Abstract
An indispensable role for oligodendrocytes in the protection of axon function and promotion of neuronal survival is strongly supported by the finding of progressive neuron/axon degeneration in human neurological diseases that affect oligodendrocytes. Imaging and pathological studies of the CNS have shown the presence of neuroaxonal injury in progressive multifocal leukoencephalopathy (PML), a demyelinating disease of the CNS, resulting from destruction of oligodendrocytes upon productive replication of the pathogenic neurotropic polyomavirus JC. Here, we examined the extracellular factors involved in communication between oligodendrocytes and neurons. Culturing cortical neurons with conditioned medium (CM) from rat CG4 oligodendrocytic cells that express the JCV agnoprotein showed that CXCL5/LIX, which is a chemokine closely related to the human CXCL5/ENA78 and CXCL6/GCP-2 chemokines, is essential for neuronal cell survival. We found that in CM from agnoprotein-producing CG-4 cells level of CXC5/LIX is decreased compared to control cells. We also demonstrated that a reduced expression of CXCL5/LIX by CG4 GFP-Agno cells triggered a cascade of signaling events in cortical neurons. Analysis of mitogen-activated protein kinases (MAPK) and glycogen synthase kinase (GSK3) pathways showed that they are involved in mechanisms of neuronal apoptosis in response to the depletion of CXCL5/LIX signaling. These data suggest that agnoprotein-induced dysregulation of chemokine production by oligodendrocytes may contribute to neuronal/axonal injury in the pathogenesis of PML lesions.
Keywords: JCV agnoprotein, chemokine, CXCL5/LIX, neuron, apoptosis
INTRODUCTION
Among demyelinating diseases, progressive multifocal leukoencephalopathy (PML), which is caused by destruction of CNS oligodendrocytes infected with the human polyomavirus JC (JCV), is one of the most prominent because it leads to rapid neurological deterioration and frequently to death (Berger, 2007). Serological studies have shown that JCV infects most people during childhood and seroconversion rates reach 70% in adults (Padgett and Walker, 1973; Kitamura et al., 1990). Latent virus has been detected in the kidneys, lymphoid tissue, and bone marrow of healthy and immunosuppressed individuals without PML (Berger et al., 1987; Yogo et al., 1990; Monaco et al., 1998; Randhawa et al., 2005; Tan et al., 2009). This state of infection when JCV DNA can be detected but expression of JCV proteins cannot be determined is considered as a state of latency, although presence of viral DNA replication below the level of detection cannot be excluded (White and Khalili, 2011). Interestingly, JCV DNA can be found in the brains of individuals whether or not they are affected by PML (Elsner and Dorries, 1992; White et al., 1992; Vago et al., 1996; Caldarelli-Stefano et al., 1999; Tan et al., 2010). However, it is not clear whether JCV first enters the central nervous system during primary infection or later (Tan et al., 2010, White and Khalili, 2011). Reactivation of the latent virus may occur in individuals with impaired immune function, including HIV-1/AIDS (which now accounts for up to 80% of PML cases), lymphoproliferative disorders, malignancies, MS and in organ transplant recipients, resulting in PML. The risk of PML is also increased in patients treated with immunosuppressive drugs and immunomodulators that inhibit migration and adhesion of leukocytes and lymphocytes (Brooks and Walker, 1984; Berger and Houff, 2006; Khalili et al., 2007; Berger, 2011; Carson et al., 2009; Clifford et al., 2010; White and Khalili, 2011).
In most cases of PML, JCV reactivation from latency is observed in the glial cells of the brain, predominantly oligodendrocytes (Hou and Major, 2000). Histology of PML reveals multiple foci of demyelination, occasionally with central necrosis, large astrocytes with bizarre, hyperchromatic nuclei and oligodendrocytes with enlarged nuclei that contain JCV inclusion bodies. Until recently, it was thought that in demyelinated lesions of PML, axon integrity is relatively unaffected. However, pathological studies have demonstrated the presence of dystrophic, transected neurites and axonal loss in both cortical and subcortical PML lesions and involvement of the grey matter (Moll et al., 2008). Neuroaxonal injury in PML is also indicated by substantial decreases in N-acetyl aspartate (NAA) levels, a marker of neuronal viability, as revealed by proton magnetic resonance spectroscopy (1H MRS) (Simmons et al., 1991; Chang et al., 1995, 1997; Simone et al., 1998; Iranzo et al., 1999). Thus it is of interest to investigate the mechanisms by which JCV and its protein products can contribute to neuronal damage.
JCV has a double-stranded circular DNA genome of 5.13 Kb and encodes regulatory proteins large T-antigen, small t-antigen and agnoprotein as well as structural capsid proteins VP1, VP2, and VP3 (Frisque, 2001; Khalili et al., 2005; Johnson, 2010). Interestingly, the expression of the viral regulatory proteins has been detected in CNS glial cells in the absence of active viral replication and PML lesions, suggesting that they may affect host cell functions in subclinical conditions (Del Valle and Khalili, 2010; Tan and Koralnik, 2010).
With regard to the role of JCV in the pathogenesis of PML and the impact of oligodendrocytic infection on neuronal integrity, it is important to note that communication between axons and myelinating glia is a reciprocal process. Stimulatory and inhibitory neuroaxonal signals, including neuregulins, neurotrophins and electrical activity, recruit glial cells to obtain trophic support and myelination (Piaton et al., 2010; Nave, 2010). Conversely, oligodendrocytes modify axonal structure via myelination, influence the formation of nodes of Ranvier, provide trophic support, and control axon extension (Mukhopadhya et al., 1994; Chen et al., 2000; Dupree et al., 2004; Chan et al., 2004; Rasband et al., 2005; Nave and Trapp 2008). It is clear that chemokines (chemoattractant cytokines) are important in these intercellular interactions. Originally described as immuno-inflammatory mediators that regulate leukocyte trafficking in response to inflammation, chemokines have been implicated in the modulation of many important biological processes in brain physiology, including migration of neuronal progenitors in the developing brain, glial proliferation and synaptic activity, and in the pathogenesis of a number of diseases of CNS that are associated with inflammation and neurodegeneration, including MS, AIDS dementia complex and Alzheimer’s disease (Bajetto et al., 2002; Cartier et al., 2005; Miller et al., 2008).
Our studies indicate that the expression of JC viral protein agnoprotein can compromise release of CXCL5/LIX. CXCL5/LIX is a member of murine ELR+ CXC chemokine family and has high sequence similarity to both human epithelial cell-derived neutrophil-activating peptide-78 CXCL5/ENA78 and granulocyte chemoattractant protein-2CXCL6/GCP-2 chemokines (Smith et al., 1997; Rovai et al., 1998). ELR+CXC chemokines contain a glutamic acid–leucine–arginine (ELR) tripeptide motif in their N-terminal domain that has been shown to be essential for receptor binding and neutrophil activation (Clark-Lewis et al., 1993), as well as for stimulation of angiogenesis (Strieter et al., 1995). ELR+CXC chemokines exert their effect on target cells via interaction with the G-protein-coupled transmembrane receptors CXCR1 and CXCR2, which are expressed at high levels by neurons and oligodendrocytes in the various regions of the brain (Horuk et al., 1997; Xia et al., 1997; Nguyen and Stangel, 2001). It has been shown that CXCR2 plays a critical role not only in neutrophil chemotaxis (Cacalano et al., 1994), but also in the recruitment of oligodendrocytes to repair lesions in MS (Omari et al., 2005) and in the positioning of OPCs in developing spinal cord (Tsai et al., 2002).
Human oligodendrocytes are not amenable to tissue culture studies but the study of oligodendrocytic functions has been facilitated by the ability of the rat CG4 progenitor cell line to develop into mature oligodendrocytes (CG4-Ol). This development is impaired and survival of differentiating oligodendrocytes is inhibited when these cells are transduced to ectopically express JCV agnoprotein (Merabova et al., 2008), which is a 71 amino-acid protein that has been shown to be involved in the regulation of many important cellular processes such as cell cycle progression, DNA damage response and DNA repair (Darbinyan et al., 2002, 2004). Here we show that the release of CXCL5/LIX by CG4-Ol is compromised by the expression of the JCV agnoprotein. As a consequence of this, exposure of neurons to medium from agnoprotein-positive CG4-Ol leads to neuronal process disintegration and neuronal death due to CXCL5/LIX depletion. Analysis of signaling pathways in these neurons implicate dysregulation of the MAPK and GSK3 pathways in the induction of apoptosis. These observations provide important information about possible mechanisms of neuronal/axonal injury associated with JCV infection.
RESULTS
Rat cortical neurons exposed to conditioned medium (CM) obtained from CG4-Ol constitutively expressing JCV agnoprotein undergo structural alterations
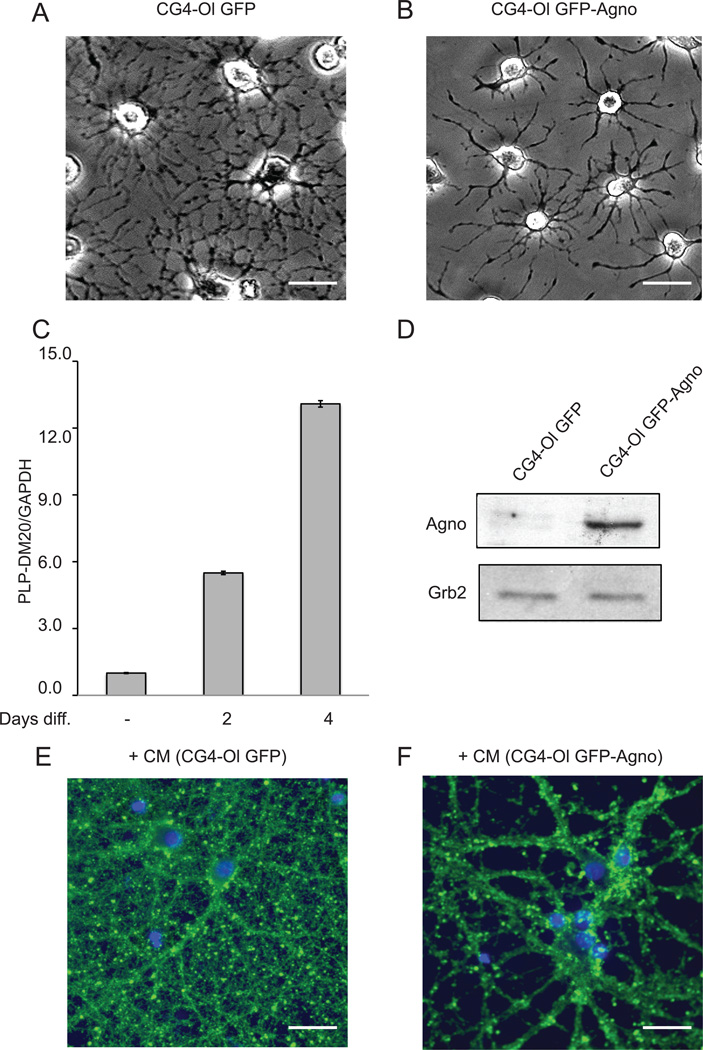
First, we examined the effect of treatment of primary cortical neurons with CM from CG4-Ol constitutively expressing JCV agnoprotein (Fig. 1). CG4, a bipotential cell line that is able to differentiate into either oligodendrocytes or astrocytes (Louis et al., 1992), was stably transduced with retroviral vectors expressing either GFP (green fluorescent protein) or GFP-Agno (JCV agnoprotein fused to GFP) as we have previously described (Merabova, 2008). Differentiation of CG4 cells into oligodendrocytic lineage was assessed by quantitative analysis of rat myelin specific PLP and DM-20 genes by quantitative polymerase chain reaction (QPCR) (Fig. 1C). Expression of agnoprotein was verified by Western blot analysis (Fig. 1D). CM from CG4 GFP or GFP-Agno cells (Fig. 1A and 1B), which had been induced to differentiate into oligodendrocyte lineage was collected after four days of differentiation and applied to primary rat cortical neurons isolated from rat embryos at embryonal day 17 (E17). Neurons were fixed after 16 hours of treatment with CM obtained from CG4-Ol and immunolabeled with antibody to class III β-tubulin. Incubation of rat cortical neurons with CG4-Ol GFP-Agno CM resulted in significantly reduced arborization and loss of neuronal processes as evident in cells stained with anti-class III β-tubulin antibody (Fig. 1F) compared to controls, which were treated with CM from agnoprotein-negative CG4-Ol cultures (Fig. 1E). Direct treatment of neurons for 16 hours with recombinant agnoprotein demonstrated no neurotoxic effects (data not shown), suggesting that soluble factors present in the CM from cells expressing agnoprotein were responsible for the toxic effects observed with primary cortical neurons.
Figure 1. Structural alterations in rat cortical neurons exposed to CM from CG4-Ol constitutively expressing JCV agnoprotein.
A and B. Phase-contrast images of CG4 cells expressing GFP and GFP-Agno induced to differentiate into oligodendrocyte lineage. Scale bar, 20 µm. C. Quantification of levels of mRNAs for PLP and DM-20 by QPCR. Relative levels of mRNAs from CG4 cells, un-induced and induced to differentiate into oligodendrocytic lineage for 2 or 4 days, were expressed as the ratio to the number of the target gene copies relative to the number of reference gene glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) copies and was referred as arbitrary units. D. Immunoblot analysis demonstrating the presence of GFP-agnoprotein using an antibody against agnoprotein. The position of the GFP-Agnoprotein band (35 kDa) is indicated by an arrow. Grb2 serves as a loading control (low panel). E and F. Rat cortical neurons isolated from rat embryos (E17) were incubated with CM from CG4-Ol cells expressing GFP or GFP-Agno. After 16 hours of incubation, neurons were fixed and immunolabeled with antibody to class III β-tubulin (green fluorescence). Nuclei were counterstained with DAPI (blue). Scale bar, 20 µm.
CM from agnoprotein-producing CG4-Ol contains reduced amount of CXCL5/LIX chemokine
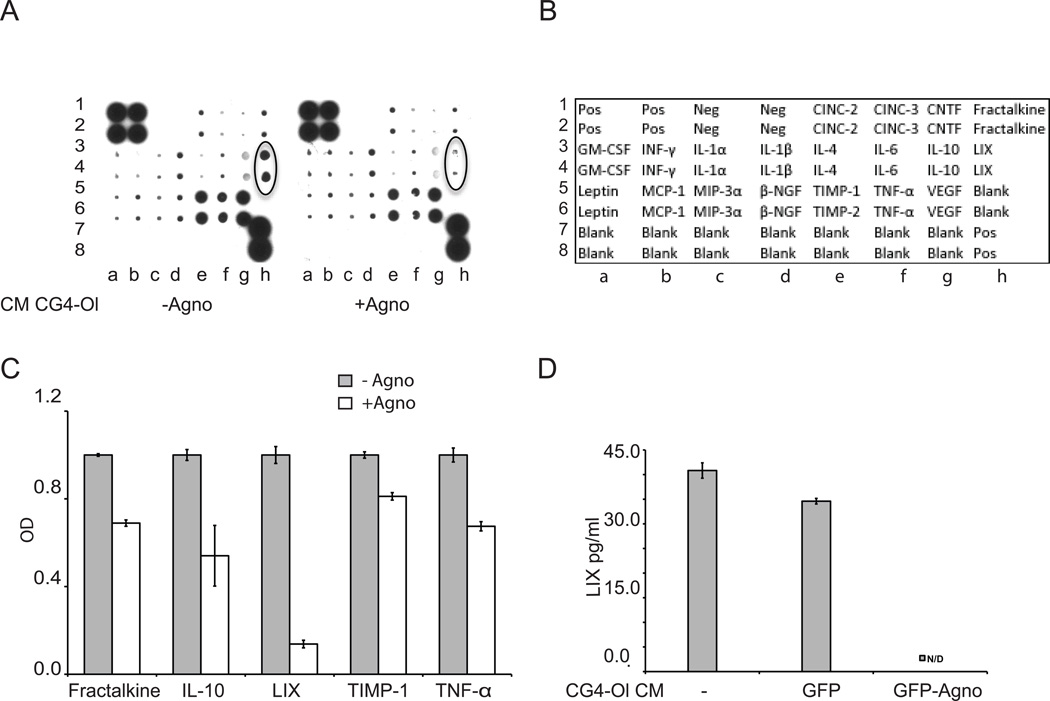
In order to identify factors involved in the observed changes in neurons, CM collected from agnoprotein-positive and agnoprotein-negative CG4-Ol were analyzed using Cytokine antibody array (RayBiotech, Inc.) for a panel of cytokines/chemokines (Fig. 2A). The positions of cytokines on the membrane are shown in the array map provided by the manufacturer (Fig. 2B). Densitometry of signal intensities was performed to quantify the differences in cytokines present in CM. Each signal was normalized to background, and the relative expression levels of cytokines/chemokines are shown (Fig. 2C). The levels of several chemokines in CM from agnoprotein-positive cells were affected including Fractalkine, IL-10, TNF-α, and TIMP-1. Of note, the most prominent decrease was found in the level of CXCL5/LIX (10 fold). Decreased release of CXCL5/LIX in CM from cells expressing GFP-Agno was also confirmed by CXCL5/LIX ELISA assay (RayBiotech, Inc.) (Fig. 2D). We conclude that the loss of neuronal processes treated with CM from agnoprotein-expressing CG4-Ol is associated with a reduced extracellular level of CXCL5/LIX.
Figure 2. Comparison of CM from agnoprotein-expressing CG4-Ol to CG4-Ol control for levels of the CXCL5/LIX chemokine.
CM collected from agnopositive and agnonegative CG4 cells at the 4th day of differentiation into oligodendrocytic lineage was analyzed by cytokine antibody array (A) and quantified (C). Positions of the cytokines on the membrane is depicted on cytokine array map provided by the Manufacturer (B). CXCL5/LIX levels in the supernatants of CG4 cells, CG4 GFP and CG4 GFP-Agno cells at the 4th day of differentiation as measured by ELISA ND - below detectable level. (D). Experiments were repeated three times.
CXCL5/LIX is essential for survival of rat cortical neurons
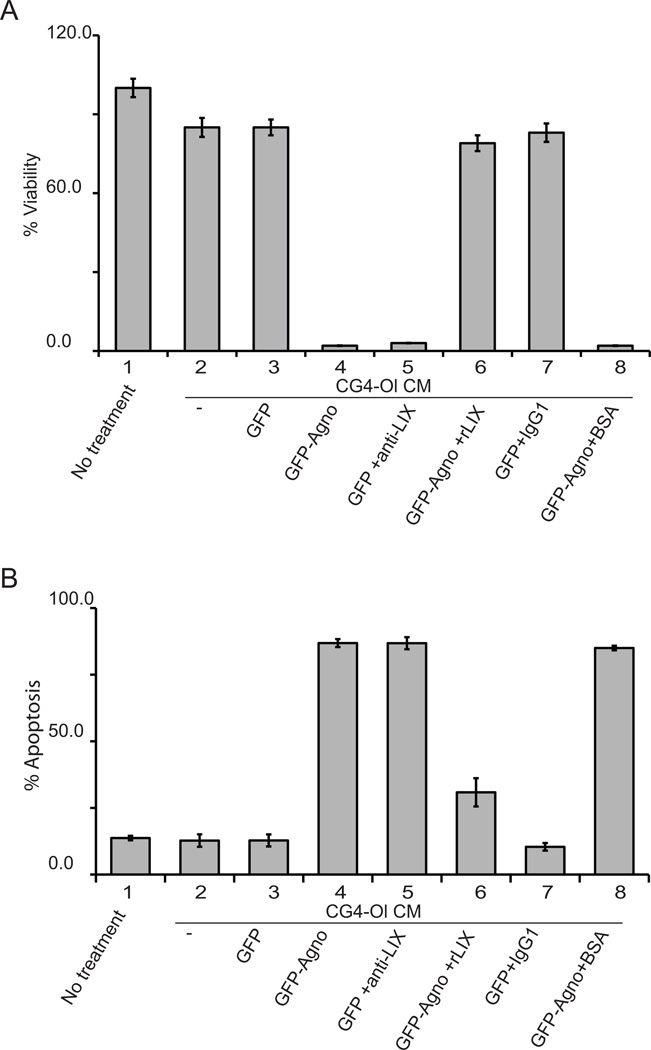
The viability of neurons that have been exposed to oligodendrocyte CG4-Ol CM with normal and reduced levels of CXCL5/LIX was examined using the MTT assay as we have previously described (Merabova et al, 2008). Rat cortical neurons were plated on 6-well plates in triplicate and treated with CM from CG4-Ol and in the presence of neutralizing anti-LIX antibody or purified recombinant LIX protein (rLIX). Bovine serum albumin (BSA) and IgG1 were used as protein and antibody controls, respectively. Neuronal viability in cultures treated with neutralizing anti-LIX antibody or rLIX was evaluated after 16 hours of treatment using MTT assay. Relative cell viability (expressed as a percentage of control) for each sample was determined as the ratio of average absorbance for each sample to cells in neuronal medium, which is shown as 100% (Fig. 3A, lane 1). The MTT assay shows a decrease in neuronal viability in samples treated with CM from cells expressing agnoprotein (Fig. 3A, lanes 4 and 8) or from control cells cultured in the presence of neutralizing anti-LIX antibody (Fig. 3A, lane 5). Reduced cell viability is CXCL5/LIX dependent, as only a small percentage of neurons are metabolically active when the amount of CXCL5/LIX is low or its function is neutralized by anti-LIX antibody. Incubation of neurons with rLIX before addition of CM from CG4-Ol GFP-Agno reduced the level of neuronal cell death (Fig. 3A lane 6). To ascertain that the observations described above resulted from apoptosis we labeled neurons with Annexin V-PE (a marker that is specific for apoptosis) and Nexin 7-AAD and analyzed the cells by flow cytometry (Fig. 3B). These data demonstrate a dramatic increase in the number of apoptotic cells identified by the presence of both Annexin V-PE and Nexin 7-AAD upon treatment with CM with reduced or neutralized by antibody CXCL5/LIX (Fig. 3B, lanes 4, 5 and 8). Pretreatment of neurons with rLIX significantly augments survival of neurons (Fig. 3B, lane 6). Thus, altered production of CXCL5/LIX by agnoprotein-expressing CG4-Ol oligodendrocytic cells is associated with reduced neuronal survival and induction of apoptosis suggesting that viability of neurons depends on CXCL5/LIX in culture medium.
Figure 3. Neuronal survival after treatment with conditioned medium from agnoprotein-expressing CG4 cells.
A. Analysis of the effect of CXCL5/LIX on the survival of rat cortical neurons in the MTT assay. Rat cortical neurons were treated with CM and cell viability was evaluated after 16 hours. The order of samples is as follows: 1. neuronal culture medium; 2. CG4-Ol CM; 3. CG4-Ol GFP CM; 4. CG4-Ol GFP-Agno CM; 5. CG4-Ol GFP CM + neutralizing anti-LIX antibodies (3 µg/ml); 6. 1h pretreatment with rLIX (100 ng/ml) + CG4-Ol GFP-Agno CM; 7. CG4-Ol GFP CM + IgG1 (3µg/ml); 8. CG4-Ol GFP-Agno CM + BSA (100ng/ml). The relative cell viability (percent) for each sample was determined as the ratio of average absorbance for treated to that for untreated cells (sample 1). B. Effect of CXCL5/ LIX on the apoptosis of rat cortical neurons in the nexin assay. Flow cytometry with Annexin V-PE and Nexin 7-ADD. Rat cortical neurons were treated with CM and cell viability was evaluated after 16 hours and presented as percentage of double-positive cells. Sample order is the same as in panel A.
Activation of apoptotic signaling pathways in rat cortical neurons upon treatment with CM from CG4-Ol with reduced level of CXCL5/LIX
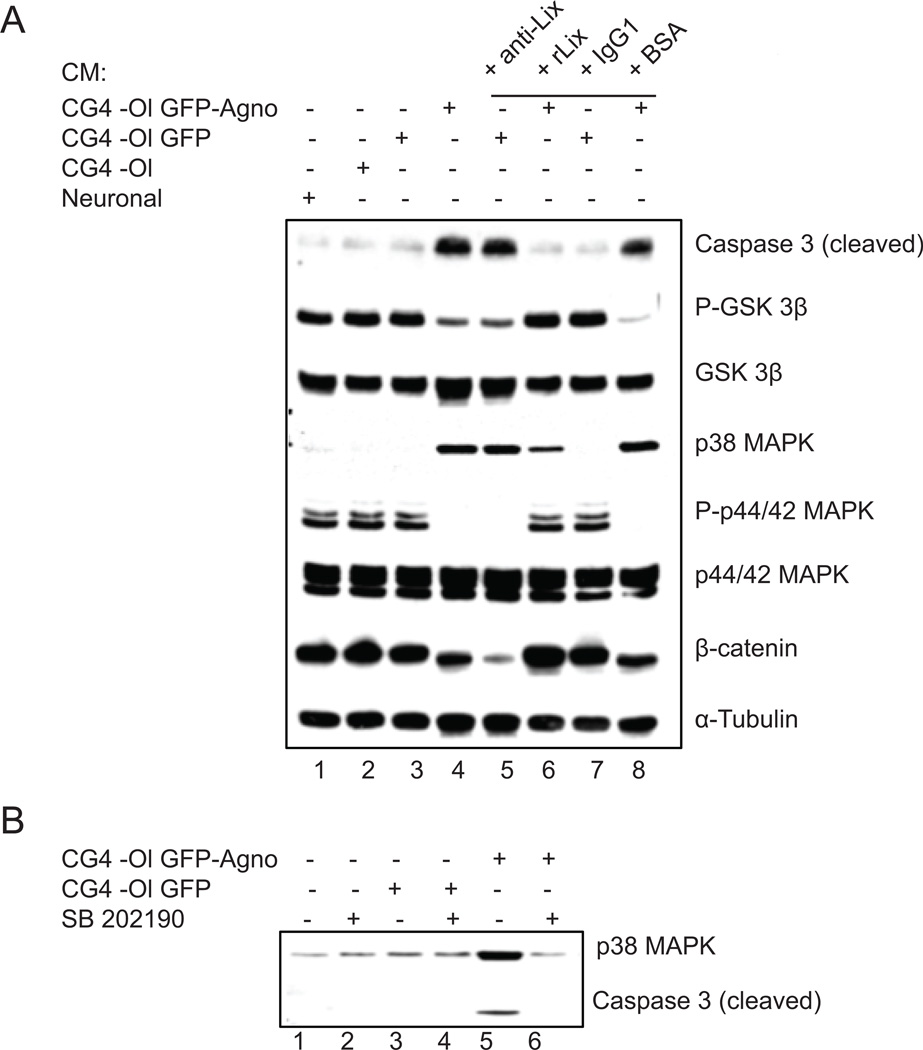
To investigate signaling pathways leading to apoptosis of cortical neurons exposed to CM from CG4-Ol, we examined the expression and phosphorylation of proteins involved in the regulation of cell survival and apoptosis (Fig. 4A). Rat cortical neurons were treated with CM from CG4-Ol as described in Figure 3 and whole-cell protein lysates prepared from neurons were analyzed by Western blotting (Fig. 4A). The final executive step in the process of apoptosis is considered to be activation of caspase-3. We analyzed the level of caspase-3 cleavage and release of the active, cleaved form of the enzyme (17 kDa). Our results indicated activation of caspase-3 in neurons exposed to CM from agnoprotein-expressing cells (Fig. 4A, lanes 4 and 8) or in the presence of CXCL5/LIX neutralizing antibodies (Fig. 4A, lane 5). Further examination of signaling pathways revealed that cleavage of caspase-3 is accompanied by activation of MAPK pathways. MAPKs, including the extracellular signal-regulated kinases (ERK1/2, p44/42), the stress-activated c-Jun N-terminal kinase (JNK), and the 38-kDa high-osmolarity glycerol response kinase (p38 MAPK) are activated in response to a wide range of extracellular stimuli and regulate a large number of physiological processes including cell survival and death (Miloso et al., 2008). Western blot analysis demonstrated that treatment of neurons with CM from CG4-Ol expressing agnoprotein caused phosphorylation (activation) of p38 MAPK expression and reduction of phospho-p44/p42 MAPK (phospho-ERK1/2) without any decrease in the total amount of p44/42 MAPK. Next, we examined GSK3, which is also an important regulator of neuronal survival. GSK3 has two isoforms, GSK3α and GSK3β and is regulated by Akt, which, through phosphorylation of GSK3β at serine 9 and GSK3α at serine 21, inhibits their activity (Nair and Olanow, 2008). Using an antibody that is specific for GSK3β phosphorylated at Ser 9, which is inhibitory, we showed that the level of inactive GSK3β was significantly lower in neurons exposed to CM from agnoprotein-positive cells (Fig. 4A, lanes 4 and 8) or with neutralized CXCL5/LIX (Fig. 4A, lane 5). As a control, the total amount of GSK3β remained unchanged. Next we examined β-catenin, one of the targets for GSK3β, and found that the level of expression of β-catenin is lower in cells where activity of GSK3β is induced (Fig. 4A, lanes 4, 5 and 8). Interestingly, Western blot analysis of proteins prepared from cortical neurons treated with recombinant LIX (rLIX) showed that an excess amount of LIX in culture medium although upregulated the expression of β-catenin in neurons, did not affect the MAPK signaling pathway (data not shown). In addition, we investigated the role of p38 MAPK in apoptotic signaling in neurons exposed to CG4-Ol CM. We found that upregulation of p38 MAPK along with activation of caspase 3 in cortical neurons treated with CM from agnoprotein-positive CG4-Ol (Fig. 4B, lane 6) was reversed by treatment of these neurons with the p38 MAPK inhibitor SB202190 (100 nM), implying that p38 plays a critical role in the induction of apoptosis in cortical neurons treated with CG4 GFP-Agno cells. Thus inhibition of ERK1/2 and concurrent stimulation of p38 MAPK signaling pathways is associated with the induction of apoptosis in neurons.
Figure 4. Effect of reduced level of CXCL5/LIX in CM on pro-survival signal transduction pathways in neurons.
A. Western blot analysis of total lysates prepared from rat cortical neurons treated with: 1. neuronal CM; 2. CG4-Ol CM; 3. CG4-Ol GFP CM; 4. CG4-Ol GFP-Agno CM; 5. CG4-Ol GFP CM + neutralizing anti-LIX antibodies (3 µg/ml); 6. 1h pretreatment with rLIX (100 ng/ml) + CG4-Ol GFP-Agno CM; 7. CG4-Ol GFP CM + IgG1 (3 µg/ml); 8. CG4-Ol GFP-Agno CM + BSA (100 ng/ml). B. Effect of SB 202190 on p38 MAPK, activation of caspase 3 and apoptotic signaling in neurons treated with CM from agnoprotein-expressing cells.
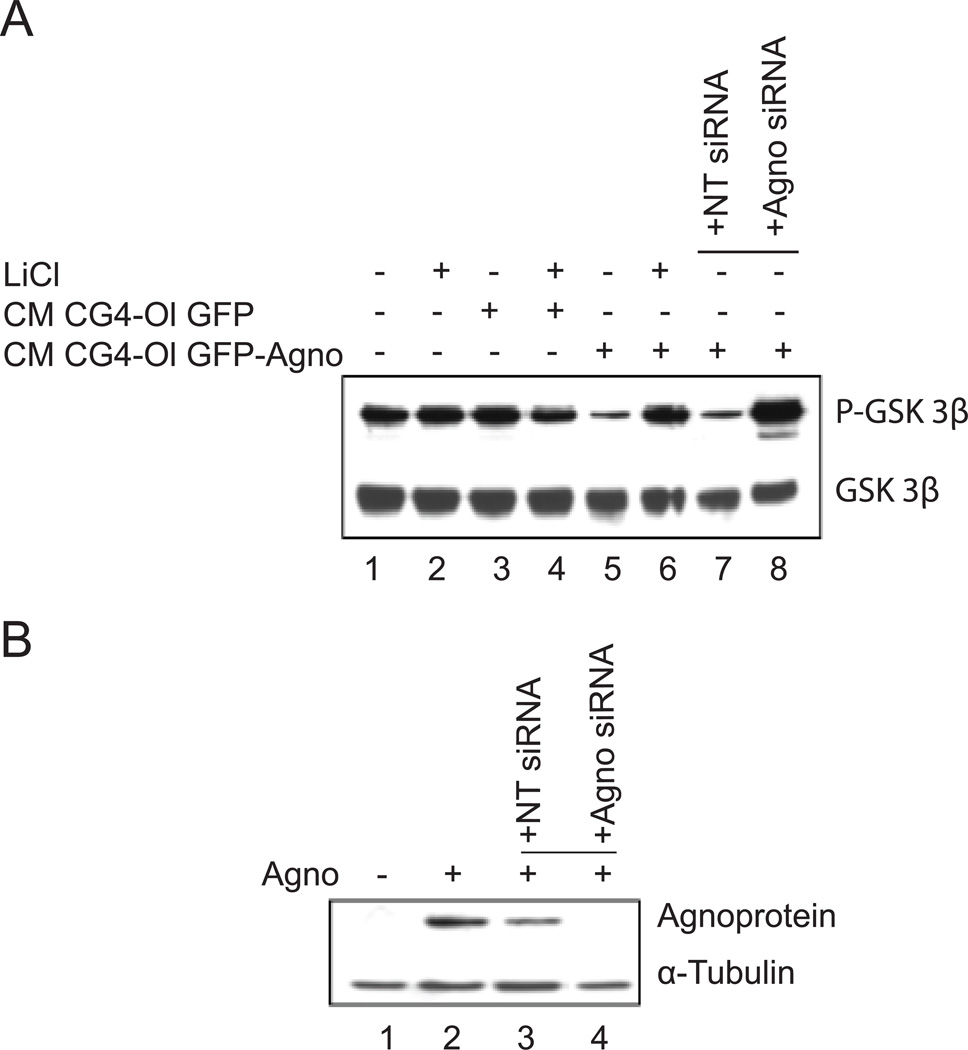
In a separate study, we analyzed the activation of GSK3β pathway in response to treatment with CM from GFP-Agno cells (Fig. 5A). Involvement of the GSK3β pathway in regulation of neuronal cell survival in response to levels of CXCL5/LIX released from oligodendrocytes was further supported by experiments where cortical neurons were incubated for 1 hour with lithium chloride (LiCl) prior to the addition of CG4 CM. LiCl, a therapeutic drug used for the treatment of bipolar mood disorder, is a direct inhibitor of GSK3 (Stambolic et al, 1996; Ryves and Harwood 2001). Expression of inactive GSK3β (phosphorylated at Ser 9) was significantly lower in neurons treated with CG4-Ol GFP-Agno CM (Fig. 5A, lane 4) compared to neurons treated with CM from CG4-Ol GFP (Fig. 5A, lane 2). Addition of LiCl to the CG4-Ol GFP-Agno CM resulted in the pronounced inhibition of GSK3β activity (Figure 5A, lane 5). Interestingly, a similar result was obtained when cortical neurons were treated with CM from CG4-Ol GFP-Agno where agnoprotein expression was silenced by agnoprotein-targeted siRNA (Fig. 5A, lane 8). Incubation of neurons with CM from CG4-Ol GFP-Agno cells treated with a non-targeting siRNA caused no difference in the level of expression of phosphorylation of GSK3β at Ser 9 (Fig. 5A, lane 7). Reduction of agnoprotein expression in CG4 GFP-Agno cells by siRNA was substantial, as measured by Western blot (Fig. 5B). Thus, treatment of neurons with CM containing reduced levels of LIX results in activation of GSK3β signaling.
Figure 5. Effect of agnoprotein on GSK3β activity.
A. Treatment of neurons with CM from CG4 cells with silenced agnoprotein and LiCl, an inhibitor of GSK3. The level of expression of total GSK3β is also shown. B. Western blot for agnoprotein expression in the cells used in Panel A is shown together with α-Tubulin as a loading control.
DISCUSSION
Our data demonstrate that exposure of rat primary cortical neurons to CM obtained from rat OPCs expressing JCV agnoprotein results in reduced viability of neurons. Activation of apoptotic signaling in neurons exposed to CG4-Ol CM was associated with lack of CXCL5/LIX or inhibition of its activity. We have previously shown that JCV agnoprotein affects several important cellular processes (Darbinyan et al., 2002, 2004). Importantly, expression of JCV agnoprotein in bipotential CG4 progenitor cells impairs their ability to develop into mature oligodendrocytes (Merabova et al., 2008). Here we demonstrate that expression of JCV agnoprotein in the absence of other viral proteins in differentiated rat OPCs alters CXCL5/LIX release. Furthermore, our findings show that survival of neurons depends on CXCL5/LIX signaling.
Although several CXCR1/2 ligands have been implicated in neuroprotection (Limatola et al., 2000; Watson and Fan, 2005; Semple et al., 2010; Hosking et al., 2010) and neurotoxicity (De Paola 2007; Valles et al., 2006), there is limited information about the functions of CXCL5/LIX in promoting survival or death signals and its role in neuron-glial communication. Activation of CXCR1 and CXCR2 leads to the formation of distinct second messengers, which activate several signaling pathways through protein kinases and phospholipases (Atta et al. 1999). The interaction of a chemokine with its specific G-protein-coupled receptor triggers a series of signaling events in target cells including activation of protein kinase-C (PKC), Akt/PKB, mitogen-activated protein kinase (MAPK) and Rb protein cascades (Wu et al., 1993; Jones et al., 1995; Ganju et al., 1998, Khan et al., 2008). ERK1/2, JNK and p38 MAPK pathways play central roles in survival signaling and neuronal apoptosis (Harper and LoGrasso 2001). It has been reported that inactivation of ERKs together with the activation of p38 may be critical for apoptosis, and an increase in ERK activity has been correlated to increased neuronal cell survival (Xia et al., 1995). Activation of apoptotic signaling in neurons treated with CM with reduced levels or neutralized CXCL5/LIX was associated with stimulation of expression of p38 MAPK and inhibition of ERK1/2 activity. Our studies on GSK3β demonstrated that in neurons exposed to a low level of CXCL5/LIX, the activity of GSK3β was significantly higher compared to cells treated with CM from control cells. Interestingly, activation of GSK3β induced by treatment of neurons with CM from agnoprotein-expressing cells was reversed by incubation of neurons with LiCl, which has been reported to have a neuroprotective function against a variety of toxic insults (Grimes and Jope, 2001). GSK3 controls diverse signaling pathways in neurons, including Wnt, insulin-like growth factor (IGF-1) and neurotrophic factor signaling pathways and regulates many transcription factors, such as cyclic AMP response element binding protein (CREB), heat shock factor −1 (HSF-1), Myc and β-catenin (Grimes and Jope, 2001). GSK3β also suppresses the activities of the HSF-1 and CREB transcriptional factors, which promote cell survival. In the Wnt signaling pathway, where the main target is β-catenin, active GSK3β phosphorylates β-catenin at the N-terminal region and enhances β-catenin degradation (Yost et al., 1996), thereby preventing the association of β-catenin with nuclear transcription factors and activation of target gene expression such as cyclin D1 and c-myc (He et al., 1998; Galceran et al., 1999; Logan and Nusse, 2004). Of note sequestration of endogenous β-catenin decreases dendritic arborization (Yu and Malenka, 2003). Our studies show that GSK3β/β-catenin signaling is activated in neurons in response to treatment with CM with anti-LIX antibodies or obtained from cells that express agnoprotein, suggesting a role for CXCL5/LIX in stimulation of this pathway.
JCV agnoprotein-induced alterations in chemokine release were associated with pronounced dysregulation of MAPK signaling in neurons leading to cell death. Inhibition of ERK, stimulation of p38 MAPK and GSK3β, followed by activation of caspase 3 may be central mechanisms of neuronal apoptosis in response to reduced levels of CXCL5/LIX. MAPK and GSK3β have been linked to neurodegenerative processes associated with neuronal loss, including Alzheimer’s and Parkinson’s neurodegeneration (Miloso et al., 2008; Grimes and Jope, 2001). We have previously described the activation of apoptotic signaling in JCV agnoprotein-expressing rat oligodendrocyte progenitors upon differentiation into the oligodendrocytic lineage (Merabova et al., 2008). Our earlier studies demonstrated the involvement of agnoprotein in the signaling pathways, which regulate the cell cycle and the DNA damage response (Darbinyan et al., 2002, 2004). Nevertheless, we could not exclude possible autocrine effects of CXCL5/LIX-CXCR2 signaling on oligodendrocytes. The demonstration of the agnoprotein-induced alteration of chemokine expression associated with inhibition of neuronal survival in these studies implies a new role for JCV agnoprotein in the pathogenesis of PML. The identification of mechanisms of neuronal injury associated with JC viral proteins may be instructive for the development of therapeutic neuroprotective strategies. Further studies of the role of JCV agnoprotein in CXCL5 chemokine expression and release are thus warranted.
In related studies, recent findings in CXCR2−/− mice and mice with acute encephalomyelitis resulting from inoculation of the neurotropic JHMV strain of mouse hepatitis virus emphasize the importance of rapid neutrophil recruitment to the CNS in response to viral infection in CNS to enhance host defense and facilitate control of viral replication (Hosking et al, 2009). These studies suggested that ELR+ chemokines contribute to the disruption of the blood brain barrier by recruiting neutrophils and release of proteases and facilitate access of the anti-viral T-lymphocytes to the CNS during viral-induced encephalomyelitis (Hosking et al, 2009). Our observations highlight the importance of further studies on the consequences of alterations of function of CXC chemokines in response to JCV infection in CNS and new aspects for possible strategies for neuroprotection.
MATERIALS AND METHODS
Cell culture
(i) CG4 cells. The CG4 (central glia-4) cell line was maintained as described previously (Louis, 1992). Briefly, cells were propagated on poly-L-ornithine (Sigma, St. Louis, MO)-coated plates in 70% Dulbecco’s minimal essential medium (DMEM) containing 2 mM glutamine, N1 supplement (50 µg/ml transferrin, 5 µg/ml insulin, 100 µM putrescine, 20 nM progesterone, and 30 nM selenium), 10 ng/ml biotin, and 30% conditioned medium from the B104 neuroblastoma cell line. Cells were induced to differentiate into oligodendrocytes by withdrawal of mitogens (without the addition of B104-conditioned medium) under serum-free conditions in the presence of 2 mM L-glutamine, N1 supplement, and insulin for up to 4 days. Withdrawal of the growth factors in the B104-conditioned medium results in the cessation of cell division and the initiation of cell differentiation. (ii) B104 neuroblastoma cells. B104 neuroblastoma cells (Interlab cell line, Genoa, Italy) were grown in DMEM containing 10% (vol/vol) heat-inactivated fetal calf serum (Invitrogen) and 2 mM L-glutamine until 70% confluent and then conditioned with modified Sato medium (see above) for 3 days. (iii) Stable cell lines. Stable cell lines were produced by the retroviral transduction as previously described (Merabova, 2008). Briefly, the Phoenix retroviral packaging cell line (Orbigen, San Diego, CA) was transfected with pLEGFP-C1-Agno, or pLEGFP-C1 plasmids by calcium phosphate precipitation as we have previously described (Darbinyan et al., 2004). Conditioned medium containing virus was collected 48 h posttransfection and used to infect CG4 progenitor cells in the presence of 10 µg/ml Polybrene (Millipore, Billerica, MA). Twenty-four hours posttransduction, cells were subcultured and selected with 700 µg/ml G418 (Invitrogen, Carlsbad, CA). Expression of the transgene was verified by Western blot analysis using anti-agnoprotein antibody or Living Colors full-length polyclonal antibody (BD Biosciences, Clontech), which recognizes enhanced green fluorescent protein (EGFP). (iv) Rat cortical neurons. Primary cultures of rat cortical neurons were prepared from rat embryos (E17) of 17-day pregnant Sprague-Dawley rats as described previously. Cortices were dissected out in dissecting medium (1.6 mM sucrose, 2.2 mM glucose, 1 mM HEPES, 16 mM NaCl, 0.5 mM KCl, 0.1 mM Na2HPO4, and 0.022 mM KH2PO4) and placed in Hibernate E medium (BrainBits, Springfield, IL). After careful removal of the meninges, the intact tissue was incubated with Tryple Express enzyme (Invitrogen, Carlsbad, CA) at 37°C for 10 min, followed by three washes with Hibernate E medium. Tissue trituration was performed in culture medium (see below) using a fire-polished glass Pasteur pipette, and single cell suspension was diluted with culturing medium. Finally, cells were plated on 60 mm poly-D-lysine-coated dishes (Sigma) at a density of 2.5 × 106 cell per 60 mm2 dishes and cultured in Neurobasal medium containing B27 supplement, 0.25 mM Glutamax, and 0.25 mM L-glutamine (all from Invitrogen). Cytosine arabinoside (Ara-C, Sigma) (final concentration 1 µM) was added after 16 hours for two days to reduce glial proliferation. Treatment of neuronal cultures with Ara-C (48 hours) efficiently depletes proliferating cells. At day 10 or 12 of in vitro culturing more than 98% of cells are positive for neuronal marker class III β-tubulin (verified by immunocytochemical analysis).
Treatment of cells with siRNA targeting agnoprotein
Cells were treated with either agnoprotein siRNA or non-targeting siRNA using Oligofectamine according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). The JCV agnoprotein siRNA targeted nt 324 to 342 of the Mad-1 isolate of JCV (sense strand, 5′-AACCUGGAGUGGAACUAAAdTdT-3′), non- targeting siRNA (siGENOME non-targeting siRNA #1) were obtained from Dharmacon (Lafayette, CO) and were used at a final concentration of 100 nM. In all experiments, Western blot analysis for agnoprotein was performed with protein extracts from control cells and cells transfected with agnoprotein-specific siRNA to verify agnoprotein knock-down with anti-α- tubulin antibody used as a loading control.
Preparation of protein extracts and immunoblot analysis
For the preparation of whole-cell extract, cells were lysed for 30 min on ice in LB1 buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1% Triton X-100) containing 1 µg/ml leupeptin, 1 µg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, and 0.2 mM Na-orthovanadate. Cell debris was removed by centrifugation at 14,000 rpm for 15 min at 4°C. The supernatant was assayed for protein content by Bradford analysis (Bio-Rad) and was either used immediately or stored at −80°C. For immunoblots, 50-µg aliquots of total cell protein were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and immunoblotted with antibody. Bound antibody was detected using the ECL enhanced chemiluminescence detection kit (GE Healthcare Life Sciences, Piscataway, NJ) according to the Manufacturer’s recommendations.
Methylthiazoletetrazolium (MTT) assay
The Cell Proliferation Kit I (MTT) was used according to the manufacturer’s protocol (Roche). Neurons were plated onto poly-D-lysine-coated 6-well plates in triplicate in eight sets at a density of 1 × 105 cells/well, cultured 10 days and treated according to the experimental design. After 16 hours, MTT (5 mg/ml) was added to the wells (final concentration, 0.5 mg/ml) for 4 h, and the reaction was stopped by the addition of solubilization solution. The tetrazolium salt MTT is cleaved to form a formazan dye by mitochondrial reductase enzymes that are active only in viable cells and not in dead cells. The amount of formazan generated is directly proportional to the number of metabolically active cells. The spectrophotometrical absorbance of neurons in each condition was measured using a microplate (enzyme-linked immunosorbent assay) reader at 570 nm with a reference wavelength of 650 nm.
Treatment of rat primary neurons with LiCl and p38 MAPK inhibitor
Rat cortical neurons were incubated with LiCl (5 mM final concentration) for 1 hour prior to the addition of CM from CG4 cells. Cortical neurons were pretreated with p38MAPK inhibitor SB202190 (100 nM final concentration) for 1 hour prior to the addition of CM obtained from CG4 cells.
Nexin assay
Rat cortical neurons were plated in duplicate in Neurobasal medium on poly-D-lysine coated 60 mm2 dishes and treated with CM, obtained from CG4 cells producing agnoprotein and control agnoprotein-negative cells at the 4th day of induction to differentiate towards oligodendrocytes. After 16 hours cells were harvested, pelleted by centrifugation and labeled with Annexin V-PE and Nexin 7-AAD according to Guava Nexin™ kit protocol (Guava Technologies, Hayward, CA). Annexin V is a protein with high affinity for phosphatidylserine (PS), a membrane component normally localized to the internal face of the cell membrane. Early in the apoptotic pathway, PS molecules are translocated to the outer surface of the cell membrane where they are bound by Annexin V. The cell impermeant dye, 7-AAD, serves as an indicator of membrane structural integrity. It is excluded from healthy cells and early apoptotic cells, but permeates late stage apoptotic and dead cells. Cells positive for both markers comprising the population of late stage apoptotic cells were identified by flow cytometric analysis.
Flow cytometric analysis
Cells were harvested, rinsed with PBS, and fixed in suspension in 73% ethanol in PBS for at least 16 to 20 h at −20°C. After incubation for 24 h at −20°C, the cells were washed with PBS containing 1% bovine serum albumin, stained with propidium iodide (10 µg/ml) in PBS containing 250 µg of RNase A/ml, and incubated at 37°C for 30 min in the dark before analysis by fluorescence-activated cell sorting. Cell cycle distribution was analyzed with the GuavaEasy Cyte mini system and using the Guava CytoSoft cell cycle program according to the manufacturer’s instructions (Guava). The DNA content determination was based on the intensity of the PI fluorescence.
LIX ELISA
LIX ELISA was performed using RayBio® Rat LIX ELISA kit protocol (RayBiotech, Inc., Norcross, GA). Briefly, CG4 GFP and CG4 GFP-Agno cells were induced to differentiate for 4 days and CM was collected and centrifuged to remove cell debris. Samples and standards were added into appropriate wells of LIX microplate coated with anti-rat LIX antibody and were incubated overnight at room temperature. Wells were washed and biotinylated anti-rat LIX antibody was added to each well. After washing away unbound biotinylated antibody, HRP-conjugated streptavidin was added to the wells. The wells were washed again and TMB substrate solution was applied. Color develops in proportion to the amount of LIX bound. The intensity of the color was measured at 450 nm.
Immunocytochemistry for class III β-tubulin
Primary rat fetal cortical neurons were prepared and seeded on poly-D-lysine (10 µg/ml final concentration) and laminin (5µg/ml final concentration) coated glass chamber slides. After 9 days, cells were incubated with CM from differentiated CG4 GFP and CG4 GFP-Agno cells and fixed with 4% paraformaldehyde. Fixed cells were blocked with 5% BSA in PBS for 1 h and incubated with mouse monoclonal antibody to class III-β-tubulin for 16 hr. Control cells were incubated without primary antibody. Cells were then washed three times with PBS-0.01% Tween-20 and incubated with FITC conjugated anti-mouse secondary antibody for 45 min. Slides were washed three times with PBS, mounted, and examined by fluorescence microscopy. Nuclear DNA was labeled with DAPI. Immunofluorescence was analyzed by fluorescence microscopy.
Reverse transcription (RT) and quantitative real-time polymerase chain reaction (QPCR)
Total RNA was isolated using RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s directions. The RT reaction was performed using random primers (p(dN)6; Roche) and M-MuLV RT enzyme. For the analysis of rat myelin specific PLP and DM-20 genes by QPCR the following primers were used: forward, 5’-ggCCgAgggCTTCTACACCAC-3’, reverse, 5’-CAggAgCCCACTgTggAgCAA-3’ (Milner et al., 1985). For relative quantification, the expression level of genes was normalized to the housekeeping gene glyceraldehyde-3-phosphate-dehydrogenase (GAPDH), and was referred as arbitrary units.
Antibodies and recombinant proteins
Monoclonal anti-rat LIX antibody and mouse IgG1 isotype control antibody were purchased from R&D Systems (R&D Systems, Minneapolis, MN). Rabbit polyclonal antibody against JCV agnoprotein was previously described (Darbinyan et al., 2007). Anti-α-tubulin, clone B512, and anti-β-tubulin isotype III, clone SDL.3D10, were obtained from Sigma-Aldrich (Sigma-Aldrich Inc.). Anti-phospho-glycogen synthase kinase 3β (GSK3β) (Ser9) antibody, rabbit polyclonal (#9336), anti-phospho-p44/42 mitogen-activated protein kinase (MAPK) (Thr202/Tyr204) (#4376), β-catenin (E-5), mouse monoclonal antibody (sc-7963) and anti-caspase-3 rabbit polyclonal (sc-7148) were obtained from Santa Cruz (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Cleaved caspase-3 (Asp175) (# 9661), anti-p44/42 MAPK (#9102) and anti-GSK3β (27C10) rabbit polyclonal antibodies were purchased from Cell Signaling (Cell Signaling Technology, Inc., Danvers, MA). Recombinant rat LIX was purchased from R&D Systems (#543-RL).
ACKNOWLEDGMENTS
We thank past and present members of the Center for Neurovirology and the Department of Neuroscience, in particular Martyn White, for their insightful discussion and sharing of ideas and reagents. We also wish to thank C. Papaleo for editorial assistance. This work was supported by grants awarded by the NIH to KK.
REFERENCES
- 1.Atta UR, Harvey K, Siddiqui RA. Interleukin-8: an autocrine inflammatory mediator. Curr Pharm Des. 1999;5:241–253. [PubMed] [Google Scholar]
- 2.Bajetto A, Bonavia R, Barbero S, Schettini G. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. J. Neurochem. 2002;82:1311–1329. doi: 10.1046/j.1471-4159.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- 3.Berger JR, Kaszovitz B, Post MJ, Dickinson G. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann. Intern Med. 1987;107:78–87. doi: 10.7326/0003-4819-107-1-78. [DOI] [PubMed] [Google Scholar]
- 4.Berger JR, Houff S. Progressive multifocal leukoencephalopathy: lessons from AIDS and natalizumab. Neurol Res. 2006;28:299–305. doi: 10.1179/016164106X98198. [DOI] [PubMed] [Google Scholar]
- 5.Berger JR. Progressive multifocal leukoencephalopathy. Curr Neurol Neurosci Rep. 2007;7:461–469. doi: 10.1007/s11910-007-0072-9. [DOI] [PubMed] [Google Scholar]
- 6.Berger JR. The basis for modeling progressive multifocal leukoencephalopathy pathogenesis. Curr Opin Neurol. 2011;24(3):262–267. doi: 10.1097/WCO.0b013e328346d2a3. [DOI] [PubMed] [Google Scholar]
- 7.Brooks BR, Walker DL. Progressive multifocal leukoencephalopathy. Neurol Clin. 1984;2:299–313. [PubMed] [Google Scholar]
- 8.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1995;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 9.Caldarelli-Stefano R, Vago L, Omodeo-Zorini E, Mediati M, Losciale L, Nebuloni M, Costanzi G, Ferrante P. Detection and typing of JC virus in autopsy brains and extraneural organs of AIDS patients and nonimmunocompromised individuals. J. Neurovirol. 1999;5:125–133. doi: 10.3109/13550289909021994. [DOI] [PubMed] [Google Scholar]
- 10.Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, Laubach J, Bawn SD, Gordon LI, Winter JN, Furman RR, Vose JM, Zelenetz AD, Mamtani R, Raisch DW, Dorshimer GW, Rosen ST, Muro K, Gottardi-Littell NR, Talley RL, Sartor O, Green D, Major EO, Bennett CL. Progressive multifocal leukoencephalopathy following rituximab therapy in HIV negative patients: a report of 57 cases from the Research on Adverse Drug Event and Reports (RADAR) project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, Shooter EM, Barres BA. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004 Jul 22;43(2):183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang L, Ernst T, Tornatore C, Aronow H, Melchor R, Walot I, Singer E, Cornford M. Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology. 1997;48(4):836–845. doi: 10.1212/wnl.48.4.836. [DOI] [PubMed] [Google Scholar]
- 14.Chang L, Miller BL, McBride D, et al. Brain lesions in patients with AIDS: H-1 MR spectroscopy. Radiology. 1995;197:525–531. doi: 10.1148/radiology.197.2.7480706. [DOI] [PubMed] [Google Scholar]
- 15.Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 16.Clark-Lewis I, Dewald B, Geiser T, Moser B, Baggiolini M. Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc Natl Acad Sci U S A. 1993;90(8):3574–3577. doi: 10.1073/pnas.90.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9(4):438–446. doi: 10.1016/S1474-4422(10)70028-4. Review. [DOI] [PubMed] [Google Scholar]
- 18.Darbinyan A, Darbinian N, Safak M, Radhakrishnan S, Giordano A, Khalili K. Evidence for dysregulation of cell cycle by human polyomavirus, JCV, late auxiliary protein. Oncogene. 2002;21(36):5574–5581. doi: 10.1038/sj.onc.1205744. [DOI] [PubMed] [Google Scholar]
- 19.Darbinyan A, Siddiqui KM, Slonina D, Darbinian N, Amini S, White MK, Khalili K. Role of JC virus Agnoprotein in DNA repair. J Virol. 2004;78(16):8593–8600. doi: 10.1128/JVI.78.16.8593-8600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darbinyan A, White MK, Akan S, Radhakrishnan S, Del Valle L, Amini S, Khalili K. Alterations of DNA damage repair pathways resulting from JCV infection. Virology. 2007;364(1):73–86. doi: 10.1016/j.virol.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Paola M, Buanne P, Biordi L, Bertini R, Ghezzi P, Mennini T. Chemokine MIP-2/CXCL2, acting on CXCR2, induces motor neuron death in primary cultures. Neuroimmunomodulation. 2007;14(6):310–316. doi: 10.1159/000123834. [DOI] [PubMed] [Google Scholar]
- 22.Del Valle L, Khalili K. Detection of human polyomavirus proteins, T-antigen and agnoprotein, in human tumor tissue arrays. J Med Virol. 2010;82(5):806–811. doi: 10.1002/jmv.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupree JL, Mason JL, Marcus JR, Stull M, Levinson R, Matsushima GK, Popko B. Oligodendrocytes assist in the maintenance of sodium channel clusters independent of the myelin sheath. Neuron Glia Biol. 2004;1:179–192. doi: 10.1017/S1740925X04000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elsner C, Dorries K. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology. 1992;191:72–80. doi: 10.1016/0042-6822(92)90167-n. [DOI] [PubMed] [Google Scholar]
- 25.Frisque RJ. Structure and function of JC virus T’ proteins. J Neurovirol. 2001;7(4):293–297. doi: 10.1080/13550280152537120. Review. [DOI] [PubMed] [Google Scholar]
- 26.Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13(6):13709–13717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J. Biol. Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 28.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 29.Harper SJ, LoGrasso P. Signalling for survival and death in neurones: the role of stress-activated kinases, JNK and p38. Cell Signal. 2001;13:299–310. doi: 10.1016/s0898-6568(01)00148-6. [DOI] [PubMed] [Google Scholar]
- 30.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 31.Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez HD, Kim J, Parker J, Hadley TJ, Peiper SC. Expression of chemokine receptors by subsets of neurons in the central nervous system. J. Immunol. 1997;158:2882–2890. [PubMed] [Google Scholar]
- 32.Hosking MP, Liu L, Ransohoff RM, Lane TE. A protective role for ELR+ chemokines during acute viral encephalomyelitis. PLoS Pathog. 2009;5(11):e1000648. doi: 10.1371/journal.ppat.1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosking MP, Tirotta E, Ransohoff RM, Lane TE. CXCR2 signaling protects oligodendrocytes and restricts demyelination in a mouse model of viral-induced demyelination. PLoS One. 2010;5(6):e11340. doi: 10.1371/journal.pone.0011340. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou J, Major EO. Progressive multifocal leukoencephalopathy: JC virus induced demyelination in the immune compromised host. J. Neurovirol. 2000:6S98–S100. [PubMed] [Google Scholar]
- 35.Iranzo A, Moreno A, Pujol J, Marti-Fabregas J, Domingo P, Molet J, Ris J, Cadafalch J. Proton magnetic resonance spectroscopy pattern of progressive multifocal leukoencephalopathy in AIDS. J Neurol Neurosurg Psychiatry. 1999;66(4):520–523. doi: 10.1136/jnnp.66.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones SA, Moser B, Thelen M. A comparison of post-receptor signal transduction events in Jurkat cells transfected with either IL-8R1 or IL-8R2. Chemokine mediated activation of p42/p44 MAP-kinase (ERK-2), FEBS Lett. 1995;364:211–214. doi: 10.1016/0014-5793(95)00397-r. [DOI] [PubMed] [Google Scholar]
- 37.Johnson EM. Structural evaluation of new human polyomaviruses provides clues to pathobiology. Trends Microbiol. 2010;18(5):215–223. doi: 10.1016/j.tim.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalili K, White MK, Sawa H, Nagashima K, Safak M. The agnoprotein of polyomaviruses: a multifunctional auxiliary protein. J Cell Physiol. 2005;204(1):1–7. doi: 10.1002/jcp.20266. Review. [DOI] [PubMed] [Google Scholar]
- 39.Khalili K, White MK, Lublin F, Ferrante P, Berger JR. Reactivation of JC virus and development of PML in patients with multiple sclerosis. Neurology. 2007;68:985–990. doi: 10.1212/01.wnl.0000257832.38943.2b. [DOI] [PubMed] [Google Scholar]
- 40.Khan MZ, Brandimarti R, Shimizu S, Nicolai J, Crowe E, Meucci O. The chemokine CXCL12 promotes survival of postmitotic neurons by regulating Rb protein. Cell Death Differ. 2008;15(10):1663–1672. doi: 10.1038/cdd.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitamura T, Aso Y, Kuniyoshi N, Hara K, Yogo Y. High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. J. Infect. Dis. 1990;161:1128–1133. doi: 10.1093/infdis/161.6.1128. [DOI] [PubMed] [Google Scholar]
- 42.Limatola C, Ciotti MT, Mercanti D, Vacca F, Ragozzino D, Giovannelli A, Santoni A, Eusebi F, Miledi R. The chemokine growth-related gene product beta protects rat cerebellar granule cells from apoptotic cell death through alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6197–6201. doi: 10.1073/pnas.090105997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 44.Louis JC, Magal E, Muir D, Manthorpe M, Varon S. CG4, a new bipotential glial cell line from rat brain, is capable of differentiating in vitro into either mature oligodendrocytes or type-2 astrocytes. J. Neurosci. Res. 1992;31(1):193–204. doi: 10.1002/jnr.490310125. [DOI] [PubMed] [Google Scholar]
- 45.Merabova N, Kaniowska D, Kaminski R, Deshmane SL, White MK, Amini S, Darbinyan A, Khalili K. JC virus Agnoprotein inhibits in vitro differentiation of oligodendrocytes and promotes apoptosis. J Virol. 2008;82:1558–1569. doi: 10.1128/JVI.01680-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller RJ, Rostene W, Apartis E, Banisadr G, Biber K, Milligan ED, White FA, Zhang J. Chemokine action in the nervous system. J Neurosci. 2008;28(46):11792–11795. doi: 10.1523/JNEUROSCI.3588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milner RJ, Lai C, Nave KA, Lenoir D, Ogata J, Sutcliffe JG. Nucleotide sequences of two mRNAs for rat brain myelin proteolipid protein. Cell. 1985;42(3):931–939. doi: 10.1016/0092-8674(85)90289-2. [DOI] [PubMed] [Google Scholar]
- 48.Miloso M, Scuteri A, Foudah D, Tredici G. MAPKs as mediators of cell fate determination: an approach to neurodegenerative diseases. Curr Med Chem. 2008;15(6):538–548. doi: 10.2174/092986708783769731. [DOI] [PubMed] [Google Scholar]
- 49.Moll NM, Rietsch AM, Ransohoff AJ, et al. Cortical demyelination in PML and MS: similarities and differences. Neurology. 2008;70:336–343. doi: 10.1212/01.WNL.0000284601.54436.e4. [DOI] [PubMed] [Google Scholar]
- 50.Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol. 1998;72:9918–9923. doi: 10.1128/jvi.72.12.9918-9923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 52.Nair VD, Olanow CW. Differential modulation of Akt/glycogen synthase kinase-3beta pathway regulates apoptotic and cytoprotective signaling responses. J Biol Chem. 2008;283:15469–15478. doi: 10.1074/jbc.M707238200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- 54.Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11(4):275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen D, Stangel M. Expression of the chemokine receptors CXCR1 and CXCR2 in rat oligodendroglial cells. Brain Res. Dev Brain Res. 2001;128:77–81. doi: 10.1016/s0165-3806(01)00128-6. [DOI] [PubMed] [Google Scholar]
- 56.Omari KM, John GR, Sealfon SC, Raine CS. CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain. 2005;128(5):1003–1015. doi: 10.1093/brain/awh479. [DOI] [PubMed] [Google Scholar]
- 57.Padgett BL, Walker DL. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J. Infect Dis. 1973;127:467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- 58.Piaton G, Gould RM, Lubetzki C. Axon-oligodendrocyte interactions during developmental myelination, demyelination and repair. J Neurochem. 2010;114(5):1243–1260. doi: 10.1111/j.1471-4159.2010.06831.x. [DOI] [PubMed] [Google Scholar]
- 59.Randhawa P, Shapiro R, Vats A. Quantitation of DNA of polyomaviruses BK and JC in human kidneys . J. Infect Dis. 2005;192:504–509. doi: 10.1086/431522. [DOI] [PubMed] [Google Scholar]
- 60.Rasband MN, Tayler J, Kaga Y, Yang Y, Lappe-Siefke C, Nave KA, Bansal R. CNP is required for maintenance of axon-glia interactions at nodes of Ranvier in the CNS. Glia. 2005;50(1):86–90. doi: 10.1002/glia.20165. [DOI] [PubMed] [Google Scholar]
- 61.Rovai LE, Herschman HR, Smith JB. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J Leukoc Biol. 1998;64(4):494–502. doi: 10.1002/jlb.64.4.494. [DOI] [PubMed] [Google Scholar]
- 62.Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 63.Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab. 2010;30(3):459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simmons ML, Frondoza CG, Coyle JT. Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience. 1991;45(1):37–45. doi: 10.1016/0306-4522(91)90101-s. [DOI] [PubMed] [Google Scholar]
- 65.Simone IL, Federico F, Tortorella C, et al. Localised 1H-MR spectroscopy for metabolic characterisation of diffuse and focal brain lesions in patients infected with HIV. J Neurol Neurosurg Psychiatry. 1998;64:516–523. doi: 10.1136/jnnp.64.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith JB, Rovai LE, Herschman HR. Sequence similarities of a subgroup of CXC chemokines related to murine LIX: implications for the interpretation of evolutionary relationships among chemokines. J Leukoc Biol. 1997;62(5):598–603. doi: 10.1002/jlb.62.5.598. [DOI] [PubMed] [Google Scholar]
- 67.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signaling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 68.Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, Van Damme J, Kunkel SL. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. 1995;270(45):27348–27357. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- 69.Tan CS, Dezube BJ, Bhargava P, Autissier P, Wuthrich C, Miller J, Koralnik IJ. Detection of JC virus DNA and proteins in the bone marrow of HIV-positive and HIV-negative patients: implications for viral latency and neurotropic transformation. J. Infect Dis. 2009;199:881–888. doi: 10.1086/597117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan CS, Ellis LC, Wüthrich C, Ngo L, Broge TA, Jr, Saint-Aubyn J, Miller JS, Koralnik IJ. JC virus latency in the brain and extraneural organs of patients with and without progressive multifocal leukoencephalopathy. J Virol. 2010 Sep;84(18):9200–9209. doi: 10.1128/JVI.00609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai HH, Frost E, To V, Robinson S, Ffrench-Constant C, Geertman R, Ransohoff RM, Miller RH. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration. Cell. 2002;110:373–383. doi: 10.1016/s0092-8674(02)00838-3. [DOI] [PubMed] [Google Scholar]
- 73.Vago L, Nebuloni M, Sala E, Cinque P, Bonetto S, Isella A, Ottoni L, Crociati A, Costanzi G. JCV-DNA and BKV-DNA in the CNS tissue and CSF of AIDS patients and normal subjects. Study of 41 cases and review of the literature. J. Acquir. Immune Defic. Syndr. Hum Retrovirol. 1996;12:139–146. doi: 10.1097/00042560-199606010-00006. [DOI] [PubMed] [Google Scholar]
- 74.Vallès A, Grijpink-Ongering L, de Bree FM, Tuinstra T, Ronken E. Differential regulation of the CXCR2 chemokine network in rat brain trauma: Implications for neuroimmune interactions and neuronal survival. Neurobiol Dis. 2006;22(2):312–22. doi: 10.1016/j.nbd.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 75.Watson K, Fan GH. Macrophage inflammatory protein 2 inhibits beta-amyloid peptide (1–42)-mediated hippocampal neuronal apoptosis through activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Mol Pharmacol. 2005;67:757–765. doi: 10.1124/mol.104.004812. [DOI] [PubMed] [Google Scholar]
- 76.White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy - revisited. J Infect Dis. 2011;203(5):578–86. doi: 10.1093/infdis/jiq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.White FA, Ishaq M, Stoner GL, Frisque RJ. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J. Virol. 1992;66:5726–5734. doi: 10.1128/jvi.66.10.5726-5734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu D, LaRosa GJ, Melvin SI. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261:101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- 79.Xia M, Qin S, McNamara M, Mackay C, Hyman BT. Interleukin-8 receptor B immunoreactivity in brain and neuritic plaques of Alzheimer’s disease. Am. J. Pathol. 1997;150:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- 80.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 81.Yogo Y, Kitamura T, Sugimoto C, et al. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990;64:3139–43. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 83.Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat. Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]