SUMMARY
We hypothesized that compared to hydrochlorothiazide (HCTZ), the renin inhibitor aliskiren (ALISK) or amlodipine (AMLO) and their combination reduce albuminuria via reduction in renal inflammation, independent of BP changes. We studied normal and streptozotocin induced diabetic (DM) Sprague-Dawley rats treated for 6 weeks with vehicle, ALISK, HCTZ or AMLO individually and combined and evaluated effects of treatments on BP, urine albumin to creatinine ratio (UACR), renal interstitial fluid (RIF) levels of angiotensin II (Ang II), tumor necrosis factor-alpha (TNFα) and interleukin-6 (IL-6), and renal expression of TNFα, IL-6, transforming growth factor-beta1 (TGF-β1) and nuclear factor-kappa B (NF-κB). There were no differences in BP between treatments. Only ALISK and its combinations reduced RIF Ang II. UACR increased in DM rats and decreased with ALISK alone or combined with HCTZ or AMLO. HCTZ or AMLO individually and combined did not influence UACR. RIF TNFα and IL-6, and the renal expression of TNFα, IL-6, TGF-β1 and NF-κB were increased in DM rats. These renal inflammatory markers were reduced only with ALISK or AMLO individually or combined with other treatments. We conclude that ALISK alone and combined with HCTZ or AMLO reduced albuminuria in diabetes via reduction in renal inflammation, independent of BP changes.
Keywords: Aliskiren, inflammation, cytokines, diabetes, kidney, renin-angiotensin system
INTRODUCTION
The renin-angiotensin system (RAS) activity is increased in diabetes. This pathophysiological change contributes to the development and progression of diabetic nephropathy (1). The pharmacological blockade of the RAS was demonstrated to reduce renal injury in diabetes (2–3). However, although the blockade of the RAS is widely used, diabetic nephropathy remains the major cause leading to end-stage renal disease (4). Increased RAS activity is associated with enhanced renal inflammation in diabetes (5). The transcription factor nuclear factor-kappa B (NF-κB) activity is increased in diabetes that may further induce pro-inflammatory cytokines production in diabetic kidney (6–7). Previous studies from our and other laboratories reported increased renal levels of Ang II in diabetes associated with enhanced production of the inflammatory cytokines tumor necrosis factor-alpha (TNFα), interleukin-1β, interleukin-6 (IL-6), and transforming growth factor-beta 1 (TGF-β1) (8–10).
Aliskiren is a potent and highly specific long-acting inhibitor of renin activity (11). As renin catalyzes the conversion of angiotensinogen to angiotensin I, inhibition of renin by aliskiren promotes a reduced activity of all downstream components of the RAS (12). Aliskiren is an effective drug to reduce blood pressure in hypertension (13). In addition, aliskiren was shown to reduce albuminuria in both clinical (14–15) and experimental studies (12,16–18). However, the mechanisms by which aliskiren protects from renal injury in diabetes are not well understood.
Combined antihypertensive therapy is often required to optimize BP control (19). Recently, the effects of combined aliskiren with hydrochlorothiazide (HCTZ) or the calcium channel blocker amlodipine were explored in hypertensive patients (20–21). In these studies, combined aliskiren and amlodipine was reported to cause greater reduction of blood pressure than each individual treatment. However, the effects of combined therapy of aliskiren with either HCTZ or amlodipine in diabetic kidney disease are unknown.
The current study was performed to evaluate the hypothesis that compared to HCTZ, aliskiren alone or combined with HCTZ, amlodipine or both reduce albuminuria in diabetes via reduction in renal inflammation, independent of their effects on blood pressure.
METHODS
Animal preparation
The University of Virginia Animal Care and Use Committee approved all study protocols. Experiments were conducted in male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) weighing 230 to 260g. Animals were given normal-sodium diet and tap water ad libitum for the whole experiment and a minimum of one week was allowed to adjust to our animal care facility. Rats were randomly allocatedinto nine groups: control (n = 10), diabetes groups receiving vehicle (DM+V; n = 10), aliskiren (DM+ALISK; n = 10), HCTZ (DM+HCTZ, n = 10), amlodipine (DM+AMLO; n = 10), HCTZ and AMLO (DM+HCTZ+AMLO; n = 10), ALISK and HCTZ (DM+ALISK+HCTZ; n = 11), ALISK and AMLO (DM+ALISK+ AMLO; n = 12), and ALISK combined with HCTZ and AMLO (DM+ALISK+ HCTZ+AMLO; n = 11). Diabetes was induced by intraperitoneal injection of 65 mg/kg of streptozotocin (STZ; Sigma-Aldrich, Saint Louis, MO, USA). Normoglycemic ccontrol rats were injected with an equal volume of vehicle (0.9% NaCl). This diabetic rat model is characterized by normal BP and allows for evaluation of direct effects of different treatments on the kidney. Treatments were initiated the day after STZ injection and given for a period of 6 weeks. Aliskiren (Novartis, East Hanover, NJ, USA) was infused at a rate of 10 mg/kg/day via osmotic minipump (models 2ML2 and 2ML4; Alzet, Cupertino, CA, USA). HCTZ and amlodipine were provided by Novartis and administered by oral gavage at a dose of 3 and 10 mg/kg/day, respectively. The controls, and non-aliskiren treated groups were implanted with a sham osmotic minipump containing 0.9% NaCl. For minipump implantation, one day after STZ or vehicle injection, rats were anesthetized with the combination of ketamine (80 mg/kg; I.P.) and xylazine (8 mg/kg; I.P.). The osmotic minipumps were surgically implanted subcutaneously in the subscapular region of all rats. Insulin was not given to any of the animals utilized in this study.
Body weight, blood glucose, 24-h urine measurements, and systolic blood pressure monitoring
Body weight, blood glucose, 24-h urine collections, and systolic blood pressure (SBP) were obtained at baseline and at the end of study. For blood glucose determination, blood was collected from tail vein after overnight fasting and glucose was measured usinga glucometer (Bayer HealthCare, Mishawaka, IN, USA). For urine collections, rats were placed in individual metabolic cages for a period of 24-h. The volume of urine collected was determined gravimetrically and a urine sample was kept at −80°C until assayed. Urinary albumin was determined by using a sensitive rat albumin enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Harbor, MI, USA). Urine creatinine was determined by creatinine assay kit (Cayman). Urinary albumin to creatinine ratio (UACR) was used as a marker for diabetic nephropathy. UACR was calculated and presented as albumin in milligrams divided by creatinine in grams. SBP was measured in non-anesthetized rats using a tail-cuff non-invasive multi channel blood pressure system (IITC Life Sciences, Woodland Hills, CA, USA). The mean values of the recorded SBP were calculated.
In vivo renal interstitial fluid (RIF) collections
To determine the RIF levels of Ang II, TNFα, and IL-6 we constructed a microdialysis probe as previously described (5,10,22). In this technique, substances with a molecular mass > 40,000 Da cannot cross the dialysis membrane but allowing the free passage of smaller molecules. At the end of the study, RIF collections were performed in each animal under sodium pentobarbital anesthesia (50 mg/kg I.P.; Sigma). In this procedure, a dialysis probe was placed in the renal cortex of both kidneys through a midline laparotomy. In brief, a 30-gauge needle was tunneled approximately 1–2 mm from the outer renal surface for about 0.5 cm before it exited by penetrating the capsule again. The tip of the needle was then inserted into one end of the dialysis probe, and the needle was pulled together with the dialysis tube until the dialysis fiber was situated into the renal cortex. To prevent dislodging, the dialysis probe was glued to the surface of the kidney using Vetbond (3M Animal Care Products, Saint Paul, MN, USA). Thereafter, the inflow tube of the dialysis probe was connected to a gas-tight syringe filled with saline. Perfusion was done at a rate of 3 μl/min using an infusion pump. After a 60-min period for stabilization following completion of surgical procedures, the effluent was collected from the outflow tube in non-heparinized plastic tubes over ice through five periods of 60-min each with an amount of around 180μl in each sample. At the end of each experiment, animals were euthanized and kidneys were harvested and weighed. Kidney masses indexes were calculated as kidney weight in miligrams divided by body weight in grams. For histological analyses, a part of one kidney was immersed in Bouin’s fixative solution (Sigma). The remaining kidney tissues were immediately frozen in liquid nitrogen and stored at −80°C until further analysis.
RIF storage and assays
The RIF collections were immediately stored at −80°C until assayed. RIF TNFα and IL-6 were measured using an EIA kit (R&D Systems, Minneapolis, MN, USA). RIF Ang II samples were measured using an Ang II EIA kit (SPI-BIO, France). In this kit, the minimal detectable concentration of Ang II is 1 pg/ml and has cross-reactivity of 100% for Ang II, 4% for Ang I, 36% for Ang III, 33% for Ang 3–8, and <0.01% for Ang 1–7.
Determination of mRNA expression
Quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) was used to determine changes in renal expression of TNFα, IL-6, TGF-β1, and NF-κB mRNAs. RNA (n = 5, each group) was extracted using Trizol (Invitrogen, Carlsbad, CA, USA). Reverse transcription of the RNA was performed by the first strand cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). The PCR was analyzed using SYBR Green Supermix (Bio-Rad). Primer sequences were: TNFα forward, 5′-ACTCCCAGAAAAGCAAGCAA-3′, reverse, 5′-CGAGCAGGAATGAGAAGAGG-3′; IL-6 forward, 5′-CCGGAGAGGAGACTTCACAG-3′, reverse, 5′-ACAGTGCATCATCGCTGTTC-3′; TGF-β1 forward, 5′-ATACGCCTGAGTGGCTGTCT-3′, reverse, 5′-TGGGACTGATCCCATTGATT-3′; NF-κB forward, 5′-CTATAGGGCTCGAGCGGCCGC-3′, reverse, 5′-AACGGAAACGCATGCCCCGC-3′; 18S rRNA forward, 5′-CGAAAGCATTTGCCAAGAAT-3′, reverse, 5′-AGTCGGCATCGTTTATGGTC-3′. RT-PCR was performed using iCycler (Bio-Rad) andthreshold cycle number was determined using iCycler software version 3.0 (Bio-Rad). Reactions were performed in triplicate, and threshold cycle numbers were averaged. The mRNA results for specific target genes were calculated with normalization to 18S rRNA.
Western blot analysis
TGF-β1 rabbit antibody #3711 (Cell Signaling Technology, Danvers, MA, USA) was used in the Western blot. Signal detection was carried out by using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL, USA). The blots were treated using Restore Western Blot Stripping Buffer (Thermo Fisher) according to the manufacturer’s recommendation and followed by reprobing with a monoclonal antibody against β-actin (Sigma). Densitometry evaluation of the bands was done using ImageMaster TotalLab software version 2.0 (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The bands densities of target proteins were normalized to the corresponding density of β-actin. The arbitrary unit of band densities was represented as the expression level.
Renal histopathology and immunohistochemistry
This method was performed as previously described (23–24). After standard procedures, frozen sections were incubated overnight at 4°C with primary antibodies directed against mouse anti-NF-κB p65 monoclonal antibody (1:100 dilution; sc-8008, Santa Cruz Biotechnology, Santa Cruz, CA, USA). In the next day, sections were incubated for 1h with secondary antibody at room temperature. Negative controls were included by omitting the primary antibody. The immunostaining images were captured by light microscopy using a Qimaging Micropublisher 5.0 RTV camera coupled to a Zeiss Axiophot microscopy (Carl Zeiss, Jena, Germany).
Statistical analysis
Comparisons among different treatment groups were assessed by ANOVA followed by a Tukey test for post-hoc comparisons. Data are expressed as mean ± SE. P<0.05 isconsidered statistically significant.
RESULTS
Body weight, blood glucose, urine output, kidney masses indexes, and systolic blood pressure data of all rats at the end of study are presented in Table 1. At baseline there were no significant differences in these parameters between all groups. There was significant reduction in body weight in all diabetic rats. Fasting blood glucose of diabetic rats at baseline was 91 ± 1 mg/dl and increased to 339 ± 4 mg/dl (P<0.001) one week after STZ injection. There were no significant changes in body weight and blood glucose in response to any drug treatment in diabetic rats. Urine volume substantially increased in diabetic rats during the whole period of study. There were no significant differences in SBP levels between normoglycemic controls and DM rats receiving different drug treatments.
TABLE 1.
Body weight (BW), blood glucose (BG), 24-h urine volume (UV), kidney masses indexes (KMI), and systolic blood pressure (SBP) of sham and diabetic rats at the end of study.
| Variable | Sham | DIABETES
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | ALISK | HCTZ | AMLO | HCTZ+AMLO | ALISK+HCTZ | ALISK+AMLO | ALISK+HCTZ+AMLO | ||
| BW (g) | 480±11 | 300±20* | 297±11* | 304±10* | 311±13* | 285±5* | 315±14* | 293±11* | 287±10* |
| BG (mg/dl) | 88±3 | 499±29* | 473±30* | 488±12* | 488±19* | 519±24* | 490±21* | 459±25* | 445±18* |
| UV (ml/d) | 24±3 | 205±16* | 183±10* | 199±14* | 184±9* | 168±10* | 180±17* | 175±10* | 188±8* |
| KMI (mg/g) | 6.3±0.1 | 11.4±0.6* | 11.3±0.4* | 11.1±0.4* | 11.2±0.4* | 11.8±0.6* | 10.3±0.3* | 10.2±0.3* | 10.2±0.4* |
| SBP (mmHg) | 112±1 | 114±1 | 113±2 | 112±1 | 112±1 | 112±1 | 113±1 | 112±1 | 114±1 |
ALISK = aliskiren; HCTZ = hydrochlorothiazide; AMLO = amlodipine. Data are mean ± SEM. P < 0.05 vs. sham.
RIF Ang II in normal and diabetic rats
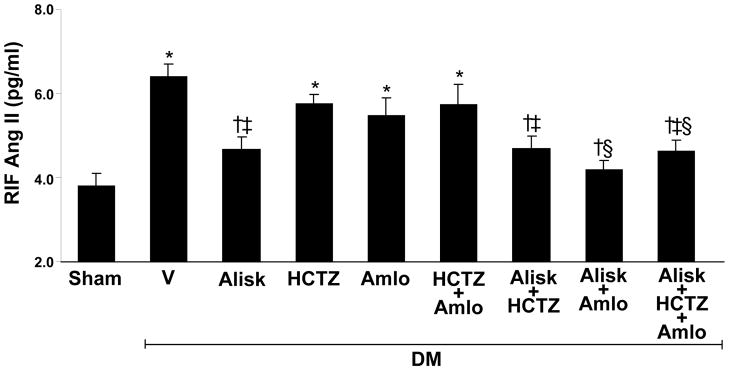
Compared to normoglycemic controls, RIF Ang II recovery levels significantly increased (69%, P<0.001) in DM+V rats (Figure 1). RIF Ang II significantly reduced in DM rats receiving ALISK (−27%, P<0.01), ALISK+HCTZ (−27%, P<0.01), ALISK+AMLO (−35, P<0.001), and ALISK+HCTZ+AMLO (−28%, P<0.001). In contrast, there were no significant differences in RIF Ang II levels between DM+V and DM rats treated with HCTZ, AMLO, or HCTZ+AMLO.
Figure 1.
Renal interstitial fluid (RIF) levels of Ang II in kidneys of sham and streptozotocin-induced diabetic (DM) rats treated with vehicle (V), aliskiren (Alisk), hydrochlorothiazide (HCTZ), amlodipine (Amlo), and their combination. Data are mean ± SEM. *P<0.05 vs. control; †P<0.05 vs. DM+V; ‡P<0.05 vs. DM+HCTZ; §P<0.05 vs. DM+Amlo.
Renal expressions of TNFα, IL-6, TGF-β1, and NF-κB
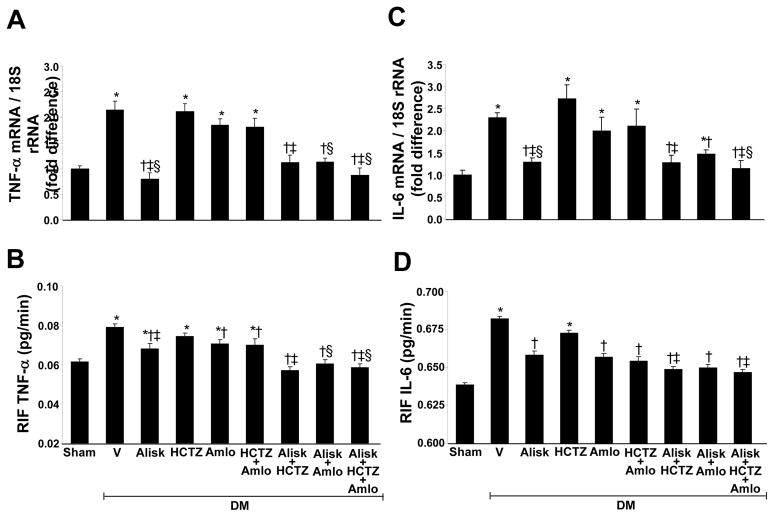
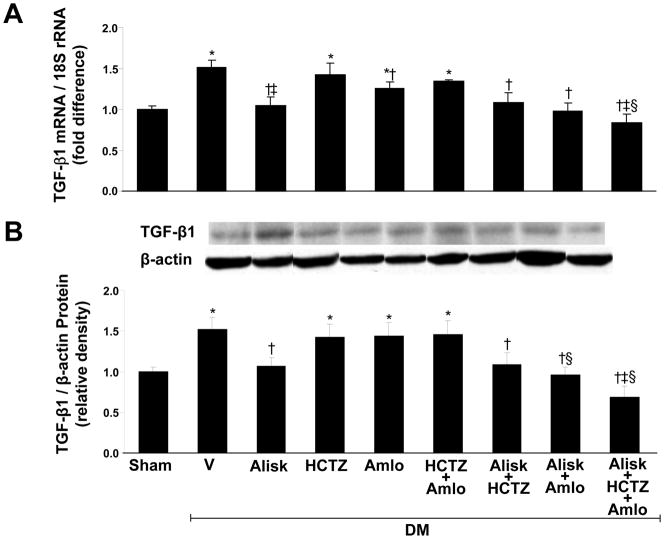
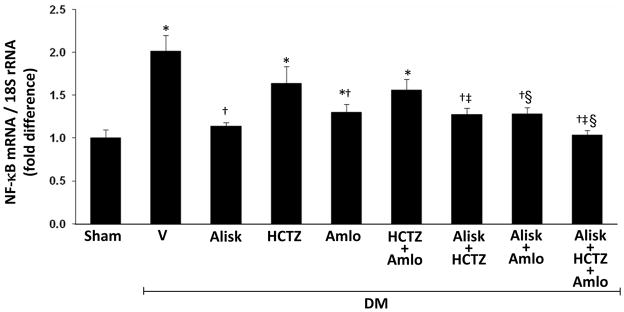
Renal TNFα (Figure 2A) and IL-6 (Figure 2C) mRNA, and the renal expression of TGF-β1 (Figure 3) and NF-κB mRNA (Figure 4) significantly increased in DM+V rats compared to normoglycemic controls. Compared to DM+V, these inflammatory markers were significantly reduced in DM rats treated with ALISK, ALISK+HCTZ, ALISK+AMLO, and ALISK+HCTZ+AMLO. TNFα, IL-6, and NF-κB mRNA did not change in DM rats treated with HCTZ or AMLO individually and combined. TGF-β1 mRNA did not change with HCTZ individually or combined with AMLO and reduced with AMLO individually. TGF-β1 protein did not change with HCTZ or AMLO treatment.
Figure 2.
Renal mRNA expression and renal interstitial fluid (RIF) levels of tumornecrosis factor alpha (TNFα) and interleukine-6 (IL-6) in kidneys of sham and streptozotocin-induced diabetic (DM) rats treated with vehicle (V), aliskiren (Alisk), hydrochlorothiazide (HCTZ), amlodipine (Amlo), and their combination. A and C, mRNA levels of TNFα and IL-6, respectively. B and D, RIF levels of TNFα and IL-6. Data are mean ± SEM. *P<0.05 vs. control; †P<0.05 vs. DM+V; ‡P<0.05 vs. DM+HCTZ; §P<0.05 vs. DM+Amlo.
Figure 3.
Renal expression of transforming growth factor-beta1 (TGF-β1) in kidneys of sham and streptozotocin-induced diabetic (DM) rats treated with vehicle (V), aliskiren (Alisk), hydrochlorothiazide (HCTZ), amlodipine (Amlo), and their combination. A, mRNA levels of TGF-β1. B, Western blot analyzes of TGF-β1. Top, Representative blots. Bottom, Quantitative results normalized to β-actin. n=5, each group. Data are mean ±SEM. *P<0.05 vs. control; †P<0.05 vs. DM+V; ‡P<0.05 vs. DM+HCTZ; §P<0.05 vs. DM+Amlo.
Figure 4.
Renal expression of NF-κB p65 mRNA in kidneys of sham and streptozotocin-induced diabetic (DM) rats treated with vehicle (V), aliskiren (Alisk), hydrochlorothiazide (HCTZ), amlodipine (Amlo), and their combination. n=5, each group. Data are mean ± SEM. *P<0.05 vs. control; †P<0.05 vs. DM+V; ‡P<0.05 vs. DM+HCTZ; §P<0.05 vs. DM+Amlo.
RIF TNFα and IL-6 in normal and diabetic rats
Figures 2B and 2D shows the RIF TNFα and RIF IL-6 of normoglycemic controls and diabetic rats. RIF TNFα and RIF IL-6 were significantly increased in DM+V (P < 0.01) compared to control animals. However, compared to DM+V, both RIF TNFα and RIF IL-6 were significantly reduced in DM rats receiving ALISK (P < 0.01), ALISK+HCTZ (P < 0.001), ALISK+AMLO (P< 0.001), ALISK+HCTZ+AMLO (P < 0.001), AMLO (P < 0.01), and HCTZ+AMLO (P < 0.03). RIF TNFα and RIF IL-6 did not change in DM rats treated with HCTZ.
NF-κB expression in normal and diabetic rat kidneys
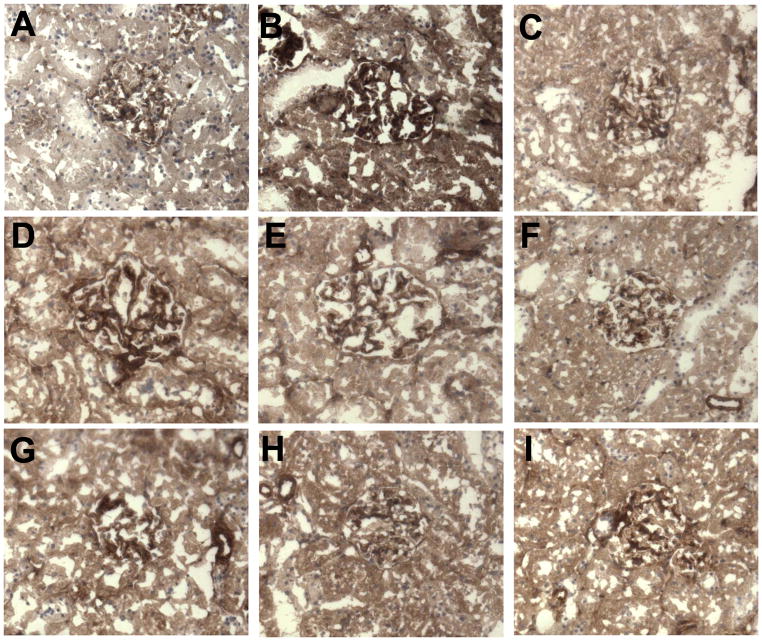
Renal NF-κB mRNA was increased in DM+V rats compared to normoglycemic controls (Figure 4). HCTZ individually or combined with AMLO treatments did not change NF-κB mRNA in diabetic rats. AMLO or ALISK individually and ALISK combined with HCTZ or AMLO significantly reduced NF-κB mRNA in diabetic rats (Figure 4). NF-κB immunostaining was mainly expressed in renal glomeruli. Compared to normoglycemic controls (Figure 5A), renal immunostaining for NF-κB increased in glomeruli of DM+V rats (Figure 5B). Treatment with ALISK individually (Figure 5C) and combined with HCTZ (Figure 5G), AMLO (Figures 5H) or both (Figure 5I) significantly reduced the renal immunostaining of NF-κB. Immunostaining for NF-κB was also reduced in DM rats treated with AMLO (Figure 5D) individually or combined with HCTZ Figure 5E) and was not influenced by HCTZ alone (Figure 5F).
Figure 5.
Representative NF-κB p65 immunostaining of renal glomeruli (brown). (A) Sham, (B–I) Streptozotocin-induced diabetic (DM) rats treated with (B) vehicle (V), (C) aliskiren (Alisk), (D) hydrochlorothiazide (HCTZ), (E) amlodipine (Amlo), (F) HCTZ+Amlo, (G) Alisk+HCTZ, (H) Alisk+Amlo, (I) Alisk+HCTZ+Amlo. NF-κB p65 immunostaining was increased in glomeruli of vehicle treated DM group. It was reduced with aliskiren and amlodipine treatments, and not affected by HCTZ. x200 magnification.
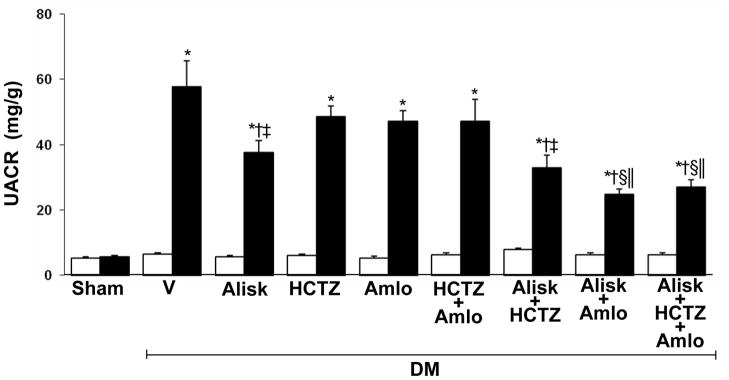
UACR in normal and diabetic rats
Compared to normoglycemic control rats, UACR significantly increased in all diabetic animals (5.5 ± 0.5 vs. 43 ± 5 mg/g; P < 0.001). At the end of study, UACR levels were significantly decreased in DM rats treated with ALISK (−35%, P < 0.04), ALISK+HCTZ (−43%, P < 0.01), ALISK+AMLO (−57%, P < 0.001), and ALIK+HCTZ+ AMLO (−53%, P < 0.01). There were no significant reductions in UACR levels in DM animals treated with HCTZ (−16%) or AMLO (−19%) individually and combined (−18%). Compared to DM+ALISK, UACR levels were significantly reduced in DM rats treated with ALISK+AMLO (−34%, P < 0.01) and ALISK+HCTZ+AMLO (−28%, P < 0.03). Compared to DM+HCTZ, UACR levels were reduced in DM rats treated with ALISK+HCTZ (−32%, P < 0.04) and ALISK+HCTZ+AMLO (−45%, P < 0.001). Similarly, compared to DM+AMLO, UACR levels were reduced in DM rats treated with ALISK+AMLO (−46%, P < 0.001) and ALISK+HCTZ+AMLO (−41%, P < 0.001) (Figure 6).
Figure 6.
Urinary albumin to creatinine ratio (UACR) of sham and streptozotocin-induced diabetic (DM) rats treated with vehicle (V), aliskiren (Alisk), hydrochlorothiazide (HCTZ), amlodipine (Amlo), and their combination at baseline (open bars) and after six weeks of STZ-induction of diabetes and drug treatments (solid bars). Data are mean ± SEM. *P<0.05 vs. control; †P<0.05 vs. DM+V; ‡P<0.05 vs. DM+HCTZ; §P<0.05 vs. DM+Amlo; ||P<0.05 vs. DM+Alisk.
DISCUSSION
The renin inhibitor aliskiren reduces blood pressure and the urine albumin excretion in diabetes (12–13). However, the mechanisms by which aliskiren improves diabetic nephropathy are not fully known. The results of this study demonstrated that aliskiren alone or aliskiren combined with either HCTZ or amlodipine reduced albuminuria in STZ-induced diabetes rats. The renal effects of aliskiren and its combination with HCTZ or amlodipine were associated with reduced production of the renal inflammatory markers TNFα, IL-6 and TGF-β1, and the transcription factor NF-κB. In contrast, HCTZ did not change albuminuria or renal levels of the inflammatory markers. Amlodipine alone also had no effect on albuminuria although it partially reduced renal inflammation. The renoprotective effects of aliskiren alone and combined with HCTZ or amlodipine were independent of blood pressure changes.
In diabetes, the increased activity of RAS plays a crucial role in the pathogenesis of diabetic nephropathy. The blockade of the RAS with angiotensin converting enzyme inhibitors or angiotensin receptor antagonists is widely used in diabetes to slow the progression of diabetic nephropathy (2–3). The renin inhibitor aliskiren was recently demonstrated to reduce renal injury in diabetes. Aliskiren treatment reduced albuminuria in diabetic patients independent of its blood pressure lowering effects (14–15). In addition, aliskiren lowered urinary albumin excretion in transgenic and diabetic rats expressing the mouse renin (16–18). Aliskiren is a highly potent inhibitor of renin ultimately leading to reduction of the RAS activity and blood pressure in hypertension. Our current data demonstrated that aliskiren treatment reduced renal levels of Ang II in STZ-induced diabetes rats. Since these experiments were performed in normotensive animals, blood pressure was not affected by aliskiren treatment.
Increased formation of Ang II enhances the release of renal inflammatory cytokines (25–26). The observed increase in these inflammatory factors is believed to contribute to the development and progression of diabetic nephropathy (27). Previous studies from our and other laboratories reported enhanced production of renal inflammatory cytokines in diabetes (8–10). Our current findings are in agreement with these studies as demonstrated by increased renal production of the inflammatory markers TNFα, IL-6, TGF-β1, and the transcription factor NF-κB in diabetes. We also demonstrated that aliskiren alone and combined with either HCTZ or amlodipine substantially reduced the renal levels of these inflammatory markers. NF-κB promotes the expression of inflammatory cytokines. Increased renal expression of NF-kB in diabetes explains the observed increase in renal TNFα and IL-6. The reduction in NF-κB with aliskiren suggests involvement of RAS in this process (6–7). As aliskiren reduces RAS activity, our findings suggest that the beneficial effects of aliskiren in diabetic nephropathy could be related to its effects in reducing renal NF-κB expression. Previous studies investigating the effects of aliskiren in diabetic nephropathy also reported reduced levels of markers of inflammation and fibrosis with aliskiren (18). These studies were done in transgenic rats expressing mouse renin, a rat model that develops hypertension and severe nephropathy after induction of diabetes. In the present work, blood pressure was not affected by diabetes or by any of the employed pharmacological treatments and suggests that the observed results were independent of blood pressure.
Our results also demonstrated that the increased albuminuria in diabetic rats was attenuated by aliskiren treatment. The albuminuria reduction was superior with combined aliskiren and amlodipine or combined aliskiren and amlodipine and HCTZ than each individual treatment. These data suggest that concomitant administration of aliskiren and amlodipine may potentiate the beneficial effects of aliskiren to diabetic kidney. Although previous studies demonstrated reduction of albuminuria with aliskiren, (16–18) the effects of combined aliskiren and amlodipine on diabetic kidney were not studied. Amlodipine was reported to possess anti-inflammatory properties independent of its blood pressure lowering effects (28–30). The anti-inflammatory effects of amlodipine may have contributed to intensify the reduction of albuminuria in animals receiving aliskiren and amlodipine concomitantly. These data suggest that the beneficial effects of aliskiren in diabetic nephropathy may be potentiated by its combination with amlodipine.
Combined antihypertensive therapy is frequently used in hypertension for optimum blood pressure control. Although our present data did not demonstrate differences in blood pressure between different treatment groups, the beneficial effects of combined aliskiren and amlodipine on blood pressure were recently reported in hypertensive patients (21). In our current study, HCTZ alone did not promote any beneficial effect in diabetic kidney. Interestingly, HCTZ when combined with aliskiren did not reverse the renoprotective effects of aliskiren in diabetes as demonstrated by reduced albuminuria and renal inflammation.
CONCLUSIONS
We conclude that aliskiren treatment reduced albuminuria in STZ-induced diabetes rats via reduction in renal inflammation. The beneficial effects of aliskiren in diabetic nephropathy and renal inflammation were maintained during its combination with HCTZ and were potentiated by concomitant administration of amlodipine. The renoprotective effects of aliskiren alone or combined with HCTZ or amlodipine were independent of blood pressure changes. The results of the present study support the concept that blockade of the RAS, including the inhibition of renin by aliskiren, is an important therapeutic tool to prevent the development of diabetic nephropathy. The effects of aliskiren in reducing albuminuria could be related to its direct effects in reducing renal inflammation in diabetes. The observed synergistic effects of combined aliskiren, HCTZ and amlodipine suggests involvement of multiple mechanisms in development of diabetic kidney disease. Our data suggest that aliskiren either alone or combined with HCTZ or amlodipine may be beneficial in the prevention of renal damage in diabetes.
Acknowledgments
Sources of Funding: This study was supported by grants from Novartis Institutes for Biomedical Research, Inc. and NIH NIDDK-078757 and NIH HL091535 to Helmy M. Siragy.
The authors would like to thank Mr. William Pitkin for his technical assistance.
References
- 1.Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14:274–281. doi: 10.1016/s1043-2760(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 4.KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(Suppl 2):S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Siragy HM, Awad A, Abadir P, Webb R. The angiotensin II type 1 receptor mediates renal interstitial content of tumor necrosis factor-α in diabetic rats. Endocrinology. 2003;144:2229–2233. doi: 10.1210/en.2003-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee FT, Cao Z, Long DM, Panagiotopoulos S, Jerums G, Cooper ME, Forbes JM. Interactions between angiotensin II and NF-kappaB-dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J Am Soc Nephrol. 2004;15:2139–2151. doi: 10.1097/01.ASN.0000135055.61833.A8. [DOI] [PubMed] [Google Scholar]
- 7.Starkey JM, Haidacher SJ, LeJeune WS, Zhang X, Tieu BC, Choudhary S, Brasier AR, Denner LA, Tilton RG. Diabetes-Induced Activation of Canonical and Noncanonical Nuclear Factor-κB Pathways in Renal Cortex. Diabetes. 2006;55:1252–1259. doi: 10.2337/db05-1554. [DOI] [PubMed] [Google Scholar]
- 8.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 9.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. The inflammatory process in type 2 diabetes. The role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 10.Matavelli LC, Huang J, Siragy HM. (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin Exp Pharmacol Physiol. 2010;37:277–282. doi: 10.1111/j.1440-1681.2009.05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood JM, Maibaum J, Rahuel J, Grütter MG, Cohen NC, Rasetti V, Rüger H, Göschke R, Stutz S, Fuhrer W, Schilling W, Rigollier P, Yamaguchi Y, Cumin F, Baum HP, Schnell CR, Herold P, Mah R, Jensen C, O’Brien E, Stanton A, Bedigian MP. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun. 2003;308:698–705. doi: 10.1016/s0006-291x(03)01451-7. [DOI] [PubMed] [Google Scholar]
- 12.Feldman DL. New insights into the renoprotective actions of the renin inhibitor aliskiren in experimental renal disease. Hypertens Res. 2010;33:279–287. doi: 10.1038/hr.2010.19. [DOI] [PubMed] [Google Scholar]
- 13.Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation. 2005;111:1012–1018. doi: 10.1161/01.CIR.0000156466.02908.ED. [DOI] [PubMed] [Google Scholar]
- 14.Persson F, Rossing P, Schjoedt KJ, Juhl T, Tarnow L, Stehouwer CD, Schalkwijk C, Boomsma F, Frandsen E, Parving HH. Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes. Kidney Int. 2008;73:1419–1425. doi: 10.1038/ki.2008.68. [DOI] [PubMed] [Google Scholar]
- 15.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK AVOID Study Investigators. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 16.Pilz B, Shagdarsuren E, Wellner M, Fiebeler A, Dechend R, Gratze P, Meiners S, Feldman DL, Webb RL, Garrelds IM, Jan Danser AH, Luft FC, Muller DN. Aliskiren, a human renin inhibitor, ameliorates cardiac and renal damage in double-transgenic rats. Hypertension. 2005;46:569–576. doi: 10.1161/01.HYP.0000179573.91016.3f. [DOI] [PubMed] [Google Scholar]
- 17.Kelly DJ, Zhang Y, Moe G, Naik G, Gilbert RE. Aliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in rats. Diabetologia. 2007;50:2398–2404. doi: 10.1007/s00125-007-0795-9. [DOI] [PubMed] [Google Scholar]
- 18.Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W, Persohn E, Schuetz H, Jan Danser AH, Nguyen G. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension. 2008;52:130–136. doi: 10.1161/HYPERTENSIONAHA.107.108845. [DOI] [PubMed] [Google Scholar]
- 19.The Seventh Report of the Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 20.Schmieder RE, Philipp T, Guerediaga J, Gorostidi M, Smith B, Weissbach N, Maboudian M, Botha J, van Ingen H. Long-term antihypertensive efficacy and safety of the oral direct renin inhibitor aliskiren: a 12-month randomized, double-blind comparator trial with hydrochlorothiazide. Circulation. 2009;119:417–425. doi: 10.1161/CIRCULATIONAHA.107.750745. [DOI] [PubMed] [Google Scholar]
- 21.Brown MJ, McInnes GT, Papst CC, Zhang J, MacDonald TM. Aliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomised, parallel-group trial. Lancet. 2011;377:312–320. doi: 10.1016/S0140-6736(10)62003-X. [DOI] [PubMed] [Google Scholar]
- 22.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue C, Siragy HM. Local renal aldosterone system and its regulation by salt, diabetes, and angiotensin II type 1 receptor. Hypertension. 2005;46:584–590. doi: 10.1161/01.HYP.0000175814.18550.c0. [DOI] [PubMed] [Google Scholar]
- 24.Siragy HM, Xue C. Local renal aldosterone production induces inflammation and matrix formation in kidneys of diabetic rats. Exp Physiol. 2008;93:817–824. doi: 10.1113/expphysiol.2008.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han Y, Runge MS, Braiser AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-κB transcription factors. Circ Res. 1999;84:695–703. doi: 10.1161/01.res.84.6.695. [DOI] [PubMed] [Google Scholar]
- 26.Granger JP. An emerging role for inflammatory cytokines in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H923–H924. doi: 10.1152/ajpheart.01278.2005. [DOI] [PubMed] [Google Scholar]
- 27.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 28.Chou T-C, Yang S-P, Pei D. Amlodipine inhibits pro-inflammatory cytokines and free radical production and inducible nitric oxide synthase expression in lipopolysaccharide/interferon-[gamma]-stimulated cultured vascular smooth muscle cells. Jpn J Pharmacol. 2002;89:157–163. doi: 10.1254/jjp.89.157. [DOI] [PubMed] [Google Scholar]
- 29.Matsumori A, Ono K, Nishio R, Nose Y, Sasayama S. Amlodipine inhibits the production of cytokines induced by ouabain. Cytokine. 2000;12:294–297. doi: 10.1006/cyto.1999.0555. [DOI] [PubMed] [Google Scholar]
- 30.Siragy HM, Xue C, Webb RL. Beneficial effects of combined benazepril-amlodipine on cardiac nitric oxide, cGMP, and TNFalpha production after cardiac ischemia. J Cardiovasc Pharmacol. 2006;47:636–642. doi: 10.1097/01.fjc.0000211750.01326.b3. [DOI] [PubMed] [Google Scholar]