). PERG amplitude did not change in NC but decreased significantly (P<0.001) in patients (GS, −25%, EMG −23%). PERG phase become delayed in NC (− 1.6%, P=0.04) but more so in patients (GS, −2.7%, P<0.001; EMG, −6.0%, P<0.001). The proportion of patients with PERG alterations significantly (P<0.05) exceeding those occurring in age- and baseline-adjusted NC were, GS: amplitude 20%, phase 15%; EMG: amplitude 14%, phase 50%. All measures recovered baseline values after HDT.
Conclusions
Moderate HDT induces temporary worsening of RGC function in a subpopulation of GS and EMG patients. This non-invasive protocol may help disclose abnormal susceptibility of RGCs in a subset of the patients at risk of glaucoma.
Keywords: retinal ganglion cell function, pattern electroretinogram, glaucoma, IOP, body posture
Introduction
The pattern ERG (PERG) is a retinal biopotential that contains a major contribution from retinal ganglion cells (RGCs).1–5 In diseases such as glaucoma that primarily damage RGCs, abnormalities in the pattern ERG are frequently noted, and these may precede reduction of visual field sensitivity.6, 7 The magnitude of PERG signal reduction in early glaucoma may exceed the proportion expected from lost RGCs,8, 9,10 thus suggesting reduced responsiveness of anatomically present neurons.11 An abnormal PERG signal in ocular hypertension and glaucoma may be improved with IOP lowering, suggesting that RGC dysfunction can be at least in part restored.12–17 Decrease of PERG signal preceding reduction of RGC axons, as well as improvement of abnormal PERG signal after IOP lowering, have been also demonstrated in mouse models of glaucoma.18–20
Decrease of PERG signal may also be induced in normal subjects by artificially raising the intraocular pressure (IOP) with a scleral suction cup or with ophthalmodynamometry.21,22 For a comparable magnitude of induced IOP elevation, the PERG signal decreases more in patients with ocular hypertension than in normal controls.14
Physiological IOP elevation can also occur when the body position is changed from upright to head-down, the magnitude of IOP elevation increasing with increasing angle of head-down tilt (HDT).23–25 HDT may also produce significant reduction of PERG amplitude in normal subjects.21, 26 The photopic flash-ERG – reflecting the activity of outer retinal neurons – is unaffected for moderate angles of body tilt. 21
Here we test the hypothesis that moderate HDT impairs the PERG signal more in patients with either suspicion for glaucoma (GS) or early manifest glaucoma (EMG) than in control subjects. We show that this hypothesis is true for a subpopulation of patients, suggesting that they have insufficient autoregulation resulting in abnormally altered PERG signal. Head-down body tilt may therefore represent a tool as a physiological stressor to identify abnormal susceptibility of RGCs in patients at risk of glaucoma. Preliminary results of this study have been previously reported in abstract form (Lima, BD et al, IOVS 2007 48: E-Abstract 212; Ventura, LM et al, IOVS 2008 49: E-Abstract 2876).
Methods
Subjects
One hundred-nine subjects (normal controls: NC= 16, glaucoma suspects: GS= 79, early manifest glaucoma: EMG=14) participated in the study. The age of participants ranged between 30 and 80 years, the mean being not significantly different among groups (NC: 49.8 ± 12.3 years; GS: 55.3 ± 9.5 years, EMG: 57.0 ± 10.6 years; ANOVA, P=0.10).
For GS and EMG patients, eligibility was determined through a detailed medical and ocular history and a comprehensive eye examination. Eye examination included Best Corrected Visual Acuity (BCVA), refraction at distance and near, IOP with Goldmann applanation tonometry, corneal pachymetry (DGH 500 Pachette), gonioscopy, dilated fundus examination, stereophotographs of the optic disc, and Humphrey Perimeter Central 24-2 program (SAP). Clinical and demographic information were obtained as specified in the OHTS study design.27 Subjects met the following inclusion criteria. GS 28: refractive errors within −5 to +3 diopters, BCVA (Snellen) better than or equal to 20/20 (Jaeger score J1+ at 30 cm distance), normal SAP according to the OHTS criteria 27 (reliability <33% on all indices, normality >5% on all global indices in two consecutive sessions 6 months apart), and glaucomatous optic disc appearance (C/D ≥ 0.5, C/D asymmetry ≥0.2, localized thinning of the disc, splinter disc hemorrhages) or increased IOP (> 21 mm Hg). For EMG, inclusion criteria were: refractive errors within −5 to +3 diopters, BCVA ≥20/20, abnormal SAP and/or progression of optic disc abnormalities according to the OHTS criteria.27 SAP mean deviation was – 0.56 ± 1.4 dB in GS and −2.7±4.5 dB in EMG patients. Twelve out of 14 EMG patients (86 %) and 16 out of 79 GS patients (21%) were taking IOP-lowering medications at the time of testing.
For all subjects, exclusion criteria were the presence of ocular or systemic disease that may cause non-specific PERG abnormality such as age-related macular degeneration, diabetes, Parkinson’s disease, multiple sclerosis,6 and myopia higher than 5 Diopters.29, 30 Patients with previous intraocular surgery, except for uncomplicated cataract extraction, were also excluded.
The study followed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Miami. Informed written consent was obtained by all subjects after the nature of the test and possible risks were explained in detail.
Body Tilt apparatus
The Ophthalmic Biophysics Center of our Institute (JMP, WL, IN, VP), designed a system that allowed PERG recording in different conditions of body tilt (± 30 deg from horizontal – supine-position) (Figure 1). Subjects lay on an electronically adjustable tilting bed (Skytron Elite 5001), which included a foam-molded neck and shoulder restrainer that helped the subject to maintain a comfortable position and prevents sliding in the head-down tilted position. The PERG stimulus was incorporated in a balanced frame that was manually adjusted to align the stimulus with the subject’s gaze. To ensure rotational precision, the PERG stimulus display pivoted around its center of gravity. In addition, a quick release handle allowed the display to immediately move upward (away from the subject’s face) for safety reasons. Moderate (−10 deg) HDT was chosen based on previous pilot data (Lima. BD, et al, IOVS 2007 48: E-Abstract 212) in controls subjects in order to induce an IOP rise in the range of that occurring during the transition between diurnal and nocturnal periods.31–33 With this modest tilt angle, potential risks of undesirable systemic hemodynamic reactions 34, 35 were minimized. In the same pilot study in control subjects we established that the PERG did not decrease for −10 deg of HDT, whereas it did for steeper angles, and did not fully recover after tilt. We reasoned that if −10 deg of HDT was a subthreshold PERG stress in controls, then it could be a suprathreshold PERG stress in some susceptible patients.
Figure 1.
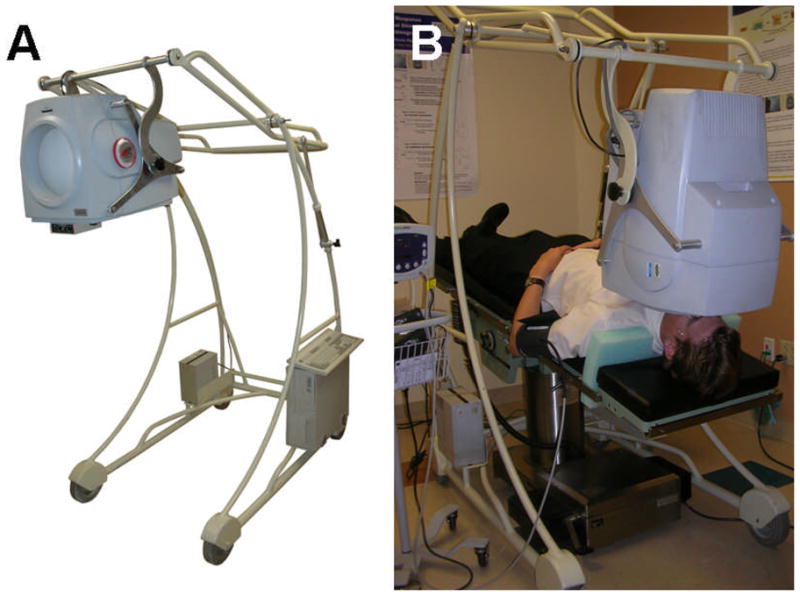
A custom made, adjustable PERG stimulus (A) in combination with a Skytron Elite surgical table (B) allowed PERG recording in subjects oriented −10° head-down.
Protocol
All subjects underwent the following protocol. IOP, systemic blood pressure, heart rate and PERG were first recorded with the subjects seated (Baseline). PERG electrodes were kept in place on the subjects’ face but unplugged from the recording system. Subjects were then asked to lie supine on the tilting bed, which was slowly adjusted to the −10 deg position (Tilted). IOP, systemic blood pressure, and heart rate were measured again in the tilted position. The PERG stimulus was aligned with the subjects’ gaze as in the seated position, the electrodes plugged again to the recording system, and a second PERG was recorded. PERG electrodes were then unplugged again, the PERG stimulus was moved away, and the subjects slowly tilted back to the seated position. A 3rd set of IOP, systemic blood pressure and PERG were then recorded with subjects in the seated position (Recovery). IOP and PERG readings in the tilted position were performed approximately 8 minutes after baseline readings. Readings in the recovery condition were performed approximately 8 minutes after tilted readings. A 8 min interval was chosen as best compromise to allow subjects adjustment to new posture and to control that all recording conditions (electrodes, trial lenses, visual stimulus) were precisely respected. Adjustment also included a time constant of about 2 minutes needed to establish the new IOP, based on published data of aqueous humor volume and turnover rate. 36, 37 The actual recording time for both IOP and PERG was about 3 minutes. We assumed that IOP remained relatively stable during this period, based on previous reports that 6 deg whole-body, head-down tilt produces a fast (within 1 min) elevation in IOP of approximately 3.5 mm Hg that persists during the first hour.26 The entire protocol lasted approximately 40 minutes. One IOP/PERG operator and one assistant operator participated in each recording. Since the beginning of the study, 4 different operators and 8 different assistant operators alternated in IOP/PERG recordings. One of the co-authors of this study (VP) served as a control subject to check that IOP and PERG were reproducible across operators. The mean within-test PERG variability of amplitude and phase reported in Table 1 includes inter-observer variability, and corresponds to previously published values.38–42 Assistant operators did not have a direct role in PERG recording.
Table 1.
Mean and SD of test-retest coefficient of variation (CV) PERG amplitude and phase, calculated from two consecutive PERG samples in individual normal controls and patients (see Methods for details)
| Normal Controls | Patients | |||
|---|---|---|---|---|
| OD amplitude CV | Mean | SD | Mean | SD |
| Baseline | 12.3 | 7.8 | 10.8 | 8.5 |
| Tilted | 14.9 | 8.1 | 13.6 | 10.6 |
| Recovery | 11.3 | 6.4 | 14.0 | 14.0 |
| OS amplitude CV | ||||
| Baseline | 14.1 | 7.6 | 11.1 | 9.5 |
| Tilted | 14.7 | 6.1 | 13.3 | 11.1 |
| Recovery | 12.9 | 5.2 | 11.7 | 7.6 |
| OD Phase CV | ||||
| Baseline | 1.1 | 0.8 | 1.6 | 1.5 |
| Tilted | 1.1 | 0.8 | 1.8 | 1.9 |
| Recovery | 0.9 | 0.8 | 2.0 | 1.6 |
| OS phase CV | ||||
| Baseline | 1.0 | 0.7 | 1.8 | 2.2 |
| Tilted | 1.5 | 1.5 | 2.4 | 2.1 |
| Recovery | 0.9 | 0.8 | 1.7 | 1.4 |
PERG recording
The PERG was simultaneously recorded from both eyes by means of standard Grass gold cup skin electrodes 10 mm diameter taped on the lower eyelids (Astro-Med West Warwick, RI, U.S.A.). Similar electrodes taped on the temples and central forehead served as reference and ground, respectively. The protocol is known as PERGLA paradigm, whose details have been previously described. 38 Reproducibility of measurements using this protocol is considered to be very good and suitable for monitoring changes over time.39–41 In brief, subjects were fitted with the appropriate add inserted in a trial frame (Oculus UB-4) which was carefully fitted to the ears and nose, and secured with surgical tape to the nose to prevent misalignment during body tilt. Subjects did not receive dilating drops, and were allowed to blink freely; they were instructed to fixate on a target at the center of a pattern stimulus placed at a viewing distance of 30 cm. The operator controlled from the side that the subjects were looking at the stimulus with eyes normally opened. The pattern stimulus was generated on a CRT display and consisted of horizontal gratings (1.7 cy/deg, 25 deg diameter circular field, 98% contrast, 40 cd/m2 mean luminance), reversing in counterphase at 8.14 Hz (16.28 reversals/s). The pattern stimulus was placed within a ganzfeld bowl to prevent reflections of ambient lighting on the stimulus screen (Figure 1). Electrical signals were band-pass filtered (1–30 Hz), amplified (100,000 fold), and averaged in synchrony with the reversal period. During signal acquisition, sweeps contaminated by eye blinks or gross eye movements were automatically rejected over a threshold voltage of 25 μV. Two successive responses of 300 artifact-free sweeps each were recorded, separated by a brief pause. The first 30 sweeps of each response were rejected to allow steady-state conditions. The software allowed visual inspection of the two consecutive responses superimposed to check for consistency, and then computed the final PERG waveform (600 artifact-free sweeps) and the coefficient of variation of amplitude and phase of the two partial averages (CV= SD/mean%), which represents a measure of within-test variability.38 As shown in Table I, CVs measured at baseline, during head down tilt, and again during recovery did not differ between normal controls and patients (GS+EMG).
Since the PERG was recorded in response to relatively fast alternating gratings, the response waveforms were typically sinusoidal-like, with a temporal period corresponding to the reversal rate (examples in Figure 4). PERG waveforms were automatically analyzed in the frequency domain by Discrete Fourier Transform (DFT) to isolate the frequency component at the contrast-reversal rate (16.28 Hz), and compute its amplitude in μV and phase in π rad. Decreasing phase values are analogues to latency delays (31 ms per 1.0 π rad).
Figure 4.
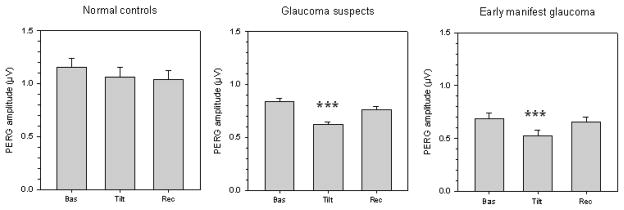
Mean PERG amplitude (average of the two eyes) measured in different conditions of body posture in normal controls and in patients with either suspicion of glaucoma or manifest glaucoma in the early stages. Labels on the x-axis: Bas, baseline seated; Tilt, whole body −10 degrees head-down; Rec, recovery seated. In all panels, errors bars represent the SEM. P-values for statistical differences with baseline are marked with asterisks (*, <0.05; **, <0.01; *** <0.001).
IOP measurement
IOP was measured with a portable, non-contact, air-pulse tonometer (Reichert PT100) for all conditions. For each IOP reading, the instrument calculates the average of three consecutive measures. Non-contact tonometry was necessary to prevent corneal irregularities that might occur after repeated readings with contact tonometers. Corneal surface irregularities produce scatter that degrade the stimulus contrast 43 and may reduce PERG amplitude.44 Air pulse tonometers yield IOP values that are comparable to those obtained with Goldmann applanation tonometry.45, 46
Systemic blood pressures measurements
Systemic blood pressure (brachial artery) and heart rate were monitored throughout the procedure with a Welch-Allyn Vital Signs monitor. For each body position, mean arterial blood pressure (MAP) was calculated from Diastolic blood pressure (DPB) and Systolic blood pressure (SBP) according to the formula MAP = DBP + 0.42 (SBP – DBP).47, 48 Ocular perfusion pressure (OPP) for different body positions were estimated using previously published coefficients derived from ophthalmodynamometric measurements obtained at different body positions and extrapolated to derive the coefficient at −10 deg body tilt.48–50,51,52 Namely, OPPseated was = (0.74*MAP) - IOP, and OPPtilted was = (0.86*MAP)-IOP. Systolic Perfusion Pressure (SPP= SBP – IOP) and Diastolic Perfusion Pressure (DBP= DBP – IOP) were also calculated.
Statistics
To simplify statistical calculations and reduce variability, analyses were performed on the averaged data of the two eyes. This approach also simplified correlations/multiple regression analyses between PERG data (two measures per patient) and vascular variables (one measure per patient). Statistical tests include univariate analyses (one-way repeated measures ANOVA and post-hoc Bonferroni t-tests) on raw IOP data and age-normalized PERG amplitude and phase data. Correlations between tilt-induced PERG amplitude/phase changes and IOP/vascular variables, as well as multiple regression analysis accounting for the role of multiple independent variables were performed. Other tests and procedures are indicated in the text as they are used.
Results
Effect of head-down tilt on IOP
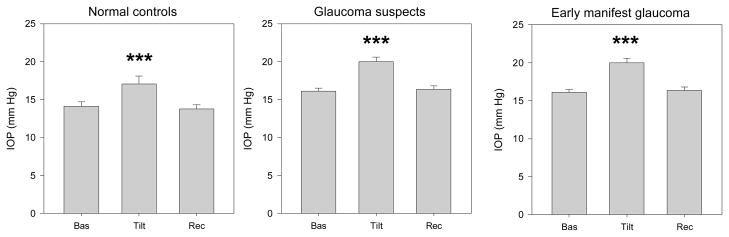
Mean IOPs measured under different posture conditions are shown (Figure 2). It can be noted that in all subjects groups IOP increased during head down tilt, and then recovered the baseline values when subjects assumed again the seated condition (repeated measures ANOVA with post-hoc least significant difference tests, P≤0.001). The magnitude of IOP elevation was approximately 3 mm Hg on average in all subject groups (NC: baseline 14.1 ± 2.3, tilted 17.3 ± 4.1, P <0.001; GS: baseline 16.1 ± 3.6, tilted 20.0 ± 5.3, P<0.001; EMG: baseline 14.4 ± 3.7, tilted 16.9 ± 4.3, P<0.001).
Figure 2.
Mean IOP (average of the two eyes) measured in different conditions of body posture in normal controls and in patients with either suspicion of glaucoma or manifest glaucoma in the early stages. Labels on the x-axis: Bas, baseline seated; Tilt, whole body −10 degrees head-down; Rec, recovery seated. In all panels, errors bars represent the SEM. P-values for statistical differences with baseline are marked with asterisks (*, <0.05; **, <0.01; *** ≤0.001).
Stepwise multiple regression analysis was performed to isolate the effect of body tilt on IOP change from other variables that might independently affect it. In the model, the magnitude of tilt-induced IOP change (tilted IOP minus baseline IOP) was the dependent variable, whereas independent variables allowed for inclusion were: Baseline IOP, Age, Disease level (1:NC, 2:GS, 3:EMG, included as a 2 degree of freedom categorical variable), baseline and tilted and the presence of IOP-lowering treatment (0:untreated, 1:treated). Baseline IOP mostly contributed to the model (the magnitude of IOP change increased with increasing baseline IOP, P<0.001). Age, Disease level, and presence of treatment did not significantly contribute to the model (P ≥ 0.35). The dependent variable IOP change could be predicted from the equation: IOP change (mm Hg) = −1.719 + (0.338 * Baseline IOP). This equation accounted for 25% of the total variance.
Effect of head-down tilt on PERG signal
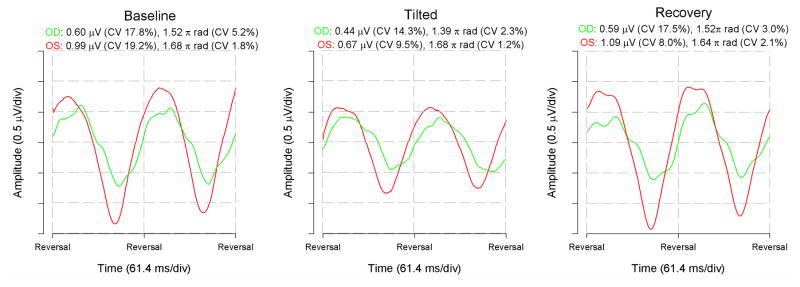
Figure 3 displays representative examples of PERG recorded from both eyes of a patient with early manifest glaucoma under seated baseline condition, 8 minutes after −10° HDT, and 8 minutes after seated repositioning. The patient was a 37 year old Caucasian female with positive family history of glaucoma not undergoing treatment. Her corrected (−4.5 spherical diopters in either eye) visual acuity was 20/20 in each eye. Her vertical cup-to-disk ratio was 0.5 in each eye. The visual field parameters (24-2 SITA standard program) were, MD: −3.33 dB OD, −1.66 dB OS, PSD: 3.06 dB OD, 2.25 dB OS; GHT: borderline OD, outside normal limits OS. Her central corneal thickness was 507 μm in the right eye and 520 μm in the left eye. Her baseline IOP was 15 mm Hg in OD and 16 mm Hg in OS, and increased to 19 and 22 mm Hg, respectively, upon HDT. Note that at baseline, the right eye has smaller amplitude and delayed phase (green waveform shifted to the right) compared to the left eye (red waveform). During HDT, the PERG amplitude decreased in both eyes while the phase further delayed in the right eye compared to the left eye. In the recovery condition, PERG waveforms were similar to those of baseline condition. Also note in the figure legends that the within-test variability (CV) of amplitude and phase is small and very similar across conditions.
Figure 3.
Example of raw PERG waveforms recorded simultaneously from both eyes of a patient with early manifest glaucoma at baseline-seated condition (left panel), during −10° head-down body tilt (middle panel), and after seated repositioning (right panel). Green waveforms represent the right eye; red waveforms represent the left eye. Waveforms were analyzed with Discrete Fourier Transform to isolate the frequency component corresponding to the reciprocal of the pattern-reversal period of 61. 4 ms (16.28 Hz) and measure its amplitude in μV and phase in π radians. In the legend, CV is the coefficient of variation of two consecutive responses whose average constitutes the waveforms shown.
PERG amplitude
Mean PERG amplitudes (average of the two eyes) measured under different posture conditions are shown (Figure 4). It can be noted that in NC the PERG amplitude displayed little change with head down tilt (repeated measures ANOVA, P=0.09). In contrast, in both GS and EMG patients the PERG amplitude decreased during head down tilt, and then recovered the baseline values when subjects assumed again the seated condition (repeated measures ANOVA, P<0.001). In GS, the magnitude of PERG amplitude reduction during HDT was 26% (baseline 0.84 ± 0.27, tilted 0.62 ± 0.23, P<0.001, post-hoc least significant difference test); in EMG the magnitude of PERG amplitude reduction during HDT was 23% (baseline 0.69 ± 0.19 μV, tilted 0.53 ± 0.20 μV, P<0.001, post-hoc least significant difference test).
Multiple regression analysis was performed to isolate the effect of body tilt on PERG amplitude from potentially contributing baseline variables. In the model, the magnitude of tilt-induced PERG amplitude change (tilted minus baseline) was the dependent variable, whereas independent variables allowed for inclusion were: Age, Baseline IOP, Baseline PERG amplitude, Disease level (0:NC, 1:GS, 2:EMG, included as a 2 degree of freedom categorical variable), and the presence of IOP-lowering treatment (0:untreated, 1:treated). Independent variables that significantly contributed to the model were Baseline PERG amplitude (P<0.001), Age (P<0.001), and Disease level (P<0.001) according to the equation: PERG amplitude change (μV) = 0.665 - (0.328 * baseline PERG amplitude) – (0.007 * Age) - 0.224 (if subject was GS) −0.19 (if subject was EMG). This equation accounted for 32% of the total variance.
PERG phase
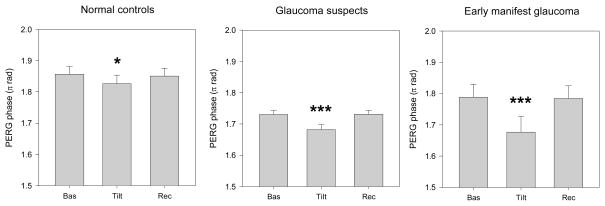
Figure 5 shows how the mean PERG phase (average of the two eyes) changes under different posture conditions. In all subject groups, the PERG phase tended to a delay during head down tilt and then recovered the baseline values when subjects assumed again the seated condition (repeated measures ANOVA: NC, P=0.032 (Greenhouse-Geisser corrected), GS, P<0.001, EMG, P=0.001). The magnitude of PERG phase delay (π rad) differed between subjects groups (NC: baseline 1.85 ± 0.09, tilted 1.83 ± 0.10, P=0.043, post-hoc least significant difference test; GS: baseline −1.73 ± 0.11, tilted −1.68 ± 0.14, P<0.001, post-hoc least significant difference test; EMG: baseline 1.79 ± 0.15, tilted 1.68 ± 0.19, P=0.001, post-hoc least significant difference test).
Figure 5.
Mean PERG phase (average of the two eyes) measured in different conditions of body posture in normal controls and in patients with either suspicion of glaucoma or manifest glaucoma in the early stages. Labels on the x-axis: Bas, baseline seated; Tilt, whole body −10 degrees head-down; Rec, recovery seated. In all panels, errors bars represent the SEM. P-values for statistical differences with baseline are marked with asterisks (*, <0.05; **, <0.01; *** <0.001).
Multiple regression analysis was performed to isolate the effect of body tilt on PERG phase from baseline variables. In the model, the magnitude of tilt-induced PERG phase change (tilted minus baseline) was the dependent variable, whereas independent variables were: Age, Baseline IOP, baseline PERG phase, Disease level (0:NC, 1:GS, 2:EMG, included as a 2 degree of freedom categorical variable), and the presence of IOP-lowering treatment (0:untreated, 1:treated). Only EMG diagnosis entered the model significantly (p=0.009), thus the average phase difference on tilting was −0.032 π rad minus an additional 0.060 if subject was EMG. This equation accounted for only 9% of the total variance.
Corneal thickness
CCT was not measured in control subjects; but was measured in most, n=72 (91%) of GS (mean ± SD, right and left eyes averaged: 544.4 ± 37.3) and all of EMG patients (mean ± SD: 523.0 ± 32.4). The difference in mean CCT between GS and EMG patients was significant (t-test, P=0.048). CCT was also assessed as a dichotomous variable with thin defined as ≤555μm.53 With this cut-off, 40/72 GS with CCT measured (55.6%) and 13/14 of EMG patients (93%) had thinner corneas. This difference between GS and EMG was significant (Fisher’s exact test, p=0.014). As in the stepwise analyses conducted above on all subjects, age and PERG amplitude at baseline were associated with PERG amplitude changes upon tilting (both p≤0.002); however, CCT did not significantly contribute to the model either as a continuous variable or dichotomous variable (both p>0.5). CCT also did not significantly contribute to the regression model for phase changes upon tilting either as a continuous variable or dichotomous variable (both p>0.6). We also analyzed the influence of thin and thick CCTs on the occurrence of abnormally high PERG amplitude/phase losses upon HDT in individual subjects (see Fig. 6 below). Four out of 33 patients with thin CCT (12%) had excessive PERG amplitude reduction upon HDT, compared with 10/53 patients (19%) with thick CCT. The difference was not significant (Fisher exact test, P=0.56). Five out of 33 patients with thin CCT (15%) had excessive PERG phase reduction upon HDT, compared with 9/53 patients (17%) with thick CCT. The difference was not significant (Fisher exact test, P=1.00). Altogether, there was no indication of an effect of thin corneas on PERG susceptibility to head down body tilt.
Figure 6.
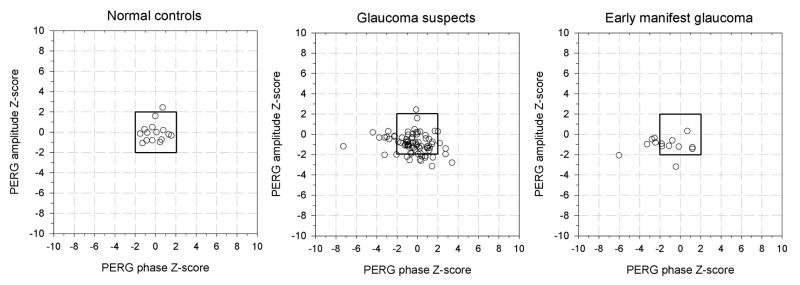
Standardized baseline and age adjusted deviations from normal (z-scores) for PERG amplitude and phase changes during −10 deg head down body tilt.
As CCT may affect IOP measurements,54 we included thin cornea as independent variable in the IOP regression model described above (Fig. 2). Inclusion of thin cornea significantly (p=0.027) contributed to the model. This, however, did not substantially change the coefficient associated with baseline IOP [IOP change (mm Hg) = −3.238 + (0.394 * Baseline IOP) + (1.006 for patients with thin corneas] compared to the equation without CCT inclusion shown in the description of Fig. 2 [IOP change (mm Hg) = −1.719 + (0.338 * Baseline IOP)].
Isolating subjects with excess PERG changes in Head Down Tilt
As shown above, baseline PERG amplitude as well as age independently contributed to PERG signal changes during HDT. To identify subjects with excessive alteration of the PERG signal during HDT, PERG measurements in patient groups were normalized to those of NC accounting for baseline PERG amplitude/phase and age as follows. HDT measurements obtained in NC were fitted to a multiple regression model including baseline measurements and age as independent variables, and corresponding prediction equations were obtained (r2 amplitude = 0.26; r2 phase = 0.35). Differences between HDT raw data and the values predicted from NC regression were calculated for each subject in all three study groups, and then divided by the SD of residuals of NC to obtain standardized baseline and age adjusted z-scores for HDT. In NC, 95% of z-scores were expected to have values between ± 1.96 with an average close to zero, whereas in patients the distribution of z-scores was expected to be more dispersed towards negative values.
Figure 6 shows z-scores of PERG amplitude plotted against corresponding z-scores of PERG phase for individual subjects of each group. As anticipated, in NC z-scores of PERG amplitude and phase were spread randomly around zero, with only 1/16 cases (6%) having a high z-score for amplitude. In the GS group, 12/79 cases (15%) had low z-scores for amplitude and 11/79 (14%) had low z-scores for phase; 2/79 cases (3%) had low z-scores for both amplitude and phase; in 5% of cases, z-scores were abnormally high for phase. In the EMG group, 2/14 cases (14%) had low z-scores for amplitude whereas 5/14 cases (36%) had low z-scores for phase; 1/14 (7%) had low z-scores for both amplitude and phase. Eighteen GS patients with low z-scores for either amplitude or phase were untreated. None of the EMG patients with decreased amplitudes were untreated. One EMG with low score for phase was untreated.
To answer the question of whether patients with low z-scores also had altered PERG recovery to baseline after HDT, we compared baseline and recovery values of subjects with low or normal z-scores. We used two-way ANOVA (Factor Condition, 2 levels: Baseline, Recovery; Factor Z-score, 2 levels: Low, Normal). GS and EMG patients were combined to have a sufficient sample in the low z-scorers. For both amplitude and phase there was a small, insignificant trend to a lower amplitude (P=0.162) or delayed phase (P=0.639) in the recovery condition compared to baseline. However, this was not different between low z-scorers and normal z-scorers (amplitude, P=0.95; phase, P=0.49).
Relationship between PERG changes and ocular/blood pressures
Correlations (Pearson’s 2-tailed) between PERG changes upon tilt (baseline and age adjusted z-scores) and IOP as well as vascular variables are summarized in Table 2. None of the vascular variables were significantly correlated to either PERG amplitude or phase z-scores. In contrast, IOP parameters were correlated with PERG phase delay significantly or borderline significantly. These were: baseline IOP (P=0.043), tilted IOP (P=0.027), delta IOP (P=0.080). The higher the IOP the larger the delay.
Table 2.
Correlations between delta PERG amplitude/phase and IOP/vascular variables.
| N=109 (NC+GS+EMG) | Z-scores PERG amplitude | Z-scores PERG phase | ||
|---|---|---|---|---|
| R | P | R | P | |
| IOP B | −0.035 | 0.722 | −0.195 | 0.043 |
| IOP T | −0.086 | 0.371 | −0.211 | 0.027 |
| IOP D | −0.135 | 0.161 | −0.168 | 0.080 |
| SBP B | 0.046 | 0.636 | −0.028 | 0.770 |
| SBP T | 0.074 | 0.447 | −0.112 | 0.247 |
| SBP D | 0.041 | 0.676 | −0.120 | 0.212 |
| DBP B | −0.033 | 0.735 | 0.046 | 0.637 |
| DBP T | −0.020 | 0.837 | 0.044 | 0.649 |
| DBP D | 0.022 | 0.819 | −0.006 | 0.952 |
| OPP B | 0.011 | 0.912 | 0.029 | 0.762 |
| OPP T | 0.037 | 0.706 | −0.024 | 0.805 |
| OPP D | 0.045 | 0.643 | −0.082 | 0.396 |
Legend. B=baseline, T= Tilted, D= delta (tilted – baseline). Significant correlations are emphasized in bold. Borderline correlations are emphasized in italic
Discussion
The aim of this study was to investigate whether moderate head-down body tilt (HDT) caused reduction of RGC electrical activity in patients suspected of glaucoma or with early manifest glaucoma. The HDT protocol, including non-contact PERG and IOP evaluation, was non-invasive and not uncomfortable.
Body tilt caused IOP elevation of the order of 3 mm Hg in both normal controls and patients groups and then recovered baseline values upon seated repositioning. IOP elevation tended to increase with increasing baseline IOP. The magnitude of IOP elevation during HDT was fairly close to that reported during the nocturnal period. 31–33
The main result of our study is that −10 deg HDT induced reversible reduction of PERG amplitude and delay of PERG phase that were on average larger in patients than in controls. PERG amplitude and phase changes upon tilt exceeded the lower 95% confidence intervals of age- and baseline-adjusted normal controls in a small percentage of patients – Amplitude: 12/79 (15%) in GS and 2/14 (14%) in EMG; Phase: 11/79 (14%) in GS, 5/14 (36%) in EMG – whose retinal function appears to be particularly susceptible to the procedure. PERG amplitude and phase reflect distinct aspects of RGC activity.5 Amplitude has a relationship with both the number and the vitality of PERG generators. The smaller the number of neurons activated by the visual stimulus the smaller the electrical signal; the less vital the neurons (and/or abnormal connectivity or gain relationships between them) the smaller the electrical signal. Phase delay may mean that underlying generators are less excitable and respond to the visual stimulus in a sluggish way.5. That the in EMG patients the effects are seen more in PERG phase than in PERG amplitude may be merely due to the fact that phase variability is typically much smaller than amplitude variability,38–41 resulting in statistically significant effects larger for phase than amplitude.
We exclude that PERG changes upon tilt were artifactual, since PERG electrodes remained taped on the skin throughout the procedure, the lens trial frame was firmly secured to the subjects’ face, and the viewing distance and orientation of the pattern stimulus was not changed. Refractive errors and accommodative changes are negligible with moderate body tilt.55 The operators, watching subjects for the side, could not detect differences in the quality of viewing between conditions. The within-participant test-retest variability of the PERG was not different between baseline and tilted condition. Altogether, this implies disease-dependent alteration of PERG generators upon HDT.
Delta PERG phase was significantly or borderline correlated with baseline IOP, tilted IOP, and delta IOP, although correlations were weak. Overall, the higher the baseline/tilted IOP and the larger the delta IOP upon tilt, the slower the PERG signals. This may suggest that IOP affects directly or indirectly the electrical responsiveness of RGCs. In addition to IOP elevation, HDT is known to be associated with a host of cardio-vascular alterations 48, 51, 56 as well as changes in cerebrospinal fluid (CSF) pressure 57 that may play a role in the development of glaucoma. 58, 59 This study does not provide estimates of CSF pressure or pressure gradient between IOP and CSF.
During HDT, the vertical heights of the columns of both venous and arterial blood gravitating on the eye are expected to increase.60 This results in increased episcleral venous pressure/IOP as well as increased central retinal artery pressure. In the present study we found a significant correlation between PERG changes and IOP but not between PERG and ocular perfusion pressure (OPP). OPP was calculated as difference between mean central artery pressure and IOP. As central retinal artery pressure was not directly measured, but derived from previous ophthalmodynamometric estimates 47, 49, the lack of correlation between OPP and PERG should be interpreted with caution.
Biomechanically-induced strains occurring at the optic nerve head during HDT may play a role.61, 62 These may be elicited by the increased IOP, increased gradient between IOP and CSF pressure, and also by increased choroidal volume. Stretch-sensitive channels in axonal membranes that contribute to neural excitability 63–65 may respond to HDT-induced deformation and alter the PERG. The short-time course (~8 min) of the effect is consistent with a biomechanical effect. It is possible that the neural effects were somewhat different if tilt were maintained for a longer time.26 Our protocol was chosen as a compromise in order to provide adequate time for development of the effects 26 while minimizing the risks to the patients associated with prolonged head-down position.
In conclusion, HDT-induced PERG changes of abnormally high magnitude occur in a relatively small subpopulation of GS and EMG. This implies that these patients, compared to NC and other patients who display relatively lesser changes, have inadequate autoregulatory ability to compensate for the disturbed homeostasis. The actual causes of PERG signal impairment are matter of speculation. A combination of factors including intraocular/cerebrospinal pressures, cardio-vascular, biomechanical, and metabolic changes may be at play during HDT. Prolonged exposure to these factors may also occur during normal nocturnal periods. This study provides proof of concept results for the use of HDT as physiological stressor to disclose altered homeostatic states in a subset of the patients at risk of glaucoma, possibly providing clues on progression of the disease.66 Were the HDT-PERG procedure sufficiently simplified (eg, using lightweight head-mounted displays, 67 then it might be tested in the clinical setting on larger patient populations also using different inclusion/exclusion criteria.
Acknowledgments
Financial support: NIH-NEI RO1 EY014957 (VP, LV, WJF), Florida Lions Eye Bank (WL, IN), Henri and Flore Lesieur Foundation (JMP), NIH center grant P30-EY014801 (VP), unrestricted grant to Bascom Palmer Eye Institute from Research to Prevent Blindness.
We would like to thank Breno Da Rocha Lima, MD, and Frank Venzara III, MD, for their contribution to data collection in the initial stages of this study.
Footnotes
Financial disclosure: None
References
- 1.Maffei L, Fiorentini A. Electroretinographic responses to alternating gratings before and after section of the optic nerve. Science. 1981;211:953–955. doi: 10.1126/science.7466369. [DOI] [PubMed] [Google Scholar]
- 2.Zrenner E. The physiological basis of the pattern electroretinogram. In: Osborne N, Chader G, editors. Progress in Retinal Research. Oxford: Pergamon Press; 1990. pp. 427–464. [Google Scholar]
- 3.Viswanathan S, Frishman LJ, Robson JG. The uniform field and pattern ERG in macaques with experimental glaucoma: removal of spiking activity. Invest Ophthalmol Vis Sci. 2000;41:2797–2810. [PubMed] [Google Scholar]
- 4.Holder GE. Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog Retin Eye Res. 2001;20:531–561. doi: 10.1016/s1350-9462(00)00030-6. [DOI] [PubMed] [Google Scholar]
- 5.Porciatti V, Ventura LM. Physiologic significance of steady-state pattern electroretinogram losses in glaucoma: clues from simulation of abnormalities in normal subjects. J Glaucoma. 2009;18:535–542. doi: 10.1097/IJG.0b013e318193c2e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ventura LM, Porciatti V. Pattern electroretinogram in glaucoma. Curr Opin Ophthalmol. 2006;17:196–202. doi: 10.1097/01.icu.0000193082.44938.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach M, Hoffmann MB. Update on the pattern electroretinogram in glaucoma. Optom Vis Sci. 2008;85:386–395. doi: 10.1097/OPX.0b013e318177ebf3. [DOI] [PubMed] [Google Scholar]
- 8.Aldebasi YH, Drasdo N, Morgan JE, North RV. S-cone, L + M-cone, and pattern, electroretinograms in ocular hypertension and glaucoma. Vision Res. 2004;44:2749–2756. doi: 10.1016/j.visres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Ventura LM, Porciatti V. Restoration of retinal ganglion cell function in early glaucoma after intraocular pressure reduction: a pilot study. Ophthalmology. 2005;112:20–27. doi: 10.1016/j.ophtha.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falsini B, Marangoni D, Salgarello T, et al. Structure-function relationship in ocular hypertension and glaucoma: interindividual and interocular analysis by OCT and pattern ERG. Graefes Arch Clin Exp Ophthalmol. 2008 doi: 10.1007/s00417-008-0808-5. [DOI] [PubMed] [Google Scholar]
- 11.Weber AJ, Harman CD. Structure-function relations of parasol cells in the normal and glaucomatous primate retina. Invest Ophthalmol Vis Sci. 2005;46:3197–3207. doi: 10.1167/iovs.04-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nesher R, Trick GL, Kass MA, Gordon MO. Steady-state pattern electroretinogram following long term unilateral administration of timolol to ocular hypertensive subjects. Doc Ophthalmol. 1990;75:101–109. doi: 10.1007/BF00146546. [DOI] [PubMed] [Google Scholar]
- 13.Falsini B, Colotto A, Porciatti V, Bolzani R, Porrello G, Giudiceandrea A. Follow-up study with Pattern ERG in ocular hypertension and glaucoma patients under timolol maleate treatment. Clin Vision Sci. 1992;7:341–347. [Google Scholar]
- 14.Colotto A, Falsini B, Salgarello T, Buzzonetti L, Cermola S, Porrello G. Transiently raised intraocular pressure reveals pattern electroretinogram losses in ocular hypertension. Invest Ophthalmol Vis Sci. 1996;37:2663–2670. [PubMed] [Google Scholar]
- 15.Ventura LM, Sorokac N, De Los Santos R, Feuer WJ, Porciatti V. The relationship between retinal ganglion cell function and retinal nerve fiber thickness in early glaucoma. Invest Ophthalmol Vis Sci. 2006;47:3904–3911. doi: 10.1167/iovs.06-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salgarello T, Falsini B, Stifano G, et al. Morpho-functional follow-up of the optic nerve in treated ocular hypertension: disc morphometry and steady-state pattern electroretinogram. Curr Eye Res. 2008;33:709–721. doi: 10.1080/02713680802277692. [DOI] [PubMed] [Google Scholar]
- 17.Sehi M, Grewal DS, Goodkin ML, Greenfield DS. Reversal of retinal ganglion cell dysfunction after surgical reduction of intraocular pressure. Ophthalmology. 2010;117:2329–2336. doi: 10.1016/j.ophtha.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 18.Saleh M, Nagaraju M, Porciatti V. Longitudinal Evaluation of Retinal Ganglion Cell Function and IOP in the DBA/2J Mouse Model of Glaucoma. Invest Ophthalmol Vis Sci. 2007;48:4564–4572. doi: 10.1167/iovs.07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaraju M, Saleh M, Porciatti V. IOP-Dependent Retinal Ganglion Cell Dysfunction in Glaucomatous DBA/2J Mice. Invest Ophthalmol Vis Sci. 2007;48:4573–4579. doi: 10.1167/iovs.07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porciatti V, Nagaraju M. Head-up tilt lowers IOP and improves RGC dysfunction in glaucomatous DBA/2J mice. Exp Eye Res. 2010;90:452–460. doi: 10.1016/j.exer.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kothe AC, Lovasik JV. A parametric evaluation of retinal vascular perfusion pressure and visual neural function in man. Electroencephalogr Clin Neurophysiol. 1990;75:185–199. doi: 10.1016/0013-4694(90)90172-g. [DOI] [PubMed] [Google Scholar]
- 22.Kremmer S, Tolksdorf-Kremmer A, Stodtmeister R. Simultaneous registration of VECP and pattern ERG during artificially raised intraocular pressure. Ophthalmologica. 1995;209:233–241. doi: 10.1159/000310622. [DOI] [PubMed] [Google Scholar]
- 23.Krieglstein GK, Waller WK, Leydhecker W. The vascular basis of the positional influence of the intraocular pressure. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978;206:99–106. doi: 10.1007/BF00414618. [DOI] [PubMed] [Google Scholar]
- 24.Weinreb RN, Cook J, Friberg TR. Effect of inverted body position on intraocular pressure. Am J Ophthalmol. 1984;98:784–787. doi: 10.1016/0002-9394(84)90698-6. [DOI] [PubMed] [Google Scholar]
- 25.Friberg TR, Sanborn G, Weinreb RN. Intraocular and episcleral venous pressure increase during inverted posture. Am J Ophthalmol. 1987;103:523–526. doi: 10.1016/s0002-9394(14)74275-8. [DOI] [PubMed] [Google Scholar]
- 26.Linder BJ, Trick GL, Wolf ML. Altering body position affects intraocular pressure and visual function. Invest Ophthalmol Vis Sci. 1988;29:1492–1497. [PubMed] [Google Scholar]
- 27.Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–583. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 28.Pakter HM, Schuman J, Hertzmark E, et al. Optical coherence tomography of the retinal fiber layer with comparison to heidelberg retina tomography optic head measurements in normal and glaucomatous eyes. In: Lemij HG, Schuman JS, editors. The shape of glaucoma: Quantitative Neural Imaging Techniques. The Hague: Kugler Publishers; 2000. pp. 149–181. [Google Scholar]
- 29.Hidajat R, Mclay J, Burley C, Elder M, Morton J, Goode D. Influence of axial length od normal eyes on PERG. Doc Ophthalmol. 2003;107:195–200. doi: 10.1023/a:1026282425885. [DOI] [PubMed] [Google Scholar]
- 30.Oner A, Gumus K, Arda H, Karakucuk S, Mirza E. Pattern electroretinographic recordings in eyes with myopia. Eye Contact Lens. 2009;35:238–241. doi: 10.1097/ICL.0b013e3181b343d9. [DOI] [PubMed] [Google Scholar]
- 31.Liu JH, Gokhale PA, Loving RT, Kripke DF, Weinreb RN. Laboratory assessment of diurnal and nocturnal ocular perfusion pressures in humans. J Ocul Pharmacol Ther. 2003;19:291–297. doi: 10.1089/108076803322279354. [DOI] [PubMed] [Google Scholar]
- 32.Liu JH, Kripke DF, Twa MD, et al. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40:2912–2917. [PubMed] [Google Scholar]
- 33.Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44:1586–1590. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- 34.Katkov VE, Chestukhin VV, Lapteva RI, et al. Central and cerebral hemodynamics and metabolism of the healthy man during head-down tilting. Aviat Space Environ Med. 1979;50:147–153. [PubMed] [Google Scholar]
- 35.Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD. Effects of head-down-tilt bed rest on cerebral hemodynamics during orthostatic stress. J Appl Physiol. 1997;83:2139–2145. doi: 10.1152/jappl.1997.83.6.2139. [DOI] [PubMed] [Google Scholar]
- 36.Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. 2008;53 (Suppl 1):S107–120. doi: 10.1016/j.survophthal.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guthoff R, Piechnick J, Jorgensen J. A new device for the measurement of episcleral venous pressure--its clinical use in the evaluation of aqueous humor circulation under physiological and pathological conditions. Fortschr Ophthalmol. 1988;85:158–160. [PubMed] [Google Scholar]
- 38.Porciatti V, Ventura LM. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology. 2004;111:161–168. doi: 10.1016/j.ophtha.2003.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang A, Swanson WH. A new pattern electroretinogram paradigm evaluated in terms of user friendliness and agreement with perimetry. Ophthalmology. 2007;114:671–679. doi: 10.1016/j.ophtha.2006.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fredette MJ, Anderson DR, Porciatti V, Feuer W. Reproducibility of pattern electroretinogram in glaucoma patients with a range of severity of disease with the new glaucoma paradigm. Ophthalmology. 2008;115:957–963. doi: 10.1016/j.ophtha.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowd C, Tafreshi A, Vizzeri G, Zangwill LM, Sample PA, Weinreb RN. Repeatability of pattern electroretinogram measurements using a new paradigm optimized for glaucoma detection. J Glaucoma. 2009;18:437–442. doi: 10.1097/IJG.0b013e31818c6f44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vizzeri G, Tafreshi A, Weinreb RN, Bowd C. Effect of Operator and Optical Defocus on the Variability of Pattern Electroretinogram Optimized for Glaucoma Detection (PERGLA) J Glaucoma. 2010;19:77–82. doi: 10.1097/IJG.0b013e31819f934e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Res. 1983;23:689–699. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- 44.Peachey NS, Seiple WH. Contrast sensitivity of the human pattern electroretinogram. Invest Ophthalmol Vis Sci. 1987;28:151–157. [PubMed] [Google Scholar]
- 45.Muller A, Godenschweger L, Lang GE, Kampmeier J. Prospective comparison of the new indentation tonometer TGdC-01, the non-contact tonometer PT100 and the conventional Goldmann applanation tonometer. Klin Monatsbl Augenheilkd. 2004;221:762–768. doi: 10.1055/s-2004-813566. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Middlemiss C, Bulsara M, et al. Telemedicine-friendly, portable tonometers: an evaluation for intraocular pressure screening. Clin Experiment Ophthalmol. 2006;34:666–670. doi: 10.1111/j.1442-9071.2006.01304.x. [DOI] [PubMed] [Google Scholar]
- 47.Sayegh FN, Weigelin E. Functional ophthalmodynamometry. Comparison between brachial and ophthalmic blood pressure in sitting and supine position. Angiology. 1983;34:176–182. doi: 10.1177/000331978303400303. [DOI] [PubMed] [Google Scholar]
- 48.Longo A, Geiser MH, Riva CE. Posture changes and subfoveal choroidal blood flow. Invest Ophthalmol Vis Sci. 2004;45:546–551. doi: 10.1167/iovs.03-0757. [DOI] [PubMed] [Google Scholar]
- 49.Sayegh FN, Weigelin E. Functional ophthalmodynamometry. Comparison between dynamometry findings of healthy subjects in sitting and supine positions. Ophthalmologica. 1983;187:196–201. doi: 10.1159/000309326. [DOI] [PubMed] [Google Scholar]
- 50.Kaeser P, Orgul S, Zawinka C, Reinhard G, Flammer J. Influence of change in body position on choroidal blood flow in normal subjects. Br J Ophthalmol. 2005;89:1302–1305. doi: 10.1136/bjo.2005.067884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kothe AC. The effect of posture on intraocular pressure and pulsatile ocular blood flow in normal and glaucomatous eyes. Surv Ophthalmol. 1994;38 (Suppl):S191–197. doi: 10.1016/0039-6257(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 52.Lovasik JV, Kothe AC, Kergoat H. Comparison of noninvasive methods to derive the mean central retinal artery pressure in man. Optom Vis Sci. 1993;70:1005–1011. doi: 10.1097/00006324-199312000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- 54.Shah S, Chatterjee A, Mathai M, et al. Relationship between corneal thickness and measured intraocular pressure in a general ophthalmology clinic. Ophthalmology. 1999;106:2154–2160. doi: 10.1016/S0161-6420(99)90498-0. [DOI] [PubMed] [Google Scholar]
- 55.Lovasik JV, Kothe AC. Ocular refraction with body orientation. Aviat Space Environ Med. 1989;60:321–328. [PubMed] [Google Scholar]
- 56.Savin E, Bailliart O, Checoury A, Bonnin P, Grossin C, Martineaud JP. Influence of posture on middle cerebral artery mean flow velocity in humans. Eur J Appl Physiol Occup Physiol. 1995;71:161–165. doi: 10.1007/BF00854974. [DOI] [PubMed] [Google Scholar]
- 57.Morgan WH, Yu DY, Alder VA, et al. The correlation between cerebrospinal fluid pressure and retrolaminar tissue pressure. Invest Ophthalmol Vis Sci. 1998;39:1419–1428. [PubMed] [Google Scholar]
- 58.Berdahl JP, Allingham RR, Johnson DH. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology. 2008;115:763–768. doi: 10.1016/j.ophtha.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 59.Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: a case-control study. Invest Ophthalmol Vis Sci. 2008;49:5412–5418. doi: 10.1167/iovs.08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friberg TR, Weinreb RN. Ocular manifestations of gravity inversion. Jama. 1985;253:1755–1757. [PubMed] [Google Scholar]
- 61.Downs JC, Roberts MD, Burgoyne CF. Mechanical environment of the optic nerve head in glaucoma. Optom Vis Sci. 2008;85:425–435. doi: 10.1097/OPX.0b013e31817841cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Modeling individual-specific human optic nerve head biomechanics. Part I: IOP-induced deformations and influence of geometry. Biomech Model Mechanobiol. 2008 doi: 10.1007/s10237-008-0120-7. [DOI] [PubMed] [Google Scholar]
- 63.Maingret F, Fosset M, Lesage F, Lazdunski M, Honore E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem. 1999;274:1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- 64.Reyes R, Lauritzen I, Lesage F, Ettaiche M, Fosset M, Lazdunski M. Immunolocalization of the arachidonic acid and mechanosensitive baseline traak potassium channel in the nervous system. Neuroscience. 2000;95:893–901. doi: 10.1016/s0306-4522(99)00484-4. [DOI] [PubMed] [Google Scholar]
- 65.Kalapesi FB, Tan JC, Coroneo MT. Stretch-activated channels: a mini-review. Are stretch-activated channels an ocular barometer? Clin Experiment Ophthalmol. 2005;33:210–217. doi: 10.1111/j.1442-9071.2005.00981.x. [DOI] [PubMed] [Google Scholar]
- 66.Kiuchi T, Motoyama Y, Oshika T. Relationship of progression of visual field damage to postural changes in intraocular pressure in patients with normal-tension glaucoma. Ophthalmology. 2006;113:2150–2155. doi: 10.1016/j.ophtha.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 67.Peli E. The visual effects of head-mounted display (HMD) are not distinguishable from those of desk-top computer display. Vision Research. 1998;38:2053–2066. doi: 10.1016/s0042-6989(97)00397-0. [DOI] [PubMed] [Google Scholar]